Abstract
Background
An ever-increasing number of cancer patients are being treated with checkpoint inhibitors such as anti-PD-1 antibodies, and a small percentage of these patients develop a psoriasis-like skin eruption or severe flares of prior psoriasis.
Objective
We investigated the role of obesity in immune checkpoint inhibitors-exacerbated psoriasiform eruption.
Methods
We fed female C57BL/6 mice a so-called Western diet (WD) or a control diet (CD). Imiquimod (IMQ) was applied topically on ears for 5 consecutive days to induce psoriasiform dermatitis (PsD). Psoriasis-related markers were examined by quantitative real-time PCR. Then we induced PsD in WD-and CD-fed mice in the presence or absence of systemic treatment of anti-PD-1 antibodies to examine if obese mice are more susceptible to anti-PD-1 related PsD than lean mice.
Results
WD-fed mice showed higher baseline mRNA expression levels of psoriasis-associated cytokines such as IL-17, S100A8, and S100A9 compared to mice fed with CD. Furthermore, WD-fed mice had more γδ low (GDL) T cells in the whole skin and higher expression of PD-1 on GDLT cells than CD-fed mice. WD-fed mice receiving anti-PD-1 had more prominent ear swelling than lean mice receiving anti-PD-1 during the 5-day IMQ course (2-fold increase, P < 0.0001 on day 5).
Conclusion
WD-induced obesity enhances IMQ-induced psoriasiform inflammation. The finding that WD-fed mice have a more dramatic response to anti-PD-1 than lean mice in terms of IMQ-induced ear swelling suggests that obesity could be a risk factor in the development of psoriasiform eruption during anti-PD-1 therapy.
Keywords: High-fat diet, Immune checkpoint, Psoriasis, Sugar, Western diet
1. Introduction
Immune checkpoint inhibitors such as anti-programmed cell death protein 1 (PD-1) and anti-programmed cell death protein ligand 1 (PD-L1) monoclonal antibodies (collectively termed anti-PD-1 therapy, APD1T) have transformed therapy for recalcitrantor advanced cancers such as melanoma and lung cancer. While treatment with these agents can induce near-to-complete regression of cancers, a growing body of literature has indicated immune-related adverse events (ir-AEs) commonly occur in multiple organs, including skin [1]. These ir-AEs often occur in skin with frequencies reported as high as 38 % in melanoma patients treated with APD1T [2]. Psoriasis-like lesions are one of the common forms of skin ir-AEs [3], and we have shown that PD-1 regulates psoriasiform dermatitis (PsD) in response to imiquimod (IMQ) [4]. Although a history of psoriasis is a risk factor in the development of psoriasis-like lesions with APD1T, nearly 30 % of patients who have psoriasis-like lesions due to APD1T do not have a prior history of psoriasis [5].
Psoriasis is a disease with systemic inflammation and multiple comorbidities [6–11]. Among these comorbidities, obesity is common and oftenprecedes psoriasis [12]. Research has indicated weight reduction improves severity of psoriasis, which implies the systemic inflammation associated with obesity may be pivotal for psoriatic inflammation [13]. In mice, the immune mechanisms underlying skin manifestations of ir-AEs due to APD1T are partly understood to occur through up-regulated expression of PD-1 on activated gd low (GDL) expressing T cells that express interleukin (IL)-17 [4]. PD-L1, which may be up-regulated on activated keratinocytes in certain conditions, presumably binds PD-1on GDL T cells and dampens T cell activation, thus stabilizing or reducing skin inflammation [4]. The immunologic consequences of obesity as well as impact of obesity on immunotherapy or immune-mediated psoriasis-like skin lesions, however, are still very poorly understood. A murine model simulating human obesity would help clarify the interactions among obesity, psoriasis, and APD1T-induced psoriasis-like lesions from an immunologic prospective.
The so-called Western diet (WD) plays a crucial role in the development of obesity in modern societies. WD is characterized by moderate to moderately high levels of fat and high sugars, especially simple sugars such as sucrose [14,15]. In the present study, we utilized a previously established model of WD-induced obesity to examine the effects of diet induced obesity on IMQ-induced PsD [16]. In that model, we showed a WD, but not a high-fat diet, predisposes mice to enhanced susceptibility of PsD, suggesting obesity alone is not sufficient to promote psoriasiform inflammation in the skin. Herein; we confirm that diet-induced obesity predisposes mice to IMQ-induced PsD. Moreover, WD-fed mice express more psoriasis-related mediators and accumulate greater numbers of GDL expressing T cells in the dermis of untreated, otherwise non-inflamed skin. Strikingly, WD-fed mice have higher expression of PD-1on GDL T cells and greater extent of IMQ-induced PsD after anti-PD-1 treatment. Considering that GDL T cells are the main sources and producers of IL-17A [17], these findings provide a mechanistic explanation for how obesity predisposes mice and humans to PsD and how obesity may enhance anti-PD-1induced psoriasiform eruption. A growing body of literature has indicated that PD-1/PD-L1 expression is a prognostic indicator for anti-PD-1 treatment [18]. In other words, if tissues express more PD-1 or PD-L1, there will have more dramatic immune response. As GDL T cells are main cells responsible for PsD in mice, our finding that WD dramatically increasePD-1 expression on GDL T cells highly suggests these mice are more susceptible to anti-PD-1 treatment compared with CD-fed mice that have low level of PD-1.
2. Materials and methods
2.1. Mice and diets
Female C57BL/6 mice were purchased from the Jackson Laboratory (Bar Harbor, ME, USA). Animal protocols were approval by the Institutional Animal Care and Use Committee at the University of California, Davis. WD (21.2 % fat, 17.3 % protein, 46.7% carbohydrate, 34.1 % sucrose, % by weight) and matched control diet (CD; 5.2 % fat, 17.3 % protein, 59.5 % carbohydrate, 12.0% sucrose, % by weight) chow was purchased from Envigo Teklad animal laboratory diets (Madison, WI, USA; catalog numbers, WD: TD.140414, CD: TD.140415) [19–21,37]. Body weight was recorded biweekly.
2.2. IMQ-induced psoriasis model
After feeding WD or CD for 12 weeks, mice were treated on each ear with 10 mg 5 % IMQ cream (Taro Pharmaceuticals, Hawthorne, NY, USA) or vehicle cream (Vanicream, Pharmaceutical Specialties, Cleveland, GA, USA) for 5 consecutive days [22], and ear thickness was measured daily using a Peacock G-1A dial thickness gauge (Ozaki MFG, Tokyo, Japan) [23–25]. For experiments combined with anti-PD-1 monoclonal antibody administration, 3.5 % IMQ cream instead of 5 % IMQ cream was applied to ears of mice for 5 consecutive days [4].
2.3. Anti-PD-1 monoclonal antibody treatment
Anti-PD-1 antibody administration protocol was modified from previous reports [4,26]. Because WD-fed mice had much higher body weight than mice fed with regular chow, we used a higher dosage of anti-PD-1 (300 μg)than previously reported [4]. Briefly, mice received intraperitoneal injections with 300 μg anti-PD-1 (clone Ј43, Bio X Cell, West Lebanon, NH, USA) diluted in PBS or PBS in a total volume of 200 μl 2 h before application of 3.5 % IMQ cream at days 0,2, and 4.3.5% IMQ cream was diluted from 5% IMQ cream (Taro Pharmaceuticals) with control vehicle cream (Vanicream; Pharmaceutical Specialties).
2.4. Histological analysis
Formaldehyde-fixed, paraffin-embedded ear skin samples were stained with hematoxylin and eosin (H&E) using standard procedures. Images were acquired using a microscope Nikon Optiphot 2 (Nikon, Tokyo, Japan). Epidermal thickness was measured with computer-assisted quantitative image analysis software (ImageJ).
2.5. Quantitative real-time PCR
Total RNA of mouse ear total skin was extracted by using an RNeasy Fibrous Tissue Kit (Qiagen, Hilden, Germany). Quantitative real-time PCR was performed using Quant Studio 3 real-time PCR system (Applied Biosystems, Foster City, CA, USA). The following primers were obtained from Integrated DNA Technologies, Inc. (Skokie, IL, USA): Gapdh (Mm.PT.39a.1), Il17a (Mm.PT.58.6531092), Il17f (Mm. PT.58.9739903), Il23 (Mm.PT.58.10594618.g), Il22 (L:5'-CTG CTT CTC ATT GCC CTG TG-3', R:5'-AGC ATA AAG GTG CGG TTG AC-3'), S100a8 (Mm.PT.58.44003402.gs), S100a9 (Mm.PT.58.41787562), Defb4 (Mm.PT.58.29993789), Defb14 (Mm.PT.58.41499310), Cxcl1 (Mm.PT.58.42076891), Cxcl2 (Mm.PT.58.10456839), Ly6g (Mm. PT.58.30498043), Itgam (Cd11b) (Mm.PT.58.14195622), Trpa1 (Mm.PT.56a.32828552), Trpv1 (Mm.PT.58.13426135), Pdcd1 (PD-1) (Mm.PT.58.29141957), Cd274 (Pdl1) (Mm.PT.58.11921659).
2.6. Flow cytometry
Monoclonal antibodies against mouse γδ-TCR (clone: eBioGL3) were purchased from eBioscience (San Diego, CA, USA). Monoclonal antibodies against mouse PD-1 (clone: RMP1–30) and CD45 (clone: 30-F11) were purchased from BioLegend (San Diego, CA, USA). Whole ear skin was minced and digested with Liberase TM (Roche, Mannheim, Germany) and DNase I. Digestion enzymes were quenched by the addition of 4% fetal bovine serum. All tissues were disaggregated by passing through Corning sterile cell strainers 100 μm nylon (Corning Life Science, Corning, NY, USA). Flow cytometry was performed and analyzed using an Accuri C6 (BD Biosciences, San Jose, CA, USA). Mouse ears were harvested on the final day of IMQ 5-day regimen. The cells were pre-gated by CD45. These CD45(+) cells were further identified by their PD-1 and γδ-TCR staining.
2.7. Statistical analysis
All data are expressed as mean ± standard deviation (SD). Data were analyzed using GraphPad Prism version 6 (GraphPad Software, San Diego, CA, USA). For two-group comparisons, a two-sidedStudent’s t-test was used. For multiple-group(more than two groups) comparisons, one-way analysis of variance (ANOVA) with Tukey post hoc test was used for multiple comparisons. Two-way ANOVA with Bonferroni post hoc test was used to analyzethe curves of body weight change and ear thickness change. A P-value less than 0.05 was considered statistically significant.
3. Results
3.1. WD-fed mice have exacerbated IMQ-induced psoriasiform dermatitis (PsD)
After confirming that WD-fed mice gained more body weight than CD-fed mice, we applied topical IMQ using a standard 5-day protocol to the CD- and WD-fed cohorts of mice and measured ear thickness as a marker of skin inflammation [22,25]. Similar to what we observed in our prior work [16], WD-fed mice had significantly more ear swelling than CD-fed mice from day 3 to day 5. Then we examined if WD-fed mice have more severe IMQ-induced PsD than CD-fed mice.
RT-PCR data showed elevated gene expression of neutrophil chemoattractant Cxcl2 and neutrophil marker Ly6g. By histological analysis, the epidermis of IMQ-treated ears of WD-fed mice was thicker than that of IMQ-treated ears of CD-fed mice while there was no difference in the thickness of vehicle-treated ears between two diet groups. Of note, more neutrophils as stained by Gr-1 and more Munro microabscesses were observed in WD-fed mice. These results were consistent with what we had previously reported and revealed WD-fed mice have an exaggerated response to IMQ-induced PsD compared to mice on CD [16].
3.2. Baseline gene expression levels of psoriasis-related cytokines are higher in WD-fed mice than CD-fed mice
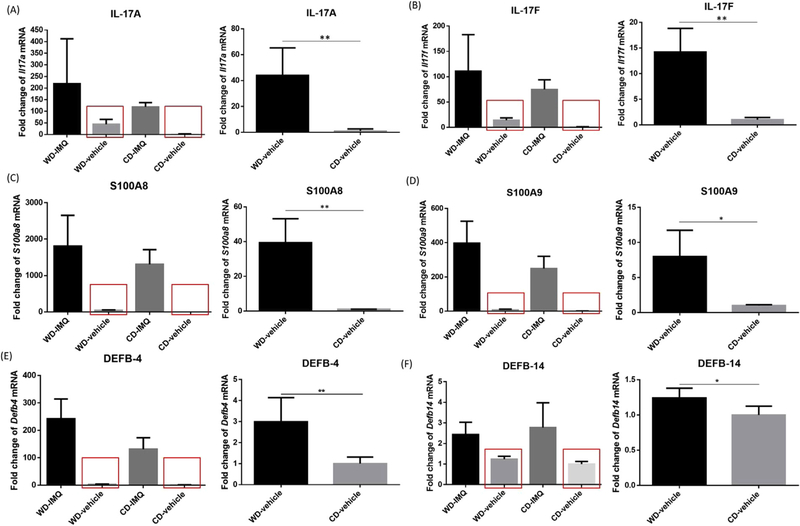
We next asked if Th17-associated cytokines are responsible for the different extent of IMQ-induced ear swelling between WD-and CD-fed mice. Similar to what we observed in our prior work [16], WD-fed (vs. CD-fed) mice showed moderately elevated baseline mRNA levels of Il17a (44-fold, P=0.007, Fig. 1A) and Il17f (14-fold, P=0.001,Fig. 1B) in vehicle-treated ears while there were no statistical differences in expression levels of Th17-associated cytokines between WD-fed and CD-fed mice in IMQ-treated ears. Gene expression levels of S100A8 and S100A9, which are proteins that are typically elevated in psoriatic lesions, were also increased in vehicle-treated ears (Fig. 1C, D). S100a8 and S100a9 had 39-fold (P=0.002) and 7-fold (P=0.010) baseline increase in WD-fed mice compared with CD-fed mice, respectively. Baseline gene expression levels of Defb4 and Defb14, whose human homologs are β-defensin-2 (HBD-2) and β-defensin-3 (HBD-3), respectively, were also higher in WD-fed mice compared with CD-fed mice (Fig. 1E, F). Taken together, WD-fed mice showed remarkably higher baseline expression of psoriasis-associated markers, including Il17a, Il17f, S100a8, S100a9, Defb4, and Defb14 than CD-fed mice.
Fig. 1.
Increased baseline expression of psoriasis-associated markers in WD-fed mice. In vehicle-treated ears, the baseline expression levels of IL-17A (A), IL-17F (B), S100A8 (C), S100A9 (D), and DEFB-4(E) were significantly higher in WD-fed mice compared with CD-fed mice. Baseline DEFB-14 (F)is also slightly higher in WD-fed mice than CD-fed mice. WD, Western diet. CD, control diet. * P<0.05. ** P<0.01. N=4.
3.3. The role of TRPA1, TRPV1 and PD-1 in WD facilitated PsD
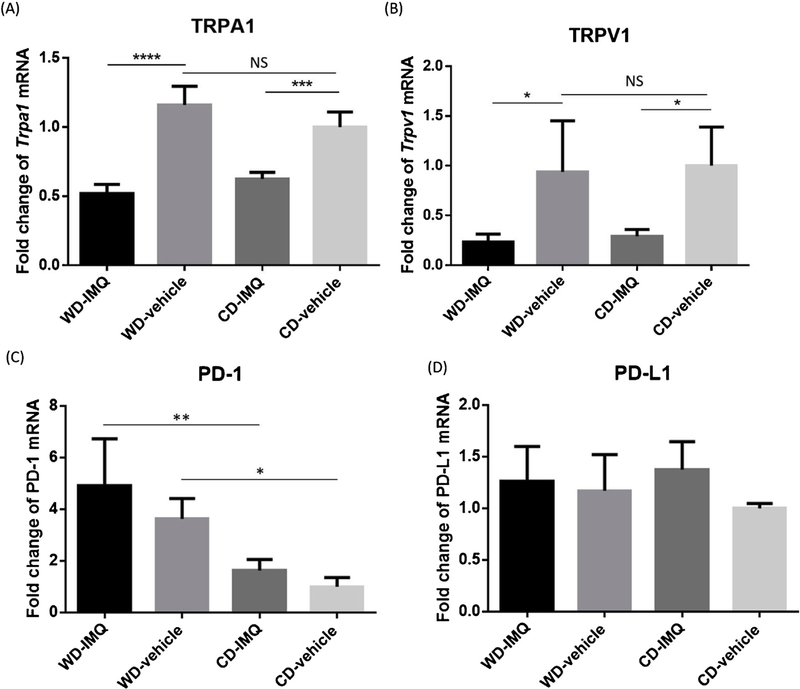
Because TRPA1 [27,28], TRPV1 [29–32], and PD-1 [4,33] were recently identified to play a crucial role in modulating psoriatic inflammation, we further examined if diet-induced obesity contributes to exacerbated PsDvia TRPA1, TRPV1, or PD-1 signaling. IMQ stimulation significantly reduced expression levels of TRPA1 (0.45-fold, WD-IMQ vs. WD-vehicle, P<0.0001; 0.63-fold, CD-IMQ vs. CD-vehicle, P=0.001) and TRPV1 (0.25-fold, WD-IMQvs. WD-vehicle, P=0.042; 0.29-fold, CD-IMQ vs. CD-vehicle, P = 0.042) mRNA while there is no difference in baselines expression of TRPA1 (1.16-fold, P=0.113) and TRPV1 (0.939-fold, P=0.857) mRNA between WD-fed and CD-fed mice (Fig. 2A, B). These results indicated that diet-induced obesity does not mediate psoriatic inflammation via TRPA1 and TRPV1 signaling. We next asked if diet-induced obesity influences PD-1 expression and response to anti-PD-1 treatment in our IMQ-mediated model of PsD. First, we assessed PD-1 (Pdcd1) and PD-L1 (Cd274) mRNA in IMQ- and vehicle-treated skin. WD-fed mice had higher expression level of PD-1 in both IMQ-treated and vehicle-treated ears (WD-IMQ vs. CD-IMQ, P=0.003; WD-vehicle vs. CD-vehicle, P=0.016) (Fig. 2C), while expression of PD-L1 was not different between WD-fed mice and CD-fed mice (Fig. 2D).
Fig. 2.
Expression levels of TRPA1, TRPV1, PD-1, and PD-L1 in WD-fed and CD-fed mice. Both TRPA1 and TRPV1 are recently shown to promote psoriasiform inflammation in murine IMQ-induced PsD model. However, WD didn’t induce upregulation of TRPA1 (A) and TRPV1 (B). In both IMQ-treated and vehicle-treated groups, WD-fed micehad significantly higher expression of PD-1 than CD-fed mice (C). On the contrary, there was no difference in PD-L1 expression between WD-fed and CD-fed mice (D). WD, Western diet. CD, control diet. IMQ, imiquimod. PsD, psoriasiform dermatitis. * P<0.05. ** P<0.01, *** P<0.001, **** P<0.0001. N=4.
3.4. WD-fed mice have more GDL T cells and more PD-1 expressing GDL T cells than CD-fed mice
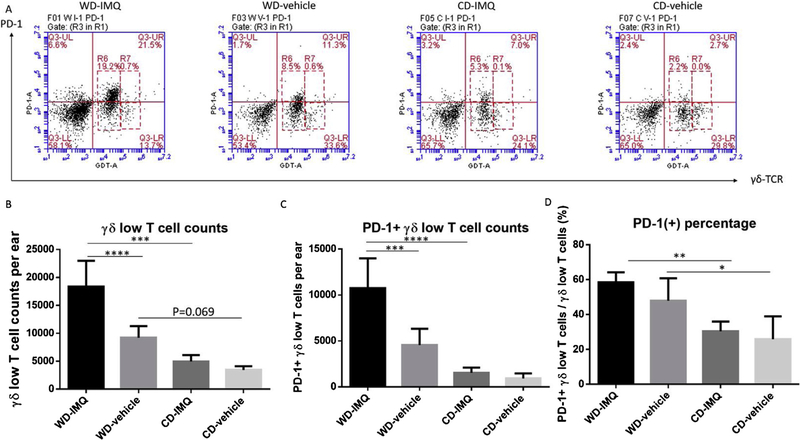
Because GDL T cells are the major producer of IL-17A and are crucial for the development of PsD in mice [17,24], we quantified GDL T cell numbers per ear by flow cytometry (Fig. 3A). Strikingly, WD-fed mice had significantly more GDL T cells in IMQ-treated ears compared with CD-fed mice at both baseline and after treatment with IMQ (Fig. 3B). Next, we examined PD-1 expression on GDL T cells. There were significantly more PD-1 positive GDL T cells in IMQ-treated ears of WD-fed mice compared with CD-fed mice (P<0.0001) (Fig. 3C). WD-fed mice had higher percentage of PD-1 expression on GDL T cells (WD-IMQ vs. CD-IMQ, P = 0.002; WD-vehicle vs. CD-vehicle, P = 0.027) (Fig. 3D).
Fig. 3.
Expression of PD-1 and accumulation of γδ low T cells in CD and WD-fed mice after IMQ or vehicle treatment. Mouse ears were harvested on the final day of IMQ5-day regimen. The cells were pre-gated by CD45. These CD45(+) cells were further identified by their PD-1 and γδ-TCR staining. (A) Representative plots flow cytometry plots. (B) WD-fed had significantly higher expression of PD-1 in IMQ-treated and vehicle-treated ears than CD-fed mice. (C) WD-fed mice had significantly more γδ low T cells in IMQ-treated ears compared with CD-fed mice. (D) WD-fed mice had significantly more PD-1 expression on γδ low T cells in IMQ -treated ears compared with CD-fed mice. (E) WD-fed mice had higher PD-1 percentage on gd low T cells than CD-fed mice. IMQ, imiquimod. WD, Western diet. CD, control diet. PsD, psoriasiform dermatitis. * P<0.05.** P<0.01. *** P<0.001. **** P<0.0001. N=4.
3.5. WD-fed mice have enhanced response to IMQ-induced ear swelling after anti-PD-1 administration
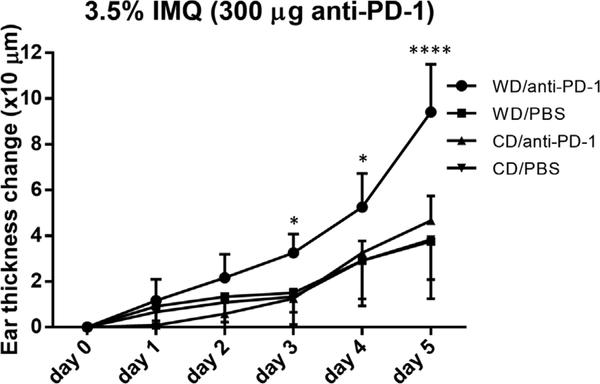
Next, we investigated if upregulation of PD-1 in WD-fed mice predisposes these obese mice to anti-PD-1 related PsD in vivo. WD-fed mice had greater ear thickness change in response to 3.5% IMQ cream after receiving anti-PD-1 antibodies, suggesting anti-PD-1 treated WD-fed mice had a greater response to IMQ treatment than did CD-fed counterparts (WD/anti-PD-1 vs. CD/anti-PD-1, P = 0.020 on day 3, P=0.020 on day 4, P<0.0001 on day 5) (Fig. 4). These findings indicate that WD-fed mice not only have more GDL T cells to both vehicle- and IMQ-treated skin but also enhances PD-1 expression on GDL T cells. Since PD-1 is important in regulation of the development of PsD in mice [4], higher PD-1 expression on GDL T cells likely explains why WD-fed obese mice have more dramatic response to anti-PD-1 than lean mice in terms of IMQ-induced PsD.
Fig. 4.
IMQ-induced swelling in CD- and WD-fed mice with anti-PD-1 treatment. WD-fed mice receiving anti-PD-1 had significantly more ear thickness change than CD-fed mice receiving anti-PD-1 from day 3 to day 5 during the IMQ 5-day course. WD, Western diet. CD, control diet. IMQ, imiquimod. PsD, psoriasiform dermatitis. * P<0.05. **** P<0.0001. N=4.
4. Discussion
The present study documents notable immunological changes in the skin of mice fed a WD (vs. CD) that might explain their predisposition PsD in the presence of anti-PD-1 antibodies. WD-fed obese mice have higher baseline expression levels of psoriasis-associated markers such as Th17-associated cytokines, S100A8, and S100A9, thus potentiating the effects of triggers such as IMQ application. It is well known that Th-17 associated cytokines and neutrophils are crucial in the IMQ murine model of PsD [22,34]. In the present study, upon IMQ stimulation, WD-fed mice showed higher expression of Ly6g than CD-fed mice, providing evidence of enhanced neutrophilic inflammation. Consistent with the Ly6g quantitative real-time PCR finding, WD-fed mice showed greater numbers of Munro microabscesses, a common histologic feature of PsD in humans and mice.
Because TRPA1 and TRPV1 were recently identified to mediate PsD in murine IMQ model [28,30], we also examined if diet-induced obesity alters expression levels of TRPA1 and TRPV1. While there are no differences in the expressions levels of TRPA1 and TRPV1 between WD-fed and CD-fed mice, IMQ treatment down-regulates mRNA of TRPA1 and TRPV1 in both dietary groups. Previous literature indicates that mRNA expression levels of TRPA1 and TRPV1 in dorsal root ganglion cells does not significantly change during the IMQ treatment course in murine PsD model [35]. In our present study, however, mRNA expression levels of TRPA1 and TRPV1 in skin significantly reduced after IMQ 5-day treatment. Considering that TRPA1 [28] and TRPV1 [30] positively regulate IMQ-induced PsD, it is likely that a negative feedback pathway exists to downregulate TRPA1 and TRPV1 in response to IMQ stimulation.
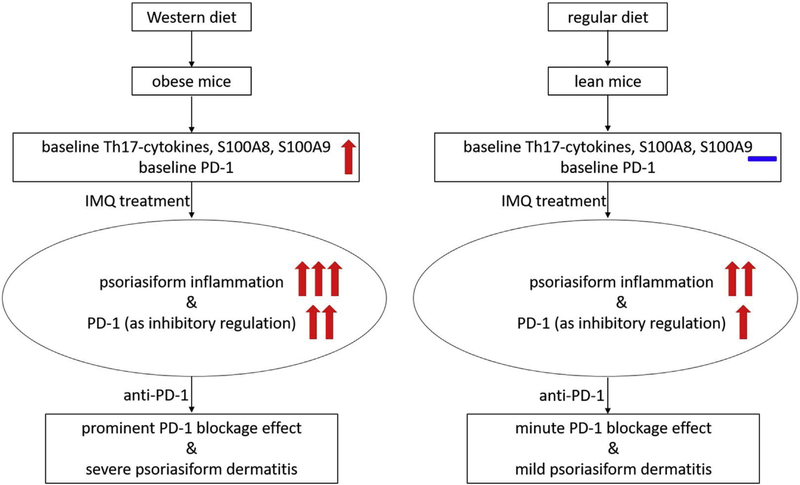
WD-fed obese mice have higher PD-1 expression and greater extent of IMQ-induced PsD, and the greater extent of IMQ-induced PsD is enhanced by anti-PD-1 administration. Previous literature has indicated that PD-1 regulates psoriasiform inflammation by inhibiting IL-17A secretion from T cells, and PD-1 knockout mice have more pronounced IMQ-induced dermatitis than wild-type mice [4,33]. Anti-PD-1 also induces more ear swelling in the murine IMQ model although the difference is less dramatic compared with PD-1 knockout mice [4]. In the present study, we asked if there is interaction between obesity and anti-PD-1. It is known that obesity exacerbates psoriatic inflammation, but obese mice also have higher expression of PD-1 to inhibit the inflammation. We hypothesize that the enhanced PD-1 expression on WD-fed mice is limiting inflammation. Consistent withthis hypothesis, we found that obese mice have a more dramatic response to anti-PD-1 than lean mice in terms of IMQ-induced ear swelling. A proposed model of interaction between obesity and PD-1 inhibition is shown in Fig. 5. Our data have clinical implications in that it suggests that diet-induced obesitymay exacerbate pre-existing psoriasis or precipitate the development of de novo psoriasis-like skin lesions in patients on anti-PD-1 treatment. Since diet and obesity can, to some extent, be controlled, recommendations to reduce obesity prior to anti-PD-1 treatment may lead to a reduction in some of the autoimmune related side effects seen with checkpoint inhibitor treatment.
Fig. 5.
Proposed schema of interactive effects of diet-induced obesity and anti-PD-1 on imiquimod-induced psoriasiform dermatitis.
Of considerable interest is our finding that WD intake increases expression of IL-17A and GDL T cells even at baseline condition prior to IMQ treatment. The presence of clearly increased IL-17A in the context of the GDL T cells suggests that the skin is “primed” to respond to triggers such as IMQ. Further studies will include an examination of the mechanisms by which GDL T cells are recruited to what appears to be otherwise uninflamed skin of WD-fed mice.
In this work, we did not mechanistically define how the WD contributes to high expression levels of PD-1. In our previous publication, however, we demonstrated that prolonged feeding with the WD resulted in dysregulation of bile acids in the skin with upregulation of specific bile acids with amelioration of spontaneous dermatitis upon feeding with cholestyramine [21]. Dysregulation of bile acids may, therefore, play a role in upregulation of PD-1 as well. Furthermore, in our previous published work [16], we showed that baseline levels of IL-17A are increased in skin after feeding with WD. While literature on the effects of IL-17A on T cells is sparse, one paper showed that (in vitro) IL-17A enhanced T cell activation along with PD-1 expression in the setting of thyroid cancer [36]. Our future work will focus on testing these hypotheses and elucidating whether obesity itself or specific components of the Western diet content leads to higher PD-1 expression.
In conclusion, WD intake predisposes mice to enhanced response to PsD and potentiates mice to more prominent anti-PD-1 induced ear swelling. In comparison with a healthy CD, WD induces more GDL T cells, the main producer of IL-17A in murine PsD, and higher expression of PD-1 on GDL T cells. This observation provides a rationale why obese mice have enhanced response to IMQ-induced PsD after anti-PD-1 injection. Further clinical investigation is warranted to validate the role of diet-induced obesity in APD1T-induced cutaneous ir-AEs in humans and to determine whether dietary intervention reduces ir-AEs in patients undertaking checkpoint inhibitor therapy.
Supplementary Material
Acknowledgements
This study was supported by a Discovery Grant from National Psoriasis Foundation to STH and YYW, grants from National Institutes of Health/National Cancer Institue (CA179582 and CA222490) to YYW, and grants from Taiwan Ministry of Science and Technology (MOST-107–2917-I-037–002 and MOST-108–2314-B-037–081) to SY.
Abbreviations
- APD1T
anti-PD-1 therapy
- CD
control diet
- GDL
γδ low
- IL
interleukin
- IMQ
imiquimod
- ir-AEs
immune-related adverse events
- PD-L1
programmed cell death protein ligand 1
- PD-1
programmed cell death protein 1
- PsD
psoriasiform dermatitis
- WD
Western diet
Footnotes
Declaration of Competing Interest
The authors have no conflict of interest to declare.
Appendix A. Supplementary data
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.jdermsci.2020.01.011.
References
- [1].Hofmann L, Forschner A, Loquai C, Goldinger SM, Zimmer L, Ugurel S, Schmidgen MI, Gutzmer R, Utikal JS, Goppner D, Hassel JC, Meier F, Tietze JK, Thomas I, Weishaupt C, Leverkus M, Wahl R, Dietrich U, Garbe C, Kirchberger MC, Eigentler T, Berking C, Gesierich A, Krackhardt AM, Schadendorf D, Schuler G, Dummer R, Heinzerling LM, Cutaneous, gastrointestinal, hepatic, endocrine, and renal side-effects of anti-PD-1 therapy, Eur. J. Cancer 60 (2016) 190–209. [DOI] [PubMed] [Google Scholar]
- [2].Sibaud V, Meyer N, Lamant L, Vigarios E, Mazieres J, Delord JP, Dermatologic complications of anti-PD-1/PD-L1 immune checkpoint antibodies, Curr. Opin. Oncol. 28 (4) (2016) 254–263. [DOI] [PubMed] [Google Scholar]
- [3].Menzies AM, Johnson DB, Ramanujam S, Atkinson VG, Wong ANM, Park JJ, McQuade JL, Shoushtari AN, Tsai KK, Eroglu Z, Klein O, Hassel JC, Sosman JA, Guminski A, Sullivan RJ, Ribas A, Carlino MS, Davies MA, Sandhu SK, Long GV, Anti-PD-1 therapy in patients with advanced melanoma and preexisting autoimmune disorders or major toxicity with ipilimumab, Ann. Oncol. 28 (2) (2017) 368–376. [DOI] [PubMed] [Google Scholar]
- [4].Imai Y, Ayithan N, Wu X, Yuan Y, Wang L, Hwang ST, Cutting edge: PD-1 regulates imiquimod-induced psoriasiform dermatitis through inhibition of IL-17A expression by innate gammadelta-Low T Cells, J. Immunol. 195 (2) (2015) 421–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Bonigen J, Raynaud-Donzel C, Hureaux J, Kramkimel N, Blom A, Jeudy G, Breton AL, Hubiche T, Bedane C, Legoupil D, Pham-Ledard A, Charles J, Perol M, Gerard E, Combemale P, Bonnet D, Sigal ML, Mahe E, P. Groupe de Recherche sur le, A. the Groupe Cancerologie Cutanee of the Societe Francaise de Dermatologie the Gem Resopso, P.-C. the Groupe Francais de, Anti-PD1- induced psoriasis: a study of 21 patients, J. Eur. Acad. Dermatol. Venereol. 31 (5) (2017) e254–e257. [DOI] [PubMed] [Google Scholar]
- [6].Hwang ST, Nijsten T, Elder JT, Recent highlights in psoriasis research, J. Invest. Dermatol. 137 (3) (2017) 550–556. [DOI] [PubMed] [Google Scholar]
- [7].Chiu HY, Wang TS, Chen PH, Hsu SH, Tsai YC, Tsai TF, Psoriasis in Taiwan: from epidemiology to new treatments, Dermatol. Sin. 36 (3) (2018) 115–123. [Google Scholar]
- [8].Yu S, Tu HP, Yu CL, Lee CH, Hong CH, Is psoriasis an independent risk factor of renal disease? A nationwide retrospective cohort study from 1996 to 2010, Dermatol. Sin. 35 (2) (2017) 78–84. [Google Scholar]
- [9].Tu HP, Yu CL, Lan CCE, Yu S, Prevalence of schizophrenia in patients with psoriasis: a nationwide study, Dermatol. Sin. 35 (1) (2017) 1–6. [Google Scholar]
- [10].Yu S, Yu CL, Huang YC, Tu HP, Lan CCE, Risk of developing psoriasis in patients with schizophrenia: a nationwide retrospective cohort study, J. Eur. Acad. Dermatol. 31 (9) (2017) 1497–1504. [DOI] [PubMed] [Google Scholar]
- [11].Yu S, Tu HP, Huang YC, Lan CE, The incidence of anxiety may not be correlated with severity of psoriasis: a prospective pilot study, Med. Hypotheses 130 (2019)109254. [DOI] [PubMed] [Google Scholar]
- [12].Debbaneh M, Millsop JW, Bhatia BK, Koo J, Liao W, Diet and psoriasis, part I: impact of weight loss interventions, J. Am. Acad. Dermatol. 71 (1) (2014) 133–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Hossler EW, Wood GC, Still CD, Mowad CM, Maroon MS, The effect of weight loss surgery on the severity of psoriasis, Br. J. Dermatol. 168 (3) (2013) 660–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Kanoski SE, Davidson TL, Western diet consumption and cognitive impairment: links to hippocampal dysfunction and obesity, Physiol. Behav. 103 (1) (2011) 59–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Sheng L, Jena PK, Hu Y, Liu HX, Nagar N, Kalanetra KM, French SW, French SW, Mills DA, Wan YY, Hepatic inflammation caused by dysregulated bile acid synthesis is reversible by butyrate supplementation, J. Pathol. 243 (4) (2017) 431–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Yu S, Wu X, Zhou Y, Sheng L, Jena PK, Han D, Yvonne Wan YJ, Hwang ST, A western diet, but not a high-fat and low-sugar diet, predisposes mice to enhanced susceptibility to imiquimod-induced psoriasiform dermatitis, J. Invest. Dermatol. 139 (6) (2019) 1404–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Mabuchi T, Takekoshi T, Hwang ST, Epidermal CCR6+ gammadelta T cells are major producers of IL-22 and IL-17 in a murine model of psoriasiform dermatitis, J. Immunol. 187 (10) (2011) 5026–5031. [DOI] [PubMed] [Google Scholar]
- [18].Ott PA, Bang YJ, Piha-Paul SA, Razak ARA, Bennouna J, Soria JC, Rugo HS, Cohen RB, O’Neil BH, Mehnert JM, Lopez J, Doi T, van Brummelen EMJ, Cristescu R, Yang P, Emancipator K, Stein K, Ayers M, Joe AK, Lunceford JK, T-cell-Inflamed gene-expression profile, programmed death ligand 1 expression, and tumor mutational burden predict efficacy in patients treated with pembrolizumab across 20 cancers: KEYNOTE-028, J. Clin. Oncol. 37 (4) (2019) 318–327. [DOI] [PubMed] [Google Scholar]
- [19].Jena PK, Sheng L, Liu HX, Kalanetra KM, Mirsoian A, Murphy WJ, French SW, Krishnan VV, Mills DA, Wan YY, Western diet-induced dysbiosis in Farnesoid X receptor knockout mice causes persistent hepatic inflammation after antibiotic treatment, Am. J. Pathol. 187 (8) (2017) 1800–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Sheng L, Jena PK, Liu HX, Kalanetra KM, Gonzalez FJ, French SW, Krishnan VV, Mills DA, Wan YY, Gender differences in bile acids and microbiota in relationship with gender dissimilarity in steatosis induced by diet and FXR inactivation, Sci. Rep. 7 (1) (2017) 1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Jena PK, Sheng L, McNeil K, Chau TQ, Yu S, Kiuru M, Fung MA, Hwang ST, Wan YY, Long-term Western diet intake leads to dysregulated bile acid signaling and dermatitis with Th2 and Th17 pathway features in mice, J. Dermatol. Sci. 95 (1) (2019) 13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].van der Fits L, Mourits S, Voerman JS, Kant M, Boon L, Laman JD, Cornelissen F, Mus AM, Florencia E, Prens EP, Lubberts E, Imiquimod-induced psoriasis-like skin inflammation in mice is mediated via the IL-23/IL-17 axis, J. Immunol. 182 (9) (2009) 5836–5845. [DOI] [PubMed] [Google Scholar]
- [23].Hedrick MN, Lonsdorf AS, Shirakawa AK, Richard Lee CC, Liao F, Singh SP, Zhang HH, Grinberg A, Love PE, Hwang ST, Farber JM, CCR6 is required for IL-23-induced psoriasis-like inflammation in mice, J. Clin. Invest. 119 (8) (2009) 2317–2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Mabuchi T, Singh TP, Takekoshi T, Jia GF, Wu X, Kao MC, Weiss I, Farber JM, Hwang ST, CCR6 is required for epidermal trafficking of gammadelta-T cells in an IL-23-induced model of psoriasiform dermatitis, J. Invest. Dermatol. 133 (1) (2013) 164–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Yu S, Wu X, Zhou Y, Han D, Anderson LS, Simon SI, Hwang ST, Imai Y, Is CCR6 Required for the Development of Psoriasiform Dermatitis in Mice? J. Invest. Dermatol. 139 (2) (2019) 485–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Sarraj B, Ye J, Akl AI, Chen G, Wang JJ, Zhang Z, Abadja F, Abecassis M, Miller SD, Kansas GS, Ansari MJ, Impaired selectin-dependent leukocyte recruitment induces T-cell exhaustion and prevents chronic allograft vasculopathy and rejection, Proc. Natl. Acad. Sci. U. S. A. 111 (33) (2014) 12145–12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Kemeny A, Kodji X, Horvath S, Komlodi R, Szoke E, Sandor Z, Perkecz A, Gyomorei C, Setalo G, Kelemen B, Biro T, Toth BI, Brain SD, Pinter E, Gyulai R, TRPA1 acts in a protective manner in imiquimod-induced psoriasiform dermatitis in mice, J. Invest. Dermatol. 138 (8) (2018) 1774–1784. [DOI] [PubMed] [Google Scholar]
- [28].Zhou Y, Han D, Follansbee T, Wu X, Yu S, Wang B, Shi Z, Domocos DT, Carstens M, Carstens E, Hwang ST, Transient receptor potential ankyrin 1 (TRPA1) positively regulates imiquimod-induced, psoriasiform dermal inflammation in mice, J. Cell. Mol. Med. 23 (7) (2019) 4819–4828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Riol-Blanco L, Ordovas-Montanes J, Perro M, Naval E, Thiriot A, Alvarez D, Paust S, Wood JN, von Andrian UH, Nociceptive sensory neurons drive interleukin-23-mediated psoriasiform skin inflammation, Nature 510 (7503) (2014) 157–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Zhou Y, Follansbee T, Wu X, Han D, Yu S, Domocos DT, Shi Z, Carstens M, Carstens E, Hwang ST, TRPV1 mediates inflammation and hyperplasia in imiquimod (IMQ)-induced psoriasiform dermatitis (PsD) in mice, J. Dermatol. Sci. 92 (3) (2018) 264–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Yu S, Li Y, Zhou Y, Follansbee T, Hwang ST, Immune mediators and therapies for pruritus in atopic dermatitis and psoriasis, J. Cutan. Immunol. Allergy 2 (1) (2019) 4–14. [Google Scholar]
- [32].Cohen JA, Edwards TN, Liu AW, Hirai T, Jones MR, Wu J, Li Y, Zhang S, Ho J, Davis BM, Albers KM, Kaplan DH, Cutaneous TRPV1(+) neurons trigger protective innate type 17 anticipatory immunity, Cell 178 (4) (2019) 919–932 e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Kim JH, Choi YJ, Lee BH, Song MY, Ban CY, Kim J, Park J, Kim SE, Kim TG, Park SH, Kim HP, Sung YC, Kim SC, Shin EC, Programmed cell death ligand 1 alleviates psoriatic inflammation by suppressing IL-17A production from programmed cell death 1-high T cells, J. Allergy Clin. Immunol. 137 (5) (2016) 1466–1476 e3. [DOI] [PubMed] [Google Scholar]
- [34].Sumida H, Yanagida K, Kita Y, Abe J, Matsushima K, Nakamura M, Ishii S, Sato S, Shimizu T, Interplay between CXCR2 and BLT1 facilitates neutrophil infiltration and resultant keratinocyte activation in a murine model of imiquimod-induced psoriasis, J. Immunol. 192 (9) (2014) 4361–4369. [DOI] [PubMed] [Google Scholar]
- [35].Sakai K, Sanders KM, Youssef MR, Yanushefski KM, Jensen L, Yosipovitch G, Akiyama T, Mouse model of imiquimod-induced psoriatic itch, Pain 157 (11) (2016) 2536–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Han LT, Hu JQ, Ma B, Wen D, Zhang TT, Lu ZW, Wei WJ, Wang YL, Wang Y, Liao T, Ji QH, IL-17A increases MHC class I expression and promotes T cell activation in papillary thyroid cancer patients with coexistent Hashimoto’s thyroiditis, Diagn. Pathol. 14 (1) (2019) 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Shi Z, Wu X, Yu S, Huynh M, Jena PK, Nguyen M, Wan YY, Hwang ST, Short-term exposure to a Western diet induces psoriasiform dermatitis by promoting accumulation of IL-17A-producing gd T cells, J. Invest. Dermatol. (2020) In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.