Abstract
Background
Poor access to healthcare in rural communities causes many people to seek herbalists who use medicinal plants for the treatment of various disease conditions. Most knowledge of traditional herbal medicine makes use of indigenous remedies which are often undocumented and are at risk of being lost. The preservation of this knowledge may facilitate scientific inquiry into promising new therapeutic molecules.
Methods
Semi-structured questionnaires were used to collect the sociodemographic information of 30 herbalists in Kisumu East Sub County. The local names of medicinal plants used in managing illnesses of the respiratory system, their habit, active parts, indications, methods of preparation, routes of administration, scientific identity, and conservation status were also recorded. Other reported traditional uses, pharmacological activities, and toxicological data were identified via a literature search.
Results
Most herbalists were female (86.7%), aged between 61 and 70 years (43.3%) with no formal education (56.7%), and had 21–30 years of practice (30%). 44 plant species, belonging to 43 genera and 28 families were identified. Leguminosae and Rutaceae plant families were predominant, leaves were frequently used (33%), and trees were the most common habit (44.4%). Most plants were collected in the wild (79.2%), preparation was mainly by decoction (68.8%), and the administration was mainly orally. The main indication was cough and 79.5% of all documented plant species had previously been reported to have a pharmacological activity relevant to the mitigation of respiratory illnesses. Toxicological data was available for 84.1% of the plant species identified.
Conclusions
The predominant use of roots, root barks, and root tubers by herbalists in Kisumu East Sub County threatens to negatively impact the ecological survival of some plant species. The preservation of herbalists’ knowledge of medicinal plants in the study area is a pressing concern considering their advanced age and little formal education. There is a need to conserve some of the medicinal plants documented in this study. The medicinal claims made by herbalists also warrant scientific scrutiny.
Keywords: Ethnopharmacology, Medicinal plants, Kisumu East, Luo, Ethnomedicinal, Ethnobotanical, Respiratory diseases, Cough
Background
The global burden of respiratory diseases makes for daunting reading. Lower respiratory tract infections (LRTI) and chronic obstructive pulmonary disease (COPD) reportedly claimed 6 million human lives in 2016 [1]. The prevalence of COPD in Sub Saharan Africa has been reported to be between 4 and 25% and > 100,000 deaths have been linked to non-communicable diseases including those of the respiratory system [2, 3]. Diseases of the respiratory system hurt individual productivity and are responsible for more than 10% of all disability-adjusted life years [4].
According to a 2013 Kenya National Bureau of Statistics (KNBS) economic survey, pneumonia, and tuberculosis were responsible for 13.7% of all total deaths in the Nyanza region [5]. It is important to note that illnesses of the upper respiratory tract are the second leading cause of death in Kisumu County [6]. Poor access to healthcare and scarcity of health resources in rural areas such as many parts of Kisumu East Sub County causes many inhabitants of such areas to rely on indigenous plant resources to manage common diseases including those that affect the respiratory system. Plant-based indigenous remedies may be key in the future management of respiratory system diseases [7]. However, the potential of this resource is largely untapped due to inadequate documentation by the herbalists who prepare the remedies.
The rapid development of infrastructure in Sub Saharan Africa including Kenya threatens to destroy cultural lands where medicinal plants are cultivated. This is problematic given that the knowledge of these plant resources is mostly an extension of people’s culture [8, 9]. Herbalists are usually the custodians of medicinal plants in these communities. By documenting the knowledge held by herbalists, vital information on the medicinal plants may be preserved. The current study aimed to collect ethnobotanical data on medicinal plants used by herbalists in the management of respiratory diseases in Kisumu East Sub County.
Materials and methods
Ethical approval and consent to participate in the study
Ethical approval for the study was obtained from the Biosafety, Animal Use and Ethics committee of the University of Nairobi (Ref: FVM BAUEC/2019/210). Approval was additionally sought from regional administrators (the area chief and assistant chief) who were duly notified of the study’s objectives. The scope, possible benefits, and risks of the study were explained to willing participants (herbalists) and consent forms were made available to them for signing.
Study area
The study was conducted in Kisumu East Sub County in Western Kenya (Fig. 1). The study area is approximately 365 km from Nairobi (the administrative capital of Kenya) and covers an area of approximately 135 km2. It lies within latitudes 0° 20′ South and 0° 50′ South and longitudes 33° 20′ E and 35° 20′ E and comprises of several administrative wards including Kolwa Central, Kolwa East, Manyatta B, Nyalenda A, and Kajulu East and West [10]. Moreover, the population in this area is about 220,977 according to the 2019 Kenya Population and Housing Census [5]. It receives an annual relief rainfall of between 1200 and 1300 mm and annual temperatures range between 20 and 35 °C. The major economic activities of residents include fish farming, and agriculture (sugar, livestock, and poultry farming) [10].
Fig. 1.
Map of Kenya showing Kisumu County and Kisumu East Sub County
Data collection
The study was conducted between March and September 2019. Ethnobotanical data were obtained by using semi-structured questionnaires. The target respondents were local herbalists with good ethnobotanical knowledge of the plants used in managing respiratory diseases and related symptoms. Thirty local herbalists were selected for interviews which were conducted both in Kiswahili and Luo dialect with the aid of a botanist familiar with the languages. Each of the respondents was interviewed individually to ensure confidentiality. The interviews sought to answer the following questions;
Which plant parts are most commonly used in preparing the indigenous remedies indicated for respiratory illnesses?
Which methods are adopted in preparing the indigenous remedies?
Which respiratory illnesses are most commonly treated with medicinal plants in the study area?
Which plant species are used in the preparation of the remedies?
How are the indigenous remedies administered? See Additional file 1.
Collection and identification of plant specimens
Several trips were made to the homesteads of the herbalists where voucher specimens were collected and pressed and later identified by a botanist before being deposited at the University of Nairobi Herbarium. Information on the vernacular name, plant part used, plant habit (i.e. the general appearance, growth form, or architecture), plant status, method of preparation, and route of administration were collected.
Literature search strategy
A literature search was conducted on MEDLINE, PubMed, PubMed Central (PMC), Google Scholar, the Directory of Open Access Journals (DOAJ), The Journal Author Name Estimator (JANE), University repositories, and from grey literature to identify relevant articles/theses/ reference material containing information on previously reported traditional uses, pharmacological/chemical activities, and toxicological data on the medicinal plants indicated for the management of respiratory illnesses in Kisumu East Sub County. Studies were excluded if they were not in English.
Data analysis
Frequencies and percentages were used to analyze the sociodemographic data of the herbalists. The relative frequency of citation (RFC) was used to evaluate the ethnobotanical data.
Relative frequency of citation (RFC)
This was done to determine the number of herbalists who considered particular plant species were worth mentioning in the management of diseases of the respiratory system. The value was calculated using the formula described by Tardio and Santayana [11];
where Fc is the number of herbalists who cited a particular species and N is the total number of herbalists (Table 1).
Table 1.
Demographic characteristics of herbalists interviewed in Kisumu East Sub County (n = 30) during the study period
| Variable (n = 30) | Frequency (percentage) |
|---|---|
| Gender | |
| Male | 4 (13.3) |
| Female | 26 (86.7) |
| Age | |
| 31–40 | 5 (16.7) |
| 41–50 | 1 (3.3) |
| 51–60 | 5 (16.7) |
| 61–70 | 13 (43.3) |
| > 70 | 6 (20) |
| Education | |
| None | 17 (56.7) |
| Basic | 12 (40) |
| Secondary | 1 (3.3) |
| Years of experience | |
| 1–10 | 5 (16.7) |
| 11–20 | 8 (26.7) |
| 21–30 | 9 (30) |
| 31–40 | 4 (13.3) |
| 41–50 | 2 (6.7) |
| > 50 | 2 (6.7) |
Results
Socio-demographic characteristics of the herbalists who were interviewed
86.7% of all herbalists were female, and aged between 61 and 70 years of age (43.3%) (Table 1). The average age of the female herbalist was 61.6 years while the average age of their male counterparts was 51.5 years of age. Seventeen of the herbalists (56.7%) had no formal education while only 1 had secondary education (Table 1). It was observed that both male and female herbalists had extensive years of practice. The mean years of practice for male and female herbalists in the study area were 27 years and 25 years for male and female herbalists respectively.
Diversity of medicinal plants identified and their use
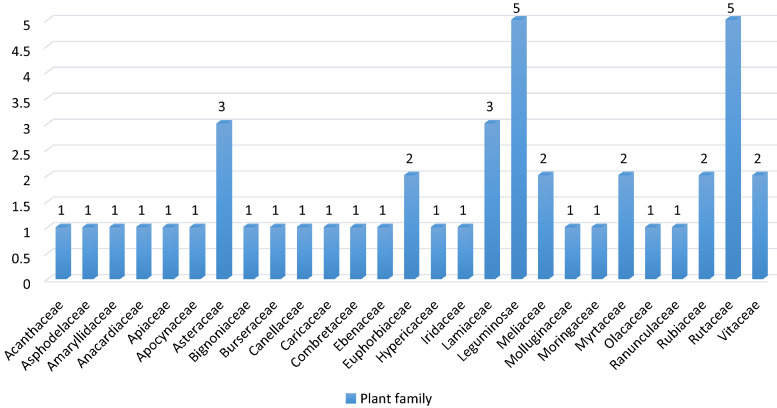
Table 2 is a summary of the family, scientific name, local name, voucher number, habit, status, and the part used, indication, method of preparation, route of administration and relative frequency of citation of medicinal plants used in managing respiratory diseases by herbalists in Kisumu East Sub County. Forty-four plant species belonging to 43 genera distributed among 28 families were reportedly used in herbal preparations for the management of respiratory infections (Table 2). Leguminosae and Rutaceae families predominated with 5 species each, followed by Asteraceae and Lamiaceae families with 3 species each (Fig. 2). Euphorbiaceae, Meliaceae, Myrtaceae, Rubiaceae, and Vitaceae family had 2 species each (Fig. 2). The other families had 1 species only. The identified 44 species comprised of trees (44.4%), shrubs (37.8%), herbs (8.9%), climbers (6.7%), and corms (2.2%) (Table 2). A majority of the plants were sourced from the wild (79.2%) while some were grown in the homestead (20.8%). The most cited plants were Euclea divinorum, Tylosema fassoglensis, Carissa edulis, Harrisonia abyssinica, Zanthoxylum gilletii, and Warburgia salutaris with RFC values of 0.73, 0.67, 0.67, 0.6, 0.5, 0.47 and 0.47 respectively (Table 2).
Table 2.
Plants used in managing diseases of the respiratory system among the Luo community of Kisumu East Sub County
| Family | Scientific name | Local name | Voucher no. | Habit | Status | Part used | Condition managed | Mode of preparation | Route of administration | RFC |
|---|---|---|---|---|---|---|---|---|---|---|
| Acanthaceae | Acanthus polystachyus Delile | Not provided | JM2019/284/003 | Shrub | Wild | Roots | Cough | Decoction | Oral | 0.07 |
| Asphodelaceae | Aloe kedongensis Reynolds | Ogaka | JM2019/194/030 | Shrub | Wild | Leaves | Asthma, Pneumonia | Concoction | Oral | 0.23 |
| Amaryllidaceae | Allium sativum L. | Otungu | JM2019/194/031 | Herb | Cultivated | Bulb | Allergies | Chewing or as a concoction | Oral | 0.03 |
| Anacardiaceae | Rhus natalensis Bernh. | Sagla | JM2019/194/021 | Shrub | Wild | Roots | Asthma | Concoction | Oral | 0.07 |
| Apiaceae | Steganotaenia araliacea Hochst. | Nyaniang-liech | JM2019/118/006 | Tree | Wild | Roots or stem bark | Pneumonia | Decoction | Oral | 0.03 |
| Apocynaceae | Carissa edulis (Forssk.) Vahl | Ochuoga | JM2019/194/022 | Shrub | Wild | Roots | Common cold, pneumonia, asthma | Decoction | Oral | 0.67 |
| Asteraceae | Artemisia annua L. | Nyumba | JM2019/269/001 | Herb | Wild or cultivated | Leaves | Asthma | Decoction | Oral | 0.03 |
| Microglossa pyrifolia (Lam.) Kuntze | Nyabung-odide | JM2019/194/006 | Shrub | Wild | Leaves or roots | Cough | Maceration or as a concoction | Oral | 0.07 | |
| Tithonia diversifolia (Hemsl.) A. Gray | Mafua/maua | JM2019/194/012 | Shrub | Wild | Stem bark or leaves | Asthma | Concoction | Oral | 0.03 | |
| Bignoniaceae | Kigelia africana (Lam.) Benth. | Yago | JM2019/194/003 | Tree | Wild or cultivated | Fruit or stem bark | Pneumonia | Decoction | Oral | 0.3 |
| Burseraceae | Commiphora africana (A.Rich.) Engl. | Arupiny | JM2019/194/007 | Tree | Wild | Roots | Pneumonia | Decoction | Oral | 0.17 |
| Canellaceae | Warburgia salutaris (G.Bertol) Chiov | Abaki | JM2019/244/001 | Tree | Wild or cultivated | Stem bark | Asthma, allergy, chest pain, pneumonia | Decoction | Oral | 0.47 |
| Caricaceae | Carica papaya L. | Apoyo | JM2019/269/002 | Tree | Cultivated | Roots or leaves | Bronchitis | Decoction | Oral | 0.07 |
| Combretaceae | Terminalia brownii Fresen | Minera/Manera | JM2019/058/016 | Tree | Wild or cultivated | Stem bark | Asthma, pneumonia, common cold | Decoction | Oral | 0.2 |
| Convolvulaceae | Ipomoea kituiensis Var | Obinju | JM2019/194/028 | Shrub | Wild | Leaves | Cough | Decoction | Oral | 0.03 |
| Ebenaceae | Euclea divinorum Hiern. | Ochol | JM2019/194/023 | Shrub | Wild | Roots | Pneumonia, asthma | Decoction | Oral | 0.73 |
| Euphorbiaceae | Croton megalocarpus Del. | Ofunja muri | JM2019/194/015 | Tree | Wild | Leaves | Pneumonia | Decoction | Oral | 0.17 |
| Croton dichogamous Pax | Rachar | JM2019/178/001 | Tree | Wild | Roots | Asthma | Decoction | Oral | 0.1 | |
| Hypericaceae | Harungana madagascariensis Lam. Ex Poir | Aremo | JM2019/058/005 | Tree | Wild | Leaves | Cough | Decoction | Oral | 0.2 |
| Iridaceae | Gladiolus dalenii Van Geel | Obuya | JM2019/284/001 | Corm | Wild | Corm | Asthma, allergy | Powdered | Inhalation | 0.1 |
| Lamiaceae | Clerodendrum myricoides (Hochst.) R.Br.ex Vatke | Okwerogweno/sangla | JM2019/058/021 | Shrub | Wild | Roots or leaves | Pneumonia, asthma | Decoction | Oral | 0.17 |
| Plectranthus barbatus Andr. | Okita | JM2019/058/009 | Shrub | Wild | Leaves | Asthma, pneumonia, allergy | Decoction | Oral | 0.33 | |
| Vitex doniana Sweet | Kalemba | JM2019/194/009 | Tree | Wild | Leaves or stem bark | Allergies, common cold | Decoction | Oral | 0.03 | |
| Leguminosae | Acacia robusta Burch. | Otiep | JM2019/214/001 | Tree | Wild | Stem bark or root bark | Bronchial obstruction | Concoction | Oral | 0.03 |
| Albizia zygia (DC.) J.J.Macbr. | Oturbam | JM2019/224/002 | Tree | Wild | Stem bark | Pneumonia | Decoction | Oral | 0.1 | |
| Rhynchosia elegans var. elegans | Jandarusi/Jandalusi | JM2019/284/002 | Herb | Wild | Root tubers | Cough | Concoction | Oral | 0.03 | |
| Tamarindus indica L. | Chwaa | JM2019/194/018 | Tree | Wild or cultivated | Fruit or stem bark | Cough, general body malaise | Decoction | Oral | 0.03 | |
| Tylosema fassoglense (Kotschy ex Schweinf.) Torre & Hillc. | Ombasa | JM2019/194/016 | Climber | Wild | Roots | Flu, pneumonia, asthma | Decoction | Oral | 0.67 | |
| Meliaceae | Azadirachta indica (L) Burm. | Mwarubaine | JM2019/269/003 | Tree | Wild or cultivated | Leaves | Cough | Decoction | Oral | 0.3 |
| Khaya senegalensis Desr. A. Juss | Tido | JM2019/194/019 | Tree | Wild | Stem bark | Common cold, cough | Decoction | Oral | 0.47 | |
| Molluginaceae | Mollugo nudicaulis Lam. | Ataro | JM2019/138/001 | Herb | Wild | Leaves | Cough | Chewed or as a decoction | Oral | 0.03 |
| Moringaceae | Moringa oleifera Lam. | JM2019/269/004 | Tree | Cultivated | Leaves | General body malaise | Decoction | Oral | 0.13 | |
| Myrtaceae | Eucalptus camaldulensis Dehnh | Bao | JM2019/269/005 | Tree | Wild or cultivated | Leaves | Common cold | Decoction | Oral | 0.33 |
| Syzygium cumini (L.) Skeels. | Jamna | JM2019/194/008 | Shrub | Wild | Stem bark | Cough | Concoction | Oral | 0.03 | |
| Olacaceae | Ximenia americana L. | Olemo | JM2019/269/006 | Shrub | Wild | Roots or stem bark | Cough | Concoction | Oral | 0.07 |
| Ranunculaceae | Clematis hirsuta Guill. & Perr | Achogo | JM2019/269/007 | Climber | Wild | Leaves | Common cold | Decoction | Oral | 0.1 |
| Rubiaceae | Gardenia ternifolia Schumach. & Thonn. | Rayudhi | JM2019/194/014 | Shrub | Wild | Roots | Cough, Pneumonia | Decoction | Oral | 0.13 |
| Keetia gueinzii (Sond.) Bridson | Atego | JM2019/264/001 | Shrub | Wild | Root bark | Asthma, pneumonia, coughing, allergy | Powdered | Inhalation | 0.2 | |
| Rutaceae | Harrisonia abyssinica Oliv. | Pedo | JM2019/194/001 | Shrub | Wild | Roots | Cough, pneumonia, asthma | Decoction | Oral | 0.6 |
| Teclea nobilis Del. | Madat midat | JM2019/194/024 | Tree | Wild | Roots or leaves | Asthma, common cold | Decoction | Oral | 0.2 | |
| Toddalia asiatica L. | Ajua Nyalwet-kwach | JM2019/194/017 | Shrub | Wild | Leaves or roots | Common cold, pneumonia, throat infection | Concoction | Oral | 0.33 | |
| Zanthoxylum chalybeum (Eng) Engl. | Roko | JM2019/269/008 | Tree | Wild | Stem bark or root bark | Pneumonia | Decoction | Oral | 0.03 | |
| Zanthoxylum gilletii (De Wild.) P.G Waterman | Sogo-maitha | JM2019/224/001 | Tree | Wild or cultivated | Stem bark | Asthma, pneumonia, coughing, General body malaise | Decoction | Oral | 0.5 | |
| Vitaceae | Cissus rotundifolia (Forssk.) Vahl | Minya/katera | JM2019/194/026 | Climber | Wild | Leaves | Throat infection, pneumonia, coughing | Decoction | Oral | 0.2 |
| Rhoicissus revoilii Planch | Rabong’o | JM2019/269/009 | Shrub | Wild | Root tubers | General body malaise | Decoction | Oral | 0.17 |
RFC Relative frequency of citation
Fig. 2.
Plant families used by herbalists in Kisumu East Sub County to manage diseases of the respiratory system
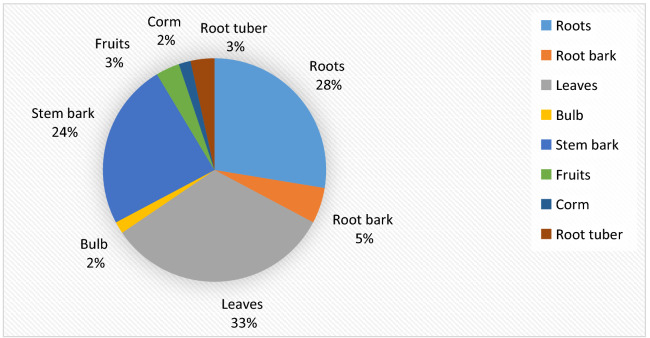
The different plant parts used by herbalists to manage respiratory illnesses in Kisumu East Sub County are summarized in Fig. 3. Leaves were the most frequently used parts (33%), followed by roots (28%) and stem bark (24%). Root bark, fruits, corms, bulbs, and root tubers accounted for 15%. Roots, root bark, root tuber, and stem bark accounted for 60% of plant parts used in the management of diseases of the respiratory system (Fig. 3).
Fig. 3.
Plant parts used by herbalists in Kisumu East Sub County to manage diseases of the respiratory system
Dosage, mode of preparation, and route of administration
Various methods were used to prepare herbal medicine used for managing diseases of the respiratory system in the study area (Table 2). The most common method was decoction (68.8%), concoction (20.8%), and chewing (4.2%) (Table 2). Other methods of preparation included cold maceration, powdering, and crushing before instillation in the nostrils which accounted for 2.1% respectively (Table 2). The main route of administration of the indigenous remedies prepared by the traditional medicine practitioners was oral (Table 2).
Pharmacological and toxicological reports on the medicinal plants documented in this study
Of the 44 plant species documented in this study, 95.5% had studies that had reported their pharmacological/chemical activity (Table 3). Moreover, 79.5% (35/44) of the documented medicinal plants had previously been reported to be effective against microorganisms that are associated with respiratory illnesses and 84.1% of the plant species had toxicological data (Table 3).
Table 3.
Previously reported traditional uses, documented pharmacological/chemical activity, and toxicological data on the medicinal plants indicated for managing diseases of the respiratory system by herbalists in Kisumu East Sub County
| Plant name | Previously reported traditional use | Reported pharmacological/chemical activity | Type of study | Toxicological data |
|---|---|---|---|---|
| Acanthus polystachyus Delile |
Malaria [12], scorpion bite [13] |
Antimalarial activity [14] | In vivo (Swiss albino mice) [14] | The methanol leaf extract was reported to be non-toxic in mice with a median lethal dose of > 2000 mg/kg [14] |
| Aloe kedongensis Reynolds | Malaria [15] | Antiplasmodial activity (aqueous leaf extract), leishmanicidal activity (aqueous and methanol extracts) [16] | In vitro (semi-automated microdilution assay, anti-leishmanial assay, anti-promastigote assay, anti-amastigote assay, MTT assay) [16] | The aqueous and methanol leaf extracts were reported to have low cytotoxicity against human embryonic lung fibroblast (HELF) cell lines (CC50 > 500 µg/mL) [16] |
| Allium sativum L. |
Malaria, wound disinfectant, intestinal infections [17], cold [18], aphrodisiac [19] |
Chemoprophylaxis against lead nitrate induced toxicity in mice [20], increase in the weight of seminal vesicles and epididymis of male animals and elevation of sperm count [21], antibacterial and antifungal activity (essential oil extracts) [22] |
In vivo (Swiss albino mice) [20], in vivo (Swiss albino mice) [21], in vitro (disc diffusion and yeast glucose Chloramphenicol Agar method) [22] |
The LD50 in rabbits was reported to be 3034 mg/kg with a maximum tolerated dose of 2200 mg/kg [23]. Mortality in rabbits was recorded at 3200 and 4200 mg/kg. Anorexia and paralysis were observed in rabbits at high doses [23] The aqueous extract at a 300 mg/kg dose was reported to have mild toxicity symptoms in Wistar rats, but doses of 600 mg/kg and 1200 mg/kg were reported to elevate biochemical parameters. No toxicity was reported up to a dose of 2500 mg/kg and LD50 was reported to be > 5000 mg/kg [24] |
| Rhus natalensis Bernh. | Diarrhea, influenza [25] Respiratory disorders, Malaria [26] |
Antinociceptive activity (dichloromethane-methanol extract) [27], antibacterial activity (aqueous extract) [25] |
In vivo (Swiss albino mice) [27], in vitro (Standard plate count method) [25] |
3-(Z)-heptadec-14-enyl) benzene-1-ol isolated from the ethyl acetate root extract of R. natalensis was reported to be toxic in brine shrimp larvae (LC50 = 7.25 µg/mL), induced apoptosis, and caused cell cycle arrest [28] |
| Steganotaenia araliacea Hochst. | Skin diseases [29], tuberculosis [30] |
Antibacterial activity (aqueous and methanol root extracts) [31], uterotonic activity in uterine strips of pregnant rats [32], diuretic activity (aqueous, methanol, and ethanol stem bark extracts) [33] |
In vitro (Agar well diffusion method) [31], ex vivo (Wistar rats; organ bath) [32], in vivo (Wistar rats) [33] |
The 80% ethanolic stem bark extract was reported to be cytotoxic against MDA-MB-231 (breast), PANC-1 (pancreas), and HT-29 (colon) cancer cell lines [34] Dibenzocyclo-Octadiene, a lignan constituent was reported to have antimitotic activity [35]. Steganacin (an isolated compound) was reported to inhibit the polymerization of tubulin and to slow the depolymerization of pre-formed microtubules in the sea urchin egg assay [36] |
| Carissa edulis (Forssk.) Vahl | Respiratory infections [37], chest pains [38, 39] | Anti-bacterial activity (S. aureus, E. coli) [40] | In vitro (Agar well diffusion method) [40] |
No acute toxicity was observed in mice at oral therapeutic doses of up to 250 mg/kg [41]. The methanol root bark and the aqueous and methanol root extracts were reported to be cytotoxic to brine shrimp larvae (LC50 = 255.06 µg/mL, 260.34 µg/mL, and 186.71 µg/mL respectively) [42, 43] |
| Artemisia annua L. | Fever [18] |
Antimicrobial activity [44] antioxidant activity [45], |
In vitro (Agar well diffusion method) [44] In vitro (total phenolic content assay, total flavonoid content assay, Ferric reducing antioxidant power assay, Trolox equivalent antioxidant capacity assay) [45], in vitro |
The dichloromethane and methanol extracts were reported to be cytotoxic against Trypanosoma brucei brucei (TC221 cells) [50] Artemisinin and quercetagetin 6,7,3′,4′-tetramethyl ether were reported to be cytotoxic against P-388, A-549, HT-29, MCF-7, and KB tumor cells [47]. The ethanol extract was reported to be cytotoxic against Molt-4 human leukemia cells and normal leukocytes [48]. The methanol extract was reported to be cytotoxic and genotoxic against meristem cells of Allium cepa [49] |
| Microglossa pyrifolia (Lam.) Kuntze |
Ovarian cysts [17], |
Antioxidant activity (leaf extracts) [52], cancer cell line cytotoxicity [53], antiplasmodial activity (dichloromethane leaf extract) [54] |
In vitro (2,2-diphenyl picryl hydrazyl (DPPH) assay) [52], In vitro (Resazurin assay) [53], In vitro (lactate dehydrogenase assay) [54] |
The organic leaf extract was reported to be cytotoxic against CCRF-CEM leukemia and decreased cell growth by 48% [53] |
| Tithonia diversifolia (Hemsl.) A. Gray |
abscesses, snake bite [56] |
Antiplasmodial activity (ethanol leaf extracts) [57], antibacterial and antifungal activity (aqueous and ethanol leaf extracts) [58], antiplasmodial activity [59] |
In vivo (Swiss albino mice) [57], in vitro (Agar diffusion method) [58], In vitro (Semi-automated microdilution technique) [59] |
Sesquiterpenoids isolated form the 80% ethanol extract of aerial parts were reported to be cytotoxic against HL-60 leukemia cells [60] Acetyltagitinin E and Tagitinine-F (leaf isolated compounds) were reported to be selectively cytotoxic against Hep G2 human hepatocellular carcinoma cells [61]. Tagitinin C (isolated from the leaves) was reported to be cytotoxic against colon cancer, other malignant cell lines [62, 63], and brine shrimp larvae [64] |
| Kigelia africana (Lam.) Benth. |
Pneumonia [65], tuberculosis [30], measles in children [39] |
Antibacterial activity (ethanol stem bark and fruit extracts) [66], antifungal activity [67], antibacterial, antifungal, antigiardial, and anticancer properties (Aqueous and methanol fruit extracts) [68] |
In vitro (Micro titre plate bioassay) [66], in vitro (Agar diffusion method) [67], in vitro (Modified disc diffusion method) [68] |
A 2000 mg/kg oral dose of the aqueous extract of the fruit was reported to cause hepatorenal toxic effects in Wistar rats [69] An 80% methanol extract of the fruit and roots was reported to be cytotoxic to brine shrimp larvae (LC50 = 240 µg/mL and 7.2 µg/mL respectively) [70] The aqueous bark extract was reported to be toxic to the African catfish (Clarias gariepinus) [71] The aqueous fruit extract was reported to be toxic to Artemia franciscana nauplii toxic with an LC50 value of 477 µg/mL [68] Compounds isolated from the hexane fraction of the stem bark were reported to be toxic against LLC/MK2 (monkey kidney epithelial cells) [72] The aqueous stem bark extract had a dose-dependent mortality on culet mosquito larvae [73] The ethanol stem bark extract was reported to be nontoxic to brine shrimp larvae (LC50 > 1000 µg/mL) [74] |
| Commiphora africana (A.Rich.) Engl. |
Malaria, fever [75], swollen testicles, and abdominal pains [39], pneumonia [25] |
Antifungal and antibacterial activity (Ethanolic root extract) [76] | in vitro (Agar diffusion technique) [76] |
The 95% ethanol extract was reported to be nontoxic in mice and no mortality was observed even at concentrations of up to 5000 mg/kg. However, drowsiness in doses between 1200 and 5000 mg/kg was reported [77] The compounds isolated from the methanol stem bark fraction (resveratrol derivatives) were reported to have low cytotoxicity on prostate cancer cell lines [78]. The ethanol root extract was reported to be nontoxic in brine shrimp larvae [74] |
| Warburgia salutaris (G.Bertol) Chiov |
Chest complaints, cough, fever, pneumonia [79], yellow fever [80], common cold, malaria [81], Aspergillosis [82] |
Fungicidal activity against Fusarium species (Acetone extract) [83] antimycobacterial activity against S. aureus, B. subtilis, S. epidermis, M. luteus, E. coli, and K. pneumoniae) [84] |
in vitro (Hole plate diffusion method, microdilution method) [83], in vitro (Bioautography assay) [84] |
The acetone leaf extract was reported to be cytotoxic against cancer cell lines [85] |
| Carica papaya L. |
Malaria, liver disease [12], tuberculosis [30], malaria, [86, 87], fever [18] |
Antibacterial activity (Methanol root extract) [88], antitumour activity and immunomodulatory effects (Aqueous leaf extract) [89] |
in vitro (Cup plate agar diffusion method) [88], in vitro (Cell viability assay, caspase assay, microarray analysis) [89] |
The aqueous and ethanol leaf extracts were reported to be cytotoxic on human oral squamous cell carcinoma SCC25 cell lines [90] The aqueous leaf extract was reported to disrupt cell division and to induce mitotic spindle disturbance in Allium cepa [91] The methanol leaf extract was reported to be cytotoxic against LLC-MK2 cell lines [92] The aqueous leaf extract was reported to be non-toxic in Sprague Dawley rats at a 2000 mg/kg dose [93] No morphological alterations were reported in Sprague Dawley rats treated with a 28-day repeated oral dose of 2000 mg/kg [94] Aqueous and ethanol leaf extracts were reported to be nontoxic at doses of up to 5000 mg/kg [95] The methanol leaf, root, and stem bark extracts were reported to be nontoxic against MRC-5 cell lines (CC50 > 32 µg/mL) [96] |
| Terminalia brownii Fresen |
Cough, bronchitis [97, 98], allergy, diabetes, malaria [25, 98], clotting agent, coughs and joint stiffness [99] |
Anti-fertility effect (Ethyl acetate extracts) [100], antibacterial activity against S. aureus, E. coli, and B. subtilis (Aqueous bark extract) [25] |
in vivo (Swiss mice) [100], in vitro (Standard plate count method) [25] |
Doses of between 500 and 1000 mg/kg of the methanol root bark extracts were reported to cause dullness and decreased activity of Swiss albino rats [101] |
| Ipomoea kituiensis Var | Constipation, digestive disorders [99] | Acaricidal activity (Methanol:DCM (1:1 v/v) leaf extract) [102] | in vivo (Modified larval packet test) [102] | The aqueous extract was reported to be moderately toxic to brine shrimp larvae (LC50 = 136.96 µg/mL) [102] |
| Euclea divinorum Hiern. | Contractile activity of isolated rabbit uterine strips (aqueous and ethanol root bark extracts) [106] | ex vivo (Organ bath; Swiss white rabbits) [106] |
The aqueous and organic root extracts were reported to cause retarded growth and altered biochemical parameters in mice [107] The methanol root extract was reported to be cytotoxic against MEC-5 fibroblast cells (IC50 = 27.5 ± 3.6 µg/mL) [108] |
|
| Croton megalocarpus Del. | Influenza, pneumonia, wounds, family planning, typhoid, over bleeding during menstruation cycle and birth [105] |
Antibacterial and antifungal activities (petroleum ether and aqueous leaf extracts) [109], antifungal activity (The methanol leaf extract) [110] |
in vitro (Agar well and disc diffusion assays) [109], in vitro (Agar well diffusion technique) [110] |
The LC50 was reported to be < 250 µg/mL in the brine shrimp lethality assay [111] |
| Croton dichogamous Pax |
Chest congestion (wheezing) [112] Polio like-symptoms, gonorrhea, chest pains [39] Threatened abortion, infertility [113] Pesticidal activity [114] |
No reports | No reports | No reports |
| Harungana madagascariensis Lam. Ex Poir | Gastrointestinal disorders [115] |
Antibacterial activity against B. subtilis, S. aureus, E. coli, P. aeruginosa (Aqueous leaf extract) [115], antibacterial activity against S. typhi, S. paratyphi, S. paratyphi B and S. typhimurium (Aqueous extracts) [116], antibacterial activity (Astilbin or 3-O-α-l-rhamnoside-5,73,4′-tetrahydroxydihydroflavonol) [117] |
in vitro (Modified agar well diffusion method) [115] In vitro (Broth dilution technique) [116], in vitro (Solid dilution method, bioautography) [117] |
The aqueous leaf extract was reported to induce liver damage at high doses of > 100 mg/kg and > 200 mg/kg in female and male rats respectively [118]. A 400 mg/kg dose of the iso saline leaf extract administered intraperitoneally in Sprague-Dawley rats significantly elevated serum levels of alanine and aspartate aminotransferase, and significantly lowered the blood glucose levels [119]. |
| Gladiolus dalenii Van Geel | Epilepsy, diarrhea, nasopharyngeal infection, intestinal spams [120] |
Antibacterial activity against S. pyogenes, K. pneumoniae (95% ethanolic extract) [121] Antifungal activity against Aspergillus niger (1:1 dichloromethane/methanol (1:1) extract) [122] |
in vitro (Agar well diffusion method) [121], in vitro (Disc diffusion method) [122] |
Reported to contain cytotoxic substances that affect mitotic active tissue [123]. There was no indication of mutagenesis when dichloromethane and 70% ethanol extracts were tested on S. typhimurium (Ames test) (TA98) [124] |
| Clerodendrum myricoides (Hochst.) R.Br.ex Vatke |
Malaria [125] Febrile convulsions, Abdominal colic [126] Respiratory infections [37] Pneumonia [25] |
Antibacterial and antifungal activity (Organic root extract) [127], antibacterial activity (Aqueous and methanol leaf extract) [128], antiplasmodial activity (Methanol leaf extract) [129] |
in vitro (Agar disc diffusion method) [127], in vitro (agar diffusion method) [128], in vivo (Swiss albino mice) [129] |
The dichloromethane root bark extract was reported to be nontoxic on L6 cells (IC50 > 90 µg/mL) [130]. The methanol root extract was reported to be toxic to brine shrimp [131] |
| Plectranthus barbatus Andr. |
Abdominal pain, diarrhea [132], tuberculosis [30], malaria [133], wounds, swelling, |
Larvicidal properties (Eugenol, α-pinene and β-caryophyllene l) [136], anticonvulsant activity (Hydroalcoholic leaf extract) [137], inhibition of HIV-1 enzymes, antioxidant and anti-inflammatory activities (Ethanol leaf extract) [138] |
in vivo (Third instar mosquito larvae) [136], in vivo (Swiss albino mice) [137], in vitro (MTT assay, flow cytometric analysis, HIV-1 protease fluorogenic assay, HIV-1 transcriptase colorimetric assay, DPPH free radical scavenging assay) [138] |
The ethanol extract was reported to have low cytotoxicity against PBMCs and TZM-bl cell lines (IC50 values = 83.7 and 50.4 µg/mL respectively) [138] The methanol leaf extract was reported to be toxic to Artemia salina (LC50 = 186.33 µg/mL) [139] The chloroform aerial part extract was reported to reduce the viability of undifferentiated/anaplastic thyroid cancer cell lines [140] |
| Vitex doniana Sweet |
Hypertension, diabetes, ulcers [141], malaria, measles [142], gastroenteritis, diarrhea [143], diuretic, diabetes [144] |
Antimicrobial activity (Methanol stem bark extract) [145, 146], antioxidant activity (Aqueous leaf extract) [147], wound healing properties (Hydroalcoholic stem bark extract) [148] |
in vitro (Paper disc assay method, Agar well diffusion method) [145, 146], in vitro (DPPH assay) and in vivo (Swiss albino mice) [147], in vivo (ICR mice) [148] |
The organic leaf and bark extracts were reported to be non-toxic to mammalian L6 cell lines (IC50 > 90 µg/mL) [149] |
| Acacia robusta Burch. | Malaria [150], fibroids [113] | Antifungal activity (Methanol root bark extract) [151] | in vitro (Broth dilution) [151] | The methanol stem bark extract was reported to be toxic to brine shrimp (LC50 = 108.5 µg/mL) [70] |
| Albizia zygia (DC.) J.J.Macbr. |
Antimalarial activity [152, 153], anticancer [154], cough, fever, aphrodisiac, counter female sterility [155], bronchial disease, fever [156] |
Antimicrobial activity (Methanol and hexane extracts) [155], anti-inflammatory and antioxidant activity (Ethanol stem bark extract) [157] |
in vitro (Agar diffusion) [155], in vivo (chicks), and in vitro (DPPH) [157] |
The ethanol stem bark extract was reported to be nontoxic against MRC-5 cells (> 64 µg/mL) [96] The methanol extract was reported to be more toxic to brine shrimp than the non-polar n-hexane extract (LC50 1.70 µg/mL compared to 174.19 µg/mL) [155] |
| Rhynchosia elegans var. elegans | Malaria, common cold, fever [12] | No reports | No reports | No reports |
| Tamarindus indica L. |
Malaria [158, 159], constipation, jaundice [97], aphrodisiac [19], general wellbeing |
Antibacterial activity against P. mirabilis (Acetone stem bark extract) [160], antibacterial activity against S. aureus, E. coli, and P.aeurigenosa (Aqueous pulp extract) [161] |
in vitro (Paper disc diffusion method) [160], in vitro (disc diffusion method) [161] |
The LD50 values of various crude extracts and 25–50% fractions were reported to be in the range of between 832 and 5019 µg/mL [162] The acute oral toxicity studies of the pulp extract at 3000 mg/kg and 5000 mg/kg body weight resulted in no mortality in Wistar albino rats [163] |
| Tylosema fassoglense (Kotschy ex Schweinf.) Torre & Hillc. | Epilepsy, infertility in women, renal disease, cancer [132] |
Antibacterial activity (Methanol extracts) [164], antifungal activity, and cytotoxicity (Ethyl acetate extracts) [165] |
in vitro (disk-diffusion assay) [164], in vitro (Broth microdilution method) and in vivo (brine shrimp cytotoxicity) [165] |
The dichloromethane, ethyl acetate, and aqueous extracts were reported to be toxic to brine shrimp (LC50 = 203.66 µg/mL, 7.58 µg/mL, and 17.57 µg/mL respectively) [165] |
| Azadirachta indica (L) Burm. |
control blood sugar levels [167], tuberculosis [30] |
Antibacterial activity against S. typhi and antifungal activity against C. albicans (n-hexane extract) [168], antioxidant and antibacterial properties (50% ethanol leaf extract) [169] |
in vitro (Ditch well diffusion method) [168], in vitro (Agar well diffusion method [169] |
The aqueous and methanol leaf extracts were reported to be non-toxic against MRC-5 cells (CC50 > 32 µg/mL) [96] The methanol leaf extract was reported to be toxic to brine shrimp larvae (LC50 = 233.061 µg/mL) [42] The aqueous and methanol leaf extracts were reported to be toxic to brine shrimp larvae (LC50 = 101.26 and 61.43 µg/mL respectively) [43]. |
| Khaya senegalensis Desr. A. Juss |
Diabetes, hypertension [170], hepatic inflammations, sinusitis [97]. malaria [87] |
Antibacterial activity against S. enterica subsp. Enterica serovar typhi (50% ethanolic leaf extract) [171], in vivo hypoglycemic activity (Ethyl acetate extract) [172], hepatoprotective effects [173], antioxidant activity (Ethanolic extract) [174] |
in vitro (Agar well diffusion method) [171], in vivo (rats) [172], in vivo (rats) [173], in vitro (DPPH radical scavenging assay, deoxyribose assay, Nitric oxide radical scavenging assay) [174] |
Orally administered ethanol stem bark extract in rats at a dose of 2 mg/kg for 18 days was reported to induce the synthesis of liver enzymes [175]. The subchronic administration of the aqueous stem bark extract to rats was reported to affect the cellular integrity of vital organs of the body [176]. Sub-chronic administration of the aqueous stem bark extract in albino rats was reported to cause the elevation of liver enzymes, and to Increase plasma total protein, blood urea, and creatinine [177]. |
| Mollugo nudicaulis Lam. | Whooping cough and jaundice [178] |
Antioxidant and antibacterial activity(Methanol leaf extract) [179], antidiabetic properties (Ethanolic whole-plant extract) [180] |
in vitro (Total phenolic content assay, total flavonoid content assay, ABTS scavenging activity assay, DPPH radical scavenging assay, agar disc diffusion assay) [179] in vivo (Wistar rats) [180] |
No reports |
| Moringa oleifera Lam. |
Malnutrition [75], tuberculosis [30], loss of memory, prostate cancer [105], flu, asthma, hypertension, malaria [181] |
Antibacterial activity against P. aeruginosa and S. aureus (Fresh leaf juice and aqueous seed extracts) [182], chemoprophylaxis against Artesunate-amodiaquine induced liver damage (aqueous-methanol leaf extracts) [183] |
in vitro (Paper disc diffusion method) [182], in vivo (Wistar rats) [183] |
The aqueous leaf extract was reported to increase the cytotoxic effect of chemotherapy on pancreatic cancer cells [184] The organic leaf extract was reported to be toxic to brine shrimp larvae [185] The aqueous extract was reported to be strongly cytotoxic on Hela cells [186] |
| Eucalyptus camaldulensis Dehnh |
Tuberculosis [30], malaria, liver disorders [75], respiratory tract congestion, chronic bronchitis, coughing, tuberculosis [187] |
Antibacterial activity (Essential oil from the leaves) [188], antibacterial activity against H. pylori (N-hexane and chloroform leaf extract) [189], antimycobacterial activity against M. tuberculosis and M. bovis strains (Methanol extracts) [190] |
in vitro (Aromatogram, micro atmosphere test, broth dilution method [188], in vitro (Agar disc diffusion) [189], in vitro (Resazurin microtiter assay) [190] |
The aqueous-acetone extract was reported to be cytotoxic on MCF-7 and HCT-116 cell lines [191] The essential oils from fresh leaves were reported to inhibit egg hatchability and to suppress the second stage juvenile viability of root-knot nematode Meloidogyne incognita [192] The methanol leaf extract was reported to be cytotoxic against human breast cancer cell lines (MCF 7 and MDA-MB-231) cell lines [193] The methanol leaf extract was reported to be cytotoxic on P19 embryonal carcinoma cells [194] |
| Syzygium cumini (L.) Skeels. |
Asthma, bronchitis, sore throat [195], coughing, diabetes, dysentery, ringworms, inflammation [196], diarrhea, dysentery, wounds, constipation [167] |
Anti-inflammatory activity in mice (Ethanol bark extract) [197], hypoglycemic activity (Aqueous bark extract) [198] |
in vivo (mice) [197] in vivo (rats) [198] |
The methanol extract was reported to have an LD50 value of > 5000 mg/kg in mice [199] The ethanol extract was reported to be nontoxic to rats at doses of up to 5000 mg/kg [200] The ethanol bark extract was reported to be nontoxic in mice at doses of up to 10.125 g/kg [197] |
| Ximenia americana L. | Throat infection, amenorrhea, wound healing, pain [201] |
Antimicrobial activity against E. coli, P. aeruginosa, and P. vulgaris (bark, leaf, and root extracts) [202], antioxidant activity (Methanol stem bark extract) [203] |
in vitro (cup-plate agar diffusion method) [202], in vitro (DPPH radical scavenging assay) [203] |
The methanol stem bark extract was reported to be nontoxic against MRC-5 cell lines (CC50 = 64 µg/mL) [96] |
| Clematis hirsuta Guill. & Perr | Colds, cleanser [105], chest problems [134] | Antifungal activity against C. albicans [204] | in vitro (Liquid dilution method) [204] | The oral administration of an 80% methanol leaf extract did not result in any physical signs e.g. depression, decrease in feeding activity, and hair erection in Swiss albino mice [205] |
| Gardenia ternifolia Schumach. & Thonn. |
Hypertension [170] Treat dysentery, urinary tract infections [206] |
Antimicrobial activity against C. coli, C. jejuni, S. aureus (Aqueous extract) [206], antiplasmodial activity (80% methanol root bark extract) [207], viricidal activity against African Swine Fever Virus (Ethanol root extract) [208] |
in vitro (disc diffusion method) [206], in vivo (Swiss albino mice) [207], in vitro (Plaque titration technique) [208] |
The ethanol root extract was reported to be non-toxic on human carcinoma cell lines [209] |
| Keetia gueinzii (Sond.) Bridson | Malaria [166] | Antimycobacterial activity against pathogenic and non-pathogenic Mycobacterium species [210] | in vitro (Bioautography and the modified two-fold serial dilution microplate method; anti mycobacterial activity) [210], in vitro cytotoxicity; MTT assay [210] | The acetone leaf extract was reported to have an LC50 of 0.142 in vero cell lines and 0.063 in SI C3 A cell lines [210] |
| Harrisonia abyssinica Oliv. |
Arthritis, sexually transmitted infections [26], stomach ache, |
Antifungal activity [211], antiviral, antifungal, antibacterial, and molluscicidal activity [212] |
in vitro (Agar well diffusion method) [211], in vivo (Molluscs) [212] |
The methanol root bark extract was reported to be cytotoxic in brine shrimp (LC50 = 198.498 µg/mL) [42] |
| Teclea nobilis Del. |
Antipyretic [213], malaria, headache, joint pains, common cold, pneumonia, intestinal worms, chest pain [134], arthritis [39] |
Antipyretic and analgesic activity and found to be weakly active against carrageenan edema (Ethanol leaf extract) [214], anti-inflammatory, analgesic, and antipyretic activities (Acetonitrile leaf extract, hexane leaf extract, and Lupeol) [215], anti-caseinolytic activity against B. arietans venom (Methanol root extract) [216] |
in vivo (Wistar-Nossan rats) [214], in vivo (Wistar rats) [215], in vitro (Spectrophotometry) [216] |
The dichloromethane and ethanol extracts of aerial parts were reported to be cytotoxic to brine shrimp (LC50 = 75.5 µg/mL and 156.6 µg/mL respectively) [217] |
| Toddalia asiatica L. |
Sore throat, Malaria [218], fever, stomach ache [219], abdominal pains, gynecologic disorders including infertility, common colds, cancer, renal disorders [132], tuberculosis, [30], common cold, fever, malaria, pneumonia, chest pain [134], colds, respiratory diseases e.g. cold, asthma, chest pain, toothache [105], malaria and bark for respiratory disorders [39] |
Larvicidal activity (Hexane, acetone, and methanol leaf extracts) [220], antifungal activity against Candida albicans (Ethyl acetate leaf extracts) [221], antinociceptive and anti-inflammatory effects (1:1 dichloromethane-methanol root extract) [222] |
in vivo (Aedes egyptii and Culex quinquefasciatus) [220], in vitro (Agar well diffusion method) [221], in vivo (Swiss albino mice) [222] |
Compound 13 isolated from the root was reported to be cytotoxic against the MCF-7 cell line (IC50 = 8.7 µg/mL) but was inactive on Vero cells. Alkaloid 11 was reported to be cytotoxic against KB, NCI-H187, MCF-7, and vero cell lines (IC50 values ranging from 0.8 to 11.6 µg/mL) [223] Essential oils from the leaves were reported to be cytotoxic against breast (MCF-7) and colorectal (HT-29) cancer cell lines [224] (IC50 values = 7.80 µg/mL and 100.0 µg/mL respectively). Benzo[c]phenanthridine and secobenzo[c]phenantridine alkaloids isolated from the ethanol root extract was reported to be cytotoxic on tumor cell lines [225] The acute toxicity and cytotoxicity of the aqueous, ethyl acetate, and methanol leaf extract and root extracts were reported to be > 1000 mg/kg (LD50) and > 100 µg/mL (CC50) respectively [219] The alkaloid (1,3)benzodioxolo(5,6-c)phenanthridine, 12,13-dihydro-2,3-dimethoxy-12-methyl-(dihydronitidine) was reported to be highly cytotoxic to human lung adenocarcinoma (A549) cells [226] |
| Zanthoxylum chalybeum (Eng) Engl. |
Tuberculosis [30], malaria [166], pneumonia, [134], cough, cervical cancer [227] |
Antibacterial activity against S. aureus (Methanol extracts) [128], antihyperglycemic activity (Aqueous stem bark extract) [228], antimicrobial activity against B. cereus and MRSA (Aqueous root bark extract) [229], antiplasmodial activity (Aqueous root bark extract) [230] |
in vitro (Agar well diffusion method) [128], in vivo (Wistar rats) [228], in vitro (Agar well diffusion method) [229], in vivo (Swiss albino mice) [230] |
The methanol root bark extract was reported to be toxic to brine shrimp (LC50 = 68.9 µg/mL) [70] The ethanol root extract was reported to be toxic in brine shrimp larvae (38.51 µg/mL) [74] The organic root extract of Zanthoxylum chalybeum (Eng) Engl. (Rutaceae) was reported to be cytotoxic in brine shrimp (LC50 = 11 µg/mL) [231] A 2000 mg/kg dose of the aqueous and organic extracts were reported to be nontoxic in mice [230] The organic extract was reported to be toxic in brine shrimp larvae (LC50 = 42.73 µg/mL) [230] |
| Zanthoxylum gilletii (De Wild.) P.G.Waterman | Malaria [51] | Antiplasmodial activity against P. falciparum (50% MeOH in CH2Cl2 extract) [232] | in vitro (non-radioactive Malaria SYBR Green I assay) [232] |
Lupeol (an isolated compound) was reported to be cytotoxic against a panel of drug-sensitive and MDR tumor cells via multiple mechanisms with marginal or no effect on normal cells at similar doses [233]. The ethanol stem bark extract was reported to be cytotoxic on leukemia CCRF-CEM cells (IC50=9.04 µg/mL) [234]. |
| Cissus rotundifolia (Forssk.) Vahl |
Threatened abortion/contraception [113] Pain [128] Malaria, liver disease and otitis [235] Malaria [159] |
Antibacterial activity (Buffered methanol (80% methanol and 20% PBS) and acetone) [236], hypoglycemic activity(Aqueous leaf extracts) [237] |
in vitro (Agar well disc diffusion assay) [236], in vivo (Wistar rats) [237] |
The methanol (70%) extract of aerial parts was reported to be more cytotoxic on MCF-7 (breast cancer) cell lines than doxorubicin (IC50 = 0.77 µg/mL and 3.45 µg/mL respectively) [238] |
| Rhoicissus revoilii Planch | Pneumonia, tonsillitis [239] | Antifungal activity against C. albicans (Ethanol extract) [239] | in vitro (Agar well disc diffusion assay) [239] | No reports |
Discussion
Socio-demographic information of herbalists in the study area
Many of the herbalists interviewed in this study were older members of the society. It has previously been reported that traditional herbal practice is usually a preserve of the older members of the society [240, 241]. It is also important to note that it is often harder for the younger generation of herbalists to be accepted by their communities as they are considered to be inexperienced in key tenets of traditional herbal medicine [240, 241]. The observation that many of the interviewed herbalists had not received any formal education seems to agree with what has been observed by other authors [241].
Diversity of medicinal plants identified in the study area and their use
The Leguminosae plant family was the most dominant family indicated for respiratory illnesses in the study area. According to Christenhusz and colleagues, Leguminosae has a large global distribution and is the 3rd largest plant family in the world (after Orchidae and Asteraceae) [242]. The worldwide distribution of this plant family may have some influence on the decision of herbalists to use the plants from this family [243].
The predominance of trees as a source of herbal therapies may have something to do with their abundance, easy availability throughout the year, and resistance to drought and seasonal variations [243–245]. Leaves are considered by herbalists to be important photosynthetic organs [241, 243]. Thus, it is not surprising that they were the most frequently used plant parts in the study area.
It was disturbing to note that many of the herbalists in the area were uprooting the plants that they used for making some of the indigenous remedies. Furthermore, in the course of the interview, some of the herbalists had reported that Warburgia salutaris and Zanthoxylum gilletii were no longer available in some parts of Kisumu East Sub County owing to poor conservation practices. According to Maroyi, it is not advisable to over use the roots and stem barks of plants for medicinal value as this may sabotage plant conservation efforts [246]. Notwithstanding, some herbalists reported that they only collected plant parts in quantities that were enough for their work and which would not hamper conservation efforts. It is also worth mentioning that a local name for Acanthus polystachyus was not available. Instead, there was a consensus among the interviewed herbalists that ‘Nyanandi’ was the closest semblance to a name that this plant could be given on account of the assertion that it may have originally have been brought in from Nandi County which happens to be an immediate neighbor of Kisumu County.
Dosage, mode of preparation, and route of administration
Teaspoons and tablespoons were used for measuring the dosages of powdered plant materials such as barks, stems, or roots while glasses or cups were used for measuring doses of concoctions or decoctions. While the use of 300/500 mL cups was commonly recommended by the herbalists as a means of measuring the dosages of concoctions/decoctions to be used, there was ambiguity in how this was applied. This trend was also observed in a previous report where medicinal plants used for maternal healthcare in Katsina state, Nigeria were surveyed [18].
Decoctions and concoctions were the most common method of preparing indigenous remedies and was done by the herbalist or by the patient who was given instructions on how to make the preparation. The process often involved harvesting the plants, drying them in the sun or in the house for a period of several days, and crushing them into powder with the aid of a homemade mortar and pestle.
The preparations would then be stored in plastic soda bottles that varied between 500 mL and 2 L and sold to the patients directly or in the market. Powdered plant parts could be included in tea and administered orally.
The route of administration was majorly orally. In the case of Eucalyptus camaldulensis, decoctions were prepared by boiling the leaves in an earthen pot and the patient was advised to cover themselves with a blanket such that the emanating steam completely engulfed them. This was done over a period of time and the patient would later be advised to take 2 teaspoons of the decoction in the event that they had a common cold. Patients were asked to revert back to the herbalist for further directions in case they did not feel better. It is worth noting that many of the interviewed herbalists were of the opinion that their remedies rarely failed. In the minds of the herbalists, the failure of the remedies to work was largely due to the incapacity of the patients to follow the instructions issued by the herbalists.
The interviewed herbalists were of the opinion that their remedies had minimal side effects. However, it is not clear whether these herbalists had the capacity to identify any adverse events or whether they had any mechanisms to report such cases whenever they occurred.
Pharmacological reports and toxicology of the medicinal plants documented in this study
To the best of our knowledge, this is the first study to document the medicinal plants used in the management of respiratory illnesses by herbalists in Kisumu East Sub County. It is interesting to note that up to 84.1% of the medicinal plants documented in this study have previously been reported to be effective against Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa, Aspergillus spp, and Candida albicans. These microorganisms have been associated with pneumonia and tonsillopharyngitis [247].
The most cited plants in this study were Warburgia salutaris, Zanthoxylum gilletii, Carissa edulis, Tylosema fassoglensis, and Harrisonia abyssinica. Carissa edulis and Clerodendrum myricoides have been reported to be useful in the management of asthma, cough, and cold [37, 105]. The similarity of our observations to those made by previous authors seems to suggest that there may in fact be a consensus among herbalists from different communities with regard to the usefulness of some of the medicinal plants in their environment.
Toxicological data was not available for 4 species of plants including Croton dichogamous, Rhynchosia elegans, Mollugo nudicaulis, and Rhoicissus revoilii. Moreover, there was no pharmacological data on Croton dichogamus, and Rhynchosia elegans. This may be a potential gap that may need filling in the future.
Conclusions
The predominant use of roots, root barks, and root tubers in preparing decoctions by herbalists in the study area threatens the ecological survival of some of the plant species used. The preservation of ethno medicinal knowledge in the study area is a pressing concern considering the advanced age and little formal education of the herbalists interviewed. Plans to conserve some of the medicinal plants documented in this study should be initiated. There is a need to scientifically scrutinize the medicinal claims made by the herbalists interviewed in this study.
Limitations
The dosage frequency, duration of treatment, and storage condition of the powdered plant material, decoctions, or concoctions were not captured during the interviews. Information on the duration of treatment was also not captured.
Supplementary information
Additional file 1. Summary of the questionnaire used to interview herbalists in Kisumu East Sub County.
Acknowledgements
The authors are grateful for the support of Mr. Peter Olewe and Mr. Kimeu Musembi for their assistance in data collection and identification of the medicinal plants collected from the study area.
Abbreviations
- LRTI
Lower respiratory tract infections
- COPD
Chronic obstructive pulmonary disease
- WHO
World Health Organization
- BAUEC
Biosafety animal use and ethics committee
- RFC
Relative frequency of citation
Authors’ contributions
JKM: Conceptualization, data curation, formal analysis, investigation, methodology, project administration, resources, visualization, writing original draft, and writing review and editing. JMN: Conceptualization, investigation, methodology, supervision, validation, writing review and editing. JMM: Conceptualization, investigation, methodology, supervision, validation, writing review and editing. MOO: Formal analysis, investigation, validation, visualization, writing original draft, writing review and editing. All authors read and approved the final manuscript.
Funding
This work did not receive any external funding.
Availability of data and materials
All data generated or analyzed during this study are included in the text.
Ethics approval and consent to participate
Ethical approval for the study was obtained from the Biosafety, Animal Use and Ethics committee of the University of Nairobi (Ref: FVM BAUEC/2019/210). Approval was additionally sought from regional administrators (the area chief and assistant chief) who were also duly made aware of the study’s objectives. The scope, possible benefits and risks of the study were explained to all willing participants (practitioners of traditional medicine) and consent forms were made available to them for signing.
Consent for publication
Not applicable.
Competing interests
The authors declare no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s13020-020-00374-2.
References
- 1.European Respiratory Society . The global impact of respiratory disease. 2. Sheffield: Forum of International Respiratory Societies; 2017. [Google Scholar]
- 2.Finney LJ, Feary JR, Gordon SB, Mortimer K. Chronic obstructive pulmonary disease in sub-Saharan Africa : a systematic review. Int J Tuberc Lung Dis. 2013;17:583–589. doi: 10.5588/ijtld.12.0619. [DOI] [PubMed] [Google Scholar]
- 3.Murray CJL, Ezzati M, Flaxman AD, Lim S, Lozano R, Michaud C, et al. GBD 2010: a multi-investigator collaboration for global comparative descriptive epidemiology. The Lancet. 2012;380:2055–2058. doi: 10.1016/s0140-6736(12)62134-5. [DOI] [PubMed] [Google Scholar]
- 4.Wang H, Naghavi M, Allen C, Barber RM, Bhutta ZA, Carter A, et al. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015. The Lancet. 2016;388:1459–1544. doi: 10.1016/S0140-6736(16)31012-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Home—Kenya National Bureau of Statistics, Nairobi, Kenya. https://www.knbs.or.ke/. Accessed 13 Mar 2020.
- 6.County K. Kisumu County Integrated Development Plan II, Vision: a peaceful and prosperous County where all citizens enjoy a high-quality life and a sense of belonging. Mission: To realize the full potential of devolution and meet the development aspirations of. 2018;2018:22.
- 7.Agyei-Baffour P, Kudolo A, Quansah DY, Boateng D. Integrating herbal medicine into mainstream healthcare in Ghana: clients’ acceptability, perceptions and disclosure of use. BMC Complement Altern Med. 2017 doi: 10.1186/s12906-017-2025-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Omoruyi BE. Ethnomedicinal survey of medicinal plants used for the management of HIV/AIDS infection among local communities of Nkonkobe Municipality, Eastern Cape, South Africa. J Med Plants Res. 2012 doi: 10.5897/jmpr12.541. [DOI] [Google Scholar]
- 9.Meragiaw M. Wild useful plants with emphasis on traditional use of medicinal and edible plants by the people of Aba’ala, North-eastern Ethiopia. J Med Plant Herb Ther Res. 2016;4:1–16. [Google Scholar]
- 10.Kisumu County–Kisumu. https://www.kisumu.go.ke/. Accessed 13 Mar 2020.
- 11.Tardio J, Pardo-de-Santayana M. Cultural importance indices: a comparative analysis based on the useful wild plants of Southern Cantabria (Northern Spain) Econ Bot. 2008;62:24–39. doi: 10.1007/s12231-007-9004-5. [DOI] [Google Scholar]
- 12.Asnake S, Teklehaymanot T, Hymete A, Erko B, Giday M. Antimalarial medicinal plants used by Gumuz people of mandura woreda, Benishangul-Gumuz regional state, Ethiopia. India: NISCAIR-CSIR; 2016. [Google Scholar]
- 13.Demilew W, Adinew GM, Asrade S. Evaluation of the wound healing activity of the crude extract of leaves of Acanthus polystachyus Delile (Acanthaceae) Evid Based Complement Altern Med. 2018;2018:1–9. doi: 10.1155/2018/2047896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Derebe D, Wubetu M. Antimalarial activity of hydroalcoholic root extract of Acanthus polystachyus Delile (Acanthaceae) against Plasmodium berghei-infected mice. J Evid Based Integr Med. 2019;24:2515690X19885322. doi: 10.1177/2515690X19885322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pascaline J, Charles M, George O, Lukhoba C. An inventory of medicinal plants that the people of Nandi use to treat malaria. J Anim Plant Sci. 2011;9:1192–1200. [Google Scholar]
- 16.Kigondu EVM, Rukunga GM, Keriko JM, Tonui WK, Gathirwa JW, Kirira PG, et al. Anti-parasitic activity and cytotoxicity of selected medicinal plants from Kenya. J Ethnopharmacol. 2009;123:504–509. doi: 10.1016/j.jep.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 17.Kasali FM, Mahano AO, Kadima NJ, Mpiana PT, Ngbolua KN, Tshibangu TSD. Ethnopharmacological survey of medicinal plants used against malaria in Butembo City (DR Congo) J Adv Bot Zool. 2014;1:1–11. [Google Scholar]
- 18.Kankara SS, Ibrahim MH, Mustafa M, Go R. Ethnobotanical survey of medicinal plants used for traditional maternal healthcare in Katsina state, Nigeria. S Afr J Bot. 2015;97:165–175. doi: 10.1016/j.sajb.2015.01.007. [DOI] [Google Scholar]
- 19.Singh R, Singh S, Jeyabalan G, Ali A. An overview on traditional medicinal plants as aphrodisiac agent. J Pharmacogn Phytochem. 2012;1:43–56. [Google Scholar]
- 20.Sharma V, Sharma A, Kansal L. The effect of oral administration of Allium sativum extracts on lead nitrate induced toxicity in male mice. Food Chem Toxicol. 2010;48:928–936. doi: 10.1016/j.fct.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 21.Al-Bekairi AM, Shah AH, Qureshi S. Effect of Allium sativum on epididymal spermatozoa, estradiol-treated mice and general toxicity. J Ethnopharmacol. 1990;29:117–125. doi: 10.1016/0378-8741(90)90049-y. [DOI] [PubMed] [Google Scholar]
- 22.Benkeblia N. Antimicrobial activity of essential oil extracts of various onions (Allium cepa) and garlic (Allium sativum) LWT Food Sci Technol. 2004;37:263–268. doi: 10.1016/j.lwt.2003.09.001. [DOI] [Google Scholar]
- 23.Mikail HG. Phytochemical screening, elemental analysis and acute toxicity of aqueous extract of Allium sativum L. bulbs in experimental rabbits. J Med Plants Res. 2010;4:322–326. [Google Scholar]
- 24.Lawal B, Shittu OK, Oibiokpa FI, Mohammed H, Umar SI, Haruna GM. Antimicrobial evaluation, acute and sub-acute toxicity studies of Allium sativum. J Acute Dis. 2016;5:296–301. [Google Scholar]
- 25.Kareru PG, Gachanja AN, Keriko JMKGM. Antimicrobial activity of some medicinal plants used by herbalists. Afr J Tradit Complement Altern Med. 2008;5:51–55. doi: 10.4314/ajtcam.v5i1.31256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kimondo J, Miaron J, Mutai P, Njogu P. Ethnobotanical survey of food and medicinal plants of the Ilkisonko Maasai community in Kenya. J Ethnopharmacol. 2015;175:463–469. doi: 10.1016/j.jep.2015.10.013. [DOI] [PubMed] [Google Scholar]
- 27.Kariuki HN, Kanui TI, Yenesew A, Mbugua PM, Patel NB. Antinociceptive activity of the root extracts of Rhus natalensis Kraus and Senna singueana. Phytopharmacology. 2012;2:312–317. [Google Scholar]
- 28.Matata DZ, Moshi MJ, Machumi F, Ngassapa OD, Swanepoel B, Oosthuizen K, et al. Isolation of a new cytotoxic compound, 3-((Z)-heptadec-14-enyl) benzene-1-ol from Rhus natalensis root extract. Phytochem Lett. 2020;36:120–126. [Google Scholar]
- 29.Taddese S, Asres K, Gebre-Mariam T. In vitro antimicrobial activities of some selected topically applied medicinal plants of Ethiopia. Ethiop Pharm J. 2003;21:39–46. [Google Scholar]
- 30.Bunalema L, Obakiro S, Tabuti JRS, Waako P. Knowledge on plants used traditionally in the treatment of tuberculosis in Uganda. J Ethnopharmacol. 2014;151:999–1004. doi: 10.1016/j.jep.2013.12.020. [DOI] [PubMed] [Google Scholar]
- 31.Lino A, Deogracious O. The in-vitro antibacterial activity of Annona senegalensis, Securidacca longipendiculata and Steganotaenia araliacea-Ugandan medicinal plants. Afr Health Sci. 2006;6:31–35. doi: 10.5555/afhs.2006.6.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goma FM, Ezeala C, Nyirenda J, Chuba D, Prashar L, Simfukwe N, et al. Extraction and demonstration of uterotonic activity from the root of steganotaenia araliacea hochst. Med J Zambia. 2017;44:125–132. [Google Scholar]
- 33.Agunu A, Abdurahman EM, Andrew GO, Muhammed Z. Diuretic activity of the stem-bark extracts of Steganotaenia araliacea hochst [Apiaceae] J Ethnopharmacol. 2005;96:471–475. doi: 10.1016/j.jep.2004.09.045. [DOI] [PubMed] [Google Scholar]
- 34.Capistrano IR, Wouters A, Foubert K, Balde AM, Apers S, Lardon F, et al. Phytochemical characterisation of a cytotoxic stem bark extract of Steganotaenia araliacea and identification of a protoflavanone by LC–SPE–NMR. Phytochem Lett. 2015;12:119–124. [Google Scholar]
- 35.Wickramaratne DBM, Pengsuparp T, Mar W, Chai H-B, Chagwedera TE, Beecher CWW, et al. Novel antimitotic dibenzocyclo-octadiene lignan constituents of the stem bark of Steganotaenia araliacea. J Nat Prod. 1993;56:2083–2090. doi: 10.1021/np50102a009. [DOI] [PubMed] [Google Scholar]
- 36.Wang RW-J, Rebhun LI, Kupchan SM. Antimitotic and antitubulin activity of the tumor inhibitor steganacin. Cancer Res. 1977;37:3071–3079. [PubMed] [Google Scholar]
- 37.Kariuki AC, Njoroge GN. Ethnobotanical and antimicrobial studies of some plants used in kibwezi (Kenya) for management of lower respiratory tract infections. Afr J Tradit Complement Altern Med. 2011;8:144–149. doi: 10.4314/ajtcam.v8i2.63201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nedi T, Mekonnen N, Urga K. Diuretic effect of the crude extracts of Carissa edulis in rats. J Ethnopharmacol. 2004;95:57–61. doi: 10.1016/j.jep.2004.06.017. [DOI] [PubMed] [Google Scholar]
- 39.Kigen G, Kamuren Z, Njiru E, Wanjohi B, Kipkore W. Ethnomedical survey of the plants used by traditional healers in Narok County, Kenya. Evid Based Complement Altern Med. 2019;2019:1–8. doi: 10.1155/2019/8976937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Abdu KB, Khan ME, Rumah MM. Antimicrobial activity and phytochemical screening of extracts from the root bark of Carissa edulis, against human/animal pathogens. Cont J Trop Med. 2008;2:1. [Google Scholar]
- 41.Tolo FM, Rukunga GM, Muli FW, Njagi ENM, Njue W, Kumon K, et al. Anti-viral activity of the extracts of a Kenyan medicinal plant Carissa edulis against herpes simplex virus. J Ethnopharmacol. 2006;104:92–99. doi: 10.1016/j.jep.2005.08.053. [DOI] [PubMed] [Google Scholar]
- 42.Leonard O, Robert S, Hoseah A, Charles M, Chepkorir R, Ngoci N. Phytochemical characterization and cytotoxicity of Carissa edulis, Azadirachta indica, Cassia siamea and Harrisonia abyssinica from Masumbi Village, Siaya County-Kenya. J Sci Res Rep. 2016;10:1–10. [Google Scholar]
- 43.Kirira PG, Rukunga GM, Wanyonyi AW, Muregi FM, Gathirwa JW, Muthaura CN, et al. Anti-plasmodial activity and toxicity of extracts of plants used in traditional malaria therapy in Meru and Kilifi Districts of Kenya. J Ethnopharmacol. 2006;106:403–407. doi: 10.1016/j.jep.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 44.Tajehmiri A, Issapour F, Moslem MN, Lakeh MT, Kolavani MH. vitro antimicrobial activity of artemisia annua leaf extracts against pathogenic bacteria. Adv Stud Biol. 2014;6:93–97. doi: 10.12988/asb.2014.4525. [DOI] [Google Scholar]
- 45.Iqbal S, Younas U, Chan KW, Zia-Ul-Haq M, Ismail M. Chemical composition of Artemisia annua L. leaves and antioxidant potential of extracts as a function of extraction solvents. Molecules. 2012;17:6020–6032. doi: 10.3390/molecules17056020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nibret E, Wink M. Volatile components of four Ethiopian Artemisia species extracts and their in vitro antitrypanosomal and cytotoxic activities. Phytomedicine. 2010;17:369–374. doi: 10.1016/j.phymed.2009.07.016. [DOI] [PubMed] [Google Scholar]
- 47.Zheng G-Q. Cytotoxic terpenoids and flavonoids from Artemisia annua. Planta Med. 1994;60:54–57. doi: 10.1055/s-2006-959408. [DOI] [PubMed] [Google Scholar]
- 48.Singh NP, Ferreira JFS, Park JS, Lai HC. Cytotoxicity of ethanolic extracts of Artemisia annua to Molt-4 human leukemia cells. Planta Med. 2011;77:1788–1793. doi: 10.1055/s-0030-1271157. [DOI] [PubMed] [Google Scholar]
- 49.Karaismailoglu MC. Investigation of the cytotoxic and genotoxic effects of Artemisia annua methanol extract with the Allium test. Ekoloji. 2014;23:64–74. [Google Scholar]
- 50.Efferth T, Herrmann F, Tahrani A, Wink M. Cytotoxic activity of secondary metabolites derived from Artemisia annua L. towards cancer cells in comparison to its designated active constituent artemisinin. Phytomedicine. 2011;18:959–969. doi: 10.1016/j.phymed.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 51.Guédé NZ, N’Guessan K, Dibié TE, Grellier P. Ethnopharmacological study of plants used to treat malaria, in traditional medicine, by Bete Populations of Issia (Côte d’ivoire) J Pharm Sci Res. 2010;2:216–227. [Google Scholar]
- 52.Akimanya A, Midiwo JO, Matasyoh J, Okanga F, Masila VM, Walker L, et al. Two polymethoxylated flavonoids with antioxidant activities and a rearranged clerodane diterpenoid from the leaf exudates of Microglossa pyrifolia. Phytochem Lett. 2015;11:183–187. doi: 10.1016/j.phytol.2014.12.008. [DOI] [Google Scholar]
- 53.Ochwang’i DO, Kimwele CN, Oduma JA, Gathumbi PK, Kiama SG, Efferth T. Cytotoxic activity of medicinal plants of the Kakamega County (Kenya) against drug-sensitive and multidrug-resistant cancer cells. J Ethnopharmacol. 2018;215:233–240. doi: 10.1016/j.jep.2018.01.004. [DOI] [PubMed] [Google Scholar]
- 54.Muganga R, Angenot L, Tits M, Frederich M. Antiplasmodial and cytotoxic activities of Rwandan medicinal plants used in the treatment of malaria. J Ethnopharmacol. 2010;128:52–57. doi: 10.1016/j.jep.2009.12.023. [DOI] [PubMed] [Google Scholar]
- 55.Mwanauta RW, Mtei KA, Ndakidemi PA. Prospective bioactive compounds from Vernonia amygdalina, Lippia javanica, Dysphania ambrosioides and Tithonia diversifolia in controlling legume insect pests. Agric Sci. 2014;05:1129–1139. [Google Scholar]
- 56.Passoni FD, Oliveira RB, Chagas-Paula DA, Gobbo-Neto L, Da Costa FB. Repeated-dose toxicological studies of Tithonia diversifolia (Hemsl.) A. gray and identification of the toxic compounds. J Ethnopharmacol. 2013;147:389–394. doi: 10.1016/j.jep.2013.03.024. [DOI] [PubMed] [Google Scholar]
- 57.Elufioye TO, Agbedahunsi JM. Antimalarial activities of Tithonia diversifolia (Asteraceae) and Crossopteryx febrifuga (Rubiaceae) on mice in vivo. J Ethnopharmacol. 2004;93:167–171. doi: 10.1016/j.jep.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 58.Liasu MO, Ayandele AA. Antimicrobial activity of aqueous and ethanolic extracts from Tithonia diversifolia and Bryum coronatum collected from Ogbomoso, Oyo State, Nigeria. Adv Nat Appl Sci. 2008;2:31–34. [Google Scholar]
- 59.Goffin E, Ziemons E, De Mol P, De Madureira MDC, Martins AP, Proença da Cunha A, et al. In vitro antiplasmodial activity of Tithonia diversifolia and identification of its main active constituent: Tagitinin C. Planta Med. 2002;68:543–545. doi: 10.1055/s-2002-32552. [DOI] [PubMed] [Google Scholar]
- 60.Kuroda M, Yokosuka A, Kobayashi R, Jitsuno M, Kando H, Nosaka K, et al. Sesquiterpenoids and flavonoids from the aerial parts of Tithonia diversifolia and their cytotoxic activity. Chem Pharm Bull. 2007;55:1240–1244. doi: 10.1248/cpb.55.1240. [DOI] [PubMed] [Google Scholar]
- 61.Wu TS, Shi LS, Kuo PC, Leu YL, Liou MJ, Wu PL, et al. Cytotoxic principles from the leaves of Tithonia diversifolia. Chin Pharm J. 2001;53:217–223. [Google Scholar]
- 62.Wahyuningsih MSH, Wijayanti MA, Budiyanto A, Hanafi M. Isolation and identification of potential cytotoxic compound from kembang bulan [Tithonia diversifolia (Hemsley) A. Gray] leaves. Int J Pharm Pharm Sci. 2015;7:298–301. [Google Scholar]
- 63.Liao M-H, Lin W-C, Wen H-C, Pu H-F. Tithonia diversifolia and its main active component tagitinin C induce survivin inhibition and G2/M arrest in human malignant glioblastoma cells. Fitoterapia. 2011;82:331–341. doi: 10.1016/j.fitote.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 64.Chavez PI, Sánchez IA, Gonzalez FA, Rodríguez JL, Axelrod F. Cytotoxicity correlations of Puerto Rican plants using a simplified brine shrimp lethality screening procedure. Int J Pharmacogn. 1997;35:222–226. [Google Scholar]
- 65.Chenia H. Anti-quorum sensing potential of crude Kigelia africana fruit extracts. Sensors. 2013;13:2802–2817. doi: 10.3390/s130302802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Grace OM, Light ME, Lindsey KL, Mulholland DA, van Staden J, Jager AK. Antibacterial activity and isolation of active compounds from fruit of the traditional African medicinal tree Kigelia africana. S Afr J Bot. 2002;68:220–222. doi: 10.1016/s0254-6299(15)30424-5. [DOI] [Google Scholar]
- 67.Owolabi OJ, Omogbai EKI, Obasuyi O. Antifungal and antibacterial activities of the ethanolic and aqueous extract of Kigelia africana (Bignoniaceae) stem bark. Afr J Biotechnol. 2007;6.
- 68.Arkhipov A, Sirdaarta J, Rayab P, McDonell PA, Cock IE. An examination of the antibacterial, antifungal, anti-Giardial and anticancer properties of Kigelia africana fruit extracts. Pharmacogn Commun. 2014;4:62–76. doi: 10.5530/pc.2014.3.7. [DOI] [Google Scholar]
- 69.Farah HM, El Hussein AM, Khalid HE, Osman HM. Toxicity of Kigelia africana fruit in rats. Adv Res. 2017 doi: 10.9734/AIR/2017/38539. [DOI] [Google Scholar]
- 70.Moshi MJ, Van den Beukel CJ, Hamza OJM, Mbwambo ZH, Nondo ROS, Masimba PJ, et al. Brine shrimp toxicity evaluation of some Tanzanian plants used traditionally for the treatment of fungal infections. Afr J Tradit Complement Altern Med. 2007;4:219–225. doi: 10.4314/ajtcam.v4i2.31211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Onusiriuka BC, Ufodike EBC. Effects of sub-lethal concentrations of Akee Apple, Blighia sapida and Sausage Plant, Kigelia africana on Tissue Chemistry of African Catfish, Clarias gariepinus (L) J Aquat Sci. 2000;15:47–50. [Google Scholar]
- 72.Zofou D, Kengne ABO, Tene M, Ngemenya MN, Tane P, Titanji VPK. In vitro antiplasmodial activity and cytotoxicity of crude extracts and compounds from the stem bark of Kigelia africana (Lam) Benth (Bignoniaceae) Parasitol Res. 2011;108:1383–1390. doi: 10.1007/s00436-011-2363-y. [DOI] [PubMed] [Google Scholar]
- 73.Taura DW, Mukhtar MD, Adoum OA. Lethality of the aqeous extracts of Acacia nilotica, Guiera senegalensis, Kigelia africana and Securidaca longepedunculata on culex mosquito larva. Ife J Sci. 2004;6:115–118. [Google Scholar]
- 74.Mbunde MVN, Innocent E, Mabiki F, Andersson PG. Ethnobotanical survey and toxicity evaluation of medicinal plants used for fungal remedy in the Southern Highlands of Tanzania. J Intercult Ethnopharmacol. 2017;6:84. doi: 10.5455/jice.20161222103956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nadembega P, Boussim JI, Nikiema JB, Poli F, Antognoni F. Medicinal plants in Baskoure, Kourittenga Province, Burkina Faso: an ethnobotanical study. J Ethnopharmacol. 2011;133:378–395. doi: 10.1016/j.jep.2010.10.010. [DOI] [PubMed] [Google Scholar]
- 76.Akor JS, Anjorin TS. Phytochemical and antimicrobial studies of Commiphora africana root extracts. Int J Agric Biol. 2009;11:795–797. [Google Scholar]
- 77.Okwute SK, Ochi IO. Phytochemical analysis and cytotoxic activity of the root extract of Commiphora africana (Caesalpiniaceae) J Pharmacogn Phytochem. 2017;6:451–454. [Google Scholar]
- 78.Segun PA, Ogbole OO, Ismail FMD, Nahar L, Evans AR, Ajaiyeoba EO, et al. Resveratrol derivatives from Commiphora africana (A. Rich.) Endl. display cytotoxicity and selectivity against several human cancer cell lines. Phytother Res. 2019;33:159–166. doi: 10.1002/ptr.6209. [DOI] [PubMed] [Google Scholar]
- 79.Maroyi A. Warburgia salutaris (Bertol. f.) Chiov.: a multi-use ethnomedicinal plant species. J Med Plants Res. 2013;7:53–60. [Google Scholar]
- 80.Kuglerova M, Tesarova H, Grade JT, Halamova K, Wanyana-Maganyi O, Van Damme P, et al. Antimicrobial and antioxidative effects of Ugandan medicinal barks. Afr J Biotechnol. 2011;10:3628–3632. [Google Scholar]
- 81.Coopoosamy RM, Naidoo KK. An ethnobotanical study of medicinal plants used by traditional healers in Durban, South Africa. Afr J Pharm Pharmacol. 2012;6:818–823. [Google Scholar]
- 82.Otang WM, Grierson DS, Ndip N. Ethnobotanical survey of medicinal plants used in the management of opportunistic fungal infections in HIV/AIDS patients in the Amathole District of the Eastern Cape Province, South Africa. J Med Plants Res. 2012;6:2071–2080. [Google Scholar]
- 83.Samie A, Mashau F. Antifungal activities of fifteen Southern African medicinal plants against five Fusarium species. J Med Plants Res. 2013;7:1839–1848. [Google Scholar]
- 84.Rabe T, van Staden J. Isolation of an antibacterial sesquiterpenoid from Warburgia salutaris. J Ethnopharmacol. 2000;73:171–174. doi: 10.1016/s0378-8741(00)00293-2. [DOI] [PubMed] [Google Scholar]
- 85.Soyingbe OS, Mongalo NI, Makhafola TJ. In vitro antibacterial and cytotoxic activity of leaf extracts of Centella asiatica (L.) Urb, Warburgia salutaris (Bertol. F.) Chiov and Curtisia dentata (Burm. F.) CA Sm-medicinal plants used in South Africa. BMC Complement Altern Med. 2018;18:1–10. doi: 10.1186/s12906-018-2378-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ngarivhume T, van’t Klooster CIEA, de Jong JTVM, Van der Westhuizen JH. Medicinal plants used by traditional healers for the treatment of malaria in the Chipinge district in Zimbabwe. J Ethnopharmacol. 2015;159:224–237. doi: 10.1016/j.jep.2014.11.011. [DOI] [PubMed] [Google Scholar]
- 87.Karou S, Agbodeka K, Gbekley H, Anani K, Agbonon A, Tchacondo T, et al. Ethnobotanical study of medicinal plants used for the treatment of malaria in the plateau region, Togo. Pharmacogn Res. 2016;8:12. doi: 10.4103/0974-8490.178646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Doughari JH, Elmahmood AM, Manzara S. Studies on the antibacterial activity of root extracts of Carica papaya L. Afr J Microbiol Res. 2007;1:37–41. [Google Scholar]
- 89.Otsuki N, Dang NH, Kumagai E, Kondo A, Iwata S, Morimoto C. Aqueous extract of Carica papaya leaves exhibits anti-tumor activity and immunomodulatory effects. J Ethnopharmacol. 2010;127:760–767. doi: 10.1016/j.jep.2009.11.024. [DOI] [PubMed] [Google Scholar]
- 90.Nguyen TT, Parat M-O, Hodson MP, Pan J, Shaw PN, Hewavitharana AK. Chemical characterization and in vitro cytotoxicity on squamous cell carcinoma cells of Carica papaya leaf extracts. Toxins. 2016;8:7. doi: 10.3390/toxins8010007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Akinboro A, Bakare AA. Cytotoxic and genotoxic effects of aqueous extracts of five medicinal plants on Allium cepa Linn. J Ethnopharmacol. 2007;112:470–475. doi: 10.1016/j.jep.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 92.Joseph B, Sankarganesh P, Ichiyama K, Yamamoto N. In vitro study on cytotoxic effect and anti-DENV2 activity of Carica papaya L. leaf. Front Life Sci. 2015;8:18–22. [Google Scholar]
- 93.Halim SZ, Abdullah NR, Afzan A, Rashid BAA, Jantan I, Ismail Z. Acute toxicity study of Carica papaya leaf extract in Sprague Dawley rats. J Med Plants Res. 2011;5:1867–1872. [Google Scholar]
- 94.Afzan A, Abdullah NR, Halim SZ, Rashid BA, Semail RHR, Abdullah N, et al. Repeated dose 28-days oral toxicity study of Carica papaya L. leaf extract in Sprague Dawley rats. Molecules. 2012;17:4326–4342. doi: 10.3390/molecules17044326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yamthe LR, David K, Ngadena YM. Acute and chronic toxicity studies of the aqueous and ethanol leaf extracts of Carica papaya Linn in Wistar rats. J Nat Prod Plant Resour. 2012;2:617–627. [Google Scholar]
- 96.Traore MS, Diane S, Diallo MST, Balde ES, Balde MA, Camara A, et al. In vitro antiprotozoal and cytotoxic activity of ethnopharmacologically selected guinean plants. Planta Med. 2014;80:1340–1344. doi: 10.1055/s-0034-1383047. [DOI] [PubMed] [Google Scholar]
- 97.Khalid H, Abdalla WE, Abdelgadir H, Opatz T, Efferth T. Gems from traditional north-African medicine: medicinal and aromatic plants from Sudan. Nat Prod Bioprospect. 2012;2:92–103. doi: 10.1007/s13659-012-0015-2. [DOI] [Google Scholar]
- 98.Salih EYA, Kanninen M, Sipi M, Luukkanen O, Hiltunen R, Vuorela H, et al. Tannins, flavonoids and stilbenes in extracts of African savanna woodland trees Terminalia brownii, Terminalia laxiflora and Anogeissus leiocarpus showing promising antibacterial potential. S Afr J Bot. 2017;108:370–386. doi: 10.1016/j.sajb.2016.08.020. [DOI] [Google Scholar]
- 99.Kaigongi MM, Musila FM. Ethnobotanical study of medicinal plants used by Tharaka people of Kenya. Int J Ethnobiol Ethnomed. 2015;1:1–8. [Google Scholar]
- 100.Kamita MK, Matu EN, Njenga EW, Wanga J, Amalemba G, Kigondu EVM. In vivo antifertility activity and phytochemical screening of selected Kenyan medicinal plants. Afr J Pharmacol Ther. 2014;3.
- 101.Thoria OO, Galal MA, Ashour NA, Hussain AM, Abdelrahman SH. Acute toxicity of the methanolic extracts of Terminalia brownii bark in rats. Res Opin Anim Vet Sci. 2012;2:122–126. [Google Scholar]
- 102.Onyango AO. An ethnobotanical, phytochemical, toxicity and efficacy study of selected antiblue-tick (boophilus decoloratus) herbal remedies for cattle of Suba sub-county. Kenya: University of Nairobi; 2016. [Google Scholar]
- 103.Kidane B, van Andel T, van der Maesen LJG, Asfaw Z. Use and management of traditional medicinal plants by Maale and Ari ethnic communities in southern Ethiopia. J Ethnobiol Ethnomed. 2014;10:46. doi: 10.1186/1746-4269-10-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Cheikhyoussef A, Shapi M, Matengu K, Ashekele HM. Ethnobotanical study of indigenous knowledge on medicinal plant use by traditional healers in Oshikoto region, Namibia. J Ethnobiol Ethnomed. 2011;7:10. doi: 10.1186/1746-4269-7-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kamau LN, Mbaabu PM, Mbaria JM, Gathumbi PK, Kiama SG. Ethnobotanical survey and threats to medicinal plants traditionally used for the management of human diseases in Nyeri County, Kenya. Tang Humanit Med. 2016;6:21.1–21.15. doi: 10.5667/tang.2016.0007. [DOI] [Google Scholar]
- 106.Kaingu CK, Oduma JA, Kanui T. Preliminary investigation of contractile activity of Ricinus communis and Euclea divinorum extracts on isolated rabbit uterine strips. J Ethnopharmacol. 2012;142:496–502. doi: 10.1016/j.jep.2012.05.026. [DOI] [PubMed] [Google Scholar]
- 107.Ngari FW, Gikonyo NK, Wanjau RN, Njagi EM. Safety and antimicrobial properties of Euclea divinorum Hiern, chewing sticks used for management of oral health in Nairobi County, Kenya. J Pharm Biomed Sci. 2013;3:1–8. [Google Scholar]
- 108.Mothana RA, Al-Musayeib NM, Matheeussen A, Cos P, Maes L. Assessment of the in vitro antiprotozoal and cytotoxic potential of 20 selected medicinal plants from the island of Soqotra. Molecules. 2012;17:14349–14360. doi: 10.3390/molecules171214349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kisangau DP, Hosea KM, Joseph CC, Lyaruu HVM. In vitro antimicrobial assay of plants used in traditional medicine in Bukoba Rural District, Tanzania. Afr J Tradit Complement Altern Med. 2008;4:510. doi: 10.4314/ajtcam.v4i4.31245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kiswii TM, Monda EO, Okemo PO, Bii C, Alakonya AE. Efficacy of selected medicinal plants from Eastern Kenya against Aspergillus flavus. J Plant Sci. 2014;2:226–231. [Google Scholar]
- 111.Mwangi JW, Masengo W, Thoithi GN, Kibwage IO. Screening of some Kenyan medicinal plants using the brine shrimp lethality test. 1999.
- 112.Kipkore W, Wanjohi B, Rono H, Kigen G. A study of the medicinal plants used by the Marakwet Community in Kenya. J Ethnobiol Ethnomed. 2014;10:24. doi: 10.1186/1746-4269-10-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kaingu CK, Oduma JA, Mbaria JM, Kiama SG. Medicinal plants traditionally used for the management of female reproductive health dysfunction in Tana River County, Kenya. Tang Humanit Med. 2013;3(2):1–10. [Google Scholar]
- 114.Muhi MQ, Mihale MJ, Mugoyela V, Henry L, Qwarse M, Mihale MJ, et al. Ethnobotanical survey of medicinal and pesticidal plants used by agro-pastoral communities in Mbulu District, Tanzania. Tanzan J Sci Technol. 2018;1:22–35. [Google Scholar]
- 115.Okoli AS, Okeke MI, Iroegbu CU, Ebo PU. Antibacterial activity of Harungana madagascariensis leaf extracts. Phytother Res. 2002;16:174–179. doi: 10.1002/ptr.991. [DOI] [PubMed] [Google Scholar]
- 116.Kengni F, Tala D, Djimeli M, Fodouop S, Kodjio N, Magnifouet H, et al. In vitro antimicrobial activity of Harungana madagascriensis and Euphorbia prostrata extracts against some pathogenic Salmonella sp. Int J Biol Chem Sci. 2013;7:1106. [Google Scholar]
- 117.Moulari B, Pellequer Y, Lboutounne H, Girard C, Chaumont J-P, Millet J, et al. Isolation and in vitro antibacterial activity of astilbin, the bioactive flavanone from the leaves of Harungana madagascariensis Lam. ex Poir. (Hypericaceae) J Ethnopharmacol. 2006;106:272–278. doi: 10.1016/j.jep.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 118.Kengni F, Fodouop SPC, Tala DS, Djimeli MN, Fokunang C, Gatsing D. Antityphoid properties and toxicity evaluation of Harungana madagascariensis Lam (Hypericaceae) aqueous leaf extract. J Ethnopharmacol. 2016;179:137–145. doi: 10.1016/j.jep.2015.12.037. [DOI] [PubMed] [Google Scholar]
- 119.Olagunju JA, Ogunfeibo AB, Ogunbosi AO, Taiwo OA. Biochemical changes elicited by isosaline leaf and stem-bark extracts of Harungana madagascariensis in the rat. Phytother Res. 2004;18:588–591. doi: 10.1002/ptr.978. [DOI] [PubMed] [Google Scholar]
- 120.Ngoupaye GT, Bum EN, Taiwe GS, Moto FCO, Talla E. Antidepressant properties of aqueous acerate from Gladiolus Dalenii corms. Afr J Tradit Complement Altern Med. 2014;11:53–61. [PMC free article] [PubMed] [Google Scholar]
- 121.Gbadamosi IT. Evaluation of antibacterial activity of six ethnobotanicals used in the treatment of infectious diseases in Nigeria. Bot Res Int. 2012;5:83–89. [Google Scholar]
- 122.Odhiambo JA, Siboe GM, Lukhoba CW, Dossaji SF. Antifungal activity of crude extracts of gladiolus dalenii van geel (Iridaceae) Afr J Tradit Complement Altern Med. 2010;7:53–58. doi: 10.4314/ajtcam.v7i1.57254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Van Dyk S, Gerritsma-Van der Vijver LM, Van Der Nest DG. The toxicity of Gladiolus dalenii van Geel. Suid-Afrikaanse Tydskrif vir Natuurwetenskap en Tegnologie. 1994;13:125–128. [Google Scholar]
- 124.Fawole OA, Finnie JF, Van Staden J. Antimicrobial activity and mutagenic effects of twelve traditional medicinal plants used to treat ailments related to the gastro-intestinal tract in South Africa. S Afr J Bot. 2009;75:356–362. [Google Scholar]
- 125.Moshi MJ, Otieno DF, Mbabazi PK, Weisheit A. Ethnomedicine of the Kagera Region, north western Tanzania. Part 2: The medicinal plants used in Katoro Ward, Bukoba District. J Ethnobiol Ethnomed. 2010;6:19. doi: 10.1186/1746-4269-6-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Moshi MJ, Otieno DF, Weisheit A. Ethnomedicine of the Kagera Region, north western Tanzania. Part 3: plants used in traditional medicine in Kikuku village, Muleba District. J Ethnobiol Ethnomed. 2012;8:14. doi: 10.1186/1746-4269-8-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Njeru S, Obonyo M, Nyambati S, Ngari S, Mwakubambanya R, Mavura H. Antimicrobial and cytotoxicity properties of the organic solvent fractions of Clerodendrum myricoides Kenyan traditional medicinal plant. J Intercult Ethnopharmacol. 2016;5:226. doi: 10.5455/jice.20160416122003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Matu EN, van Staden J. Antibacterial and anti-inflammatory activities of some plants used for medicinal purposes in Kenya. J Ethnopharmacol. 2003;87:35–41. doi: 10.1016/s0378-8741(03)00107-7. [DOI] [PubMed] [Google Scholar]
- 129.Deressa T, Mekonnen Y, Animut A. In vivo anti-malarial activities of Clerodendrum myricoides, Dodonea angustifolia and Aloe debrana against Plasmodium berghei. Ethiop J Health Dev. 2010;24:25–29. [Google Scholar]
- 130.Irungu BN, Rukunga GM, Mungai GM, Muthaura CN. In vitro antiplasmodial and cytotoxicity activities of 14 medicinal plants from Kenya. S Afr J Bot. 2007;73:204–207. [Google Scholar]
- 131.Oryema C, Ziraba RB, Odyek O, Omagor N, Opio A. Phytochemical properties and toxicity to brine shrimp of medicinal plants in Erute county, Lira district, Uganda. J Med Plants Res. 2011;5:5450–5457. [Google Scholar]
- 132.Kigen G, Maritim A, Some F, Kibosia J, Rono H, Chepkwony S, et al. Ethnopharmacological survey of the medicinal plants used in Tindiret, Nandi County, Kenya. Afr J Tradit Complement Altern Med. 2016;13:156. doi: 10.4314/ajtcam.v13i3.19. [DOI] [Google Scholar]
- 133.Nguta JM, Mbaria JM, Gakuya DW, Gathumbi PK, Kiama SG. Antimalarial herbal remedies of Msambweni, Kenya. J Ethnopharmacol. 2010;128:424–432. doi: 10.1016/j.jep.2010.01.033. [DOI] [PubMed] [Google Scholar]
- 134.Ngari EW. Ethnomedicne of Ogiek of River Njoro watershed, Nakuru, Kenya. Ethnobot Res Appl. 2010;8:135. doi: 10.17348/era.8.0.135-152. [DOI] [Google Scholar]
- 135.Yashaswini S, Vasundhara M. Coleus (Plectranthus barbatus)—a multipurpose medicinal herb. Int Res J Pharm. 2011;2:47–58. [Google Scholar]
- 136.Govindarajan M, Rajeswary M, Hoti SL, Bhattacharyya A, Benelli G. Eugenol, α-pinene and β-caryophyllene from Plectranthus barbatus essential oil as eco-friendly larvicides against malaria, dengue and Japanese encephalitis mosquito vectors. Parasitol Res. 2016;115:807–815. doi: 10.1007/s00436-015-4809-0. [DOI] [PubMed] [Google Scholar]
- 137.Borges Fernandes LC, Campos Câmara C, Soto-Blanco B. Anticonvulsant activity of extracts of Plectranthus barbatus leaves in mice. Evid Based Complement Altern Med. 2012 doi: 10.1155/2012/860153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kapewangolo P, Hussein AA, Meyer D. Inhibition of HIV-1 enzymes, antioxidant and anti-inflammatory activities of Plectranthus barbatus. J Ethnopharmacol. 2013;149:184–190. doi: 10.1016/j.jep.2013.06.019. [DOI] [PubMed] [Google Scholar]
- 139.Lawi Y, Saria J, Kidukuli AW. Brine shrimp cytotoxicity, phytochemical screening and larvicidal activities of Plectranthus barbatus extracts. Res Rev Insights. 2018;2:1–4. [Google Scholar]
- 140.Amina M, Al-Musayeib NM, Alam P, Aleanizy FS, Alqahtni FY, Al-Said MS, et al. Cytotoxic evaluation and concurrent analysis of two diterpenes in the chloroform extract of Plectranthus barbatus using a validated HPTLC-UV method. Bull Chem Soc Ethiop. 2018;32:407–419. [Google Scholar]
- 141.Osuagwu GGE, Eme CF. The phytochemical composition and antimicrobial activity of Dialium guineense, Vitex doniana and Dennettia tripetala leaves. Asian J Nat Appl Sci. 2013;2:69–81. [Google Scholar]
- 142.Lagnika L, Amoussa M, Adjovi Y, Sanni A. Antifungal, antibacterial and antioxidant properties of Adansonia digitata and Vitex doniana from Bénin pharmacopeia. J Pharmacogn Phytother. 2012;4:44–52. [Google Scholar]
- 143.Fadeyi SA, Fadeyi OO, Adejumo AA, Okoro C, Myles EL. In vitro anticancer screening of 24 locally used Nigerian medicinal plants. BMC Complement Altern Med. 2013 doi: 10.1186/1472-6882-13-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Muanda F, Koné D, Dicko A, Soulimani R, Younos C. Phytochemical composition and antioxidant capacity of three malian medicinal plant parts. Evid Based Complement Altern Med. 2011;2011:1–8. doi: 10.1093/ecam/nep109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kilani AM. Antibacterial assessment of whole stem bark of Vitex doniana against some enterobactriaceae. Afr J Biotechnol. 2006;5.
- 146.Ali M, Aminu F, Ibrahim IS. In-vitro assessment of antibacterial activity and phytochemical screening of Vitex doniana on clinical isolate of Salmonella typhi. Int J Adv Acad Res. 2017;3:9–16. [Google Scholar]
- 147.Agbafor KN, Nwachukwu N. Phytochemical analysis and antioxidant property of leaf extracts of Vitex doniana and Mucuna pruriens. Biochem Res Int. 2011 doi: 10.1155/2011/459839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Amégbor K, Metowogo K, Eklu-Gadegbeku K, Agbonon A, Aklikokou KA, Napo-Koura G, et al. Preliminary evaluation of the wound healing effect of Vitex doniana sweet (Verbenaceae) in mice. Afr J Tradit Complement Altern Med. 2012;9:584–590. doi: 10.4314/ajtcam.v9i4.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Abiodun O, Gbotosho G, Ajaiyeoba E, Happi T, Falade M, Wittlin S, et al. In vitro antiplasmodial activity and toxicity assessment of some plants from Nigerian ethnomedicine. Pharm Biol. 2011;49:9–14. doi: 10.3109/13880209.2010.490224. [DOI] [PubMed] [Google Scholar]
- 150.Belayneh A, Asfaw Z, Demissew S, Bussa NF. Medicinal plants potential and use by pastoral and agro-pastoral communities in Erer Valley of Babile Wereda, Eastern Ethiopia. J Ethnobiol Ethnomed. 2012;8:42. doi: 10.1186/1746-4269-8-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Hamza OJM, van den Bout-van den Beukel CJP, Matee MIN, Moshi MJ, Mikx FHM, Selemani HO, et al. Antifungal activity of some Tanzanian plants used traditionally for the treatment of fungal infections. J Ethnopharmacol. 2006;108:124–132. doi: 10.1016/j.jep.2006.04.026. [DOI] [PubMed] [Google Scholar]
- 152.Kokila K, Priyadharshini SD, Sujatha V. Phytopharmacological properties of Albizia species: a review. Int J Pharm Pharm Sci. 2013;5:70–73. [Google Scholar]
- 153.Abdalla MA, Laatsch H. Flavonoids from Sudanese Albizia zygia (Leguminosae, subfamily Mimosoideae), a plant with antimalarial potency. Afr J Tradit Complement Altern Med. 2012;9:56–58. doi: 10.4314/ajtcam.v9i1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Appiah-Opong R, Asante IK, Safo DO, Tuffour I, Ofori-Attah E, Uto T, et al. Cytotoxic effects of Albizia zygia (DC) J. F. Macbr, a Ghanaian medicinal plant, against human T-lymphoblast-like leukemia, prostate and breast cancer cell lines. Int J Pharm Pharm Sci. 2016;8:392–396. [Google Scholar]
- 155.Oloyede GK, Ogunlade AO. Phytochemical screening, antioxidant, antimicrobial and toxicity activities of polar and non-polar extracts of Albizia zygia (DC) stem-bark. Ann Res Rev Biol. 2013;3:1020–1031. [Google Scholar]
- 156.Okpo SO, Igwealor CO, Eze GI. Sub-acute toxicity study on the aqueous extract of Albizia zygia stem bark. J Pharm Bioresour. 2016;13:32. [Google Scholar]
- 157.Olarbi AA, Bekoe EO, Agyare C, Osafo N, Boamah VE. In vivo anti-inflammatory and antioxidant properties of Albizia zygia D. C. Macbr. Planta Med. 2016;81:S1–S381. doi: 10.1055/s-0036-1596353. [DOI] [Google Scholar]
- 158.Pierre S, Alex NN, Jean M. Medicinal plants used in traditional treatment of malaria in Cameroon. J Ecol Nat Environ. 2011;3:104–117. [Google Scholar]
- 159.Ali A, Al-rahwi K, Lindequist U. Some medicinal plants used in Yemeni herbal medicine to treat malaria. Afr J Tradit Complement Altern Med. 2004;1:72–76. [Google Scholar]
- 160.Doughari JH. Antimicrobial activity of Tamarindus indica Linn. Trop J Pharm Res. 2006;5:597–603. [Google Scholar]
- 161.Abukakar MG, Ukwuani AN, Shehu RA. Phytochemical screening and antibacterial activity of Tamarindus indica pulp extract. Asian J Biochem. 2008;3:134–138. doi: 10.3923/ajb.2008.134.138. [DOI] [Google Scholar]
- 162.Nwodo UU, Ngene AA, Anaga AO, Chigor VN, Henrietta II, Okoh AI. Acute toxicity and hepatotoxicokinetic studies of Tamarindus indica extract. Molecules. 2011;16:7415–7427. doi: 10.3390/molecules16097415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Abubakar MG, Yerima MB, Zahriya AG, Ukwuani AN. Acute toxicity and antifungal studies of ethanolic leaves, stem and pulp extract of Tamarindus indica. Res J Pharm Biol Chem Sci. 2010;1:104–111. [Google Scholar]
- 164.Adongo JO, Omolo JO, Njue AW, Matofari JW. Antimicrobial activity of the root extracts of Tylosema fassoglensis Schweinf. Torre & Hillc (Caesalpiniaceae) Sci J Microbiol. 2012 doi: 10.7237/sjmb/209. [DOI] [Google Scholar]
- 165.Ochanga O, Chacha M. Antifungal and cytotoxicity activity of plants used as herbal teas in Tanzania. Eur J Med Plants. 2016;16:1–8. doi: 10.9734/ejmp/2016/29475. [DOI] [Google Scholar]
- 166.Njoroge GN, Bussmann RW. Diversity and utilization of antimalarial ethnophytotherapeutic remedies among the Kikuyus (Central Kenya) J Ethnobiol Ethnomed. 2006;2:1–7. doi: 10.1186/1746-4269-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Singh A, Kumar A, Tewari D. An ethnobotanical survey of medicinal plants used in Terai forest of western Nepal. J Ethnobiol Ethnomed. 2012;8:19. doi: 10.1186/1746-4269-8-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Akpuaka A, Ekwenchi MM, Dashak DA, Dildar A. Biological activities of characterized isolates of n-hexane extract of Azadirachta indica A. Juss (Neem) leaves. N Y Sci J. 2013;6:119–124. [Google Scholar]
- 169.Pandey G, Verma K, Singh M. Evaluation of phytochemical, antibacterial and free radical scavenging properties of Azadirachta indica (neem) leaves. Int J Pharm Pharm Sci. 2014;6:444–447. [Google Scholar]
- 170.Karou SD, Tchacondo T, Tchibozo MAD, Abdoul-Rahaman S, Anani K, Koudouvo K, et al. Ethnobotanical study of medicinal plants used in the management of diabetes mellitus and hypertension in the Central Region of Togo. Pharm Biol. 2011;49:1286–1297. doi: 10.3109/13880209.2011.621959. [DOI] [PubMed] [Google Scholar]
- 171.Ugoh SC, Agarry OO, Garba SA. Studies on the antibacterial activity of Khaya senegalensis [(Desr.) A. Juss)] stem bark extract on Salmonella enterica subsp. enterica serovar Typhi [(ex Kauffmann and Edwards) Le Minor and Popoff] Asian Pac J Trop Biomed. 2014;4:S279–S283. doi: 10.12980/apjtb.4.2014c636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Muhammad I, Alhassan A, Sule M, Idi A, Mohammed A, Taalu AE, et al. Anti-hyperglycemic activity of solvents extract of Khaya senegalensis stem bark in alloxan induced diabetic rats. J Adv Biol Biotechnol. 2016;6:1–8. doi: 10.9734/jabb/2016/25986. [DOI] [Google Scholar]
- 173.Muhammad I, Alhassan A, Wudil A, Jarumi I. Toxicological and protective effect of aqueous stem bark extract of Khaya senegalensis ({ASBEKS}) on liver of experimental rat. Br J Appl Sci Technol. 2015;9:600–605. doi: 10.9734/bjast/2015/16545. [DOI] [Google Scholar]
- 174.Ibrahim MA, Koorbanally NA, Islam MS. Antioxidative activity and inhibition of key enzymes linked to type-2 diabetes (α-Glucosidase and α-Amylase) by Khaya senegalensis. Acta Pharm. 2014;64:311–324. doi: 10.2478/acph-2014-0025. [DOI] [PubMed] [Google Scholar]
- 175.Yakubu MT, Adebayo OJ, Egwim EC, Owoyele VB. Increased liver alkaline phosphatase and aminotransferase activities following administration of ethanolic extract of Khaya senegalensis stem bark to rats. Nigerian Society for Experimental Biology; 2005.
- 176.Onu A, Saidu Y, Ladan MJ, Bilbis LS, Aliero AA, Sahabi SM. Effect of aqueous stem bark extract of Khaya senegalensis on some biochemical, haematological, and histopathological parameters of rats. J Toxicol. 2013 doi: 10.1155/2013/803835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Takin MC, Attindehou S, Sezan A, Attakpa SE, Lamine B-M. Bioactivity, therapeutic utility and toxicological risks of Khaya senegalensis. Indian J Pharm Biol Res. 2013;1:122–129. [Google Scholar]
- 178.Nagesh KS, Shanthamma C. Micropropagation and antioxidant activity of Mollugo nudicaulis Lam. J Med Plants Res. 2011;5:895–902. [Google Scholar]
- 179.Rameshkumar A, Sivasudha T. In vitro antioxidant and antibacterial activity of aqueous and methanolic extract of Mollugo nudicaulis Lam. leaves. Asian Pac J Trop Biomed. 2012;2:S895–S900. doi: 10.1016/s2221-1691(12)60332-3. [DOI] [Google Scholar]
- 180.Sindhu T, Rajamanikandan S, Ragavendran P, Sophia D, Meenakshi P, Durgapriya D, et al. Antidiabetic activity of Mollugo nudicaulis against alloxan induced diabetic rats. Int J Appl Biol Pharm Technol. 2010;1:511–519. [Google Scholar]
- 181.Kasolo JN, Bimenya GS, Ojok L, Ochieng J, Ogwal-Okeng JW. Phytochemicals and uses of Moringa oleifera leaves in Ugandan rural communities. J Med Plants Res. 2010;4:753–757. [Google Scholar]
- 182.Caceres A, Cabrera O, Morales O, Mollinedo P, Mendia P. Pharmacological properties of Moringa oleifera. 1: Preliminary screening for antimicrobial activity. J Ethnopharmacol. 1991;33:213–216. doi: 10.1016/0378-8741(91)90078-r. [DOI] [PubMed] [Google Scholar]
- 183.Okumu MO, Ochola FO, Mbaria JM, Kanja LW, Gakuya DW, Kinyua AW, et al. Mitigative effects of Moringa oleifera against liver injury induced by artesunate-amodiaquine antimalarial combination in wistar rats. Clin Phytosci. 2017;3:18. doi: 10.1186/s40816-017-0052-9. [DOI] [Google Scholar]
- 184.Berkovich L, Earon G, Ron I, Rimmon A, Vexler A, Lev-Ari S. Moringa oleifera aqueous leaf extract down-regulates nuclear factor-kappaB and increases cytotoxic effect of chemotherapy in pancreatic cancer cells. BMC Complement Altern Med. 2013;13:212. doi: 10.1186/1472-6882-13-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Shahriar M, Hossain MI, Bahar ANM, Akhter S, Haque MA, Bhuiyan MA. Preliminary phytochemical screening, in-vitro antioxidant and cytotoxic activity of five different extracts of Moringa oleifera leaf. J Appl Pharm Sci. 2012;2:65. [Google Scholar]
- 186.Nair S, Varalakshmi KN. Anticancer, cytotoxic potential of Moringa oleifera extracts on HeLa cell line. J Nat Pharm. 2011;2:138–142. [Google Scholar]
- 187.Basak SS, Candan F. Chemical composition and in vitro antioxidant and antidiabetic activities of Eucalyptus Camaldulensis Dehnh. essential oil. J Iran Chem Soc. 2010;7:216–226. doi: 10.1007/bf03245882. [DOI] [Google Scholar]
- 188.Ghalem BR, Mohamed B. Antibacterial activity of leaf essential oils of Eucalyptus globulus and Eucalyptus camaldulensis. Afr J Pharm Pharmacol. 2008;2:211–215. [Google Scholar]
- 189.Adeniyi CBA, Lawal TO, Mahady GB. In vitro susceptibility of Helicobacter pylori to extracts of Eucalyptus camaldulensis and Eucalyptus torelliana. Pharm Biol. 2009;47:99–102. doi: 10.1080/13880200802448708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Gemechu A, Giday M, Worku A, Ameni G. In vitro anti-mycobacterial activity of selected medicinal plants against Mycobacterium tuberculosis and Mycobacterium bovis strains. BMC Complement Altern Med. 2013 doi: 10.1186/1472-6882-13-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Singab A-N, Ayoub N, Al-Sayed E, Martiskainen O, Sinkkonen J, Pihlaja K. Phenolic constituents of Eucalyptus camaldulensis Dehnh, with potential antioxidant and cytotoxic activities. Rec Nat Prod. 2011;5:271–280. [PubMed] [Google Scholar]
- 192.El-Baha AM, El-Sherbiny AA, Salem MZM, Sharrawy NMM, Mohamed NH. Toxicity of essential oils extracted from Corymbia citriodora and Eucalyptus camaldulensis leaves against Meloidogyne incognita under laboratory conditions. Pak J Nematol. 2017;35:93–104. [Google Scholar]
- 193.Hrubik JD, Kaišarević SN, Glišić BD, Jovin EĐ, Mimica-Dukić NM, Kovačević RZ. Myrtus comunis and Eucalyptus camaldulensis cytotoxicity on breast cancer cells. Zbornik Matice srpske za prirodne nauke. 2012;123:65–73. [Google Scholar]
- 194.Soltanian S, Sheikhbahaei M, Mohamadi N. Cytotoxicity evaluation of methanol extracts of some medicinal plants on P19 embryonal carcinoma cells. J Appl Pharm Sci. 2017;7:142–149. [Google Scholar]
- 195.Ayyanar M, Subash-babu P. Syzygium cumini (L.) skeels: a review of its phytochemical constituents and traditional uses. Asian Pac J Trop Biomed. 2012;2:240–246. doi: 10.1016/S2221-1691(12)60050-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Swami SB, Thakor NSJ, Patil MM, Haldankar PM. Jamun. Syzygium cumini: a review of its food and medicinal uses. Food Nutr Sci. 2012;03:1100–1117. [Google Scholar]
- 197.Muruganandan S, Srinivasan K, Chandra S, Tandan SK, Lal J, Raviprakash V. Anti-inflammatory activity of Syzygium cumini bark. Fitoterapia. 2001;72:369–375. doi: 10.1016/s0367-326x(00)00325-7. [DOI] [PubMed] [Google Scholar]
- 198.Saravanan G, Pari L. Hypoglycaemic and antihyperglycaemic effect of Syzygium cumini bark in streptozotocin-induced diabetic rats. J Pharmacol Toxicol. 2008;3:1–10. doi: 10.3923/jpt.2008.1.10. [DOI] [Google Scholar]
- 199.Ugbabe GE, Ezeunala MN, Edmond IN, Apev J, Salawu OA. Preliminary phytochemical, antimicrobial and acute toxicity studies of the stem, bark and the leaves of a cultivated Syzygium cumini Linn. (Family: Myrtaceae) in Nigeria. Afr J Biotechnol. 2010;9:6747–6943. [Google Scholar]
- 200.Prasad M, Venugopal SP, Alagarsamy V, Sridevi C. The preliminary phytochemical analysis and oral acute toxicity study of stem bark of Syzygium cumini. Int J Pharm Pharm Sci. 2016;8:209–213. [Google Scholar]
- 201.Le NHT, Malterud KE, Diallo D, Paulsen BS, Nergård CS, Wangensteen H. Bioactive polyphenols in Ximenia americana and the traditional use among Malian healers. J Ethnopharmacol. 2012;139:858–862. doi: 10.1016/j.jep.2011.12.031. [DOI] [PubMed] [Google Scholar]
- 202.Omer MEFA, Elnima EI. Antimicrobial activity of Ximenia americana. Fitoterapia. 2003;74:122–126. doi: 10.1016/s0367-326x(02)00302-7. [DOI] [PubMed] [Google Scholar]
- 203.Maikai VA, Kobo PI, Maikai BVO. Antioxidant properties of Ximenia americana. Afr J Biotechnol. 2010;9:7744–7746. [Google Scholar]
- 204.Cos P, Hermans N, De Bruyne T, Apers S, Sindambiwe JB, Vanden Berghe D, et al. Further evaluation of Rwandan medicinal plant extracts for their antimicrobial and antiviral activities. J Ethnopharmacol. 2002;79:155–163. doi: 10.1016/s0378-8741(01)00362-2. [DOI] [PubMed] [Google Scholar]
- 205.Habtamu A, Mekonnen Y. Antibacterial potential of the 80% methanol and chloroform extracts of Clematis hirsuta. Afr J Pharm Pharmacol. 2017;11:204–208. [Google Scholar]
- 206.Silva O, Duarte A, Cabrita J, Pimentel M, Diniz A, Gomes E. Antimicrobial activity of Guinea-Bissau traditional remedies. J Ethnopharmacol. 1996;50:55–59. doi: 10.1016/0378-8741(95)01323-7. [DOI] [PubMed] [Google Scholar]
- 207.Nureye D, Assefa S, Nedi T, Engidawork E. In vivo antimalarial activity of the 80% methanolic root bark extract and solvent fractions of Gardenia ternifolia Schumach. & Thonn. (Rubiaceae) against Plasmodium berghei. Evid Based Complement Altern Med. 2018 doi: 10.1155/2018/9217835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Silva O, Barbosa S, Diniz A, Valdeira ML, Gomes E. Plant extracts antiviral activity against herpes simplex virus type 1 and African swine fever virus. Int J Pharmacogn. 1997;35:12–16. doi: 10.1076/phbi.35.1.12.13264. [DOI] [Google Scholar]
- 209.Moshi MJ, Kamuhabwa A, Mbwambo Z, De Witte P. Cytotoxic screening of some Tanzania medicinal plants. East Cent Afr J Pharm Sci. 2003;6:52–56. [Google Scholar]
- 210.Aro AO, Dzoyem JP, Hlokwe TM, Madoroba E, Jacobus N, Mcgaw LJ. Some South African Rubiaceae Tree Leaf Extracts Have Antimycobacterial Activity Against Pathogenic and Non-pathogenic Mycobacterium species. Phytother Res. 2015;29:1004–1010. doi: 10.1002/ptr.5338. [DOI] [PubMed] [Google Scholar]
- 211.Fabry W, Okemo P, Ansorg R. Fungistatic and fungicidal activity of East African medicinal plants: Fungistatische und fungizide Wirksamkeit ostafrikanischer Heilpflanzen. Mycoses. 1996;39:67–70. doi: 10.1111/j.1439-0507.1996.tb00087.x. [DOI] [PubMed] [Google Scholar]
- 212.Balde AM, Pieters L, De Bruyne T, Geerts S, Berghe D, Vlietinck A. Biological investigations on Harrisonia abyssinica. Phytomedicine. 1995;1:299–302. doi: 10.1016/s0944-7113(11)80006-1. [DOI] [PubMed] [Google Scholar]
- 213.Wabe N, Mohammed M, Raju N. An ethnobotanical survey of medicinal plants in the Southeast Ethiopia used in traditional medicine. Spatula. 2011;1:153. doi: 10.5455/spatula.20110921101924. [DOI] [Google Scholar]
- 214.Mascolo N, Pinto A, Capasso F, Yenesew A, Dagne E. Antipyretic and analgesic studies of the ethanalic extract of Teclea nobilis delile. Phytother Res. 1988;2:154–156. [Google Scholar]
- 215.Al Rehaily AJ, El Tahir KEH, Mossa JS, Rafatullah S. Pharmacological studies of various extracts and the major constituent, lupeol, obtained from hexane extract of Teclea nobilis in rodents. Nat Prod Sci. 2001;7:76–82. [Google Scholar]
- 216.Chayamiti T, Mwenje E, Mahamadi C. Spectrophotometric study of the anti-caseinolytic activity of root extracts of Teclea nobilis and Vepris zambesiaca on Bitis arietans venom. Afr J Pharm Pharmacol. 2013;7:1420–1425. [Google Scholar]
- 217.Moshi MJ, Innocent E, Magadula JJ, Otieno DF, Weisheit A, Mbabazi PK, et al. Brine shrimp toxicity of some plants used as traditional medicines in Kagera region, north western Tanzania. Tanzan J Health Res. 2010;12:63–67. doi: 10.4314/thrb.v12i1.56287. [DOI] [PubMed] [Google Scholar]
- 218.Orwa JA, Jondiko IJO, Minja RJA, Bekunda M. The use of Toddalia asiatica (L) Lam. (Rutaceae) in traditional medicine practice in East Africa. J Ethnopharmacol. 2008;115:257–262. doi: 10.1016/j.jep.2007.09.024. [DOI] [PubMed] [Google Scholar]
- 219.Orwa JA, Ngeny L, Mwikwabe NM, Ondicho J, Jondiko IJO. Antimalarial and safety evaluation of extracts from Toddalia asiatica (L) Lam. (Rutaceae) J Ethnopharmacol. 2013;145:587–590. doi: 10.1016/j.jep.2012.11.034. [DOI] [PubMed] [Google Scholar]
- 220.Borah R, Kalita MC, Kar A, Talukdar AK. Larvicidal efficacy of Toddalia asiatica (Linn.) Lam against two mosquito vectors Aedes aegypti and Culex quinquefasciatus. Afr J Biotechnol. 2010;9:2527–2530. [Google Scholar]
- 221.Maobe MAG, Gitu L, Gatebe E, Rotich H, Karanja PN, Votha DM, et al. Antifungal activity of eight selected medicinal herbs used for the treatment of diabetes, malaria and pneumonia in Kisii Region, Southwest Kenya. World J Med Sci. 2013;8:74–78. [Google Scholar]
- 222.Kariuki H, Kanui T, Yenesew A, Patel N, Mbugua P. Antinocieptive and anti-inflammatory effects of Toddalia asiatica (L) Lam. (Rutaceae) root extract in Swiss albino mice. Pan Afr Med J. 2013 doi: 10.11604/pamj.2013.14.133.2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Hirunwong C, Sukieum S, Phatchana R, Yenjai C. Cytotoxic and antimalarial constituents from the roots of Toddalia asiatica. Phytochem Lett. 2016;17:242–246. [Google Scholar]
- 224.Thirugnanasampandan R, Jayakumar R, Prabhakaran M. Analysis of chemical composition and evaluation of antigenotoxic, cytotoxic and antioxidant activities of essential oil of Toddalia asiatica (L.) Lam. Asian Pac J Trop Biomed. 2012;2:S1276–S1279. [Google Scholar]
- 225.Hu J, Shi X, Chen J, Mao X, Zhu L, Yu L, et al. Alkaloids from Toddalia asiatica and their cytotoxic, antimicrobial and antifungal activities. Food Chem. 2014;148:437–444. doi: 10.1016/j.foodchem.2012.12.058. [DOI] [PubMed] [Google Scholar]
- 226.Iwasaki H, Oku H, Takara R, Miyahira H, Hanashiro K, Yoshida Y, et al. The tumor specific cytotoxicity of dihydronitidine from Toddalia asiatica Lam. Cancer Chemother Pharmacol. 2006;58:451–459. doi: 10.1007/s00280-005-0183-4. [DOI] [PubMed] [Google Scholar]
- 227.Tugume P, Kakudidi EK, Buyinza M, Namaalwa J, Kamatenesi M, Mucunguzi P, et al. Ethnobotanical survey of medicinal plant species used by communities around Mabira Central Forest Reserve, Uganda. J Ethnobiol Ethnomed. 2016 doi: 10.1186/s13002-015-0077-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Kimani CN, Mbaria JM, Suleiman M, Gakuya D, Kiama SG. Antihyperglycemic activity of Zanthoxylum chalybeum stem bark extract in diabetic rats. J Phytopharmacol. 2015;4:183–189. [Google Scholar]
- 229.Nguta JM, Kiraithe MN. In vitro antimicrobial activity of aqueous extracts of Ocimum suave Willd., Plectranthus barbatus andrews and Zanthoxylum chalybeum Engl. against selected pathogenic bacteria. Biomed Biotechnol Res J. 2019;3:30. [Google Scholar]
- 230.Kiraithe MN, Nguta JM, Mbaria JM, Kiama SG. Evaluation of the use of Ocimum suave Willd. (Lamiaceae), Plectranthus barbatus Andrews (Lamiaceae) and Zanthoxylum chalybeum Engl. (Rutaceae) as antimalarial remedies in Kenyan folk medicine. J Ethnopharmacol. 2016;178:266–271. doi: 10.1016/j.jep.2015.12.013. [DOI] [PubMed] [Google Scholar]
- 231.Nguta JM, Mbaria JM, Gathumbi PK, Kabasa JD, Kiama SG. Biological screening of Kenya medicinal plants using artemia salina (ARTEMIIDAE). University of Nairobi Research Archive. 2011. http://erepository.uonbi.ac.ke/handle/11295/9787.
- 232.Omosa LK, Okemwa EK. Antiplasmodial activities of the stem bark extract and compounds of Zanthoxylum gilletii (De wild) P.G. Waterman. Pharmacogn Commun. 2017;7:41–46. doi: 10.5530/pc.2017.1.6. [DOI] [Google Scholar]
- 233.Nyaboke HO, Moraa M, Omosa LK, Mbaveng AT, Vaderament-Alexe N-N, Masila V, et al. Cytotoxicity of lupeol from the stem bark of Zanthoxylum gilletii against multi-factorial drug resistant cancer cell lines. Invest Med Chem Pharmacol. 2018;1:10. [Google Scholar]
- 234.Omosa LK, Midiwo JO, Masila VM, Gisacho BM, Munayi R, Chemutai KP, et al. Cytotoxicity of 91 Kenyan indigenous medicinal plants towards human CCRF-CEM leukemia cells. J Ethnopharmacol. 2016;179:177–196. doi: 10.1016/j.jep.2015.12.028. [DOI] [PubMed] [Google Scholar]
- 235.Hussein S, Dhabe A. Ethnobotanical study of folk medicinal plants used by villagers in Hajjah district, Republic of Yemen. J Med Plants Stud. 2018;6:24–30. [Google Scholar]
- 236.Alzoreky NS, Nakahara K. Antibacterial activity of extracts from some edible plants commonly consumed in Asia. Int J Food Microbiol. 2003;80:223–230. doi: 10.1016/s0168-1605(02)00169-1. [DOI] [PubMed] [Google Scholar]
- 237.Al-Mehdar AA, Al-Battah AM. Evaluation of hypoglycemic activity of boswellia carterii and cissus rotundifolia in streptozotocin/nicotinamide-induced diabetic rats. Yemeni J Med Sci. 2016;10:30–38. [Google Scholar]
- 238.Said A, Aboutabl EA, Melek FR, Abdel Jaleel Raheem Abdel Jaleel G, Raslan M. Phytoconstituents profiling of Cissus rotundifolia (Forssk.) Vahl. by HPLC–MS/MS, and evaluation of its free radical scavenging activity (DPPH) and cytotoxicity. Trends Phytochem Res. 2018;2:65–74. [Google Scholar]
- 239.Maima AO, Ndwigah SN, Thoithi GN, Kamau FN, Kibwage IO. Antimicrobial properties of some medicinal plants of the Luo Community of Kenya. Afr J Pharmacol Ther. 2014;3:112–115. [Google Scholar]
- 240.Lambert J, Omindi-ogaja E, Gatheru G, Mirangi T, Owara J, Herbst CH, et al. The contribution of traditional herbal medicine practitioners to Kenyan Health Care Delivery Results from Community Health-Seeking Behavior Vignettes and a Traditional Herbal Medicine Practitioner Survey. 2011.
- 241.Mukungu N, Abuga K, Okalebo F, Ingwela R, Mwangi J. Medicinal plants used for management of malaria among the Luhya community of Kakamega East sub-County, Kenya. J Ethnopharmacol. 2016;194:98–107. doi: 10.1016/j.jep.2016.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Christenhusz MJM, Byng JW. The number of known plants species in the world and its annual increase. Phytotaxa. Magnolia Press; 2016. pp. 201–17. http://biotaxa.org/Phytotaxa/article/download/phytotaxa.261.3.1/20598.
- 243.Alamgeer, Younis W, Asif H, Sharif A, Riaz H, Bukhari IA, et al. Traditional medicinal plants used for respiratory disorders in Pakistan: a review of the ethno-medicinal and pharmacological evidence Milen Georgiev, Ruibing Wang. Chin Med. 2018 doi: 10.1186/s13020-018-0204-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Ahmed AA, Bassuony NI. Importance of medical herbs in animal feeding. World J Agric Sci. 2009;5:456–465. [Google Scholar]
- 245.Khan SM, Page S, Ahmad H, Harper D. Identifying plant species and communities across environmental gradients in the Western Himalayas: method development and conservation use. Ecol Inform. 2013;14:99–103. [Google Scholar]
- 246.Maroyi A. Traditional use of medicinal plants in South-Central Zimbabwe: review and perspectives. J Ethnobiol Ethnomed. 2013;9:1–18. doi: 10.1186/1746-4269-9-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Dasaraju PV, Liu C. Infections of the respiratory system. In: Medical microbiology. 4th edition. Galveston: University of Texas Medical Branch; 1996. [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Summary of the questionnaire used to interview herbalists in Kisumu East Sub County.
Data Availability Statement
All data generated or analyzed during this study are included in the text.