ABSTRACT
The overall structure and composition of human centromeres have been well reported, but how these elements vary between individual chromosomes and influence the chromosome-specific behavior during mitosis remains untested. In our study, we discover the existence of heterogeneity of centromeric DNA features that dictates the chromosome segregation fidelity during mitosis.
KEYWORDS: Centromere, cell division, aneuploidy
Correct chromosome segregation during mitosis and meiosis is essential to ensure faithful transmission of genetic material to daughter cells and avoid aneuploidy. During cell division, chromosomes are linked to microtubules at a specific region, the centromere. This region is characterized by the enrichment of a histone H3 variant, named CENtromere Protein A (CENP-A), and long stretches of repetitive DNA, called alpha-satellites in humans.1 These repeats contain a 17-nucleotide motif, the CENP-B box, that is recognized by the only centromeric DNA sequence-specific binding protein, CENP-B. Numerous proteins are then assembled on the centromeres, forming the Constitutive Centromere Associated Network (CCAN) complex, at the interface between the kinetochore (KT)–microtubule assembly point.
In our manuscript,2 we performed a quantitative analysis of centromere composition at individual chromosomes in stable, non-transformed hTERT-immortalized Retinal Pigment Epithelial (RPE-1) diploid cells. We also measured the chromosome mis-segregation profile in physiological conditions or following short (two cell cycles) CENP-A removal using the Auxin-Inducible Degron (AID) system.3,4 The advantage of this system is that, in the absence of CENP-A, chromosome segregation fidelity depends exclusively on CENP-B as the sole source of centromere/kinetochore interaction via CENP-C.4
Our experiments revealed a nonrandom mis-segregation pattern of different chromosomes, observed using three different techniques [Single-cell sequencing, ImageStream cytometry, and Fluorescence In Situ Hybridization (FISH) analysis] combined with statistical modeling. In parallel, we characterized the centromere composition of each chromosome using whole-genome sequencing along with mapping on centromere reference models, imaging (combining Immunofluorescence, FISH, and/or multicolor FISH), and the Cleavage Under Targets & Release Using Nuclease (CUT&RUN) method. We analyzed variations in various centromere DNA-dependent features such as length of alpha satellite, abundance of CENP-B boxes, and level of some CCAN/KT proteins.
We then correlated the quantitative data with the aneuploidy profiles we obtained. Interestingly, all of the linear regressions were significantly negative, except the one for centromere length. This indicates that, for example, a given chromosome with a low density of CENP-B boxes has a higher probability to mis-segregate – mainly following centromere perturbation (by CENP-A removal) – revealing a weakened “centromere strength.” These correlations were even more significant when the whole-chromosome length was taken into consideration. Bigger chromosomes with a low amount of centromere components are more likely to undergo chromosome mis-segregation (e.g., chromosomes 3, 6, and X) compared to smaller chromosomes with a high density of CENP-B boxes, CENP-B, and CENP-C (e.g., 17, 19, and 20). Importantly, CENP-B knock-out considerably reduced the bias in aneuploidy observed in CENP-B-positive cells following CENP-A depletion.
Variation in centromere composition was not only observed at the inter-chromosome level but also between homologs. We have found that the centromeres of chromosome 3 homologs harbor different levels of CENP-B boxes. Analysis of their mis-segregation frequency revealed that the homolog with the lowest amount of CENP-B boxes was preferentially lost into micronuclei. This is a natural demonstration of the positive impact of centromere DNA on the strength of centromeres, necessary to sustain faithful chromosome segregation.
To evaluate if the variation in centromere composition is translated into the heterogeneity of microtubule-binding capacity, we treated the cells with low doses of a microtubule polymerization inhibitor to reduce the number of microtubules attached to a given kinetochore.5 Analysis of the frequency of mis-segregation into micronuclei of candidate chromosomes revealed the same susceptibility pattern as for CENP-A depletion.
Our findings have so far suggested that modulation of centromere DNA-associated features should, in principle, control the outcome of chromosome segregation. To test this hypothesis, we engineered an inducible dead CRISPR-associated protein 9 (dCas9) fused to CENP-B and targeted it using sgRNAs specific to the centromere of the Y chromosome, the only chromosome devoid of CENP-B boxes. Induction of the dCas9-CENP-B could significantly reduce the high mis-segregation rate of the chromosome Y into micronuclei following CENP-A depletion.
It is very likely that additional factors other than centromere protein abundance influence the segregation/mis-segregation of specific chromosomes in a particular environment. First of all, cell selection is a key process that drives the maintenance of aneuploidy in cancer cells. In this regard, by analyzing the chromosome segregation profile of a pseudo-diploid colorectal human male cancer cell line (DLD-1), we have found that its complex and instable karyotype is mainly mediated by driver-mutations and/or rearrangements, besides differences in “centromere strength.” Aside from the specificities of cancerous cells, other factors may influence the fate of each chromosome during segregation in a kinetochore-independent manner. For example, a recent study of drug-induced cohesion fatigue revealed a nonrandom chromosome mis-segregation profile in non-transformed RPE-1 cells.6
Altogether, our data support a model in which the heterogeneity of centromeric DNA features dictates the fidelity of chromosome segregation during mitosis (Figure 1), a hypothesis only formulated for the asymmetric segregation of female meiosis (meiotic drive).7 This has broad implications for human chromosome segregation. For example, the segregation bias we observe might help to explain why female meiosis shows a high incidence of aneuploidy in association with aging, a relationship that will require further investigation. Our findings further suggest that biological differences between different chromosomes are behind genetic diseases involving alterations in whole-chromosome numbers, DNA breaks or chromosome rearrangements,8 and certain types of aging-associated cancers (e.g., Y chromosome loss9). Association of human disease and centromere DNA variation has been proposed previously, given that chromosome structural variation can influence the susceptibility of each centromere to DNA breaks.10 However, to date, the knowledge that we have on centromere DNA is incomplete. A telomere to telomere map of each human chromosome will be soon within reach and will considerably help to understand inter- and intra-chromosome centromere variations.
Figure 1.
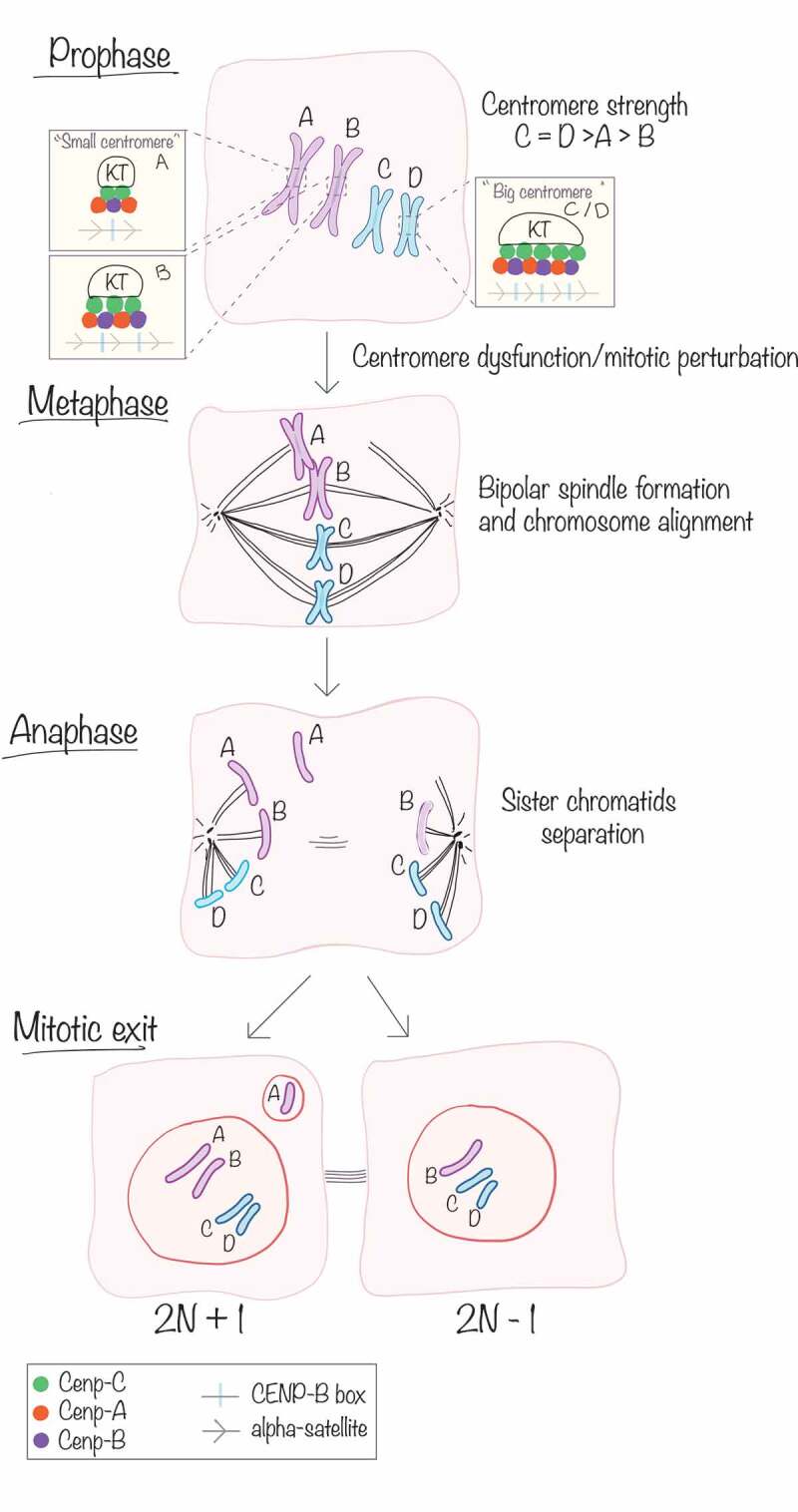
Heterogeneity in centromere composition impacts chromosome segregation fidelity during mitosis. Centromere DNA (alpha-satellite and CENP-B boxes) and the abundance of CENtromere Proteins (CENP-A, CENP-B, CENP-C) vary between chromosomes (inter-chromosome variability A/B vs C/D) and also between homologs (intra-chromosome variability, A and B). During prophase, kinetochore (KT) proteins are recruited at the centromeres to mediate the attachment of the microtubules. During metaphase, correctly attached chromosomes are aligned to the metaphase plate, while bigger chromosomes harboring a small centromere (A) can be mis-aligned following mitotic perturbation. Sister chromatids are then separated and pulled apart to the opposite poles of the cell during anaphase, and at mitotic exit, the nuclear membrane is reformed to give rise to two daughter cells. The mis-aligned chromosome bearing a “smaller” centromere (A) was not equally separated and could be encapsulated into a micronucleus, giving rise to aneuploidy in one of the two daughter cells.
Acknowledgments
D.F. receives salary support from the CNRS. M.D.’s salary is covered by the Emergence program from the city of Paris and by an HFSP grant.
References
- 1.Fukagawa T, Earnshaw WC.. The centromere: chromatin foundation for the kinetochore machinery. Dev Cell. 2014;30(5):1–3. doi: 10.1016/j.devcel.2014.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dumont M, Gamba R, Gestraud P, Klaasen S, Worrall JT, De Vries SG, Boudreau V, Salinas‐Luypaert C, Maddox PS, Lens SM, Kops GJ. et al. Human chromosome‐specific aneuploidy is influenced by DNA‐dependent centromeric features. Embo J. 2019;178:1132. doi: 10.15252/embj.2019102924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nishimura K, Fukagawa T, Takisawa H, Kakimoto T, Kanemaki M. An auxin-based degron system for the rapid depletion of proteins in nonplant cells. Nat Methods. 2009;6(12):917–922. doi: 10.1038/nmeth.1401. [DOI] [PubMed] [Google Scholar]
- 4.Hoffmann S, Dumont M, Barra V, Ly P, Nechemia-Arbely Y, McMahon MA, Hervé S, Cleveland DW, Fachinetti D. CENP-A is dispensable for mitotic centromere function after initial centromere/kinetochore assembly. Cell Rep. 2016;17(9):2394–2404. doi: 10.1016/j.celrep.2016.10.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dudka D, Noatynska A, Smith CA, Liaudet N, McAinsh AD, Meraldi P. Complete microtubule-kinetochore occupancy favours the segregation of merotelic attachments. Nat Commun. 2018;9(1):2042. doi: 10.1038/s41467-018-04427-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Worrall JT, Tamura N, Mazzagatti A, Shaikh N, van Lingen T, Bakker B, Spierings DCJ, Vladimirou E, Foijer F, McClelland SE, et al. Non-random mis-segregation of human chromosomes. Cell Rep. 2018;23(11):3366–3380. doi: 10.1016/j.celrep.2018.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Henikoff S, Malik HS. Centromeres: selfish drivers. Nature. 2002;417(6886):227. doi: 10.1038/417227a. [DOI] [PubMed] [Google Scholar]
- 8.Barra V, Fachinetti D. The dark side of centromeres: types, causes and consequences of structural abnormalities implicating centromeric DNA. Nat Commun. 2018;9(1):4340. doi: 10.1038/s41467-018-06545-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Forsberg LA. Loss of chromosome Y (LOY) in blood cells is associated with increased risk for disease and mortality in aging men. Hum Genet. 2017;136(5):657–663. doi: 10.1007/s00439-017-1799-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miga KH. Centromeric satellite DNAs: hidden sequence variation in the human population. Genes. 2019;6(10):267–298. 2015. doi: 10.3390/genes10050352. [DOI] [PMC free article] [PubMed] [Google Scholar]


