ABSTRACT
In contrast to stress-induced macroautophagy/autophagy that happens during nutrient deprivation and other environmental challenges, basal autophagy is thought to be an important mechanism that cells utilize for homeostatic purposes. For instance, basal autophagy is used to recycle damaged and malfunctioning organelles and proteins to provide the building blocks for the generation of new ones throughout life. In addition, specialized autophagic processes, such as lipophagy, the autophagy-induced breakdown of lipid droplets (LDs), and glycophagy (breakdown of glycogen), are employed to maintain proper energy levels in the cell. The importance of autophagy in the regulation of stem cell behavior has been the focus of recent studies. However, the upstream signals that control autophagic activity in stem cells and the precise role of autophagy in stem cells are only starting to be elucidated. In a recent publication, we described how the Egfr (epidermal growth factor receptor) pathway stimulates basal autophagy to support the maintenance of somatic cyst stem cells (CySCs) and to control lipid levels in the Drosophila testis.
KEYWORDS: Autophagy, cyst stem cell, Drosophila, Egfr, fatty acid oxidation, lipid droplet
Adult (tissue) stem cells comprise an important pool of progenitor cells capable of self-renewal and differentiating into specialized cell types that regenerate tissues and organs throughout life. Active autophagy is necessary for the maintenance and activation of adult stem cells, such as in the blood and the muscle. In our recently published work [1], we revealed that basal autophagy is active and required for the maintenance of CySCs in the Drosophila testis. CySCs, along with germline stem cells (GSCs), make up the resident stem cell populations in the Drosophila testis. Surprisingly, autophagy appears to be active and required in somatic cyst cells, but not in GSCs, under homeostatic conditions. CySCs and cyst cells, the differentiated descendants of CySCs, comprise the supporting stroma of the developing germline, similar to Sertoli cells in the mammalian testis. Thus, we hypothesize that autophagy is needed in somatic cells to serve the metabolic needs of the adjacent germ cells to support germline maintenance and proper differentiation.
In addition to demonstrating that autophagy is important for CySC maintenance, we also discovered an unexpected, positive relationship between the Egfr pathway and autophagy, in which Egfr stimulates autophagy in CySCs for the maintenance of these cells. In most cases where a relationship has been described in mammals, EGFR negatively regulates autophagy. However, mammalian EGFR signaling controls multiple pathways downstream of the receptor, including the MAP kinase (MAPK) cascade and the PI3K-MTOR pathways. Given that MTOR activation negatively regulates autophagy, a “competition” between the MAPK and PI3K-MTOR branches of the pathway may preclude the observation that EGFR-MAPK acts to stimulate autophagy in specific contexts. In fact, in some cancer models, both EGFR and MAPK components positively regulate autophagy. Possible explanations could be that certain mutations in EGFR may favor the activation of the MAPK branch or that additional mutations in the PI3K-MTOR branch may hinder the suppression of autophagy. Because Egfr acts primarily through the MAPK pathway in CySCs, we think that our work provides a system in which this previously unanticipated role of Egfr in controlling autophagy was revealed. Interestingly, the adjacent germ cells are the source of the Egfr ligand Spi (Spitz) that activates Egfr in somatic cyst cells, again pointing to the possible metabolic cross-talk between these two cells types in order to regulate their metabolic and developmental needs (Figure 1).
Figure 1.
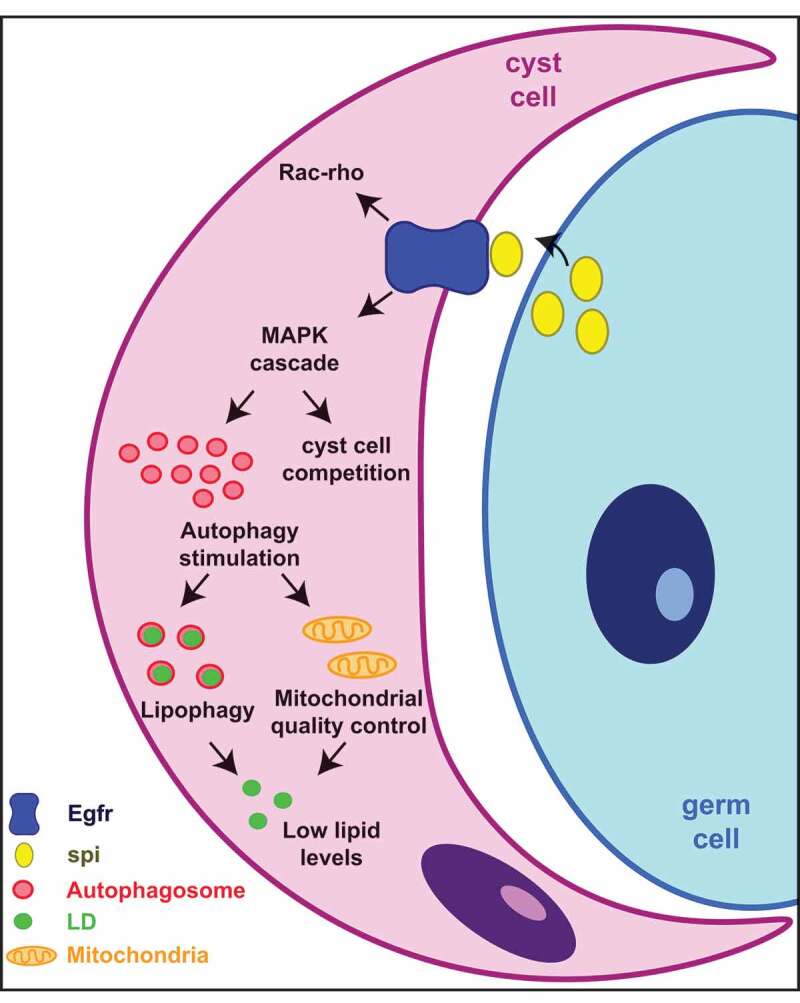
Egfr stimulates autophagy to control lipid levels and CySC maintenance. Schematics of the Egfr signaling pathway in CySCs. Upon Spi binding, Egfr will activate the MAPK signaling pathway to promote the activation of autophagy. In addition to MAPK, Egfr signaling also controls the balance between Rac and rho activation for cyst cell membrane elongation. Other downstream targets of MAPK, unrelated to autophagy, are likely present as well to control cyst cell behavior, including CySC competition for the niche. One of the roles of autophagy in cyst cells is to prevent the accumulation of lipids, which can be detrimental to stem cell maintenance. Lipid balance can be achieved through the autophagy-mediated quality control of mitochondria, as well as through the direct degradation of LDs through lipophagy.
Our work also suggests that autophagy is temporarily suppressed, through the activation of the InR (insulin-like receptor)-PI3K-MTOR pathway, to permit CySC differentiation. When CySCs divide, daughter cells can either repopulate the niche and retain CySC identity or encounter a burst of insulin signaling that promotes cyst cell differentiation. Indeed, when InR-MTOR is inhibited, CySC-like cells accumulate due to the failure in differentiation. However, when autophagy and InR-MTOR activity are inhibited simultaneously, cyst cells proceed to differentiate. These data indicate that although CySCs require active autophagy for maintenance, autophagy needs to be suppressed for the initiation of differentiation. Interestingly, active autophagy appears to be required, once again, in more differentiated cyst cells to allow for the proper regulation of germline differentiation. Altogether, our study suggests that autophagy must be fine-tuned in the testis to control somatic stem cell behavior and coordinate soma-germline interactions. In addition, metabolic “re-wiring” is needed in order for cyst cells to differentiate from CySCs.
An outstanding question from our findings is what the autophagic cargo may be. Using a combination of ex vivo culture and genetic assays, we demonstrated that lipids accumulate in lipid droplets (LDs) when autophagy-related genes are downregulated in early cyst cells. In addition, we showed that autophagosomes colocalize with LDs in cyst cells. Interestingly, enhancing mitochondrial fatty acid oxidation reduces the lipid accumulation that occurs when autophagy is downregulated, which correlates with a suppression of cyst cell loss. Therefore, one future goal is to characterize the mechanism(s) by which autophagy regulates lipid levels. One possible scenario would be through the direct breakdown of LDs through lipophagy. However, LDs could also arise as a consequence of the accumulation of mitochondrial damage due to loss of mitophagy, resulting in a decline in fatty acid oxidation and a consequent increase in lipid levels. Regardless of the precise mechanism(s) by which lipids accumulate when autophagy is disrupted, our data indicate that autophagy is important for somatic stem cell maintenance. In addition, autophagy regulates lipid levels in somatic cells to provide a supportive niche for germ cell differentiation (Figure 1).
Of course, there are other possible roles for autophagy in the somatic cells of the testis. Upon nutrient stress, there is some evidence that cyst cells may act as nonprofessional phagocytes to eliminate dead germline cysts and provide recycled nutrients to the surviving cells; indeed, autophagic markers are upregulated in this context. In addition, organelle quality control is likely key for maintenance of CySC function in aging animals. Hence, basal autophagy is likely to act through mitophagy and other cargo-specific pathways to maintain a pristine stem cell pool. Taken together, these data from our lab and published data from others highlight the importance of autophagy in regulating stem cell behavior via both cell autonomous and cell non-autonomous mechanisms to maintain tissue homeostasis.
Funding Statement
This work was supported by the Eli and Edythe Broad Center of Regenerative Medicine and Stem Cell Research, University of California Los Angeles [Postdoctoral training award]; Eli and Edythe Broad Center of Regenerative Medicine and Stem Cell Research, University of California Los Angeles [Innovation Award]; National Institute on Aging [AG040288, AG028092, AG052732].
Disclosure statement
No potential conflict of interest was reported.
Reference
- [1].Sênos Demarco R, Uyemura BS, Jones D. Leanne. EGFR signaling stimulates autophagy to regulate stem cell maintenance and lipid homeostasis in the Drosophila testis. Cell Rep. 2020;30(4):1101–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]


