ABSTRACT
Macroautophagy/autophagy induction, i.e., the formation of autophagosomes, is robust following many forms of muscle injury. Autophagy inhibition studies strongly indicate that autophagy is necessary for successful muscle fiber recovery. Now, there are accumulating pieces of evidence indicating that autophagosome clearance, i.e., autophagy flux, does not increase to match the burden of accumulating damaged proteins and organelles after muscle fiber damage, creating a bottleneck effect. Some potential consequences of the bottleneck effect are reduced regenerative capacity marked by the inadequate activation of muscle stem cells (i.e., satellite cells) and a lesser commitment toward differentiation due to a deficiency in energetic substrates and/or molecular signaling pathways. These findings highlight an emerging area of investigation for both autophagy and muscle regeneration fields. The identification of the molecular mechanisms governing autophagy and autophagy flux may serve as targets for future therapies to enhance the recovery of its function in healthy and diseased muscle.
Abbreviations
BNIP3: BCL2/adenovirus E1B interacting protein 3; CQ: chloroquine; DMD: Duchenne muscular dystrophy; MAP1LC3/LC3: microtubule-associated protein 1 light chain 3; ULK1: unc-51 like kinase 1.
KEYWORDS: Mitochondria, mitophagy, muscle regeneration, muscle strength, satellite cell, two-photon microscopy, ULK1
Introduction
Skeletal muscle injury caused by exercise or trauma (e.g., contusion, crush, blast, freezing, penetration, laceration) results in the physical disruption of the force-generating and/or force-transducing proteins, thereby causing an immediate loss of strength [1]. The functional recovery of skeletal muscle is accomplished through a sequence of phases: degradation, inflammation, regeneration, and remodeling phases [2,3]. The importance of these phases is emphasized by the fact that 1) disruption of any one of these phases results in a protracted recovery, and 2) regenerative medicine approaches commonly seek to augment these phases to expedite muscle healing. Autophagy is recognized across many tissue types and cellular conditions as a process for maintaining cellular homeostasis through the removal of damaged proteins and/or organelles. While it is not surprising that autophagy should play a role in skeletal muscle recovery, the area remains a topic of open investigation and intrigue.
Over the past decade, the association between muscle damage and autophagy has been well established in the literature. Strenuous bouts of exercise, which can cause the most common type of contraction-induced muscle injury, have been shown to increase autophagy protein content in the human and rodent skeletal muscle [4,5]. More direct examples linking autophagy to muscle damage come from the field of muscular dystrophy, i.e., Duchenne muscular dystrophy (DMD). Basil Petrof’s group showed that dmd/mdx (dystrophin, muscular dystrophy) mice, the most common animal model for DMD that presents with continuous muscle damage and recovery, have greater ULK1, LC3, and BNIP3 protein content compared to the wild-type control mice. With further stimutation of autophagy using the AMP-activated protein kinase (AMPK) agonist AICAR, the result was an increase in skeletal muscle contractility and mitochondrial content in dmd mice [6]. The rationale is that greater clearance of damaged and/or dysfunctional proteins via autophagy stimulation improves muscle strength. In agreement, Bibee and colleagues stimulated autophagy using rapamycin nanoparticles, relieving MTORC1-mediated autophagy suppression and reported greater skeletal and cardiac muscle contractility in dmd mice [7]. Clearly, there was evidence linking muscle injury and recovery processes with autophagy, but definitive studies on the necessity of autophagy were still lacking.
In 2016, we reported that 3-methyladenine-treated mice had significantly less recovery of muscle strength and less recovery of mitochondrial enzyme activities two weeks post-injury [8]. Also, Kahana and colleagues demonstrated that CQ impaired muscle regeneration in zebrafish [9]. Both studies provide evidence that chemical-induced autophagy inhibition negatively affects muscle fiber repair. Indeed, more direct evidence comes from knockout mouse studies of autophagy proteins. For instance, skeletal muscle-specific knockout of Ulk1, responsible for autophagy initiation, slows the recovery of muscle strength in mice after injury [10]. Ketan Patel’s research group reported that mice with genetically altered Atg16l1 (autophagy related 16-like 1 [S. cerevisiae]) [11], which participates in autophagosome elongation, had impaired muscle regeneration after an injury as well [12]. Importantly, autophagy induction occurs during myoblast differentiation and after muscle injuries, including eccentric contraction-induced injury, myotoxic injury (i.e., cardiotoxin), ischemia-reperfusion injury, freeze injury, ischemia, and rotator cuff injury [8,10,13–15]. These observations are important to acknowledge because the cellular mechanisms of recovery are injury-dependent [16], yet autophagy is common among them all. Therefore, these studies collectively demonstrate that autophagy is a consistent response to skeletal muscle damage and a necessary component for a successful recovery.
The process of autophagy is complete when assembled autophagosomes fuse with the lysosomes, and the degraded end-products (e.g., nucleic, amino, and fatty acids) are released into the cell (Figure 1). Our recent results indicate that autophagosome clearance, i.e., autophagy flux, does not increase to match the burden even if muscle damage leads to an abundance of autophagosomes. By utilizing the GFP-LC3-RFP-LC3ΔG fluorescent probe (Addgene, 84572; deposited by Noboru Mizushima) and 2-photon microscopy, we detected an abundance of autophagosomes in injured skeletal muscle cells, yet there was a decrease in autophagy flux compared to uninjured muscle cells [13]. Abhinav Diwan and colleagues reported a similar finding in cardiomyocytes when ischemia-reperfusion injury resulted in the accumulation of BECN1 and reduction of LAMP2 (lysosomal-associated membrane protein 2), indicating slowed autophagy flux [17]. We can also return to the DMD literature where Lucia Latella’s research group showed deficient autophagy flux in the dmd mouse during the disease progression [18]. These studies illustrate the presence of an autophagy bottleneck in the recovering muscle cells as autophagy is mounting a response to clear damaged proteins and organelles but is limited in its capacity to degrade those autophagosomes (Figure 1). Clearly, therapeutic strategies targeting autophagy flux are warranted and could impact muscle repair across organs (e.g., skeletal and cardiac muscle) and pathologies.
Figure 1.
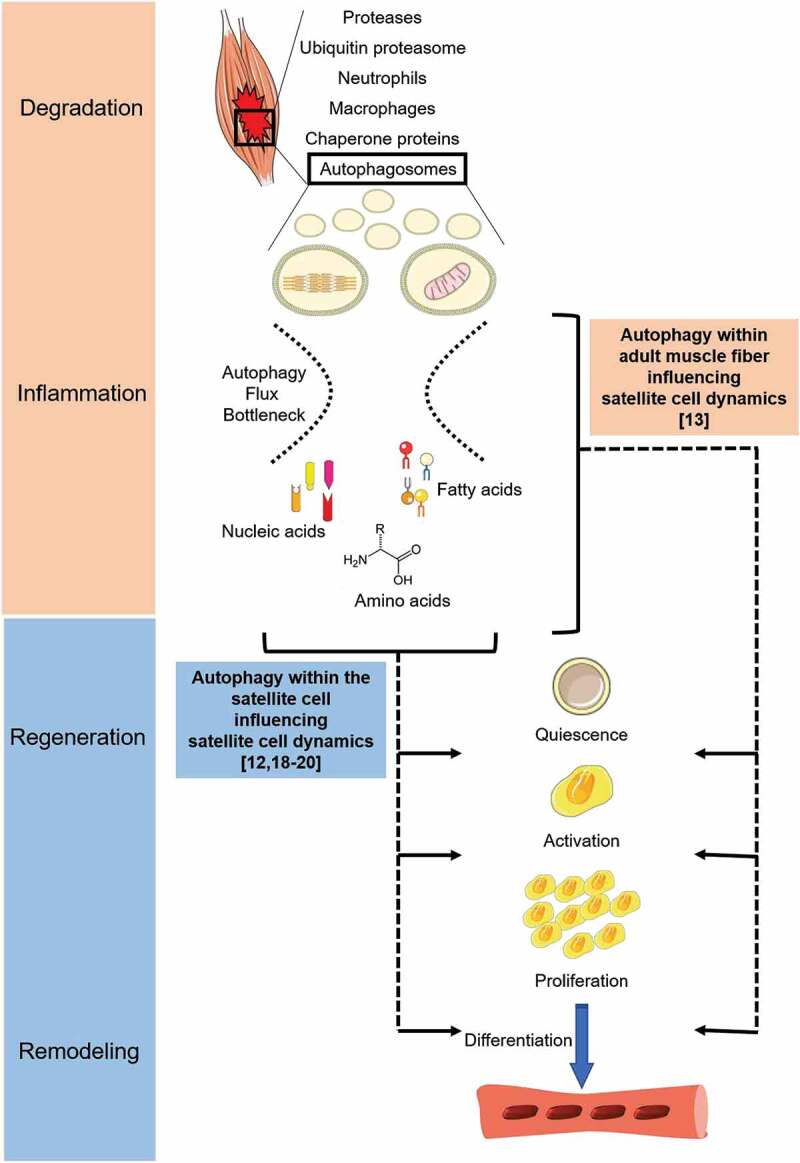
The timing of the autophagic response to muscle damage places it among the degradation and inflammation phases of muscle recovery. Autophagy is among the important cellular processes for the removal of damaged proteins and/or organelles after muscle damage. Both contractile proteins and mitochondria can be removed by autophagy. Recent work discussed within this commentary highlights two possible limitations of autophagy during muscle recovery: First, an inability to match autophagy flux with autophagosome numbers creating an autophagy bottleneck; and second, insufficient autophagy within the adult muscle fiber and/or the muscle stem cell negatively influences stem cell behaviors, such as proliferation and commitment to differentiation.
The end-products of successful autophagosome clearance can be used as molecular building blocks (e.g., DNA replication), metabolic fuels (e.g., fatty acid oxidation), and/or in molecular signaling. Interestingly, Lucia Latella’s research also shows that muscle biopsies from boys with DMD have a reduced autophagic response during disease progression that corresponds with a reduction in muscle stem cell, i.e., satellite cell, activity [18]. Additionally, CQ-treated dmd mice have reduced satellite cell commitment to differentiation and repair. Because CQ was administered i.p., autophagy within the muscle fiber must also be considered to potentially influence satellite cell activation. A finding from our recent work sheds more light on the subject that satellite cells may be influenced by autophagy signaling within the adult muscle fiber. Specifically, we used a Myog (myogenin)-Cre model to drive the Ulk1 genomic deletion, thereby eliminating ULK1 protein in adult muscle fibers, but not in satellite cells. Despite this, we reported less satellite cell activation and commitment to differentiation following muscle damage in our ULK1-deficient animals [13]. In the last four years, there have been several reports highlighting the specific role of autophagy within satellite cells [12,18–20]. The consensus is that autophagy is critical for helping to provide energy for the energetically demanding proliferative phase, and by providing a method for cellular remodeling during differentiation. Our work extends the role of autophagy for satellite cell function to the molecular signaling of the adult myofiber (Figure 1).
In summary, research conducted primarily in the last ten years has advanced our appreciation for autophagy during muscle fiber repair. Autophagy is robust and occurs following multiple types of muscle injuries. However, there are apparent limitations during the autophagic process, and data from our lab and others strongly indicate late-stage autophagy, i.e., autophagosome clearance, is not adequate to match the burden of damaged proteins and organelles resulting from muscle damage and this potentially has negative consequences on satellite cell activation. Autophagic insufficiency is reported in pathologies ranging from aging to DMD. Defining the molecular regulation of autophagy flux and autophagy-linked satellite cell signaling are achievable goals for the next ten years of research. Such advancements may help develop future regenerative medicine approaches to bolster muscle repair in otherwise healthy muscle and diseased muscle alike.
Disclosure statement
No potential conflict of interest was reported by the authors
References
- [1].Warren GL, Lowe DA, Armstrong RB.. Measurement tools used in the study of eccentric contraction-induced injury. Sports Med. 1999;27:43–59. [DOI] [PubMed] [Google Scholar]
- [2].Grounds MD. The need to more precisely define aspects of skeletal muscle regeneration. Int J Biochem Cell Biol. 2014;56:56–65. [DOI] [PubMed] [Google Scholar]
- [3].Jarvinen TA, Jarvinen TL, Kaariainen M, et al. Muscle injuries: biology and treatment. Am J Sports Med. 2005;33:745–764. [DOI] [PubMed] [Google Scholar]
- [4].Fry CS, Drummond MJ, Glynn EL, et al. Skeletal muscle autophagy and protein breakdown following resistance exercise are similar in younger and older adults. J Gerontol A Biol Sci Med Sci. 2013;68:599–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Salminen A, Vihko V. Autophagic response to strenuous exercise in mouse skeletal muscle fibers. Virchows Archiv B. 1984;45:97–106. [DOI] [PubMed] [Google Scholar]
- [6].Pauly M, Daussin F, Burelle Y, et al. AMPK activation stimulates autophagy and ameliorates muscular dystrophy in the mdx mouse diaphragm. Am J Pathol. 2012;181:583–592. [DOI] [PubMed] [Google Scholar]
- [7].Bibee KP, Cheng YJ, Ching JK, et al. Rapamycin nanoparticles target defective autophagy in muscular dystrophy to enhance both strength and cardiac function. Faseb J. 2014;28:2047–2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Nichenko AS, Southern WM, Atuan M, et al. Mitochondrial maintenance via autophagy contributes to functional skeletal muscle regeneration and remodeling. Am J Physiol Cell Physiol. 2016;311:C190–200. [DOI] [PubMed] [Google Scholar]
- [9].Saera-Vila A, Kish PE, Louie KW, et al. Autophagy regulates cytoplasmic remodeling during cell reprogramming in a zebrafish model of muscle regeneration. Autophagy. 2016;12:1864–1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Call JA, Wilson RJ, Laker RC, et al. Ulk1-mediated autophagy plays an essential role in mitochondrial remodeling and functional regeneration of skeletal muscle. Am J Physiol Cell Physiol. 2017;312:C724–C32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Cadwell K, Liu JY, Brown SL, et al. A key role for autophagy and the autophagy gene Atg16l1 in mouse and human intestinal Paneth cells. Nature. 2008;456:259–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Paolini A, Omairi S, Mitchell R, et al. Attenuation of autophagy impacts on muscle fibre development, starvation induced stress and fibre regeneration following acute injury. Sci Rep. 2018;8:9062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Nichenko AS, Southern WM, Tehrani KF, et al. Mitochondrial-specific autophagy linked to mitochondrial dysfunction following traumatic freeze injury in mice. Am J Physiol Cell Physiol. 2020;318:C242–C252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].McClung JM, McCord TJ, Ryan TE, et al. BAG3 (Bcl-2-associated athanogene-3) coding variant in mice determines susceptibility to ischemic limb muscle myopathy by directing autophagy. Circulation. 2017;136:281–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Gumucio JP, Davis ME, Bradley JR, et al. Rotator cuff tear reduces muscle fiber specific force production and induces macrophage accumulation and autophagy. J Orthop Res. 2012;30:1963–1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Warren GL, Summan M, Gao X, et al. Mechanisms of skeletal muscle injury and repair revealed by gene expression studies in mouse models. J Physiol. 2007;582:825–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Ma X, Liu H, Foyil SR, et al. Impaired autophagosome clearance contributes to cardiomyocyte death in ischemia/reperfusion injury. Circulation. 2012;125:3170–3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Fiacco E, Castagnetti F, Bianconi V, et al. Autophagy regulates satellite cell ability to regenerate normal and dystrophic muscles. Cell Death Differ. 2016;23:1839–1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Garcia-Prat L, Martinez-Vicente M, Perdiguero E, et al. Autophagy maintains stemness by preventing senescence. Nature. 2016;529:37–42. [DOI] [PubMed] [Google Scholar]
- [20].Tang AH, Rando TA. Induction of autophagy supports the bioenergetic demands of quiescent muscle stem cell activation. Embo J. 2014;33:2782–2797. [DOI] [PMC free article] [PubMed] [Google Scholar]


