Abstract
Background
National Comprehensive Cancer Network guidelines consider 18F-fluciclovine PET-CT for prostate cancer biochemical recurrence localisation after radical prostatectomy, whereas European Association of Urology guidelines recommend prostate-specific membrane antigen (PSMA) PET-CT. To the best of our knowledge, no prospective head-to-head comparison between these tests has been done so far. The aim of this study was to compare prospectively paired 18F-fluciclovine and PSMA PET-CT scans for localising biochemical recurrence of prostate cancer after radical prostatectomy in patients with low prostate-specific antigen (PSA) concentrations (<2·0 ng/mL).
Methods
This was a prospective, single-centre, open-label, single-arm comparative study done at University of California Los Angeles (Los Angeles, CA, USA). Patients older than 18 years of age with prostate cancer biochemical recurrence after radical prostatectomy and PSA levels ranging from 0·2 to 2·0 ng/mL without any prior salvage therapy and with a Karnofsky performance status of at least 50 were eligible. Patients underwent 18F-fluciclovine (reference test) and PSMA (index test) PET-CT scans within 15 days. Detection rate of biochemical recurrence at the patient level and by anatomical region was the primary endpoint. A statistical power analysis demonstrated that a sample size of 50 patients was needed to show a 22% difference in detection rates in favour of PSMA (test for superiority). Each PET scan was interpreted by three independent masked readers and a consensus majority interpretation was generated (two vs one) to determine positive findings. This study is registered with ClinicalTrials.gov, number NCT03515577, and is complete.
Findings
Between Feb 26, 2018, and Sept 20, 2018, 143 patients were screened for eligibility, of whom 50 patients were enrolled into the study. Median follow-up was 8 months (IQR 7–9). The primary endpoint was met; detection rates were significantly lower with 18F-fluciclovine PET-CT (13 [26%; 95% CI 15–40] of 50) than with PSMA PET-CT (28 [56%; 41–70] of 50), with an odds ratio (OR) of 4·8 (95% CI 1·6–19·2; p=0·0026) at the patient level; in the subanalysis of the pelvic nodes region (four [8%; 2–19] with 18F-fluciclovine vs 15 [30%; 18–45] with PSMA PET-CT; OR 12·0 [1·8–513·0], p=0·0034); and in the subanalysis of any extrapelvic lesions (none [0%; 0–6] vs eight [16%; 7–29]; OR non-estimable [95% CI non-estimable], p=0·0078).
Interpretation
With higher detection rates, PSMA should be the PET tracer of choice when PET-CT imaging is considered for subsequent treatment management decisions in patients with prostate cancer and biochemical recurrence after radical prostatectomy and low PSA concentrations (≤2·0 ng/mL). Further research is needed to investigate whether higher detection rates translate into improved oncological outcomes.
Introduction
Treatment of patients with biochemical recurrence of prostate cancer is guided by disease location and extent.1,2 Whole-body PET-CT imaging can depict increased L-amino-acid-transporter-1 (LAT1) activity with 18F-fluciclovine or overexpressed cell-surface proteins such as prostate-specific membrane antigen (PSMA) with 68Ga-PSMA-11. Both 18F-fluciclovine and PSMA PET-CT localise biochemical recurrence with higher detection rates and sensitivity than conventional imaging (eg, CT, bone scanning, and MRI) and choline PET-CT.3,4 For biochemical recurrence localisation, National Comprehensive Cancer Network (NCCN) guidelines recommend the Food and Drug Administration-approved 18F-fluciclovine PET-CT, whereas European Association of Urology guidelines recommend PSMA PET-CT.1,2 Preliminary reports suggest superior detection rates of PSMA PET-CT compared with 18F-fluciclovine PET-CT.5 However, these imaging tests have not been compared prospectively and directly.
Here, we present a prospective head-to-head comparison between 18F-fluciclovine and PSMA PET-CT for localising biochemical recurrence after radical prostatectomy in patients with low prostate-specific antigen (PSA) concentrations (≤2·0 ng/mL). Validation of imaging findings is rarely available in patients with biochemical recurrence. Therefore, assessments of true test sensitivity and specificity for biochemical recurrence detection is difficult, if not impossible. In this setting, the most relevant performance parameter is the detection rate (the proportion of patients with PET-positive findings) that approximates the test sensitivity for prostate cancer detection.6 Although some false-positive findings have been reported (eg, mistaken identification of ganglia and ribs trauma as prostate cancer),7–9 the positive predictive value (PPV) of PSMA PET-CT with experienced readers is high (>85%).6,10 Hence, we aimed to compare the detection rates of 18F-fluciclovine and PSMA PET-CT, at the patient level and by anatomical region (pelvic and extra-pelvic localisations). Based on published data3,11–15 the hypothesis was a detection rate difference of at least 22% between the two tests in favour of PSMA.
Methods
Study design and participants
This was a prospective, single-centre, open-label, single-arm comparative imaging study done at University of California Los Angeles (UCLA; Los Angeles, CA, USA) using external, anonymised, masked, and inde pendent interpretations of 50 consecutive paired 18F-fluciclovine and PSMA PET-CT studies. The study was done under an investigational new drug approval protocol (IND#130649; appendix pp 18–41), approved by the local institutional review board (IRB#17–001885).
Inclusion criteria were histopathologically proven prostate cancer; biochemical recurrence after radical prostatectomy with PSA values of 0·2–2·0 ng/mL at the time of imaging; no previous salvage therapies (including salvage radiotherapy or salvage lymph node dissection); 18F-fluciclovine and PSMA PET-CT done within 15 days of each other; no change in prostate cancer treatment between the two scans; ability to understand and sign the written informed consent form; age older than 18 years; and Karnofsky performance status of at least 50. Patients were enrolled irrespective of previous conventional imaging findings. Informed written and oral consent was obtained from all patients.
Procedures
All patients had standard-of-care 18F-fluciclovine and investigational PSMA PET-CT according to guidelines within a maximum time interval of 15 days between the two scans.16,17 Patients were asked to fast for more than 4 h and avoid substantial exercise for more than 24 h before 18F-fluciclovine tracer administration. 68Ga-PSMA-11 (Glu-NH-CO-NH-Lys-(Ahx)-[68Ga(HBED-CC)]) was used as the PSMA ligand18 and was obtained from the Biomedical Cyclotron Facility at UCLA. Oral and intravenous CT-contrast was administered for both tests unless obtained at outside institutions or contraindicated. A 5-mm slice thickness CT scan was used. All PET images acquired from pelvis to vertex were corrected for attenuation, dead time, random events, and scatter. The time per bed position was based on patient weight.19
PET-CT scans were each interpreted by three independent masked experts (18F-fluciclovine experts were TB-G, CN, and BS-B; PSMA experts were MSH, TAH, and CR) who were not involved in study design or data acquisition. The fluciclovine experts did not read the PSMA scans and the PSMA experts did not read the fluciclovine scans. Details of PET-CT experience of each reader are in the appendix (p 1).
Each reader was masked to the interpretations of the five other readers. Anonymised datasets included CT and attenuation-corrected PET images, prostate cancer history, and a spreadsheet with interpretation guidelines (appendix p 17). Readers were instructed to first characterise PET lesions as suspicious or non-suspicious for prostate cancer lesions. CT correlates of the PET-positive lesions were then analysed for disease localisation and to rule out pitfalls.7–9 Readers assessed the presence of prostate cancer (positive vs negative) for five regions according to interpretation guidelines:7,20,21 prostate bed (T), pelvic lymph nodes (N), extrapelvic nodes (M1a), bone (M1b), or other organ (M1c).
In cases of reader disagreement, regions were rated on the basis of a consensus majority rule (2:1). PET-CT scans were considered positive if any of the five regions were rated positive by a 2:1 majority.
All patients were followed for subsequent biopsies, imaging studies, PSA measurements, and disease management. Treatment decisions were not standardised and were made at the discretion of the referring physician on the basis of all available clinical information, including the non-masked local reports of both PET scans and any other imaging findings. If available, PET-positive regions were categorised by the non-masked UCLA investigators as true or false positive by a composite reference standard (appendix p 41). This composite reference standard included histopathology, follow-up imaging, or PSA decrease after PET-positive lesion-directed therapy without systemic therapy or without whole-pelvic lymph node radiotherapy. PET-negative regions by majority consensus but with subsequently confirmed prostate cancer were considered false negatives. True negative was not defined.
Outcomes
The primary outcome was the detection rate (proportion of patients with PET-positive findings) of 18F-fluciclovine (reference test) and PSMA PET-CT (index test) for the identification of tumour locations, at the patient level and by anatomical region. The secondary outcomes were detection rates of 18F-fluciclovine and PSMA PET-CT stratified by PSA value (0·2–0·5 ng/mL vs 0·51–1·0 ng/mL vs 1·01–2·0 ng/mL); the positive predictive value and sensitivity of 18F-fluciclovine and PSMA PET-CT in patients with available lesion validation by the composite reference standard; and the inter-reader agreement of 18F-fluciclovine and PSMA PET-CT studies.
Statistical analysis
Based on published data, detection rates at the patient level for biochemical recurrence localisation at PSA concentrations of 2 ng/mL or less are 21–59% (estimated mean 47%) for 18F-fluciclovine PET-CT3,11,12 and 61–82% (estimated mean 69%) for PSMA PET-CT.13–15 A statistical power analysis established prospectively that a sample size of 50 patients provides at least 86% power to detect the expected difference of 22% between detection rates at the patient level in favour of PSMA PET-CT (test for superiority) assuming a one-sided α of 0·05 (one-sided McNemar exact conditional test).
Descriptive statistics (median and IQR) or frequencies and percentages were computed to summarise demographic, clinical, pathological, and imaging characteristics. Detection rates per patient, per region, and sensitivity of index and reference tests based on majority consensus reads were compared using the two-sided McNemar’s test for paired proportions (with odds ratios [ORs] and 95% CIs; Clopper-Pearson Exact method) and two-sided Fisher’s exact test for independent proportions. These analyses were also done after stratifying the population by PSA concentrations (<0·5 vs 0·51–1·00 vs 1·01–2·00 ng/mL). Fleiss multirater κ statistics were computed to assess inter-reader agreement between reviewers for each imaging modality (18F-fluciclovine and PSMA). Pairwise κ coefficients were used to compare reader performance for the index and reference tests.
To establish the degree of lesion PET-tracer uptake, a post-hoc semiquantitative analysis was done in the subset of patients with concordant PSMA and 18F-fluciclovine positive findings. The maximum standardised uptake value (SUVmax) of each PET-positive lesion was measured by local investigators. Background organ SUVmean was established by placing 3D volumes of interest on the right liver lobe, the descending aorta at the carina level, and the pelvic muscle closest to the lesion. Lesion-to-background ratios were then calculated. The paired t test was used to compare SUVmax and lesion-to-background ratios.
To establish which other factors might be associated with lesion detection after accounting for PET tracer (18F-fluciclovine vs PSMA), a post-hoc analysis was done with multivariable mixed-effects logistic regression models using SAS, version 9.4. The following variables were tested with the outcome of a positive PET scan: PET tracer, ongoing androgen deprivation therapy, history of adjuvant androgen deprivation therapy, history of adjuvant radiotherapy, risk group, PSA doubling time (higher vs lower than median), PSA velocity, 18F-fluciclovine uptake time (≤3 min vs >3 min) and 18F-fluciclovine with contrast-enhanced CT. Because of sample size limitations, two predictor variables at a time were tested rather than a single full model with all terms: PET tracer (18F-fluciclovine vs PSMA) and each other variable was tested separately.
All other statistical analyses were done in R, version 3.5.1. To overcome the statistical limitation of multiple testing in a sample of modest size, an additional post-hoc Benjamini-Hochberg step-up procedure was done to obtain the overall false-discovery rate associated with the 0·05 significance level.
This study is registered on ClinicalTrials.gov, numberNCT03515577.
Role of the funding source
There was no funding for this study. The corresponding author had full access to all the data and had final responsibility to submit for publication.
Results
Patient were enrolled between Feb 26, 2018, and Sept 20, 2018. 143 patients were assessed for eligibility, of whom 93 were excluded (19 of these could not be included in the study because their insurance denied coverage of 18F-fluciclovine PET-CT) and 50 were enrolled (figure 1). Demographics and clinical characteristics of the study population are presented in table 1. Median PSA concentration at enrolment was 0·48 ng/mL (IQR 0·38–0·83). The median time interval between the two scans was 6 days (IQR 2–8). 21 (42%) of 50 patients had 18F-fluciclovine PET-CT after PSMA PET-CT and 29 (58%) had 18F-fluciclovine PET-CT before PSMA PET-CT.
Figure 1: Trial profile.
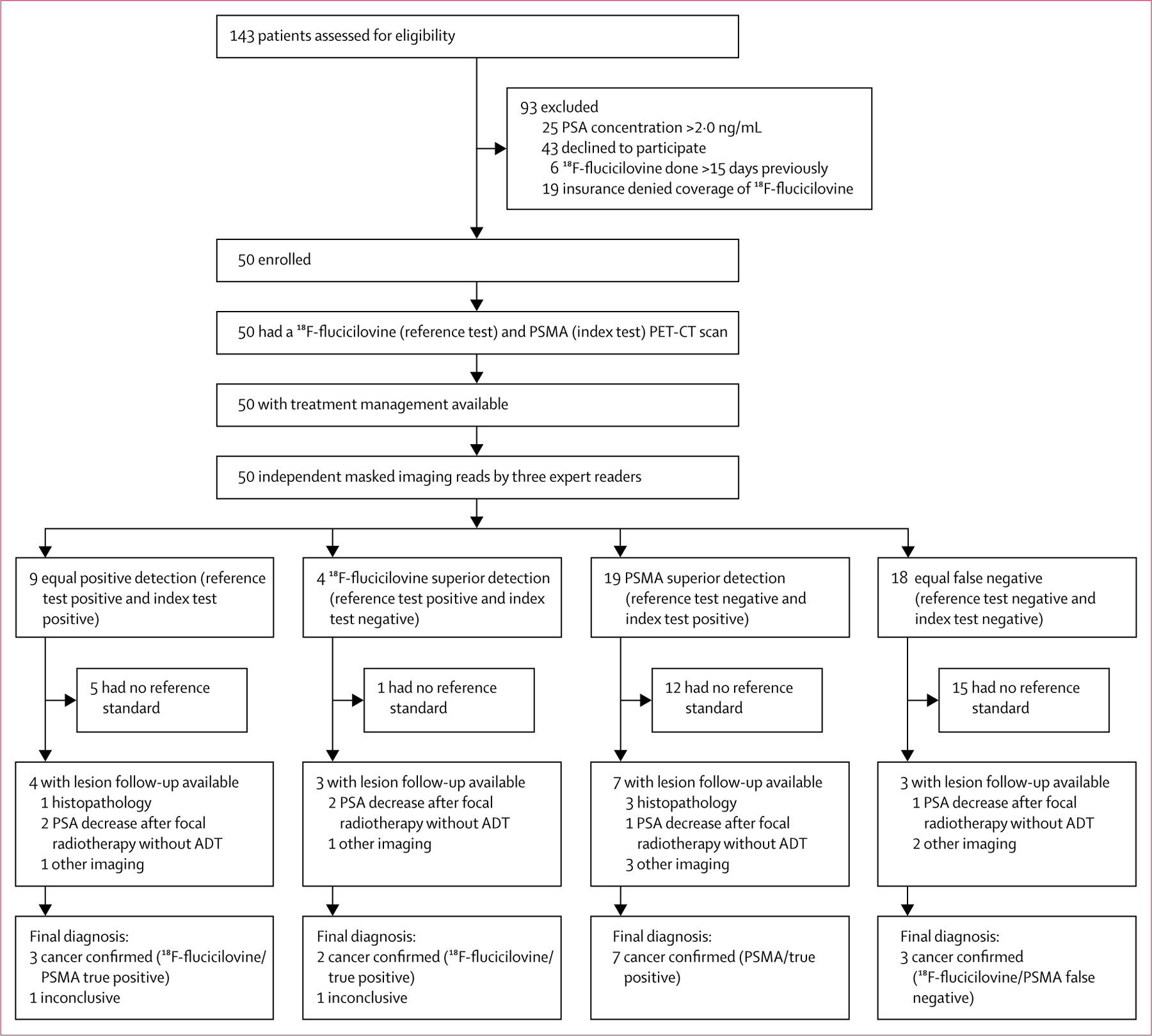
The reference standard included histopathology, follow-up imaging, or PSA concentration decrease after PET-positive lesion-directed therapy without systemic therapy or without whole-pelvic lymph node radiotherapy. Patients without a reference standard were not excluded from primary and safety analyses; this was only used for the positive predictive value and sensitivity analyses.
Table 1:
Demographic and clinicopathological characteristics
| All patients (n=50) | |
|---|---|
| National Comprehensive Cancer Network risk group | |
| Intermediate | 17 (34%) |
| High | 14 (28%) |
| Very high | 5 (10%) |
| Regional (N1) | 11 (22%) |
| Not available | 3 (6%) |
| PSA at initial diagnosis, ng/mL | |
| <10 | 25 (50%) |
| 10–20 | 9 (18%) |
| >20 | 3 (6%) |
| Not available | 13 (26%) |
| Histopathological TNM stage | |
| pT2 | 18 (36%) |
| pT3a | 12 (24%) |
| pT3b | 5 (10%) |
| pN1 | 11 (22%) |
| Not available | 4 (8%) |
| International Society of Urological Pathologists grade group | |
| 1 | 3 (6%) |
| 2 | 16 (32%) |
| 3 | 15 (30%) |
| 4 | 5 (10%) |
| 5 | 8 (16%) |
| Not available | 3 (6%) |
| Pelvic lymph node dissection | 40 (80%) |
| Margins positive (R1) | 13 (26%) |
| Adjuvant radiotherapy | 6 (12%) |
| Adjuvant androgen deprivation therapy | 10 (20%) |
| PSA persistence (never undetectable after surgery) | 12 (24%) |
| PSA recurrence (became undetectable after surgery then increased to detectable levels) | 38 (76%) |
| Ongoing androgen deprivation therapy | 7 (14%) |
| Age, years | 68 (64–74) |
| Time from radical prostatectomy to PET, years | 3(1–8) |
| Last PSA concentration before PET, ng/mL | 0.48 (0.38–0.83) |
| PSA doubling time, months | 4(3–16) |
| PSA velocity, ng/mL per year | 0.3 (0.11–1.2) |
Data are n (%) or median (IQR). PSA=prostate-specific antigen.
Scanner devices and iterative algorithms used for PET image reconstruction are listed with the technical parameters in the appendix (p 2). Standrd-of-care 18F-fluciclovine PET-CT was done at UCLA in 38 (76%) of 50 and at other institutions in 12 (24%) patients. Investigational PSMA PET-CT was done at UCLA in all 50 patients. For 18F-fluciclovine PET-CT, intravenous CT contrast was administered in 35 (70%) of 50 patients and oral CT contrast was administered in 37 (74%) patients; for PSMA PET-CT, intravenous CT contrast was administered in 48 (96%) patients and oral CT contrast in 49 (98%) of 50 patients. The median injected activity was 381 MBq (IQR 359–407) for 18F-fluciclovine and 200 MBq (192–204) for PSMA. PET images were acquired after a median uptake period of 2 min (IQR 1–3) for 18F-fluciclovine and 61 min (57–66) for PSMA.
The subsequent patient management of the entire cohort after a median follow-up of 8 months (IQR 7–9) is summarised in the appendix (p 3). Focal therapy (metastasis surgery and metastasis stereotactic body radiation therapy) was applied to PET-positive lesions in 15 (30%) of 50 patients, 30 (60%) patients received androgen deprivation therapy, and nine (18%) were managed with active surveillance. No patients were lost to follow-up or excluded from analysis.
Individual reader interpretations and the majority consensus are detailed in the appendix (pp 4–7). 18 (36%) of 50 patients had equal false-negative detection, nine (18%) had equal positive detection, four (8%) had 18F-fluciclovine superior detection, and 19 (38%) had PSMA superior detection (figure 1, appendix p 8).20 The contingency tables are in the appendix (pp 9–10).
The detection rates of biochemical recurrence per patient were significantly lower with 18F-fluciclovine (13 [26%; 95% CI 15–40] of 50) than with PSMA PET-CT (28 [56%; 41–70] of 50), with an OR of 4·8 (95% CI 1·6–19·2; p=0·0026; figure 2).
Figure 2: Detection rates per region and per patient (majority consensus reads).

PSMA=prostate-specific membrane antigen.
To assess potential bias, a post-hoc analysis was done, confirming the differences in the patient-level detection rates of biochemical recurrence between the reference and index tests in the 38 patients with 18F-fluciclovine PET-CT obtained at UCLA (12 [32%; 95% CI 18–49] of 38 with 18F-fluciclovine vs 21 [55%; 38–71] of 38 with PSMA; OR 3·3 [1·0–13·7], p=0·049), the 12 patients with 18F-fluciclovine PET-CT obtained at other institutions (one [8%; 0–38] of 12 with 18F-fluciclovine vs seven [58%; 28–85] of 12 with PSMA; OR not estimable, p=0·031), and the 35 patients in whom both studies were done with contrast-enhanced CT (11 [31%; 17–49] of 35 with 18F-fluciclovine vs 20 [57%; 40–74] of 35 with PSMA; OR 4·0 [1·08–22·1], p=0·035). Additionally, we found no significant difference between the patient-level detection rates of 18F-fluciclovine PET-CT obtained at UCLA and at other institutions (12 [32%; 17–51] of 38 at UCLA vs one [8%; 0–38] of 12 at other institutions; difference 23% [95% CI 7–41], p=0·15) or those done with versus without intravenous contrast (11 [31%; 17–49] of 35 vs two [13%; 2–40] of 15; difference 18% [10–37], p=0·29).
18F-fluciclovine detection rates of biochemical recurrence were significantly lower than PSMA detection rates for the pelvic lymph node region (N; four [8%; 95% CI 2–19] of 50 with 18F-fluciclovine vs 15 [30%; 18–45] of 50 with PSMA; OR 12·0 [95% CI 1·8–513·0], p=0·0034) and for any extrapelvic lesions (M1; none [0–6] of 50 vs eight [16%; 7–29] of 50; OR non-estimable [95% CI non-estimable], p=0·0078; figure 2). No significant differences were detected for the individual extrapelvic lesion locations (M1a, M1b, and M1c), possibly because patient numbers were too small. Detection rates for prostate bed recurrence (T) did not differ significantly between the tests (nine [18%; 9–31] of 50 vs seven [14%; 6–27] of 50; OR 0·6 [0·1–3·1], p=0·73).
In the 26 patients with PSA concentrations of 0·2–0·5 ng/mL, detection rates were seven (27%; 95% CI 12–48) for 18F-fluciclovine versus 12 (46%; 27–67) with PSMA; for the 18 with concentrations 0·51–1·00 ng/mL, rates were five (28%; 10–53) versus 12 (67%; 41–87); and for the six with concentrations 1·01–2·00 ng/mL, detection rates were one (17%; 0–64) versus four (67%; 22–96; appendix pp 11–12). No significant difference was detected between these subgroups in the analysis by patient or region (appendix p 12).
Inter-reader agreement was significantly lower for 18F-fluciclovine (κ values ≤0·20) than for PSMA (κ values ≥0·60) at the patient level (p=0·0020) and per region (p≤0·016) except for the prostate bed region (table 2; pairwise κ is in the appendix p 13).
Table 2:
Inter-reader measures of agreement
| PSMA | l8F-fluciclovine | p value | |
|---|---|---|---|
| Detection at the patient level | |||
| Overall | 0.67 (0.51 to 0.83) | 0.20 (0.04 to 0.36) | 0.0020 |
| Detection at the regional level | |||
| Prostate bed (T) | 0.65 (0.49to 0.81) | 0.43 (0.27 to 0.59) | 0.046 |
| Pelvic lymph nodes (N) | 0.76 (0.60to 0.92) | 0.05 (−0.11 to 0.21) | <0.0001 |
| Extrapelvic nodes (M1a) | 0.60 (0.44to 0.76) | −0.02 (−0.18 to 0.14) | 0.0025 |
| Bone (M1b) | 0.46 (0.30 to 0.62) | −0.03 (−0.19 to 0.13) | 0.0051 |
| Other organ (M1c) | 0.65 (0.49to 0.81) | −0.01 (−0.17 to 0.15) | 0.016 |
| Any extrapelvic lesion (M1) | 0.60 (0.44to 0.76) | −0.07 (−0.23 to 0–09) | <0.0001 |
PET findings were validated in 15 (30%) of 50 patients (five (38%) of 13 with 18F-fluciclovine-positive findings and ten (36%) of 28 with PSMA-positive findings). Reference standard included histopathology (n=4), follow-up imaging (n=7), and PSA decreases after PET-directed focal therapy without androgen deprivation therapy (n=4; appendix p 14). Five other patients had imaging follow-up (MRI or CT) but without lesion validation because follow-up scans were negative. As anticipated, only a minority of patients (16 [32%] of 50) had surgery (n=3), biopsy (n=1), or further imaging (n=12) for lesion verification. No false-positive findings occurred with either tracer in the 15 patients in whom lesions were verified (positive predictive value of 100% for both 18F-fluciclovine and PSMA findings). Per-patient sensitivity was 33% (95% CI 15–58; five true positives and ten false negatives) for 18F-fluciclovine and 66% (42–85; ten true positive and five false negative) for PSMA PET-CT (OR 3·5 [95% CI 0·67–34·5], p=0·18).
The post-hoc semiquantitative analysis of lesion PET tracer uptake was done in seven patients: three concordantly positive pelvic lymph nodes and four local recurrences (appendix p 15). The mean lesion SUVmax was 8·21 (SD 4·05) for PSMA versus 3·73 (0·85) for 18F-fluciclovine (p=0·013) and lesion-to-background ratios were 1·68 (SD 1·16) versus 0·52 (0·16) for liver (p=0·0052); 6·83 (3·60) versus 2·44 (0·60) for blood pool (p=0·0085); and 25·39 (15·3) versus 3·69 (1·87) for muscle (p=0·021); all significantly higher for PSMA than 18F-fluciclovine PET-CT.
A summary of the post-hoc multivariable logistic regression analysis is in the appendix (p 16). The only significant predictor for test positivity was the PET tracer used (18F-fluciclovine vs PSMA; ORs 3·56–3·88; p<0·05). Neither 18F-fluciclovine uptake time (≤3 min vs >3 min) nor the administration of intravenous contrast for CT imaging were confounding factors.
The Benjamini-Hochberg step-up procedure indicated that the 0·05 significance level provided a 7% false-discovery rate control (data not shown), suggesting that the observed statistical differences were generally not artefacts of multiple hypothesis testing.
Discussion
PSMA PET-CT detects biochemical recurrence sites at low PSA concentrations more frequently and with higher reader agreement than 18F-fluciclovine PET-CT. The primary endpoint of this study was met (≥22% difference between the detection rates at the patient level) in this highly relevant population of patients with early biochemical recurrence in whom focused salvage therapy can be potentially curative. Detection rates per patient, for pelvic lymph nodes, and for any extrapelvic metastasis were more than twice as high with PSMA than with 18F-fluciclovine. The inter-reader agreement was consistently higher for PSMA than 18F-fluciclovine PET-CT.
Differences in detection rates between 18F-fluciclovine and PSMA PET-CT in similar patient cohorts have been previously reported: they averaged around 45% for 18F-fluciclovine3,11,12 and 65% for PSMA PET-CT.6,10,13–15 Inter-reader agreement was also consistently higher for PSMA than for 18F-fluciclovine PET-CT.8,22 These differences were now corroborated prospectively in the same cohort of post-radical prostatectomy biochemical recurrence patients with low PSA concentrations.
Detection rates for both the index and reference tests were lower in our study than those reported previously, probably because of the retrospective nature of most previous studies with heterogeneous patient populations and absence of masked image interpretation by multiple readers in some studies. One prospective study10 reported PSMA PET-CT detection rates in patients with low PSA concentrations similar to those reported in our study. Most of our cohort (44 [88%] of 50) had PSA concentrations of 1·0 ng/mL or less. 18F-fluciclovine detection rates for similar populations ranged from 41% in a retrospective study without masked readers12 to 21% in a prospective study with two independent readers.3 Thus, our detection rate of 26% is well within the expected range.3 Detection rates stratified by PSA concentrations were not significantly different between index and reference test. These findings might be because of the low number of patients in each PSA subgroup in our analysis. With more patients included, 18F-fluciclovine might have been non-inferior to PSMA PET-CT at higher PSA concentrations (eg, >1·5 ng/mL), although this notion is speculative.
Several factors might account for the superiority of PSMA PET-CT in patients with early biochemical recurrence. First, overexpression of PSMA results in high tracer uptake. SUVmax was two times higher and lesion-to-background ratios were seven times higher for concordantly PET-positive lesions with PSMA than 18F-fluciclovine. Expression and activity of LAT1, which is responsible for transport of 18F-fluciclovine into tumour cells, is high in advanced castrate-resistant prostate cancer but low in early castrate-sensitive prostate cancer.23 By contrast, PSMA expression is increased 100–1000 times in both castrate-sensitive and castrate-resistant prostate cancer.24,25 Equally important, PSMA expression in non-target tissues is very low. By contrast, amino acid transporters are important contributors to muscle protein anabolism and LAT1 expression is associated with skeletal muscle micro vasculature.26 Blood pool activity is high at early imaging timepoints and remains high over time.27 Subsequently, 18F-fluciclovine becomes highly distributed throughout skeletal muscles.27 Thus, both target and background characteristics favour lesion detectability with PSMA PET-CT. The favourable lesion-to-background ratio explains the high agreement among PSMA readers. This advantage of PSMA over 18F-fluciclovine might be less pronounced in patients with more advanced disease, higher PSA concentrations,12 and castrate-resistant disease,23 or in the 5–10% of patients whose lesions exhibit low or no PSMA expression.24,25
PSMA PET-CT detected pelvic lymph node metastases more frequently and with greater reader confidence than 18F-fluciclovine PET-CT. This finding is important because biochemical recurrence is most frequently associated with pelvic lymph node involvement and accurate detection of pelvic disease is crucial for planning local, potentially curative treatment.
No extrapelvic metastases were detected with 18F-fluciclovine PET-CT, whereas PSMA PET-CT detected extrapelvic oligometastatic disease in eight (16%) of 50 patients. Lesion validation was available in four of these patients and prostate cancer was confirmed in all of them (appendix p 14). Unusual lesion locations became evident in our study. These regions included the penis, the inguinal canal or spermatic cord, and inguinal lymph nodes (appendix p 14). Notably, ablative therapies might benefit patients when disease is still oligometastatic.28,29
Obtaining a firm reference standard in biochemical recurrence of prostate cancer is challenging. NCCN guidelines recommend observation or ADT with or without salvage radiotherapy and histological confirmation of the PET-positive lesions when feasible.1 In patients with low PSA concentrations, PET-positive lesions are rarely targeted with biopsies because they are often small and difficult to reach anatomically (deep pelvic or abdominal lymph nodes or bone lesions without a CT correlate). Notably, 15 (30%) of 50 of the patients received PET-positive metastasis-directed focal therapy on the basis of the local non-masked clinical reads. As anticipated, only a minority of patients had surgery, biopsy, or further imaging for lesion verification. Thus, specificity and negative predictive value could not be established and lesion validation was only available in five (38%) of 13 patients with positive findings by 18F-fluciclovine and ten (36%) of 28 patients with positive findings by PSMA (appendix p 14).
The term detection rate used in this and many previous reports is not entirely correct because false-positive findings have been reported.7–9 However, the majority consensus rule (2:1) might have led to lower sensitivity but higher specificity than clinical routine interpretations,10 thus explaining the positive predictive value of 100% for both scans in the subset of patients with lesion validation. Furthermore, high positive predictive values of PSMA (>85%) reported in a meta-analysis that included only patients with biochemical recurrence with histopathological verification6 and a prospective multi-centre phase 3 trial10 justify the use of detection rates rather than positivity rates. In this study, probably because of small patient numbers with lesion validation (n=15), the sensitivity of the two scans did not differ significantly. Larger cohorts would be required to formally address this question.
This study has several limitations. Technical parameters might have been confounding factors and could have potentially introduced a bias. 18F-fluciclovine uptake time was shorter than that recommended by guidelines.16 This might have affected pelvic image quality via higher blood pool activity at the time of imaging, and thus T and N staging, but not the extrapelvic (M) staging. Some 18F-fluciclovine PET-CT scans were done without intravenous CT-contrast application. However, the differences were also confirmed in the subset of patients who had both 18F-fluciclovine and PSMA PET-CT scans with intravenous CT contrast. A post-hoc multivariate analysis of potential confoun ding factors revealed no effect of the tracer uptake time or the use of intravenous CT contrast. Only the PET tracer used (18F-fluciclovine or PSMA) was predictive of PET scan positivity.
PSMA readers had recorded a higher number of PSMA scan reads than the 18F-fluciclovine readers had recorded of 18F-fluciclovine scan reads. This difference is probably because of the more frequent clinical use of PSMA, especially in Europe. However, care was taken to select well-trained readers with extensive publication and clinical track records for both scans. Thus, a qualification bias as a confounding factor is highly unlikely.
A high rate of change in management of patients with recurrence after PSMA or 18F-fluciclovine PET-CT has been reported.30,31 Our study was not designed to assess the effect of 18F-fluciclovine or PSMA on patient management or outcome. Because both tests were done in the same patient cohort within 2 weeks, the independent effect of the tests could not be established. The effect of PET imaging findings on patient outcome is still unknown. In a randomised trial of patients with recurrent oligo metastatic disease, androgen deprivation therapy-free survival was longer with PET-positive metastasis-directed therapy than with surveillance alone.29 However, whether or not PET-positive metastasis-directed therapy improves progression-free or overall survival remains unclear.28 Furthermore, inappropriate management due to false positive findings cannot be ruled out. Even if PSMA PET-CT detects sites of recurrence earlier than 18F-fluciclovine PET-CT, the implications on hard clinical endpoints remain uncertain. Randomised clinical trials of standard salvage radiotherapy versus PSMA PET-CT-based salvage radiotherapy (NCT03582774) and 18F-fluciclovine versus PSMA PET-CT-based salvage radiotherapy (NCT03762759), both powered for outcome, are ongoing.
This prospective head-to-head comparison between 18F-fluciclovine and PSMA PET-CT in 50 patients with post-radical prostatectomy biochemical recurrence and PSA concentrations of 2·0 ng/mL or less shows superior detection rates and reader agreement with PSMA PET-CT than with 18F-fluciclovine. Primary and secondary endpoints were met: PSMA PET-CT detection rates at the patient level, and at the regional level for pelvic lymph node regions and for extra-pelvic metastasis were more than twice as high as those for 18F-fluciclovine PET-CT. However, because the PET findings could not be validated by a gold reference standard in two-thirds of patients, neither sensitivity nor specificity could be established. Nevertheless, the results of this prospective head-to-head comparison indicate that PSMA should be the PET tracer of choice when PET-CT imaging is considered for subsequent treatment management decisions in patients with biochemical recurrence and low PSA concentrations (≤2·0 ng/mL).
Supplementary Material
Research in context.
Evidence before this study
Two PET-CT imaging tests for detection and localisation of prostate cancer tumour sites in patients with biochemical recurrence have been introduced in Europe and the USA. 18F-fluciclovine exploits upregulated amino acid transporter activity whereas the second test targets the prostate-specific membrane antigen (PSMA). It is unknown which test performs better, especially in patients with biochemical recurrence at low PSA concentrations (≤2·0 ng/mL) in whom focused salvage therapy could potentially be curative. We did PubMed searches for publications in English comparing 18F-fluciclovine and PSMA PET-CT at any date using the keywords (“Fluciclovine” AND “PSMA”) OR (“FACBC” AND “PSMA”) on March 13, 2017, Oct 21, 2017, and Nov 19, 2018. No prospective direct comparison between the two PET-CT imaging tests was found.
Added value of this study
Superiority of one over the other test can only be established in a prospective head-to-head comparative study, which, to the best of our knowledge, has not been done previously. Our study is prospective, using paired studies in the same cohort of patients, and findings are based on external independent masked reads.
Implications of all the available evidence
The collective data from this prospective comparative imaging trial and published studies suggest higher detection rates and reliability of PSMA PET-CT than 18F-fluciclovine PET-CT in patients with biochemical recurrence and low serum PSA concentrations. Thus, PSMA-targeted PET-CT imaging should become the standard of care in these patients. Whether early detection of biochemical recurrence sites by PET-CT imaging affects patient outcome is the subject of ongoing randomised phase 3 clinical trials (NCT03582774 and NCT03762759).
Acknowledgments
We thank all the patients and their referring physicians whose willingness to participate made this study possible. We thank the whole staff team of the University of California Los Angeles (UCLA; Los Angeles, CA, USA) Nuclear Medicine and Theranostics Division whose hard work made this study possible. This was an investigator-initiated trial with institutional academic funding (Ahmanson Translational Theranostics Division, Department of Molecular and Medical Pharmacology, UCLA). JCa was the recipient of a grant from the Philippe Foundation Inc. (New York, NY, USA) and from the Fondation ARC pour la recherche sur le cancer (grant SAE20160604150). FC was supported by a Postdoctoral Fellowship Award from the Fondazione Umberto Veronesi (post-doctoral travel-grant 2018). JCz was the recipient of a grant from the US Department of Energy (DE SC0012353), from the Prostate Cancer Foundation (2017 Challenge Award, 17CHAL02), and from the Johnson Comprehensive Cancer Center National Institutes of Health-National Cancer Institute Cancer Center Support Grant (P30 CA016042). TAH was supported by the Prostate Cancer Foundation (2017 Jonathan Kovler Young Investigator Award) and the National Institutes of Health (NIH, grant R01CA212148). ME was supported by the SFB 824 (DFG Sonderforschungsbereich 824, Project B11) from the Deutsche Forschungsgemeinschaft (Bonn, Germany). WPF was the recipient of a scholarship from the German Research Foundation (Deutsche Forschungsgemeinschaft grant 807122), the University of Duwasburg-Essen IFORES programme, the Doktor Robert Pfleger-Stiftung, and the Wiedenfeld-Stiftung. MSH was supported by a Clinical Fellowship Award from the Peter MacCallum Foundation. HJ was supported in part by the National Institutes of Health grants (numbers R21-EB017568 and P30-CA014089).
Footnotes
See Online for appendix
Declaration of interests
JCa reports personal fees from Progenics Pharmaceuticals and RadioMedix and is a consultant for Blue Earth Diagnostics outside the submitted work. JCz is a founder, board member, and holds equity in Sofie Biosciences and Trethera Therapeutics, serves on the medical advisory board of Actinium, and is a member of the VISION trial steering committee, a clinical trial sponsored by Endocyte, outside the submitted work. WPF is a consultant for Endocyte and Ipsen and reports personal fees from Radiomedix Inc outside the submitted work. ME reports grants from ABX advanced biochemical compounds and Blue Earth Diagnostics, is a consultant for Progenics Pharmaceuticals and Janssen, and has a patent rhPSMA issued, outside the submitted work. TB-G reports grants and personal fees from Blue Earth Diagnostics outside the submitted work. BS-B reports grants and personal fees from Blue Earth Diagnostics and personal fees from Phillips outside the submitted work. CN reports grants and personal fees from Blue Earth Diagnostics outside the submitted work. TAH reports grants from Advanced Accelerator Applications and personal fees from GE Healthcare, Progenics Pharmaceuticals, Curium, and Ipsen outside the submitted work. MBR reports grants and non-financial support from Novartis, personal fees and non-financial support from Johnson & Johnson, non-financial support from Merck, Medivation, and Astellas, and has a patent “Inhibitors of the N-terminal Domain of the Androgen Receptor” pending, outside the submitted work. MSH reports grants and personal fees from Endocyte, Ipsen, and Sanofi Genzyme outside the submitted work. CR received speaker fees from GE Healthcare outside the submitted work. AUK reports personal fees from Varian Medical Systems, ViewRay, and Janssen Pharmaceuticals outside the submitted work. HJ is an investigator for ImaginAb and Subtle Medical outside the submitted work. All other authors declare no competing interests.
Data sharing
Deidentified data collected for the study have been made available to other researchers for purposes of reproducing the results and are available to others in the FACBC-PSMA PUBLIC Master Table Database Appendix.
References
- 1.National Comprehensive Cancer Network. Guidelines for treatment of cancer by site: prostate cancer. Version 4 2018. https://www.nccn.org/professionals/physician_gls/default.aspx#prostate (accessed March 13, 2019). [Google Scholar]
- 2.European Association of Urology. Prostate cancer 2018. Uroweb. https://uroweb.org/guideline/prostate-cancer/ (accessed March 13, 2019 ). [Google Scholar]
- 3.Nanni C, Zanoni L, Pultrone C, et al. (18)F-FACBC (anti1-amino-3-(18)F-fluorocyclobutane-1-carboxylic acid) versus (11)C-choline PET/CT in prostate cancer relapse: results of a prospective trial. Eur J Nucl Med Mol Imaging 2016; 43: 1601–10. [DOI] [PubMed] [Google Scholar]
- 4.Morigi JJ, Stricker PD, van Leeuwen PJ, et al. Prospective comparison of 18F-fluoromethylcholine versus 68Ga-PSMA PET/CT in prostate cancer patients who have rising PSA after curative treatment and are being considered for targeted therapy. J Nucl Med 2015; 56: 1185–90. [DOI] [PubMed] [Google Scholar]
- 5.Calais J, Fendler WP, Herrmann K, Eiber M, Ceci F. Comparison of 68Ga-PSMA-11 and 18F-fluciclovine PET/CT in a case series of 10 patients with prostate cancer recurrence. J Nucl Med 2018; 59: 789–94. [DOI] [PubMed] [Google Scholar]
- 6.Hope TA, Goodman JZ, Allen IE, Calais J, Fendler WP, Carroll PR. Meta-analysis of 68Ga-PSMA-11 PET accuracy for the detection of prostate cancer validated by histopathology. J Nucl Med 2019; 60: 786–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hofman MS, Hicks RJ, Maurer T, Eiber M. Prostate-specific membrane antigen PET: clinical utility in prostate cancer, normal patterns, pearls, and pitfalls. RadioGraphics 2018; 38: 200–17. [DOI] [PubMed] [Google Scholar]
- 8.Fendler WP, Calais J, Allen-Auerbach M, et al. 68Ga-PSMA-11 PET/CT interobserver agreement for prostate cancer assessments: an international multicenter prospective study. J Nucl Med 2017; 58: 1617–23. [DOI] [PubMed] [Google Scholar]
- 9.Rischpler C, Beck TI, Okamoto S, et al. 68Ga-PSMA-HBED-CC uptake in cervical, coeliac and sacral ganglia as an important pitfall in prostate cancer PET imaging. J Nucl Med 2018; 59: 1406–11. [DOI] [PubMed] [Google Scholar]
- 10.Fendler WP, Calais J, Eiber M, et al. Assessment of 68Ga-PSMA-11 PET accuracy in localizing recurrent prostate cancer: a prospective single-arm clinical trial. JAMA Oncol 2019; published online March 28. DOI: 10.1001/jamaoncol.2019.0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Odewole OA, Tade FI, Nieh PT, et al. Recurrent prostate cancer detection with anti-3-[(18)F]FACBC PET/CT: comparison with CT. Eur J Nucl Med Mol Imaging 2016; 43: 1773–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bach-Gansmo T, Nanni C, Nieh PT, et al. Multisite experience of the safety, detection rate and diagnostic performance of fluciclovine (18F) positron emission tomography/computerized tomography imaging in the staging of biochemically recurrent prostate cancer. J Urol 2017; 197: 676–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Afshar-Oromieh A, Holland-Letz T, Giesel FL, et al. Diagnostic performance of (68)Ga-PSMA-11 (HBED-CC) PET/CT in patients with recurrent prostate cancer: evaluation in 1007 patients. Eur J Nucl Med Mol Imaging 2017; 44: 1258–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Afshar-Oromieh A, Avtzi E, Giesel FL, et al. The diagnostic value of PET/CT imaging with the (68)Ga-labelled PSMA ligand HBED-CC in the diagnosis of recurrent prostate cancer. Eur J Nucl Med Mol Imaging 2015; 42: 197–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eiber M, Maurer T, Souvatzoglou M, et al. Evaluation of hybrid 68Ga-PSMA ligand PET/CT in 248 patients with biochemical recurrence after radical prostatectomy. J Nucl Med 2015; 56: 668–74. [DOI] [PubMed] [Google Scholar]
- 16.Savir-Baruch B, Banks KP, McConathy JE, et al. ACR-ACNM practice parameter for the performance of fluorine-18 fluciclovine-PET/CT for recurrent prostate cancer. Clin Nucl Med 2018; 43: 909–17. [DOI] [PubMed] [Google Scholar]
- 17.Fendler WP, Eiber M, Beheshti M, et al. 68Ga-PSMA PET/CT: joint EANM and SNMMI procedure guideline for prostate cancer imaging: version 1.0. Eur J Nucl Med Mol Imaging 2017; 44: 1014–24. [DOI] [PubMed] [Google Scholar]
- 18.Eder M, Schäfer M, Bauder-Wüst U, et al. 68Ga-complex lipophilicity and the targeting property of a urea-based PSMA inhibitor for PET imaging. Bioconjug Chem 2012; 23: 688–97. [DOI] [PubMed] [Google Scholar]
- 19.Halpern BS, Dahlbom M, Quon A, et al. Impact of patient weight and emission scan duration on PET/CT image quality and lesion detectability. J Nucl Med 2004; 45: 797–801. [PubMed] [Google Scholar]
- 20.Eiber M, Herrmann K, Calais J, et al. Prostate cancer molecular imaging standardized evaluation (PROMISE): proposed miTNM classification for the interpretation of PSMA-ligand PET/CT. J Nucl Med 2018; 59: 469–78. [DOI] [PubMed] [Google Scholar]
- 21.Axumin Schuster D. (fluciclovine F 18) image interpretation training. Society of Nuclear Medicine and Molecular Imaging, 2016. https://www.snmmilearningcenter.org/Activity/4521746/Detail.aspx (accessed March 13, 2019 ). [Google Scholar]
- 22.Miller MP, Kostakoglu L, Pryma D, et al. Reader training for the re-staging of biochemically recurrent prostate cancer using 18F-fluciclovine PET/CT. J Nucl Med 2017; 58: 1596–602. [DOI] [PubMed] [Google Scholar]
- 23.Wang Q, Bailey CG, Ng C, et al. Androgen receptor and nutrient signaling pathways coordinate the demand for increased amino acid transport during prostate cancer progression. Cancer Res 2011; 71: 7525–36. [DOI] [PubMed] [Google Scholar]
- 24.Silver DA, Pellicer I, Fair WR, Heston WD, Cordon-Cardo C. Prostate-specific membrane antigen expression in normal and malignant human tissues. Clin Cancer Res 1997; 3: 81–85. [PubMed] [Google Scholar]
- 25.Mannweiler S, Amersdorfer P, Trajanoski S, Terrett JA, King D, Mehes G. Heterogeneity of prostate-specific membrane antigen (PSMA) expression in prostate carcinoma with distant metastasis. Pathol Oncol Res 2009; 15: 167–72. [DOI] [PubMed] [Google Scholar]
- 26.Hodson N, Brown T, Joanisse S, et al. Characterisation of L-type amino acid transporter 1 (LAT1) expression in human skeletal muscle by immunofluorescent microscopy. Nutrients 2017; 10: e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McParland BJ, Wall A, Johansson S, Sørensen J. The clinical safety, biodistribution and internal radiation dosimetry of [18F]fluciclovine in healthy adult volunteers. Eur J Nucl Med Mol Imaging 2013; 40: 1256–64. [DOI] [PubMed] [Google Scholar]
- 28.Bernard B, Gershman B, Karnes RJ, Sweeney CJ, Vapiwala N. Approach to oligometastatic prostate cancer. Am Soc Clin Oncol Educ Book 2018; 36: 119–29. [DOI] [PubMed] [Google Scholar]
- 29.Ost P, Reynders D, Decaestecker K, et al. Surveillance or metastasis-directed therapy for oligometastatic prostate cancer recurrence: a prospective, randomized, multicenter phase II trial. J Clin Oncol 2018; 36: 446–53. [DOI] [PubMed] [Google Scholar]
- 30.Andriole GL, Kostakoglu L, Chau A, et al. The impact of positron emission tomography with 18F-fluciclovine on the management of patients with biochemical recurrence of prostate cancer: results from the LOCATE trial. J Urol 2019; 201: 322–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Han S, Woo S, Kim YJ, Suh CH. Impact of 68Ga-PSMA PET on the management of patients with prostate cancer: a systematic review and meta-analysis. Eur Urol 2018; 74: 179–90. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


