ABSTRACT
Macroautophagy/autophagy, an evolutionarily conserved eukaryotic bioprocess, plays an important role in the bulk degradation of intracellular macromolecules, organelles, and invading pathogens. PIK3C3/VPS34 (phosphatidylinositol 3-kinase catalytic subunit type 3) functions as a key protein in autophagy initiation and progression. The activity of PIK3C3 is tightly regulated by multiple post-translational modifications, including ubiquitination, however, the regulatory mechanisms underpinning the reversible deubiquitination of PIK3C3 remain poorly understood. Recently, we identified the E3 ubiquitin ligase NEDD4/NEDD4-1 as a positive regulator of autophagy through decreasing the K48-linked ubiquitination of PIK3C3 by recruiting USP13.
KEYWORDS: Auto-ubiquitination, autophagy, deubiquitination complex, NEDD4, PIK3C3, USP13
Autophagy, mediated by a number of autophagy-related (ATG) proteins, is an evolutionarily ancient mechanism for the recycling and degradation of cytoplasmic constituents. The ATG proteins of autophagy are tightly regulated by multiple post-translational modifications, including phosphorylation and ubiquitination. Previous studies have revealed that the E3 ubiquitin ligase NEDD4 affects autophagy at multiple levels by targeting BECN1 and SQSTM1. To gain insight into the detailed roles of NEDD4 in autophagy, we knocked down the expression of NEDD4 and found that NEDD4 depletion decreases the accumulation of GFP-LC3B puncta [1]. Ectopic expression of NEDD4 significantly enhances the accumulation of LC3B-II and results in greater consumption of SQSTM1. NEDD4 interacts with PIK3C3 complex members, but does not affect the assembly of the PIK3C3 complex. PIK3C3 plays a key role in the interaction between NEDD4 and other members of the PIK3C3 complex. NEDD4 associates with PIK3C3, and this association is increased upon starvation. NEDD4 stabilizes PIK3C3 through the ubiquitin-protease pathway. Although BECN1 is regulated by NEDD4, NEDD4 stabilizes PIK3C3 in a BECN1-indepenent manner. Moreover, we found that NEDD4 is degraded after 6–9 h of autophagy induction, which prevents the over-activation of autophagy.
To discover the detailed mechanism of NEDD4 in stabilizing PIK3C3, we analyzed different types of poly-ubiquitination of PIK3C3 and found that NEDD4 specifically inhibits K48-linked ubiquitination of PIK3C3 (Figure 1). We generated a series of PIK3C3 mutant constructs bearing single lysine (K) to arginine (R) substitution in five potential ubiquitin sites, and found that NEDD4 fails to stabilize the PIK3C3K419R mutant which displays reduced K48-linked ubiquitination. We further investigated whether K48-linked ubiquitination on K419 serves as a degradation signal for PIK3C3, and observed that the degradation rate of the PIK3C3K419R mutant is decreased compared to WT PIK3C3. Additionally, we observed that the PIK3C3K419R mutant dramatically enhances the formation of GFP-LC3B puncta and accumulation of LC3B-II.
Figure 1.
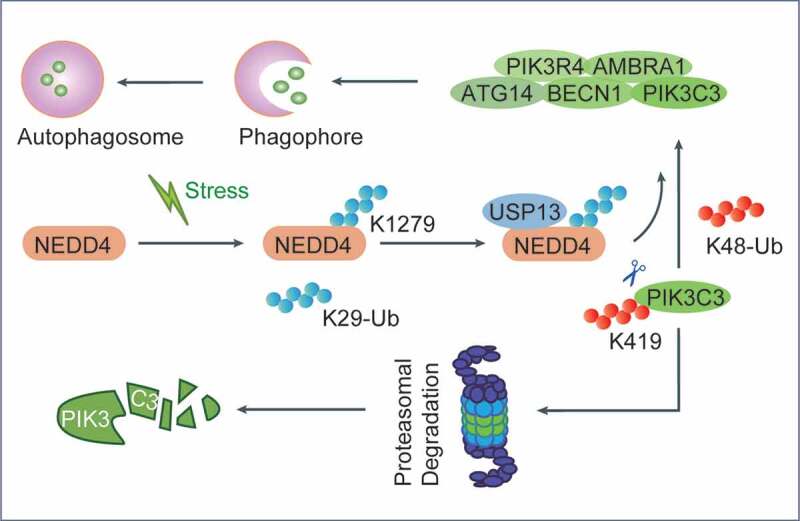
Working model for the regulatory function of NEDD4 in autophagy through PIK3C3 deubiquitination. NEDD4 undergoes K29-linked auto-ubiquitination at K1279 and serves as a scaffold for recruiting USP13 to form a NEDD4-USP13 deubiquitination complex, which subsequently cleaves the K48-linked poly-ubiquitin chains from PIK3C3 at K419, thus stabilizing PIK3C3 to promote autophagy.
As an E3 ubiquitin ligase, NEDD4 mainly catalyzes the ubiquitination of certain substrates, implying that certain deubiquitinating enzymes might cooperate with NEDD4 to cleave the K48-linked poly-ubiquitin chains on PIK3C3. We discovered that NEDD4 enhances the association between USP13 and PIK3C3, and NEDD4 fails to inhibit K48-linked ubiquitination of PIK3C3 in USP13-deficient cells. Meanwhile, an in vitro deubiquitination assay showed that USP13 inhibits the K48-linked ubiquitination of PIK3C3 in the presence of NEDD4. Interestingly, although knockdown of PIK3C3 does not affect the association between NEDD4 and USP13, the association between USP13 and PIK3C3 is completely abrogated in NEDD4-deficient cells. We observed that NEDD4 interacts with USP13 upon autophagy induction, whereas NEDD4-USP13 interacts with PIK3C3 at a later time point, indicating that NEDD4 might recruit USP13 to form a NEDD4-USP13 complex (Figure 1). Likewise, an in vitro His-affinity-isolation assay showed that purified NEDD4 directly enhances the association between USP13 and PIK3C3. We observed that knockdown of either NEDD4 or USP13 leads to decreased protein levels of PIK3C3, and that USP13 specifically stabilizes PIK3C3 in an enzyme activity-dependent manner. In addition, we found that a catalytically inactive mutant of NEDD4 (H1284A,C1286A), displays reduced accumulation of GFP-LC3B puncta and fails to interact with PIK3C3 and USP13, suggesting the E3 ubiquitin ligase activity of NEDD4 is required for the association with PIK3C3 and USP13, which promotes the formation of autophagosomes.
We next investigated how NEDD4 mediates the deubiquitination of PIK3C3. We found that NEDD4 does not affect the ubiquitination of USP13; however, NEDD4 appears to be its own substrate for ubiquitination, and the catalytic activity of NEDD4 is required for its auto-ubiquitination. Moreover, we observed that NEDD4 undergoes K29-linked ubiquitination, which is required for its auto-interaction (Figure 1). We identified two conserved lysine sites by amino acid sequence analysis, and observed that the NEDD4K1279R mutant displays reduced K29-linked ubiquitination and loses its ability to stabilize PIK3C3. Meanwhile, the NEDD4K1279R mutant fails to promote autophagy flux. The formation of GFP-LC3B puncta, the accumulation of LC3B-II, and the consumption of SQSTM1 are largely abrogated in cells expressing NEDD4K1279R mutant. Likewise, we observed that neither the NEDD4H1284A,C1286A nor the NEDD4K1279R mutant reduces the K48-linked ubiquitination of PIK3C3, or interacts with PIK3C3 and USP13.
In summary, our study suggests that the NEDD4 functions as a key positive regulator of autophagy. NEDD4 undergoes K29-linked auto-ubiquitination at K1279, which in turn serves as a scaffold for recruiting the deubiquitinating enzyme USP13 to form a NEDD4-USP13 deubiquitination complex. Subsequently, the NEDD4-USP13 axis cleaves the K48-linked poly-ubiquitin chains on PIK3C3 at K419 and promotes autophagy through suppressing the proteasomal degradation of PIK3C3. Our work provides evidence to support the function of NEDD4 in autophagy through deubiquitinating PIK3C3. These findings may promote the understanding of the molecular mechanism underlying autophagy initiation, and may provide options for clinical therapy by targeting autophagy.
Funding Statement
This work was supported by National Natural Science Foundation of China (31870862, 31970700, 31700760, and 31800751), National Key Basic Research Program of China (2015CB859800), Science and Technology Planning Project of Guangzhou, China (201804010385), and the Fundamental Research Funds for the Central Universities (18lgpy49 and 18lgpy53).
Disclosure statement
No potential conflict of interest was reported by the authors.
Reference
- [1].Xie W, Jin S, Wu Y, et al. Auto-ubiquitination of NEDD4-1 recruits USP13 to facilitate autophagy through deubiquitinating VPS34. Cell Rep. 2020;30(8):2807–2819. [DOI] [PubMed] [Google Scholar]


