ABSTRACT
Energy stress disturbs cellular homeostasis and induces cell death. Our recent study revealed that ferroptosis (a non-apoptotic cell death) is an energy-requiring process, and energy stress-mediated activation of adenosine monophosphate-activated protein kinase (AMPK) inhibits ferroptosis. Mechanistically, AMPK regulates ferroptosis through acetyl-CoA carboxylase (ACC) and polyunsaturated fatty acid (PUFA) biosynthesis.
KEYWORDS: Energy stress, ferroptosis, AMP-activated protein kinase, acetyl-CoA carboxylase, polyunsaturated fatty acid
Maintenance of energy homeostasis during metabolic limitations is critical for appropriate functioning in all eukaryotic cells. Under energy stress conditions, cells turn on adaptive responses to control energy balance. Adenosine triphosphate (ATP) is a major energy source in cells, and the hydrolysis of ATP to adenosine diphosphate (ADP) and subsequent ADP to adenosine monophosphate (AMP) releases energy. AMP-activated protein kinase (AMPK), a key regulator of cellular energy homeostasis, is activated in response to energy stress that increases either AMP: ATP or ADP:ATP ratios.1 AMPK is a serine/threonine protein kinase that reprograms cellular metabolism by phosphorylating its targets to enhance ATP preservation. AMPK inhibits ATP-consuming anabolic pathways (protein, glycogen, and fatty acid synthesis) and promotes ATP-producing catabolic pathways (glycolysis, autophagy, and fatty acid oxidation). One critical anabolic process controlled by AMPK is lipid metabolism. AMPK-mediated phosphorylation of acetyl-CoA carboxylase (ACC) on serine 79 (of ACC1) or serine 212 (of ACC2) inhibits fatty acid synthesis and promotes its oxidation. Both ACC1 and ACC2 catalyze the synthesis of malonyl-CoA, the major building block of fatty acid synthesis.2 If energy stress is prolonged and the adaptive responses are unable to maintain the energy balance, cells activate cell death pathways and eventually die.
Ferroptosis is a non-apoptotic form of regulated cell death that is characterized by iron-dependent lipid peroxidation. Lipid peroxidation preferentially occurs on polyunsaturated fatty acid-containing phospholipids (PUFA-PLs), which are structural components of lipid bilayers of cell membranes.3,4 Peroxidation of PUFA-PLs causes cellular damage by disrupting membrane integrity and generating secondary toxic products such as 4-Hydroxy-2-nonenal (4-HNE) and malondialdehyde (MDA),5 ultimately resulting in ferroptotic cell death. The induction of ferroptosis requires three major redox metabolic domains: active iron, PUFA-PLs, and loss of lipid peroxide repair systems.6 Specifically, the highly reactive hydroxyl radical produced by the iron-mediated fenton reaction extracts hydrogen atom from bis-allylic carbons in PUFAs, forming the lipid radical (L∙), which ultimately causes lipid peroxidation (LOOH). LOOHs are normally detoxified by cellular anti-ferroptosis defense systems, prominent among which is glutathione peroxidase 4 (GPX4), which reduces LOOH to less toxic lipid alcohol (LOH) at the expense of reduced glutathione. When GPX4 activity is compromised, lipid peroxides accumulate to toxic levels, leading to ferroptosis.7 Studies on oxygenated phospholipids suggest that, among PUFA-PLs, phosphatidylethanolamines (PEs) with two fatty acyls – arachidonic acid (AA) and adrenic acid (AdA) – are likely involved in ferroptosis.3,4 Ferroptosis has been associated with pathological conditions such as ischemia/reperfusion injury (IRI) and degenerative disease. Recent reports have also revealed a role of ferroptosis in tumor suppression.8,9
Dysregulation of redox metabolism is associated with ferroptosis. Since glucose starvation decreases ATP generation and increases reactive oxygen species (ROS) production, we were initially interested in studying a potential involvement of glucose starvation in promoting ferroptosis, but subsequently made an unexpected observation that glucose starvation, as well as other energy stress-inducing conditions, prevents cells from undergoing ferroptosis.10 It is important to note that, since glucose starvation or treatment with other energy stress inducers eventually causes cell death (which is not ferroptosis), our analyzes were limited in the time frames during which cells do not exhibit obvious energy stress-induced cell death. We subsequently showed that AMPK activation, by treatments that induce or mimic energy stress or overexpression of constitutively active AMPK, inhibits ferroptosis, and conversely, pharmacological or genetic inactivation of AMPK sensitizes cells to ferroptosis. In line with these in vitro data, we further revealed that pharmacological activation of AMPK by AICAR or 2DG in vivo attenuated renal IRI, a pathological condition associated with ferroptosis, and AMPK deletion in mice abolished the protective effect of these energy stress inducers on renal IRI.
Mechanistically, we provided several lines of evidence to support a model wherein AMPK regulates ferroptosis at least partly through AMPK-mediated ACC phosphorylation and lipid metabolism; ACC phosphorylation and inactivation by AMPK restrains the conversion of acetyl-CoA to malonyl-CoA, which is required for the biosynthesis of fatty acids, including the long-chain PUFAs, resulting in reduced generation of lipid peroxides and ferroptosis inhibition (Figure 1). First, treatment with the ACC inhibitor TOFA rescued ferroptosis in cell lines with low basal AMPK activation or with genetic deletion of AMPK. In addition, in cells in which AMPK is no longer capable of phosphorylating ACC (ACC double knock-in cells), the inhibitory effects of energy stress inducers on ferroptosis were attenuated or largely abolished. Finally, lipidomic analysis revealed that AMPK activation (by AMPK activator A769662 treatment) resulted in significant depletion of free PUFAs and PUFA-containing lipid species, particularly AA-PE and AdA-PE, which are susceptible to free radical attack, whereas AMPK deletion in cells with high basal AMPK activation exerted opposite effects on lipid compositions. Importantly, adding these PUFAs re-sensitized AMPK-activated/ferroptosis-resistant cells to ferroptosis.
Figure 1.
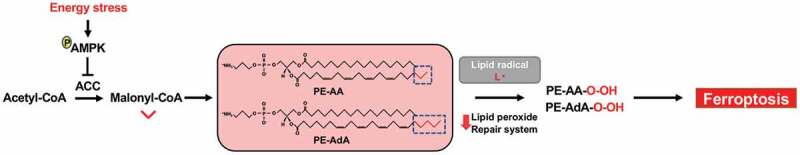
AMPK suppresses ferroptosis in the context of energy stress. Energy stress-mediated adenosine monophosphate-activated protein kinase (AMPK) activation inhibits acetyl-CoA carboxylase (ACC), blocking the conversion of acetyl-CoA to malonyl-CoA. Malonyl-CoA is used to synthesize certain polyunsaturated fatty acids, such as arachidonic acid (AA) and adrenic acid (AdA). AA and AdA are then esterified into the phosphoethanolamine (PE) to generate PE-AA and PE-AdA, which are susceptible to undergo lipid peroxidation. If the lipid peroxide repair system is defective, these oxidized lipids cause ferroptosis.
Depending on cellular contexts, AMPK can have either tumor promoting or tumor suppressive effects in cancer cells. AMPK can restrict tumor cell growth and proliferation by inhibiting anabolic pathways, resulting in tumor suppression, but can also promote tumor development by maintaining tumor cell survival under metabolic or energy stress conditions. We and others previously showed that ferroptosis represents a critical tumor suppression mechanism.9 Whether ferroptosis suppression plays any role in AMPK’s tumor promoting function remains unclear and will be an exciting topic for future studies.
Funding Statement
This work was supported by the National Institutes of Health [CA181196].
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- 1.Garcia D, Shaw RJAMPK.. Mechanisms of cellular energy sensing and restoration of metabolic balance. Mol Cell. 2017;66(6):1–2. doi: 10.1016/j.molcel.2017.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jeon SM, Chandel NS, Hay N. AMPK regulates NADPH homeostasis to promote tumour cell survival during energy stress. Nature. 2012;485(7400):661–665. doi: 10.1038/nature11066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kagan VE, Mao G, Qu F, Angeli JPF, Doll S, Croix CS, Dar HH, Liu B, Tyurin VA, Ritov VB, et al. Oxidized arachidonic/adrenic phosphatidylethanolamines navigate cells to ferroptosis. Nat Chem Biol. 2017;13(1):81. doi: 10.1038/NCHEMBIO.2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Doll S, Proneth B, Tyurina YY, Panzilius E, Kobayashi S, Ingold I, Irmler M, Beckers J, Aichler M, Walch A, et al. ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition. Nat Chem Biol. 2017;13(1):91–98. doi: 10.1038/nchembio.2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Catalá A. An overview of lipid peroxidation with emphasis in outer segments of photoreceptors and the chemiluminescence assay. Int J Biochem Cell Biol. 2006;38(9):1482–1495. doi: 10.1016/j.biocel.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 6.Dixon SJ, Stockwell BR. The hallmarks of ferroptosis. Annu Rev Cancer Biol. 2019;3(1):35–54. doi: 10.1146/annurev-cancerbio-030518-055844. [DOI] [Google Scholar]
- 7.Yang WS, Sriramaratnam R, Welsch ME, Shimada K, Skouta R, Viswanathan V, Cheah J, Clemons P, Shamji A, Clish C, et al. Regulation of ferroptotic cancer cell death by GPX4. Cell. 2014;156(1–2):317–331. doi: 10.1016/j.cell.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stockwell BR, Friedmann Angeli JP, Bayir H, Bush AI, Conrad M, Dixon SJ, Fulda S, Gascón S, Hatzios SK, Kagan VE, et al. Ferroptosis: a regulated cell death nexus linking metabolism, redox biology, and disease. Cell. 2017;171(2):273–285. doi: 10.1016/j.cell.2017.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang Y, Shi J, Liu X, Feng L, Gong Z, Koppula P, Sirohi K, Li X, Wei Y, Lee H, et al. BAP1 links metabolic regulation of ferroptosis to tumour suppression. Nat Cell Biol. 2018;20(10):1181–1192. doi: 10.1038/s41556-018-0178-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee H, Zandkarimi F, Zhang Y, Meena JK, Kim J, Zhuang L, Tyagi S, Ma L, Westbrook TF, Steinberg GR, et al. Energy-stress-mediated AMPK activation inhibits ferroptosis. Nat Cell Biol. 2020;22(2):225–234. doi: 10.1038/s41556-020-0461-8. [DOI] [PMC free article] [PubMed] [Google Scholar]


