Abstract
Ketamine, a dissociative anesthetic and psychedelic compound, has revolutionized the field of psychopharmacology by showing robust, and rapid-acting antidepressant activity in patients suffering from major depressive disorder (MDD), suicidal tendencies, and treatment-resistant depression (TRD). Ketamine’s efficacy, however, is transient, and patients must return to the clinic for repeated treatment as they experience relapse. This is cause for concern because ketamine is known for its abuse liability, and repeated exposure to drugs of abuse often leads to drug abuse/dependence. Though the mechanism(s) underlying its antidepressant activity is an area of current intense research, both clinical and preclinical evidence shows that ketamine’s effects are mediated, at least in part, by molecular adaptations resulting in long-lasting synaptic changes in mesolimbic brain regions known to regulate natural and drug reward. This review outlines our limited knowledge of ketamine’s neurobiological and biochemical underpinnings mediating its antidepressant effects and correlates them to its abuse potential. Depression and addiction share overlapping neural circuitry and molecular mechanisms, and though speculative, repeated use of ketamine for the treatment of depression could lead to the development of substance use disorder/addiction, and thus should be tempered with caution. There is much that remains to be known about the long-term effects of ketamine, and our lack of understanding of neurobiological mechanisms underlying its antidepressant effects is a clear limiting factor that needs to be addressed systematically before using repeated ketamine in the treatment of depressed patients.
Introduction
Ketamine was first discovered in the 1960s as a novel anesthetic which induced a typical state of dissociative anesthesia by its non-competitive blocking of the N-methyl-D-Aspartate (NMDA) receptor. It gained widespread popularity due to its high margin of safety and low need for intubation and monitoring. It came to be successfully used as an anesthetic in the emergency room, in pediatric and geriatric anesthesia, and is routinely used as a tranquilizer in veterinary medicine [96]. However, the use of ketamine was limited due to its strong psychotomimetic effects seen during the recovery phase from anesthesia. This side effect made ketamine extremely popular during the “rave-era” as a party or club drug. It was heavily abused by recreational drug users in order to experience the psychotomimetic phenomena which users termed as “K-hole”: a hallucinatory out of body feeling [96]. In the early 21st century, the severe, widespread, neurotoxic potential of ketamine on the developing brain was demonstrated, further decreasing its clinical use [65]. However, the recent discovery of its rapid-acting antidepressant effects in patients who typically do not respond to traditional antidepressant therapy has brought this drug renewed limelight [61]. This rediscovery of ketamine appears to have been embraced all over the world, with ketamine’s recreational use increasing dramatically over the past few years [23,24,72,112,18,106].
Here, we begin by reviewing literature on ketamine’s pharmacology, followed by evidence of its rapid acting, antidepressant efficacy, especially in patients suffering from treatment-resistant depression (TDR). Both clinical and preclinical studies are reviewed demonstrating a robust antidepressant effect with differences in response based on different dosing regimens. This is followed by a discussion of clinical and preclinical findings pointing to ketamine’s abuse potential. Based on empirical evidence demonstrating a close overlap in the dysregulation of the mesolimbic dopaminergic reward system in both depression and addiction, we hypothesize the use of ketamine as an antidepressant activates neural pathways and mechanisms closely implicated in drug addiction, leading to vulnerability for the potential development of a substance use/abuse disorder. The primary aim of this review is therefore to highlight the overlap in brain regions and neural circuits involved in drug addiction, and are also activated by ketamine, as well as outline the close overlap in the molecular mechanisms mediating both drugs of abuse and ketamine’s antidepressant effects that may lead to the development of addiction. Thus, although efficacious as a rapid-acting antidepressant, repeated ketamine use in the treatment of depression may need to be tempered with caution due to its abuse potential. More research is needed in understanding this overlap between the antidepressant and addictive effects triggered by ketamine, and pharmacological agents need to be developed that prevent its abuse potential while boosting its antidepressant effects.
Ketamine pharmacology
Ketamine was first synthesized in 1962 by Parke-Davis industries as an alternative to phencyclidine (PCP). The underlying motivation behind its development was to synthesize a drug which could have the same anesthetic and analgesic effects of PCP without the severe side effects (i.e., delirium and strong psychosis) that emerged during the recovery phase from anesthesia [96]. Ketamine succeeded in drastically reducing the duration and severity of these side effects while being a good anesthetic with analgesic properties, and a high margin of safety. In addition, it proved to be different from most barbiturate and gaseous anesthetics of that time due to its rapid-acting anesthetic effects, without compromising cardiopulmonary functioning, by reducing the chances of respiratory failure during the anesthesia phase. This property increased ketamine’s approval as an anesthetic safely administered via several routes [109]. Consequently, it became popular in the emergency room (ER) and the battle field where analgesia and safety were of utmost importance [58]. Currently, ketamine is commonly used in the ER, and in specialized pediatric and geriatric anesthesia due to its immediate sedative effects and high safety margins [49].
Pharmacokinetics and pharmacodynamics of ketamine
Ketamine has a molecular weight of 238 g/mol and is an aryl-cyclo-alkylamine with a single asymmetrical carbon. This gives rise to the two enantiomers of ketamine: S(+)-ketamine and R(−)ketamine. Ketamine is normally available and used in its racemic (optically inactive; containing equal amounts of S and R enantiomers) form as a slightly acidic (pH 3.5–5.5) aqueous solution containing benzothonium chloride or chlorbutanol as preservative [113]. Ketamine is lipid soluble and therefore can be easily and extensively distributed throughout the body. It does not bind to plasma proteins readily and hence has high bioavailability when injected intravenously (i.v.; 100% in <1 minute) or intramuscularly (i.m.; 93% in <5 minutes) [104]. Ketamine reaches its targets rapidly and induces anesthetic effects within minutes. It has a high clearance rate that equals that of liver blood flow leading to a short elimination half-life of 2–3 hours in humans, and about 30 minutes in rodents [113]. Ketamine gets metabolized to norketamine (80%), and 4- and 5-hydroxyketamine (20%) by cytochromes belonging to the P450 system and several microsomal enzymes within 30 minutes after administration [113]. Norketamine is an active metabolite that has analgesic properties of its own [155], and a longer elimination rate persisting in the system for more than 5 hours. This leads to accumulation of norketamine in the system, especially when ketamine is administered as a continuous perfusion, and it has been proposed to be the cause of tachyphylaxis seen after ketamine [64]. The majority of ketamine’s metabolism occurs in the liver [137], along with substantial metabolism occurring in the kidneys, intestines, and lungs [41]. Ketamine’s metabolism and clearance can be modified by the use of enzyme inducers like rifampicin [133], enzyme inhibitors, and benzodiazepines.
Ketamine induces a very characteristic “dissociative anesthetic state” in which there is a functional separation between the thalamo-cortical and limbic systems [26]. In this state, sensory input is received by the cortex but prevented from further propagation to the association cortices [26]. The depth of anesthesia, after a typical anesthetic dose of 1–2 mg/kg, i.v., induces a surgical plane with the absence of motor responses to nociceptive stimuli [177]. Varying the doses of ketamine has differential effects. Subanesthetic doses (0.1 – 0.8 mg/kg, i.v.), for example, induce psychological/psychedelic effects, while higher doses induce anesthetic and analgesic effects (2 – 5 mg/kg, i.v.). The psychedelic effects are observed as a side-effect during the “awakening phase” or “emergence phase” from anesthesia, and are characterized by auditory and visual hallucinations, synesthesia, impressions of unreality or depersonalization, alterations in bodily perceptions, and proprioceptive impairments [26]. Several preclinical studies have shown ketamine to induce widespread neurotoxicity when used repeatedly, specifically in the developing brain [45,137]. In addition, ketamine is also known for its relatively strong abuse potential [96,119]. These “sideeffects” have been a major reason for limiting the use of ketamine as an anesthetic and a potent analgesic. However, recent findings of ketamine’s potential as a rapid-acting antidepressant, especially for TRD [12,9], has once again put this controversial drug back into the limelight.
Mechanisms of Action
The major therapeutic effects of ketamine are believed to be mediated via selective, noncompetitive antagonism of the N-methyl-D-aspartate (NMDA) receptor. However, over the years, researchers have come to understand that ketamine’s neuropharmacology is far more complex than its mere inhibition of the NMDA receptor. Due to its complex neuropharmacology, ketamine gives rise to several effects besides anesthesia, and its neuropharmacological effects can be explained via two mechanisms of action: glutamate-dependent and glutamate-independent mechanisms.
Glutamate-dependent mechanisms
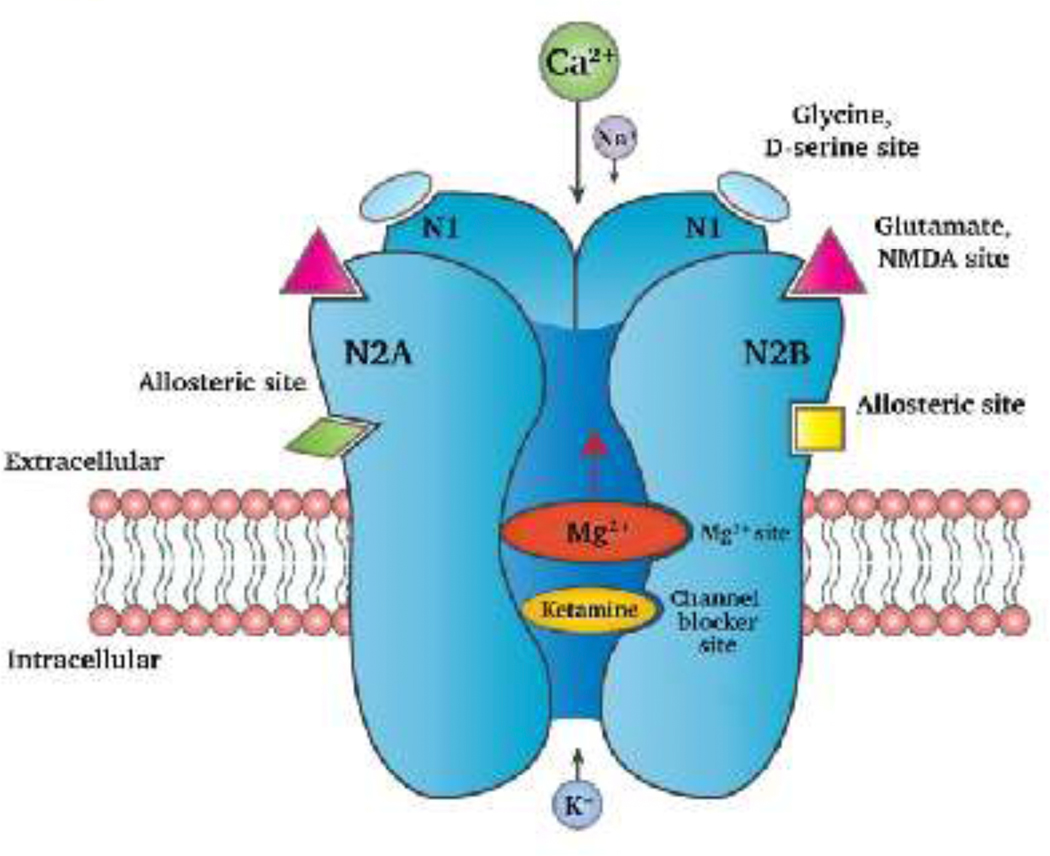
Ketamine’s most important clinical effects (i.e., anesthesia, analgesia and, recently, as antidepressant) have been explained via its interaction with the NMDA receptor and its effects on glutamatergic neurotransmission. The NMDA receptor belongs to a family of ionotropic glutamate receptors which bring about depolarization of the neuronal membrane by allowing the influx of Na2+ ions and, more predominantly, Ca2+ ions. This depolarization leads to excitation of the neuron. NMDA receptors are expressed throughout the nervous system. Functionally, they mediate sensory inputs to spinal cord, thalamus and the cerebral cortex. They also regulate emotional responses and play a major role in learning and memory, and the induction of long-term potentiation (LTP). Opening of the NMDA receptor requires the binding of glutamate and glycine simultaneously, and a membrane depolarization that releases the Mg2+ ion present within the channel of this receptor. The binding site for non-competitive antagonists, both ketamine and Mg2+, are deep within the channel pore lining areas of the NMDA receptor [72] (see Figure 1). Upon ketamine’s inhibition of the NMDA receptor, there is a net decrease in the excitatory neurotransmission which is the known basis of ketamine’s anesthetic effect. The “emergence phenomena” (i.e., delirium and psychosis) observed during ketamine-induce anesthesia is typical of NMDA receptor antagonism. NMDA receptor antagonism by ketamine also leads to hypofunction of the NMDA receptor [134], resulting in schizophrenia-like symptoms [124].
Figure 1.
The NMDA receptor comprises of two N1 sub-units and two N2A/N2B, or one N2A and one N2B subunit. Ketamine binds within the channel of the NMDA receptor and acts as a non-competitive antagonist of the NMDA receptor. Inhibition of the NMDA receptor by ketamine prevents the influx of Ca2+ and/or Na+ ions thus preventing neuronal membrane depolarization. This decreases the probability of neuronal firing, preventing further propagation of the neuronal signal, neurotransmitter release or downstream signaling mechanisms. (Figure modeled after Pham and Gardier, 2019 [138]).
The analgesic effects of ketamine can be explained by the interaction between NMDA and opioid receptors. Upon activation of opioid receptors, protein kinase C (PKC) phosphorylates the NMDA receptor. This phosphorylation relieves the Mg2+ blockade of the channel and allows for Ca2+ influx, which further activates a cascade of molecular changes involving PKC activation and transcriptional changes, leading to the development of tolerance towards opioids that results in hyperalgesia. Ketamine’s blockade of NMDA receptors prevents this phosphorylation from happening, and leads to analgesia [114,13]. In chronic pain conditions, ketamine block of the NMDA receptor results in pain sensitization even after repeated nociceptive stimulation (i.e., “wind-up”). This is useful in reducing allodynia and hyperalgesia [59].
Ketamine blockade of the NMDA receptor has also been reported to have antidepressant effects in clinical and preclinical models [7,141]. This effect has been proposed by the disinhibition hypothesis. Within the framework of this hypothesis, ketamine blockade of NMDA receptors on GABAergic interneurons prevents GABA release thereby allowing increased glutamatergic release into the synapse. This blockade of NMDA receptors leads to an increase in AMPA receptor expression and insertion into the synaptic terminal. This eventually leads to synaptic remodeling and neurogenesis, causing increased connectivity between neurons and antidepressant effects (for more extensive details see 104).
Glutamate-independent mechanisms
Potentiates inhibitory effects of GABA:
GABA is the predominant inhibitory neurotransmitter of the nervous system. Unlike other anesthetics, ketamine’s potentiation of the GABAA receptor is controversial. Although in vivo studies have suggested involvement of GABAA receptor in ketamine’s clinical effects, in vitro studies have shown that ketamine neither enhances the GABAA receptor activity nor its function [2, 31]. However, other in vitro studies have indicated that ketamine may enhance GABAA receptor mediated inhibition [67,123]. These inconsistent findings were delineated and explained by Wang et al., wherein they demonstrated that ketamine induces its clinical effects via increase in the activity of the extrasynaptic GABAA receptors in the hippocampus and cortex [174]. However, further studies need to be conducted to elucidate the exact mechanisms involved in the final outcome of the behavioral and clinical effects observed in vivo.
Interaction with monoaminergic system:
Ketamine causes the release of monoamines and inhibits their reuptake [138]. It stimulates noradrenergic neurons by inhibiting the reuptake of norepinephrine from circulation [74]. It increases serotonin release and inhibits the serotonin transporter increasing overall serotonin levels in the brain [105]. This, in turn, is believed to mediate hippocampal neurogenesis through the increased expression of insulin-like growth factor-1 (IGF-1). Ketamine also inhibits the reuptake of dopamine via mechanisms similar to those employed by cocaine [106]. These interactions have been implicated, at least partially, in the induction of the psychedelic, addictive, and antidepressant effects of ketamine (reviewed in 104).
Inhibitor of acetylcholine release:
At clinically-relevant doses, ketamine has an antagonistic effect on cholinergic neurons of the hippocampus and the striatum. This antagonism is mediated via its ability to block nicotinic and muscarinic acetylcholine receptors present on these neurons which cause release of acetylcholine. This antagonistic action of ketamine on the cholinergic neurons has been shown to give rise to the hypnotic effects of ketamine-induced psychosis [165].
Interactions with sodium-, L-type calcium- and potassium-channels:
Ketamine induces local anesthesia via its effects on Na2+ channels [47]. Some of the hallucinogenic effects of ketamine have been attributed to its interaction with L-type Ca2+ channels [79]. Neuron K+ channels have been shown to be inhibited by ketamine, and it is postulated that the neuroprotective effects of ketamine are, in part, attributed to this inhibition of the K+ channels [48,142].
Ketamine and depression
Historically, depression has been thought of as a disorder of the monoaminergic system typically because of the drugs used for its treatment. However, there now exists several alternate hypotheses, including that of the glutamatergic system’s involvement in the etiology and treatment of depression, and TRD [148]. Although ketamine’s antidepressant effects in some clinical settings had been observed since the 1970’s (reviewed in 26), a link between glutamate and depression was first reported by Trullas and Skolnick nearly 25 years ago: They demonstrated that competitive, channel-blocking and glycine-site NMDA receptor antagonists were effective in animal models of depression [167]. Thereafter, Berman et al. (2000) demonstrated rapid antidepressant effects of ketamine in patients suffering from intractable depression [8]. These studies brought a renewed interest in understanding the mechanisms by which ketamine brings about antidepressant effects. In the following section we give a brief overview of clinical and preclinical evidence demonstrating the antidepressant properties of ketamine.
Clinical evidence
Since Berman’s original 2000 study, a number of controlled clinical trials have reported the robust, short term effectiveness of i.v. infusions of ketamine in inducing rapid antidepressant effects in patients suffering from depression and TRD (reviewed in 9). However, these effects are limited by a short-term antidepressant response, high incidences of relapse, lack of established dose-response relationships, and a need for studies assessing the efficacy and safety of repeated ketamine administration in order to prolong the antidepressant response.
Several clinical studies have shown that an infusion of a single subanesthetic dose (0.5 mg/kg, i.v.) of ketamine, over a 40 minute duration, significantly reduced depressive symptoms when compared to saline or midazolam placebo controls [8,122,196]. The reduction in depressive symptoms occurred within hours of ketamine administration, with peak effects observed 24 hours post infusion, and lasting for up to seven days. These antidepressant effects, however, were shown to last a maximum of approximately two-weeks, with almost all TRD patients relapsing by day 14 post-administration. Subsequent studies have also demonstrated ketamine’s efficacy in reducing suicidal ideation [32]. A review of nine meta-analyses [10] of acute phase randomized short-term trials reported that ketamine had robust and rapid response in reducing depressive symptoms in TRD patients. However, these effects were acute, and depressive symptoms returned within two weeks of drug administration. In order to extend ketamine’s antidepressant effects, chronic administration of ketamine has been assessed. In a study conducted by aan het Rot et al. (2010), repeated infusions (six i.v. infusions administered over an 11-day period) of ketamine significantly decreased depressive symptoms when compared to single infusion [1]. These patients achieved symptom remission for up to 19 days post last ketamine infusion; however, 9 out of 11 patients relapsed soon after that. Response rates of up to 90% were reported by Murrough et al. (2013b) in controlled trials of TRD patients after repeated administration of ketamine (six treatments of 0.5 mg/kg ketamine administered i.v. thrice/weekly for two weeks) [123]. Another meta-analysis reported that serial ketamine infusions (mostly six i.v. infusions over a period of 12–14 days) induced high remission rates and even greater reduction in depressive symptoms than single infusion of ketamine at 4 and 24 hours, and at 7 and 12–14 days [27]. These effects lasted throughout the treatment period and extended for up to 20 days post last infusion, after which time close to 90% of all patients relapsed. Evidence towards a dose-related response of ketamine’s antidepressant effects was provided by a meta-analysis conducted by Xu et al. (2015). According to this report, reduction in depressive symptoms with a subthreshold dose (six trials of 0.5 mg/kg, i.v.) was significantly higher than low-doses (three separate trials of 0.1–0.4 mg/kg, i.v.; or 50 mg intranasal, and 0.1–0.5 mg/kg, i.v., i.m., or subcutaneously (s.c.)) of ketamine [189]. Another double-blind, placebo-controlled pilot study with four TRD patients revealed that the highest decrease in depressive symptoms was a result of the highest ketamine dose received (four doses of 0.4 mg/kg infused over 2–5 minutes) when compared to low dose (four doses of 0.1 mg/kg infused over 2–5 minutes) [82]. Based on these reports, it is evident that ketamine has robust acute antidepressant effects, and that prolongation of antidepressant effects requires administration of multiple doses over time and/or increases in dosing regimens. To date, however, there are limited data regarding the long-term safety and efficacy of repeated administration of ketamine for depression. No controlled or uncontrolled longitudinal clinical nor preclinical studies exist assessing long-term maintenance of ketamine’s initial antidepressant effects. This issue is of great importance because recent reports have indicated that a subset TRD patients who were repeatedly treated with ketamine developed ketamine dependence, indicative of ketamine’s abuse potential [12,94,150]. Additional safety concerns about ketamine’s long-term use as an antidepressant stem from adverse effects reported in ketamine abusers which include neurocognitive dysfunction, dizziness, nausea, anxiety, and development of urinary cystitis and adverse changes in brain structure and function [121,173,187].
Preclinical evidence
Preclinical studies utilizing different behavioral paradigms commonly used for inducing and assessing depressive-like phenotypes have paralleled clinical findings demonstrating ketamine’s antidepressant effects (see Table 1; reviewed in 105). Table 1 summarizes results from studies demonstrating ketamine’s ability to reverse increased immobility, reduce stress-induced anhedonia and novelty suppressed feeding, and ability to increase social interaction in both male and female animals after antidepressant treatment. Ketamine doses ranging from 0.5 mg/kg to up to 160 mg/kg administered intraperitoneally have been shown to have antidepressant efficacy in stress naïve, and to alleviate depressive-like symptoms in stress-induced animal models of depression (Table 1; reviewed in 14). From all of these various studies it can be concluded that ketamine’s effects were protracted, and that a single dose of the drug can produce antidepressant effects lasting from a few hours to several days (Table 1) [3,68,69,163,193]. Repeated, as well as administration of higher doses, demonstrated longer-lasting antidepressant-like effects (Table 1) [50,93,136,163]. The results from these studies parallel the clinical data and provide additional support for ketamine’s antidepressant effects. As with the clinical data, there is a severe lack of preclinical studies looking at the long-term safety and efficacy of chronic ketamine administration.
Table 1.
Summary of preclinical studies demonstrating ketamine’s antidepressant effects in vivo.
| Species/strain | Ketamine dose | Behavioral effects | |
|---|---|---|---|
| Yilmaz et al. 2002 [193] | Male Wistar rats (280–310 g) | Single dose of 1 mg/kg i.p. | Decreased immobility in the forced swim test (FST) for up to 10 days post injection. |
| Garcia et al. 2008 [50] | Male Wistar rats (300–350 g) | 5, 10, 15 mg/kg i.p. once daily for up to 12 days |
All doses decreased immobility in the FST. |
| Maeng et al. 2008 [102] | Male mice (20–25 g) | Single dose of 0.5, 2.5, 10 mg/kg i.p. | All doses rescued learned helplessness 24 h post treatment. 2.5 mg/kg dose decreased immobility in the FST 30 mins, and 2 weeks post treatment. |
| Cruz et al. 2009 [29] | Male Swiss mice (25–35 g) | Single dose of 6.35–50 mg/kg i.p. | 12.5, 25 mg/kg doses decreased immobility in the FST 24 h post treatment. 50 mg/kg dose decreased immobility in the FST and the tail suspension test (TST) 24 h post treatment. |
| Engin et al, 2009 [42] | Male Sprague Dawley rats (180–360 g) | Single dose of 10, 50 mg/kg i.p. | Both doses decreased immobility in the FST. |
| Ghasemi et al. 2010 [53] | Male NMRI mice (23–30g) | Single dose of 0.55 mg/kg i.p. | 2, 5 mg/kg doses decreased immobility in the FST 45 mins post treatment. |
| Li et al. 2010 [87] | Male Sprague Dawley rats (150–250 g) | Single dose of 10 mg/kg i.p. | Antidepressant-like effects seen 24 h post treatment as measured by the FST, learned helplessness, and novelty suppressed feeding tests. Effects lasted for up to 2 days post treatment. Increased sucrose preference 1, 3, 5, and 7 days post treatment. |
| Autry et al. 2011 [3] | Male C57BL/6 mice (6–8 weeks old) | Single dose of 3 mg/kg i.p. | Decreased immobility in the FST 30 mins post treatment effects lasting up to 1-week. |
| Bechtholt-Gompf et al. 2011 [5] | Male Swiss and C57BJ/6 mice (8 weeks) | Single dose of 0.5, 2.5, 12.5, 40, 80, 160 mg/kg i.p. | 160 mg/kg dose decreased immobility in the FST 1 h post treatment in the C57BL/6 mice, but not at 7 days post treatment. |
| Koike et al. 2011 [75] | Male ICR mice (25–35 g) | Single dose of 3–30 mg/kg i.p. | Decreased immobility in the FST only by the 30 mg/kg dose |
| Reus et al. 2011 [145] | Male adult Wistar rats (60 days old) | Single dose of 5, 10 mg/kg i.p. | Decreased immobility in the FST only the 10 mg/kg dose 1 h post treatment. |
| Wang et al. 2011 [175] | Male Wistar rats (60 days old) | Single dose of 15 mg/kg i.p. | Decreased immobility in FST 1 h post treatment. |
| Lindholm et al. 2012 [93] | Adult male C57BL/6 mice | Single dose of 20, 50 mg/kg i.p. | Decreased immobility in the FST 45 minutes post treatment, but not at 7 days. |
| Ma et al, 2012 [100] | Male C57BL/6 mice | Single dose of 10 mg/kg i.p. | Decreased immobility in the FST and TST 48 h post-treatment. Increased sucrose preference 24 h, 4, 6, and 8 days post treatment. |
| Tizabi et al. 2012 [164] | Male and female Wistar rats | 0.25–5.0 mg/kg i.p. once daily for 10 days | No acute/chronic effect on the Wistar rats. Immobility in the FST was decreased in Wistar Kyoto rats for up to 1 week by the 5.0 mg/kg dose. Low doses did not show long lasting effects in either strains. |
| Carrier & Kabbaj 2013 [16] | Male and female Sprague Dawley rats (200–270 g) | Single dose of 2.5–10 mg/kg i.p. | Decreased latency to feed 24 h post treatment in novelty suppressed feeding test. Sucrose preference was increased in males 48 h post treatment. Decreased immobility in the FST in both males and females 30 mins post treatment. |
| Gigliucci et al. 2013 [55] | Male Sprague Dawley rats (280–320 g) | Single dose of 1025 mg/kg | Decreased immobility in the FST 1 and 24 h post treatment. Pretreatment with 3 doses of ketamine (at 24, 5 and 1 h prior to testing) was ineffective. |
| Liu et al. 2013 [95] | Male Sprague Dawley rats (150–250 g) | Single dose of 1, 10 mg/kg i.p. | Decreased immobility in the FST 24 h and 1 week after 10 mg/kg. |
| Parise et al. 2013 [136] | Male Sprague Dawley rats (post-natal day 35–49) | 5, 10, 20 mg/kg i.p. twice daily for 1 or 15 days | Decreased immobility in the FST (10/20 mg/kg) 24 h after second injection. Effects lasted for up to 2 months after chronic treatment cessation. |
| Yang et al. 2013 [191] | Male Wistar rats (180–220 g) | Single dose of 10 mg/kg i.p. | Decreased Reduced immobility in the FST 30 mins post treatment. |
| Gideons et al. 2014 [54] | Male C57BL/6 mice (6–8 weeks) | Single dose of 3 mg/kg i.p. | Decreased immobility in the FST for up to 24 h post treatment. Decreased latency to feed 30 mins post treatment in novelty suppressed feeding test. |
| Donahue et al. 2014 [35] | Male C57BL/6 mice (6–8 weeks) | Single dose of 2.5 or 20 mg/kg i.p. | Increased social interaction score 24 h post treatment in animals treated with 20 mg/kg ketamine dose. |
| Francesschelli et al. 2015 [45] | Adult male and female C57BL/6 mice | Single dose of 3, 5, 10 mg/kg i.p. | For females, all three doses decreased immobility in the FST 30 mins post treatment. For males, 5 and 10 mg/kg doses decreased immobility in the FST 30 mins post treatment. At 24 h post treatment, both males and females continued to show decreased immobility in the FST at 10 mg/kg dose. |
| Jett at al. 2015 [68] | Male Sprague Dawley rats (220–300 g) | Single dose of 10 mg.kg i.p. | Decreased immobility in the FST 7 days post treatment. |
| Zanos et al. 2015 [195] | Male Swiss mice (8 weeks old) | Single dose of 10 mg/kg i.p. | Decreased immobility 1 h post treatment in the FST and TST. |
| Chiu et al. 2015 [23] | Male CD-1 mice | Single dose of 2.5, 50 mg/kg i.p. plus pre-treatment with lithium (600/1200 mg/L) | No effects with single dose of 2.5 mg/kg. Decreased immobility in the FST and TST when 2.5 mg/kg was coupled with Lithium (Li) (60/1200 mg/L). Decreased immobility in the FST and TST when 50 mg/kg was coupled with Li (60/1200 mg/L). |
| Lin et al. 2016 [91] | Male Swiss mice (30–45 g, 8 weeks old) | Single dose of 3, 10, 15, 30 mg/kg i.p. | 10 and 15 mg/kg decreased immobility in the FST 1, and 7 days post treatment. 10 mg/kg decreased latency to feed 1 h post treatment. |
| Sarkar & Kabbaj 2016 [149] | Male and female Sprague Dawley rats (200–270 g) | Single dose of 2.5, 5 mg/kg i.p. | Decreased immobility in the FST 72 h post treatment. In males, increased sucrose preference 24 h post treatment. |
| Sun et al. 2016 [160] | Male Sprague Dawley rats (250–300 g) | Single dose of 10 mg/kg i.p. | Decreased immobility in the FST and increased sucrose consumption in sucrose preference test 72 h post treatment. |
| Thelen et al. 2016 [163] | Male and female C57BL/6 mice (8–12 weeks old) | 3–10 mg/kg i.p. once daily for 21 days | No change in spontaneous locomotor activity. Decreased immobility in the FST in males (10 mg/kg). Females showed increased immobility in the FST (indicative of increase in depression-like behavior). |
| Chowdhury et al. 2017 [24] | Male Sprague Dawley rats (180–220 g) | Single dose of 3, 30, 80 mg/kg i.p. | 30 mg/kg decreased immobility in the FST 24 h post treatment. |
| Dong et al. 2017 [37] | Male C57BL/6 mice (8 weeks old, 20–25 g) | Single dose of 10 mg/kg i.p. | Decreased immobility in the TST 24 h post treatment and in the FST 48 h post treatment. Increased sucrose preference for up to 7 days post treatment. |
| Popik et al. 2017 [141] | Male Swiss mice (18–22g) | Single dose of 5, 10, 15, 25 mg/kg i.p. | Immobility was reduced in the FST for all doses except 5 mg/kg 30 mins post treatment. This effect was lost at 24 h post treatment. All doses except 5 mg/kg increased sucrose preference 48 h post treatment. |
| Donegan & Lodge 2017 [36] | Male Sprague Dawley rats (250–275 g) | Single dose of 10 mg/kg i.p. | Decreased immobility in the FST 30 mins and 7 days post treatment. |
| Jiang et al. 2017 [69] | Male Sprague Dawley rats (250–350 g) | 10 mg/kg i.p. once daily for 15 days | Decreased immobility in the FST 24 h post treatment. Reduced immobility was sustained 7 weeks post treatment. Increased sucrose preference 24 h and 7 weeks post treatment. |
| Iniguez et al. 2018 [66] | Female C57BL/6 mice (8 weeks old) | Three doses of 20 mg/kg i.p. | Increased interaction ratio in a social interaction test immediately post treatment. |
| Maciel et al. 2018 [101] | Male Wistar rats (60 days old) | Single dose of 15 mg/kg i.p. | Decreased immobility in the FST 8 days post treatment. |
| Wang et al. 2018 [176] | Male C57BL/6 mice (4-week-old, 16–19 g) | Two doses of 30 mg/kg i.p., 14 days apart | Reduced immobility in the FST 28 days post treatment. |
| Zhang et al. 2018 [197] | Male C57BL/6 mice (8 weeks old, 20–25 g) | Single dose of 10 mg/kg i.p. | Decreased immobility in the TST 4 h post treatment. Increased sucrose preference 2, and 5 days post treatment. |
| Hare et al. 2019 [60] | Male and female C57BL/6 mice | Single dose of 10 mg/kg i.p. | Decreased immobility in the FST 24 h post treatment. |
| Newman et al. 2019 [130] | Female Swiss mice (12 weeks old) | Single dose of 20 mg/kg i.p. | Increased social contact in chronically defeated female mice 24 h post treatment. |
Ketamine and addiction
Illicit use of ketamine began in the United States in the 1970s [156] and soon spread more widely aided by the emergence of the “rave” culture [67]. Recreational misuse was also expanded by the resulting delirium and psychosis (i.e., emergence phenomena) experienced during the recovery phase of ketamine anesthesia. When used at subanesthetic doses (0.5–1.5 mg/kg, i.v., or 4–5 mg/kg, i.m.), ketamine induces a “psychedelic” state of mind. This psychedelic state incentivized users to begin illicit use of ketamine. There has been a significant increase in the incidence of illicit use of ketamine globally over the past few years [22,30,31,97,143,152]. The worldwide prevalence of ketamine abuse is not known; however, lifetime incidence rates are around 0.1% in the United States [147], and about 4% in the United Kingdom [115]. Although ketamine use is not as widespread as other abused drugs, it is a preferred drug in party settings. Ketamine insufflation induces rapid psychedelic effects in abusers, and its short elimination half-life has been suggested to promote bingeing, and its increased appeal as a party drug over longer-lasting hallucinogens such as LSD or ‘magic’ mushrooms [115]. Taken together, ketamine’s psychedelic effects and short half-life make it an appealing drug for party-goers suggesting a strong potential for its abuse. Since its resurgence as a rapid acting antidepressant, understanding its abuse potential has become extremely important.
Clinical evidence of abuse
Studies with healthy volunteers demonstrated that the subjective feelings of “high” are increased upon ketamine use [80]. Morgan et al. (2004) demonstrated that i.v. infusion of subanesthetic doses (0.4 mg/kg and 0.8 mg/kg) to healthy ketamine-naïve volunteers increased the “liking” and “wanting” of ketamine in a dose-dependent manner. These effects were rapidly induced as both groups liked and wanted more ketamine shortly after drug administration. Notably, towards the end of the 80-minute infusion, the low-dose group (0.4 mg/kg) continued to like and want ketamine while the high-dose group (0.8 mg/kg) significantly reduced their ratings [120]. This demonstrated that low doses of ketamine infusion, those within antidepressant range, may have stronger abuse potential. This finding is a serious cause of concern as the lower end of the dose preferred by recreational users (0.5 mg/kg) is also being used as an antidepressant. The incidence of ketamine dependence is unknown due to the lack of studies demonstrating compulsive patterns of ketamine-seeking behavior. Most of the extant literature includes case reports or small-scale studies [63,116,135]. Within these studies, frequent users report compulsive patterns of drug using without stopping until supplies ran out. In frequent users, ketamine dose has been shown to escalate by 600% from first use to current use [117]. Hair analysis of infrequent users showed doubling of ketamine concentrations over a period of one year, whereas in frequent users, there was no change in ketamine concentrations [121]. Ketamine tolerance has also been shown to develop in users over time. However, there seems to be a maximum dose beyond which abusers do not experience the addictive effects of ketamine. This effect, including the development of ketamine tolerance, has been attributed to induction of liver enzymes and neuronal adaptations which decrease user’s sensitivity to the drug [119]. Evidence for the development of a withdrawal syndrome upon ketamine abstinence is conflicting. Cravings, anxiety, sweating and shaking have been reported to be common withdrawal symptoms in frequent users upon ketamine abstinence [28]. However, cravings for the drug are the primary cause of failure towards abstinence. In a study by Morgan et al. 28 out of 30 frequent users reported that they failed to discontinue ketamine use due to intensity of these cravings [117]. Cognitive impairments were only present in frequent ketamine users, while infrequent or recreational ketamine use did not appear to cause any long-lasting cognitive deficits [118,126]. More specifically, frequent use caused visual and spatial working memory impairments, and these deficits may be reversible as observed in ex-ketamine users who had been abstinent for at least a year [121]. Together, this evidence indicates that ketamine possesses a strong abuse potential when used at subanesthetic doses, which are within the dose range commonly used to treat depression. However, studies providing evidence demonstrating either a severe addiction, or lack thereof, for ketamine are lacking. This may be due to the fact that the prevalence use rate in the United States is about 0.1%, and does not represents a public health concern as with other abused drugs. Nevertheless, the use of ketamine at subanesthetic doses must be regarded with caution due to its obvious abuse potential.
Preclinical evidence of ketamine abuse
A recent increase in preclinical studies to evaluate ketamine’s abuse potential may be indicative of this “side-effect” as being a cause of concern. The following section summarizes some of the most recent studies demonstrating ketamine’s abuse potential.
It has been demonstrated that rodents show addictive-like behaviors upon ketamine administration under various drug administration conditions. Li et al. in 2008 and Botanas et al. in 2015 independently demonstrated that male rats showed increased preference for environments associated with ketamine exposure as measured in the conditioned place preference (CPP) paradigm when exposed to 5 and 10 mg/kg ketamine, respectively [13,86]. In another study by Schoepfer et al. both male and female rats developed CPP to ketamine at a dose of 5 mg/kg [153]. However, Parise et al. reported that adolescents male rats (postnatal day 35) did not develop CPP to a wide range of ketamine doses (5, 10, 20 mg/kg) [136]. The mechanisms underlying these discrepancies are unknown. It is possible that these differences may be age-dependent, as ketamine has been used for sedation successfully for decades in pediatric populations, potentially making it suitable for its use as an antidepressant in adolescence [33]. Increased ketamine-induced locomotor sensitization has been demonstrated in both male and female rodents at 2.5, 5 and 10 mg/kg doses after daily or weekly treatment regimens [153,159,161,179,180]. Locomotor sensitization was also observed in rats following repeated intermittent ketamine dosing [166]. In addition, both male and female rats self-administer ketamine readily, and show an increased intake of the drug [184,188] thus demonstrating the rewarding and reinforcing properties of ketamine. Thus, ketamine’s rewarding, reinforcing and locomotor-activating effects are present in both male and female rodents at clinically-relevant antidepressant doses. Additionally, some of these studies have reported sex-differences wherein females appear to be more sensitive to ketamine’s abuse potential than males, as well as showing age- and dose-dependent effects (reviewed in [16]).
Ketamine regulation of the overlapping neural circuitry between depression and addiction
It is well known that depression and addiction are highly comorbid. While much has been elucidated about the neural basis of drug abuse, and more recently depression, how and where in the brain these conditions converge is unknown. A reason for these highly comorbid conditions may be due, at least in part, to an overlap in the neural circuitry known to regulate mood and responses to natural and drug rewards which, when dysregulated, contributes to the pathophysiology of both of these disorders. The mesolimbic reward circuit, constituting of the dopaminergic projections arising in the ventral tegmental area (VTA) and projecting to the nucleus accumbens (NAc), is of special interest. This is due to this circuit’s role in the control of motivation and hedonic value under normal conditions, and alterations in reward and motivational processes play a central role in the manifestation of core symptoms of both MDD and drug addiction [44,77,125,129,185]. Dysfunction of this neural pathway, better known for mediating drug abuse/addiction, is also involved in the negative affective state (i.e., anhedonia) associated with depression. In this section, studies involved in understanding the roles of dopaminergic and glutamatergic systems in addiction and depression are reviewed to demonstrate an overlap of the neural circuitry between them. Projections from the reward circuit to the dorsal striatum, frontal cortex, hippocampus, lateral habenula and parts of the amygdala are also included in this section due to their reported involvement in both depression and addiction.
The VTA sends dopaminergic (DA) projections to the dorsal striatum, the NAc, the hippocampus, and medial prefrontal cortex (mPFC). Differential effects occurring as a function of these circuits have been observed in different animal models of depression. For example, optogenetic stimulation of VTA-NAc DA projecting neurons, but not VTA-mPFC DA projecting neurons, induced social withdrawal and anhedonia (i.e., depression-like behaviors) after exposure to subthreshold social defeat stress, while inhibition of both projections reversed these effects [20]. These differential effects are dependent on the stress levels induced by the different paradigms. For example, while a subthreshold social defeat was able to induced depressive-like behaviors due to the stimulation of VTA-NAc DA projections, under chronic mild stress conditions this stimulation produces an antidepressant-like phenotype [19,73]. The VTA-NAc projections are known for playing a prominent role in drug reward, development of cue-induced drug seeking, drug self-administration, and transition from drug use to compulsive drug taking. All drugs of abuse activate DA projections from the VTA to NAc, dorsal striatum, hippocampus, and the mPFC [77]. A couple of studies have demonstrated ketamine’s ability to increase burst firing and firing rate of VTA DA neurons and reverse depression phenotypes [7,90]. These data point to an overlap in the functioning of the VTA-NAc circuit in depression and addiction, and further shows ketamine’s ability to modulate its functioning, making it a prominent brain pathway that can be affected by ketamine in either of these disorders.
Dysregulation of the NAc has been associated with reduced motivation and increased anhedonia in models of depression. In models of drug addiction, the NAc has been shown to be involved in drug reward prediction, drug-taking behavior, and cue-elicited drug seeking and reinstatement [73,129]. Medium spiny neurons (MSNs) of the NAc are differentially enriched with DA type-1 (D1) or type-2 (D2) receptors. The activation of either receptor subtype gives rise to circuits regulating contrasting motivational states (i.e., either reward or aversion). More specifically, activation of the D1-containing MSNs leads to induction of reward-related behaviors while activation of D2-containing MSNs leads to aversion-related behaviors. The NAc also receives overlapping projections from the hippocampus, amygdala and the prefrontal cortex that mediate aspects of motivated, goal-directed behaviors such as drug seeking and social interaction [57]. Belujon and Grace (2014) demonstrated ketamine’s ability to increase the synaptic input from hippocampus to the NAc in the depressive phenotypes induced in rats exposed to the learned helplessness procedure [7]. An imbalance in the functioning of either the neuronal circuits or inputs from various input areas has been implicated in the pathophysiology of addiction and depression [19,44] thereby demonstrating a significant overlap in the functioning of the NAc in the manifestation of either of these disorders.
The hippocampus and frontal regions of the cerebral cortex, including the anterior cingulate cortex (ACC), are known to also play a significant role in depression and responses to antidepressant drugs [38,40]. Brain imaging studies have demonstrated decreased blood flow and reduced activity of the hippocampus and frontal cortex in depressed patients [108]. Neuroimaging studies have also demonstrated that depressed patients show blunted activity and decreased responses to reward-related tasks due to reduced connectivity in the reward-related networks of the prefrontal-striatal regions. These deficits in network connectivity are significantly associated with cognitive deficits (including learning and memory) and depression severity in depressed patients [56]. The addiction literature also demonstrates that prefrontal cortex attributes salience to neutral stimuli associated with drug-reward contexts, as well as maintaining control over goaldirected behaviors, while the hippocampus has been implicated in the formation of drug-context memories, drug-cue associations, and reconsolidation of drug memories [77]. Protracted abstinence from drugs of abuse leads to overactivation of the glutamatergic systems in the frontal cortex that mediates strong craving-like responses via glutamatergic activation of the NAc [110,186], thus indicative of this circuit’s importance in cravings associated with drugs of abuse. In addition, the hippocampus has been implicated in reinstatement of drug-taking behavior leading to relapse via cue and contextual triggers [81]. As previously stated, continued use of ketamine in frequent users is primarily attributed to the cravings experienced during drug abstinence. Chronic treatment with ketamine for the treatment of depression may therefore have the potential of leading to craving-induced continued use in patients [144]. However, there is a lack of empirical evidence supporting this claim and further research exploring this aspect is severely needed.
The lateral habenula has been recently identified as an important region involved in regulating depressive-like behavior. Increased activation of neurons projecting from the lateral habenula to the VTA has been observed in mice expressing depressive phenotypes following exposure to the learned helplessness and the chronic social defeat paradigms [19,85]. The addiction literature also implicates the lateral habenula in mediating physiological and behavioral responses to drugs of abuse. Several studies have demonstrated its role in controlling the firing of VTA DA neurons [77]. More recently, the lateral habenula has also been implicated in regulating DA release within the VTA via NMDA receptor blockade by ketamine. Specifically, ketamine block of glutamatergic input to the lateral habenula disinhibits VTA DA neurons thus resulting in increased DA neurotransmission [192].
Together, the reviewed evidence indicates that there is an overlap in the neural circuitry involved in both depression and addiction. An imbalance in the functioning of this circuitry gives rise to either, or both disorders (i.e., comorbidity; see Figure 2). Both depression and addiction entail long-lasting changes in the functioning of neural networks that are associated with mood, reward, and motivation. A relatively recent study also demonstrated the ability of a single, subanesthetic dose of ketamine to induce large-scale, persistent reconfigurations of the cortical and subcortical areas of the mesolimbic DA reward pathway [99]. Therefore, the use of any pharmaceutical agent with abuse liability such as ketamine has the potential to activate and induce changes in these overlapping networks. Although there is some evidence demonstrating that different subpopulations of neurons within these neural circuits are responsible for either addictive or depressive behaviors [19,44], the use of ketamine as an antidepressant has a strong potential to cause non-selective widespread effects which may lead to development of addiction in depressed patients being treated with ketamine for prolonged periods of time [128]. However, sufficient empirical data demonstrating ketamine’s effects (acute, chronic, immediate or long-lasting) on the functioning of these neurocircuitry is severely lacking. In paradigms that have applied multiple ketamine injections for up to 15 days, addictive potential/abuse liability of ketamine has not been addressed [50,69,136,163,164]. Neither have the studies demonstrating long term effects of ketamine treatment addressed its influence towards abuse liability [176]. Unless evidence indicating safety of its repeated and long-term use is obtained, the possibility of adverse addictive phenotype due to ketamine’s antidepressant use cannot be negated.
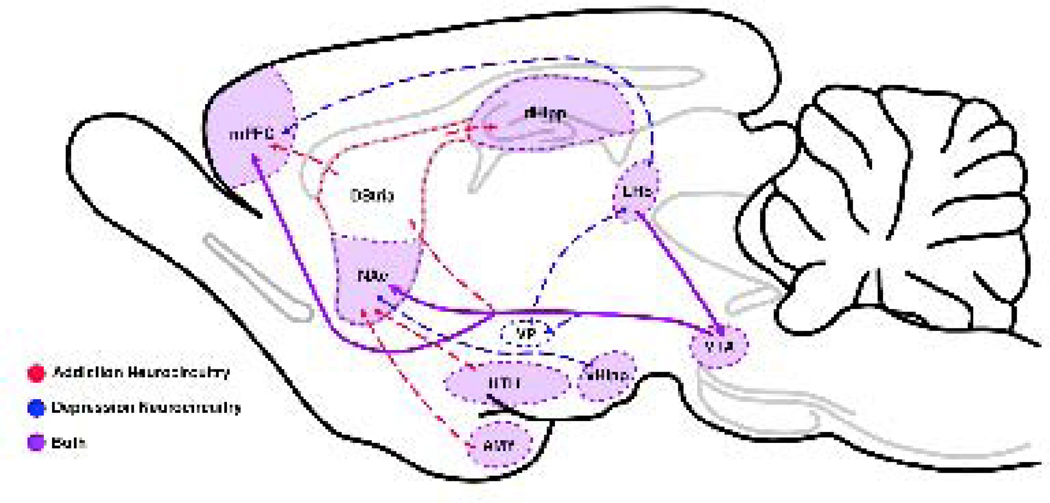
Figure 2.
Involvement of overlapping neurocircuitry in both addiction and depression. Neural changes occuring along the mesolimbic dopaminergic reward pathway in drug addiction strongly overlap with those occurring within the same areas in depression. In both addiction and depression changes occur within the medial prefrontal cortex (mPFC), nucleus accumbens (NAc), dorsal hippocampus (DHipp), ventral hippocampus (VHipp), hypothalamus (HTH), Lateral Habenula (LHb), and amygdala (AMY). Neural changes induced by ketamine mimic those induced by other drugs of abuse.
Overlap in the molecular pathways inducing antidepressant and abuse potential of ketamine
Depression is a complex disorder. Pathophysiological evidence has ascribed dysregulation of several brain regions and neurotransmitter systems including dopaminergic, glutamatergic, GABAergic and serotonin. Within the context of this review, only the changes in neurobiological mechanisms on dopaminergic and glutamatergic systems that are influenced by ketamine are discussed in the following section.
Animal models of depression show that loss in function and atrophy within mesolimbic brain regions are due to stress-induced decrease in synapses, reduction in the number and density of dendritic spines, and reduction in the number of glial cells [40]. A single subanesthetic dose of ketamine has been shown to increase synaptic activity within two hours after ketamine administration. Levels of synaptic proteins are also increased after ketamine, indicating an increase in synapse number [87]. This finding coincides with the timeline for therapeutic antidepressant effects of ketamine in both human and animal studies, and supports the conclusion that ketamine induces rapid antidepressant effects by reversing synaptic deficits, and possibly atrophy, through the revival and enhancement of synaptic connections. Interestingly, the addiction literature also ascribes the development of addictive phenotypes to synaptogenesis, increased dendritic branching, and enhanced synaptic activity of mesocorticolimbic regions [77,78,171]. Therefore, it is possible that the enhanced synaptic activity brought about by ketamine may also function to induce an addiction-vulnerability phenotype.
The neurophysiological effects of subanesthetic ketamine are long lasting, with increased cortical excitability still being evident up to four hours after drug exposure [25]. This may be indicative of lasting activation of downstream molecular pathways initiated by ketamine’s block of NMDA receptors. Tonic disinhibition of GABAergic interneurons has been proposed to be the mechanism of action underlying ketamine’s antidepressant effects. Briefly, tonic inhibition of GABAergic interneurons via ketamine block of the NMDA receptors on these interneurons causes disinhibition of the glutamatergic neurons leading to increased synaptic glutamate. This, in turn, induces synaptic insertion of AMPA receptors, eventually leading to synaptic potentiation and synaptogenesis [39]. This mechanism of action is currently the leading working hypothesis underlying ketamine’s antidepressant effects. Because of the widespread presence of glutamatergic neurotransmission within the brain, these ketamine-induced effects can be assumed to constitutively enhance glutamatergic neurotransmission within the reward circuitry and contribute to its abuse potential.
Animal models of depression have demonstrated that chronic stress leads to long lasting increase in DA neurotransmission that eventually results in dampening of emotional reactivity towards natural rewards, thus leading to anhedonia [129]. Given that DA neurotransmitter system plays a role in depression, a potential mechanism of action leading to depressive phenotypes may involve stress-induced dysregulated dopaminergic inputs from the VTA [57,140]. While the VTA-NAc reward circuit receives glutamatergic inputs from prefrontal cortex, hippocampus, amygdala, and peptidergic inputs from the hypothalamus, the VTA also sends out reciprocal dopaminergic projections to these brain regions, thus ketamine disinhibition of glutamatergic inputs may be involved in modulation of these VTA DA projections. Additionally, ketamine has a strong affinity for D2 receptors, and has been proposed to also enhance DA release via blockade of DA reuptake [71]. Hence, there seems to be a synergistic effect of ketamine on the DA neurotransmission which may, in part, play a role in ketamine’s antidepressant effects. However, a mechanistic model of ketamine’s antidepressant effect via the DA system has not yet been established and further research exploring this is needed.
Neurotrophic factors play an important role in activity-dependent synaptic plasticity in the adult brain, as well as maintenance and survival of neurons. Brain-derived neurotrophic factor (BDNF) is the most highly expressed neurotrophic factor in the brain and strongly regulates activity-dependent synaptic plasticity. Stress, and consequently depression, have been shown to directly affect levels of BDNF. Several preclinical studies have reported that stress reduces the levels of BDNF mRNA expression in the prefrontal cortex as well as overall circulating BDNF levels in the brain [40]. Ketamine’s antidepressant efficacy has been blocked in BDNF-knockout mice [3], and is also rendered ineffective in mice expressing human BDNF carrying the Val66Met mutation [21,194]. Depressed patients carrying this mutated BDNF allele also show decreased response to ketamine’s antidepressant effects [83]. This mutation blocks the activity-dependent release of BDNF, indicating that synaptic activity may be required for the release of BDNF and the subsequent antidepressant effects of ketamine [3]. These data support ketamine’s protracted effects on the increase in postsynaptic AMPARs in that AMPAR-mediated synaptic potentiation would be required for the activity-dependent release of BDNF and subsequent antidepressant effects via synaptogenesis and increased synaptic spine density. As long as these effects on BDNF release are restricted to the prefrontal cortex and hippocampus, ketamine could be considered a safe drug for use as an antidepressant. However, ketamine is a “promiscuous” drug and there are no empirical data demonstrating that its effects are specific to the prefrontal cortex and hippocampus. Increased expression of BDNF in the mesolimbic DA system has been shown to be associated with the induction of depressive symptoms seen during withdrawal in drug addicted individuals [6,11,51,89,170]. Increases in BDNF expression have also been associated with increased drug cravings [146]. Therefore, if ketamine increases BDNF expression in the mesolimbic DA system of depressed patients, it might induce craving in these patients increasing their risk for abusing ketamine (see Figure 3).
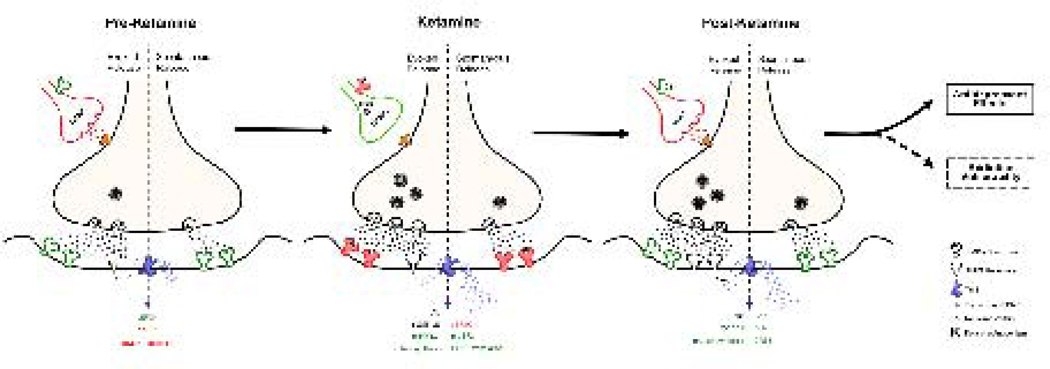
Figure 3.
Ketamine’s inhibition of the NMDA receptor results in increases in glutamatergic neurotransmission. Red indicates inhibitory effects. Green indicates disinhibitory, facilitatory effects. When on board, ketamine causes the inhibition of GABAergic interneurons by blocking the NMAD receptors on the GABAergic interneurons, resulting in disinhibition. This, in turn, increases AMPA receptor expression resulting in synaptic strengthening. Brain-derived neurotrophic factor (BDNF) synthesis and translation is mediated by evoked and spontaneous activity. During normal function, spontaneous NMDA receptor activation and Ca2+-dependent second messengers cause the eEF2 kinase (eEF2K) to dephosphorylate eEF2. Ketamine causes the inhibition of eEF2K, which induces an increase in phosphorylated eEF2 (p-eEF2), and a resultant increase in BDNF translation and protein synthesis [114]. Ketamine causes the activation of the mTOR signaling pathway, promoting synaptogenesis. All of these molecular changes appear to underly ketamine’s rapid antidepressant effects, and possibly its potential addiction liability. (Figure modeled after Monteggia et al., 2013.[114])
The glycogen synthase kinase-3 (GSK-3) is a serine/threonine kinase originally characterized as a regulator of glycogen metabolism. GSK-3 exists in the form of two functionally distinct isomers: GSK-3α and GSK-3β. Both are constitutively active and get rapidly deactivated by phosphorylation. GSK-3β has been found to be widely distributed in the CNS and has been implicated in several normal and pathological processes, including glycogen metabolism, regulation of cell proliferation, induction of long-term depression, neurodegeneration in Alzheimer’s disease, schizophrenia, and depression [103]. Levels of GSK-3β have been reported to be significantly increased in depressed patients, and ketamine treatment has been shown to increase the phosphorylation of this protein [9,88,194]. Mutant mice expressing GSK-3β, which lack the inhibitory phosphorylation sites, have been shown to be resistant to ketamine’s antidepressant effects [95]. This indicates that ketamine’s antidepressant effects may be mediated via increases in the phosphorylation of GSK-3β (p-GSK-3β). Interestingly, GSK-3β has been implicated in the activation of ketamine’s psychotomimetic effects such that co-administration of GSK-3β antagonists with a single subanesthetic dose of ketamine inhibits the psychotomimetic effects [18]. Nevertheless, previous studies have demonstrated increased expression and activity of GSK-3β upon administration of a single subanesthetic dose of other NMDA receptor antagonists such as MK-801 and PCP [18]. A possible explanation for these contradictory results may be dependent on the immediate, as well as protracted effects of ketamine on NMDA receptor and glutamatergic signaling. Immediate blockade of NMDA receptors by ketamine might be involved in increasing the activity of GSK-3β, causing the psychotomimetic effects seen early on after administration of subanesthetic ketamine, while the protracted effects on the glutamatergic transmission would be responsible for the increased phosphorylation and subsequent inhibition of GSK-3β. This kinase also plays a major role in drug addiction due to its role in modulating DA, glutamate and serotonin transmission [4]. Studies have shown that a single injection of cocaine or methamphetamine increased expression and activity of GSK-3β, while self-administration of these drugs reduced p-GSK-3β in the limbic forebrain, NAc, and amygdala. Similarly, self-administration of ketamine decreases p-GSK-3β levels in the caudate putamen, VTA, and NAc, but not in the prefrontal cortex and hippocampus [62]. Thus, the differential effects of ketamine on the expression and activity of GSK-3β may be considered to be dose and time dependent. In summary, a single subanesthetic dose of ketamine increases activity of GSK-3β immediately following administration. Subsequently, protracted effect(s) of a single subanesthetic dose of ketamine would result in decrease activity of GSK-3β, which might be responsible for ketamine’s antidepressant effects. Conversely, repeated administration of ketamine would increase GSK-3β expression and activity which is implicated in the development of addiction and addictive behaviors.
Miller et al. in 2014 showed that GluN2B-null mutant mice had increased activated mammalian target of rapamycin (mTOR) and reduced depression-like behavior, and these effects could not be enhanced further with ketamine treatment [112]. Overall, evidence from this study demonstrated that ketamine’s antagonism of the NMDA receptor may not be the only mechanism regulating its antidepressant activity. Ketamine has also been hypothesized to have direct intracellular effects by entering the cell and its different organelles (lysosomes and endoplasmic reticulum) due to its lipid-solubility and low molecular weight [84]. The predicted mechanisms for its action would be via its activation of mTORC1 branch of the mTOR pathway through entry into lysosomes. Activation of the mTOR pathway would then lead to expression of proteins involved in synaptogenesis, which would eventually induce the antidepressant effects associated with ketamine exposure [84]. Activation of the mTOR signaling pathway has also been implicated in drug addiction. Specifically, mTOR signaling gets activated in the presence of cocaine and during cocaine CPP and self-administration paradigms (reviewed in 95). Data from our laboratory implicates the mTOR signaling pathway in mediating sex-differences in drug addiction in that females show increased cocaine CPP along with increased expression of mTOR in the VTA, NAc and dorsal striatum than males [76]. Taken together, these data implicate the involvement of mTOR signaling in drug addiction (see Figure 3), in that increased mTOR signaling may underlie the neurobiological basis of addiction and addictive behaviors.
From the above discussion, it is evident that the involvement of overlapping neurotransmitter systems and molecular mechanisms in addiction and the antidepressant effects of ketamine provide a strong case towards potential of ketamine abuse when used repeatedly for the treatment of depression.
Conclusions and Future directions
Strong evidence exists supporting ketamine’s rapid acting antidepressant effects. These effects, however, are transient and do not last for more than two weeks, and in order to extend these antidepressant effects, repeated ketamine treatment appears necessary. However, clinical studies of repeated ketamine infusions have shown limited success, with depressive symptoms remaining low throughout the course of the treatment, but patients relapsing soon after treatment cessation. There also seems to be a dose-dependent effect of ketamine on depressive symptoms, with higher doses (0.4 mg/kg vs. 0.1 mg/kg) prolonging the antidepressant effects, yet higher doses have the potential to cause strong psychotomimetic effects. A recently reported case study showed development of alcohol abuse and subsequent suicide death of a patient suffering from TRD and undergoing unsupervised ketamine therapy. The patient was asked to take 0.5–1 mL of 150 mg/mL ketamine intranasally once every four hours as required. The patient reported using more than the prescribed dose of ketamine to keep the antidepressant effects longer, along with increased alcohol consumption. He also reported using ketamine even when not feeling depressed because he “liked” the “trippy” feeling that he got after consuming the drug. Ultimately, after three years of using ketamine for his depression, the patient died in a fatal car crash. Autopsy findings indicated blood alcohol levels of 0.133%, and family members reported that the patient’s death was a suicide facilitated by alcohol consumption [150]. This case study speculated that unregulated use of ketamine as an antidepressant led to alcohol dependence and subsequent death of the patient. Long term safety and efficacy of repeated ketamine treatment lacks empirical evidence and until dosing regimens and potential abuse patterns are not determined, ketamine’s antidepressant use may need to be tempered with caution. Future studies should focus on systematically understanding how repeated ketamine treatment would affect other systems, especially the mesolimbic DA reward system, and also investigate possible mechanisms or strategies that could restrict or eliminate its abuse potential.
Ketamine’s abuse potential, especially when used repeatedly as an antidepressant, can also be illustrated by its effects on VTA DA neuronal activity. The study by Belujon and Grace (2014) mentioned previously, demonstrated that repeated injections of subanesthetic doses of ketamine reversed learned helplessness behaviors, confirming the antidepressant action of ketamine. They also reported that 20 minutes after ketamine administration, the activity of the VTA DA neurons was restored back to normal levels in these animals. Two hours after ketamine administration, there was a significant increase in the VTA DA neuronal activity in both control and helplessness-induced animals indicating that ketamine’s protracted effects increased DA neuronal activity in the VTA [7]. These effects on DA neuronal activity remained consistent even 24 hours after ketamine administration. The authors attributed these changes to long-lasting effects on synaptic plasticity mediated by ketamine’s action on glutamatergic signaling. They also suggested that ketamine induces antidepressant effects by stimulating the reward pathway and reversing the hyposensitivity to rewarding stimuli [7]. How much of this reversal in reward pathway activation is sufficient for long-lasting permanent remission from depression remains unknown. Until these mechanisms have been delineated, it would be unsafe to use ketamine repeatedly for the treatment of depression due to its strong abuse potential.
Ketamine interacts with several different receptors and proteins, including opioid, serotonin, and acetylcholine receptors, and its ability to induce global “reconstruction” of neural circuitry [99] demonstrates its capacity to affect several neural systems at the same time. Therefore, understanding the effects of ketamine’s use as an antidepressant on different neurotransmitter systems is critically important. Indeed, more evidence addressing ketamine’s antidepressant use and its association to non-glutamatergic neurotransmitter systems and non-NMDA receptor proteins is much needed. It is well known that ketamine interacts with all the opioid receptors, likely contributing to the many effects seen after ketamine exposure [98]. There are three known opioid receptors: mu, kappa, and delta, which are found in high densities in limbic regions known to mediate mood and responding to natural and drug reward [98]. Mu receptors, in particular, regulate mood [157] and the rewarding properties of drugs of abuse (e.g. cocaine, amphetamine, morphine) [169], while increased activity of kappa receptors is associated with depression [15]. Two recent studies showed that, in humans, pretreatment with the mu receptor antagonist naltrexone (50 mg/kg) attenuates the antidepressant and anti-suicidal effect of 0.5 mg/kg ketamine infusion, findings potentially indicating that the antidepressant and anti-suicidal activity of ketamine is mediated through the opioid system [182,183]. These studies received a flurry of interest, and although they had several limitations and would need to be replicated with rigor, they raised concerns because ketamine acting via an opioid-mediated mechanism could potentially worsen the harm of current opioid drug epidemic [107]. Given ketamine’s history as a drug of abuse, determining whether opioid receptor stimulation mediates its antidepressant effects is of great importance. Development of anhedonia is a cardinal feature of depression and is a result of dysregulated motivation, reinforcement learning, and reward-based decision making. Thus, dysregulation of the reward pathway is a potent precursor for depression [178]. Within this framework, if ketamine reduces depression, it may do so by potentiating the reward pathway and clearing out the deficits therein via permanent synaptic changes in this pathway. This would mean that ketamine’s long-term effects in depressed patients may be occurring by permanently potentiating the reward pathway, and thus supporting the view that repeated ketamine treatment would repeatedly activate reward pathways involved in the development of drug addiction.
Interestingly, there are novel studies reporting ketamine’s efficacy in the treatment of substance use disorders (SUDs) combined with psychotherapeutic intervention (reviewed by 47). These studies have shown positive effects of ketamine on drug use rates, craving, motivation, abstinence, and withdrawal symptoms of cocaine, heroin, alcohol and cannabis. However, these studies do not report individual’s dependence or motivation for ketamine abuse/addiction. The effects presented in these studies might be a case of drug substitution [132]. Another important aspect to consider is that most of these studies have used antidepressant (0.5–0.8 mg/kg) and higher doses of ketamine (2–2.5 mg/kg), with maximum effects towards control of SUDs seen with higher doses of ketamine. Further delineation of the effects from these studies need to be carefully investigated with larger sample sizes and robust controls.
Together, this evidence indicates that ketamine’s effects can be derived from multiple mechanisms that could be working in sequence or parallel. The use of ketamine for the treatment of depression has raised serious concerns across the field, ranging from the scarcity of data on efficacy and safety, to its potential for misuse [52,151,168]. While most acknowledge these concerns and propose the use of this drug with caution [46,151], there are no current clinical guidelines for the long-term use of ketamine [162], thus clinicians may have to rely on their own expertise and responsibility to do no harm [157]. Unfortunately, ketamine is currently available, often without psychiatric monitoring, in commercial clinics across the US [128,131,154,158], and barriers such as inability to pay for treatment may turn patients to self-medicate by seeking cheaper or illegal alternatives [111,181]. More research delineating ketamine’s mechanisms of actions to find ways to regulate them during treatment to help potentiate ketamine’s antidepressant actions while inhibiting potential addictive effects is paramount. Exploring strategies using ketamine-like pharmacological agents, or co-administration of sub-effective doses of ketamine in combination with agents devoid of abuse liability such as antidepressants, or antagonists of group II metabotropic glutamate (mGlu) receptors (mGlu2/3 subtypes), which have been shown to have antidepressant properties, may open up new avenues to prevent ketamine misuse. Until there is more evidence available on the dosing and long-term safety and efficacy of its repeated use, enthusiasm about ketamine’s antidepressant effects must be tempered with caution.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].aan het Rot M, Collins KA, Murrough JW, Perez AM, Reich DL, Charney DS, Mathew SJ, Safety and Efficacy of Repeated-Dose Intravenous Ketamine for Treatment-Resistant Depression, Biol. Psychiatry 67 (2010) 139–145. [DOI] [PubMed] [Google Scholar]
- [2].Anis NA, Berry SC, Burton NR, Lodge D, The dissociative anaesthetics, ketamine and phencyclidine, selectively reduce excitation of central mammalian neurones by N-methyl-aspartate, Br. J. Pharmacol 79 (1983) 565–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Autry AE, Adachi M, Nosyreva E, Na ES, Los MF, Cheng P, Kavalali ET, Monteggia LM, NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses., Nature. 475 (2011) 91–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Beaulieu JM, A role for Akt and glycogen synthase kinase-3 as integrators of dopamine and serotonin neurotransmission in mental health, J. Psychiatry Neurosci 37 (2012) 7–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Bechtholt-Gompf AJ, Smith KL, John CS, Kang HH, Carlezon WA, Cohen BM, Öngür D, CD-1 and Balb/cJ mice do not show enduring antidepressant-like effects of ketamine in tests of acute antidepressant efficacy, Psychopharmacology (Berl). 215 (2011) 689–695. [DOI] [PubMed] [Google Scholar]
- [6].Becker JB, Chartoff E, Sex differences in neural mechanisms mediating reward and addiction, Neuropsychopharmacology. 44 (2019) 166–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Belujon P, Grace AA, Restoring Mood Balance in Depression: Ketamine Reverses Deficit in Dopamine-Dependent Synaptic Plasticity, Biol. Psychiatry 76 (2014) 927–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, Krystal JH, Antidepressant effects of ketamine in depressed patients, Biol. Psychiatry 47 (2000) 351–354. [DOI] [PubMed] [Google Scholar]
- [9].Beurel E, Song L, Jope RS, Inhibition of glycogen synthase kinase-3 is necessary for the rapid antidepressant effect of ketamine in mice., Mol. Psychiatry 16 (2011) 1068–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Bobo WV, Voort JLV, Croarkin PE, Leung JG, Tye SJ, Frye MA, Ketamine for Treatment-resistant unipolar and bipolar major depression: Critical review and implications for clinical practice, Depress. Anxiety 33 (2016) 698–710. [DOI] [PubMed] [Google Scholar]
- [11].Bolaños CA, Nestler EJ, Neurotrophic Mechanisms in Drug Addiction, NeuroMolecular Med. 5 (2004) 69–83. [DOI] [PubMed] [Google Scholar]
- [12].Bonnet U, Long-Term Ketamine Self-Injections in Major Depressive Disorder: Focus on Tolerance in Ketamine’s Antidepressant Response and the Development of Ketamine Addiction, J. Psychoactive Drugs 47 (2015) 276–285. [DOI] [PubMed] [Google Scholar]
- [13].Botanas CJ, De La Peña JB, Dela Peña IJ, Tampus R, Yoon R, Kim HJ, Lee YS, Jang CG, Cheong JH, Methoxetamine, a ketamine derivative, produced conditioned place preference and was self-administered by rats: Evidence of its abuse potential, Pharmacol. Biochem. Behav 133 (2015) 31–36. [DOI] [PubMed] [Google Scholar]
- [14].Browne CA, Lucki I, Antidepressant effects of ketamine: Mechanisms underlying fast-acting novel antidepressants, Front. Pharmacol 4 December (2013) 161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Carlezon WA, Krystal AD, Kappa-Opioid Antagonists for Psychiatric Disorders: From Bench to Clinical Trials, Depress. Anxiety 33 (2016) 895–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Carrier N, Kabbaj M, Sex differences in the antidepressant-like effects of ketamine., Neuropharmacology. 70 (2013) 27–34. [DOI] [PubMed] [Google Scholar]
- [17].Célèrier E, Rivat C, Jun Y, Laulin JP, Larcher A, Reynier P, Simonnet G, Long-lasting hyperalgesia induced by fentanyl in rats: Preventive effect of ketamine, Anesthesiology. 92 (2000) 465–472. [DOI] [PubMed] [Google Scholar]
- [18].Chan MH, Chiu PH, Lin CY, Chen HH, Inhibition of glycogen synthase kinase-3 attenuates psychotomimetic effects of ketamine, Schizophr. Res 136 (2012) 96–103. [DOI] [PubMed] [Google Scholar]
- [19].Chaudhury D, Liu H, Han M-H, Neuronal correlates of depression, Cell. Mol. Life Sci 72 (2015) 4825–4848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Chaudhury D, Walsh JJ, Friedman AK, Juarez B, Ku SM, Koo JW, Ferguson D, Tsai H-C, Pomeranz L, Christoffel DJ, Nectow AR, Ekstrand M, Domingos A, Mazei-Robison MS, Mouzon E, Lobo MK, Neve RL, Friedman JM, Russo SJ, Deisseroth K, Nestler EJ, Han M-H, Rapid regulation of depression-related behaviours by control of midbrain dopamine neurons, Nature. 493 (2013) 532–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Chen ZY, Jing D, Bath KG, Ieraci A, Khan T, Siao CJ, Herrera DG, Toth M, Yang C, McEwen BS, Hempstead BL, Lee FS, Genetic variant BDNF (Val66Met) polymorphism alters anxiety-related behavior, Science (80-.). 314 (2006) 140–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Chengzheng Z, Zhimin L, Dong Z, Yanhong L, Jianhui L, Yilang T, Zeyuan L, Jiwang Z, Drug abuse in China, in: Ann. N. Y. Acad. Sci, 2004: pp. 439–445. [Google Scholar]
- [23].Chiu CT, Scheuing L, Liu G, Liao HM, Linares GR, Lin D, Chuang DM, The mood stabilizer lithium potentiates the antidepressant-like effects and ameliorates oxidative stress induced by acute ketamine in a mouse model of stress, Int. J. Neuropsychopharmacol 18 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Chowdhury GMI, Zhang J, Thomas M, Banasr M, Ma X, Pittman B, Bristow L, Schaeffer E, Duman RS, Rothman DL, Behar KL, Sanacora G, Transiently increased glutamate cycling in rat PFC is associated with rapid onset of antidepressant-like effects, Mol. Psychiatry 22 (2017) 120–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Cornwell BR, Salvadore G, Furey M, Marquardt CA, Brutsche NE, Grillon C, Zarate CA, Synaptic potentiation is critical for rapid antidepressant response to ketamine in treatment-resistant major depression, Biol. Psychiatry 72 (2012) 555–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Corssen G, Domino EF, Dissociative anesthesia: further pharmacologic studies and first clinical experience with the phencyclidine derivative CI-581., Anesth. Analg 45 (1966) 29–40. [PubMed] [Google Scholar]
- [27].Coyle CM, Laws KR, The use of ketamine as an antidepressant: A systematic review and meta-analysis, Hum. Psychopharmacol. 30 (2015) 152–163. [DOI] [PubMed] [Google Scholar]
- [28].Critchlow DG, A case of ketamine dependence with discontinuation symptoms, Addiction. 101 (2006) 1212–1213. [DOI] [PubMed] [Google Scholar]
- [29].Cruz SL, Soberanes-Chávez P, Páez-Martinez N, López-Rubalcava C, Toluene has antidepressant-like actions in two animal models used for the screening of antidepressant drugs, Psychopharmacology (Berl). 204 (2009) 279–286. [DOI] [PubMed] [Google Scholar]
- [30].Dalgarno PJ, Shewan D, Illicit Use of Ketamine in Scotland, J. Psychoactive Drugs 28 (1996) 191–199. [DOI] [PubMed] [Google Scholar]
- [31].Degenhardt L, Dunn M, The epidemiology of GHB and ketamine use in an Australian household survey, Int. J. Drug Policy 19 (2008) 311–316. [DOI] [PubMed] [Google Scholar]
- [32].Diazgranados N, Ibrahim L, Brutsche NE, Newberg A, Kronstein P, Khalife S, Kammerer WA, Quezado Z, Luckenbaugh DA, Salvadore G, Machado-Vieira R, Manji HK, Zarate CA, A randomized add-on trial of an N-methyl-D-aspartate antagonist in treatment-resistant bipolar depression, Arch. Gen. Psychiatry 67 (2010) 793–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Dolansky G, Shah A, Mosdossy G, Rieder M, What is the evidence for the safety and efficacy of using ketamine in children?, Paediatr. Child Health (Oxford) 13 (2008) 307–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Domino EF, Taming the ketamine tiger, Anesthesiology. 113 (2010) 678–684. [DOI] [PubMed] [Google Scholar]
- [35].Donahue RJ, Muschamp JW, Russo SJ, Nestler EJ, Carlezon WA, Effects of striatal ΔFosB over expression and ketamine on social defeat stress-induced anhedonia in mice, Biol. Psychiatry 76 (2014) 550–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Donegan JJ, Lodge DJ, Hippocampal perineuronal nets are required for the sustained antidepressant effect of ketamine, Int. J. Neuropsychopharmacol 20 (2017) 354–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Dong C, Zhang J, Yao W, Ren Q, Ma M, Yang C, Chaki S, Hashimoto K, Rapid and Sustained Antidepressant Action of the mGlu2/3 Receptor Antagonist MGS0039 in the Social Defeat Stress Model: Comparison with Ketamine, Int. J. Neuropsychopharmacol (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Dranovsky A, Hen R, Hippocampal Neurogenesis: Regulation by Stress and Antidepressants, Biol. Psychiatry 59 (2006) 1136–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Duman RS, Pathophysiology of depression and innovative treatments: remodeling glutamatergic synaptic connections, Dialogues Clin. Neurosci 16 (2014) 11–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Duman RS, Monteggia LM, A Neurotrophic Model for Stress-Related Mood Disorders, Biol. Psychiatry 59 (2006) 1116–1127. [DOI] [PubMed] [Google Scholar]
- [41].Edwards SR, Mather LE, Tissue uptake of ketamine and norketamine enantiomers in the rat Indirect evidence for extrahepatic metabolic inversion, Life Sci. 69 (2001) 2051–2066. [DOI] [PubMed] [Google Scholar]
- [42].Engin E, Treit D, Dickson CT, Anxiolytic- and antidepressant-like properties of ketamine in behavioral and neurophysiological animal models, Neuroscience. 161 (2009) 359–369. [DOI] [PubMed] [Google Scholar]
- [43].Flood P, Krasowski MD, Intravenous Anesthetics Differentially Modulate Ligand-gated Ion Channels, Anesthesiology. 92 (2000) 1418–1425. [DOI] [PubMed] [Google Scholar]
- [44].Fox ME, Lobo MK, The molecular and cellular mechanisms of depression: a focus on reward circuitry, Mol. Psychiatry (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Franceschelli A, Sens J, Herchick S, Thelen C, Pitychoutis PM, Sex differences in the rapid and the sustained antidepressant-like effects of ketamine in stress-naïve and “depressed” mice exposed to chronic mild stress, Neuroscience. 290 (2015) 49–60. [DOI] [PubMed] [Google Scholar]
- [46].Freedman R, Brown AS, Cannon TD, Druss BG, Earls FJ, Escobar J, Hurd YL, Lewis DA, López-Jaramillo C, Luby J, Mayberg HS, Moffitt TE, Oquendo M, Perlis RH, Pine DS, Rush AJ, Tamminga CA, Tohen M, Vieta E, Wisner KL, Xin Y, Can a framework be established for the safe use of ketamine?, Am. J. Psychiatry 175 (2018) 587–589. [DOI] [PubMed] [Google Scholar]
- [47].Frenkel C, Urban BW, Molecular actions of racemic ketamine on human cns sodium channels, Br. J. Anaesth 69 (1992) 292–297. [DOI] [PubMed] [Google Scholar]
- [48].Friederich P, Urban BW, Interaction of intravenous anesthetics with human neuronal potassium currents in relation to clinical concentrations, Anesthesiology. 91 (1999) 1853–1860. [DOI] [PubMed] [Google Scholar]
- [49].Gao M, Rejaei D, Liu H, Ketamine use in current clinical practice, Acta Pharmacol. Sin 37 (2016) 865–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Garcia LS, Comim CM, Valvassori SS, Réus GZ, Andreazza AC, Stertz L, Fries GR, Gavioli EC, Kapczinski F, Quevedo J, Chronic administration of ketamine elicits antidepressant-like effects in rats without affecting hippocampal brain-derived neurotrophic factor protein levels., Basic Clin. Pharmacol. Toxicol 103 (2008) 502–6. [DOI] [PubMed] [Google Scholar]
- [51].Geoffroy H, Noble F, BDNF During Withdrawal, in: Vitam. Horm, Academic Press, 2017: pp. 475–496. [DOI] [PubMed] [Google Scholar]
- [52].George MS, Is there really nothing new under the sun? Is low-dose ketamine a fastacting antidepressant simply because it is an opioid?, Am. J. Psychiatry 175 (2018) 1157–1158. [DOI] [PubMed] [Google Scholar]
- [53].Ghasemi M, Raza M, Dehpour AR, NMDA receptor antagonists augment antidepressant-like effects of lithium in the mouse forced swimming test, J. Psychopharmacol 24 (2010) 585–594. [DOI] [PubMed] [Google Scholar]
- [54].Gideons ES, Kavalali ET, Monteggia LM, Mechanisms underlying differential effectiveness of memantine and ketamine in rapid antidepressant responses., Proc. Natl. Acad. Sci. U. S. A 111 (2014) 8649–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Gigliucci V, O’Dowd G, Casey S, Egan D, Gibney S, Harkin A, Ketamine elicits sustained antidepressant-like activity via a serotonin-dependent mechanism, Psychopharmacology (Berl). 228 (2013) 157–166. [DOI] [PubMed] [Google Scholar]
- [56].Gong L, Yin Y, He C, Ye Q, Bai F, Yuan Y, Zhang H, Lv L, Zhang H, Xie C, Zhang Z, Disrupted reward circuits is associated with cognitive deficits and depression severity in major depressive disorder, J. Psychiatr. Res 84 (2016) 9–17. [DOI] [PubMed] [Google Scholar]
- [57].Grace AA, Dysregulation of the dopamine system in the pathophysiology of schizophrenia and depression, Nat. Rev. Neurosci 17 (2016) 524–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Green SM, Johnson NE, Ketamine sedation for pediatric procedures: Part 2, review and implications, Ann. Emerg. Med 19 (1990) 1033–1046. [DOI] [PubMed] [Google Scholar]
- [59].Guirimand F, Dupont X, Brasseur L, Chauvin M, Bouhassira D, The effects of ketamine on the temporal summation (wind-up) of the R(III) nociceptive flexion reflex and pain in humans, Anesth. Analg 90 (2000) 408–414. [DOI] [PubMed] [Google Scholar]
- [60].Hare BD, Shinohara R, Liu RJ, Pothula S, DiLeone RJ, Duman RS, Optogenetic stimulation of medial prefrontal cortex Drd1 neurons produces rapid and long-lasting antidepressant effects, Nat. Commun 10 (2019) 223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Hashimoto K, Rapid-acting antidepressant ketamine, its metabolites and other candidates: A historical overview and future perspective, Psychiatry Clin. Neurosci 73 (2019) 613–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Huang X, Huang K, Zheng W, Beveridge TJR, Yang S, Li X, Li P, Zhou W, Liu Y, The effects of GSK-3β blockade on ketamine self-administration and relapse to drugseeking behavior in rats., Drug Alcohol Depend. 147 (2015) 257–65. [DOI] [PubMed] [Google Scholar]
- [63].Hurt PH, Ritchie EC, A case of ketamine dependence [1], Am. J. Psychiatry 151 (1994) 779. [DOI] [PubMed] [Google Scholar]
- [64].Idvall J, Ahlgren I, Aronsen KF, Stenberg P, Ketamine infusions: Pharmacokinetics and clinical effects, Br. J. Anaesth 51 (1979) 1167–1173. [DOI] [PubMed] [Google Scholar]
- [65].Ikonomidou C, Bosch F, Miksa M, Bittigau P, Vöckler J, Dikranian K, Tenkova TI, Stefovska V, Turski L, Olney JW, Blockade of NMDA receptors and apoptotic neurodegeneration in the developing brain., Science. 283 (1999) 70–74. [DOI] [PubMed] [Google Scholar]
- [66].Iñiguez SD, Flores-Ramirez FJ, Riggs LM, Alipio JB, Garcia-Carachure I, Hernandez MA, Sanchez DO, Lobo MK, Serrano PA, Braren SH, Castillo SA, Vicarious Social Defeat Stress Induces Depression-Related Outcomes in Female Mice, Biol. Psychiatry 83 (2018) 9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Jansen KLR, A review of the nonmedical use of ketamine: Use, users and consequences, J. Psychoactive Drugs 32 (2000) 419–433. [DOI] [PubMed] [Google Scholar]
- [68].Jett JD, Boley AM, Girotti M, Shah A, Lodge DJ, Morilak DA, Antidepressant-like cognitive and behavioral effects of acute ketamine administration associated with plasticity in the ventral hippocampus to medial prefrontal cortex pathway, Psychopharmacology (Berl). 232 (2015) 3123–3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Jiang Y, Wang Y, Sun X, Lian B, Sun H, Wang G, Du Z, Li Q, Sun L, Short- and long-term antidepressant effects of ketamine in a rat chronic unpredictable stress model, Brain Behav. 7 (2017) e00749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Jones JL, Mateus CF, Malcolm RJ, Brady KT, Back SE, Efficacy of ketamine in the treatment of substance use disorders: A systematic review, Front. Psychiatry 9 (2018) 277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Kapur S, Seeman P, NMDA receptor antagonists ketamine and PCP have direct effects on the dopamine D2 and serotonin 5-HT2 receptors - Implications for models of schizophrenia, Mol. Psychiatry 7 (2002) 837–844. [DOI] [PubMed] [Google Scholar]
- [72].Kashiwagi K, Masuko T, Nguyen CD, Kuno T, Tanaka I, Igarashi K, Williams K, Channel Blockers Acting at N-Methyl-d-aspartate Receptors: Differential Effects of Mutations in the Vestibule and Ion Channel Pore, Mol. Pharmacol 61 (2002) 533 LP – 545. [DOI] [PubMed] [Google Scholar]
- [73].Knowland D, Lim BK, Circuit-based frameworks of depressive behaviors: The role of reward circuitry and beyond, Pharmacol. Biochem. Behav 174 (2018) 42–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Kohrs R, Durieux ME, Ketamine: Teaching an old drug new tricks, Anesth. Analg 87 (1998) 1186–1193. [DOI] [PubMed] [Google Scholar]
- [75].Koike H, Iijima M, Chaki S, Involvement of AMPA receptor in both the rapid and sustained antidepressant-like effects of ketamine in animal models of depression., Behav. Brain Res 224 (2011) 107–11. [DOI] [PubMed] [Google Scholar]
- [76].Kokane SS, Hoch AC, Armant RJ, Perrotti LI, Increased phosphorylation of mTOR in the dorsal striatum and nucleus accumbens shell underlies the estradiol-induced augmentation of cocaine place preference., in: Annu. Meet. Int. Behav. Neurosci. Soc, Cairns, QLD, Australia, 2019. [Google Scholar]
- [77].Koob GF, Volkow ND, Neurobiology of addiction: a neurocircuitry analysis, The Lancet Psychiatry. 3 (2016) 760–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Korpi ER, den Hollander B, Farooq U, Vashchinkina E, Rajkumar R, Nutt DJ, Hyytiä P, Dawe GS, Mechanisms of action and persistent neuroplasticity by drugs of abuse, Pharmacol. Rev 67 (2015) 872–1004. [DOI] [PubMed] [Google Scholar]
- [79].Krupitsky EM, Burakov AM, Romanova TN, Grinenko NI, Grinenko AY, Fletcher J, Petrakis IL, Krystal JH, Attenuation of ketamine effects by nimodipine pretreatment in recovering ethanol dependent men: Psychopharmacologic implications of the interaction of NMDA and L-type calcium channel antagonists, Neuropsychopharmacology. 25 (2001) 936–947. [DOI] [PubMed] [Google Scholar]
- [80].Krystal JH, D’Souza DC, Karper LP, Bennett A, Abi-Dargham A, Abi-Saab D, Cassello K, Bowers MB, Vegso S, Heninger GR, Charney DS, Interactive effects of subanesthetic ketamine and haloperidol in healthy humans., Psychopharmacology (Berl). 145 (1999) 193–204. [DOI] [PubMed] [Google Scholar]
- [81].Kutlu MG, Gould TJ , Effects of drugs of abuse on hippocampal plasticity and hippocampus-dependent learning and memory: Contributions to development and maintenance of addiction, Learn. Mem 23 (2016) 515–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Lai R, Katalinic N, Glue P, Somogyi AA, Mitchell PB, Leyden J, Harper S, Loo CK, Pilot dose-response trial of I.V. ketamine in treatment-resistant depression, World J. Biol. Psychiatry 15 (2014) 579–584. [DOI] [PubMed] [Google Scholar]
- [83].Laje G, Lally N, Mathews D, Brutsche N, Chemerinski A, Akula N, Kelmendi B, Simen A, McMahon FJ, Sanacora G, Zarate C, Brain-derived neurotrophic factor Val66Met polymorphism and antidepressant efficacy of ketamine in depressed patients, Biol. Psychiatry 72 (2012) e27–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Lester HA, Lavis LD, Dougherty DA, Ketamine Inside Neurons?, Http://Dx.Doi.Org/10.1176/Appi.Ajp.2015.14121537. (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Li B, Piriz J, Mirrione M, Chung C, Proulx CD, Schulz D, Henn F, Malinow R, Synaptic potentiation onto habenula neurons in the learned helplessness model of depression., Nature. 470 (2011) 535–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Li F, Fang Q, Liu Y, Zhao M, Li D, Wang J, Lu L, Cannabinoid CB1 receptor antagonist rimonabant attenuates reinstatement of ketamine conditioned place preference in rats, Eur. J. Pharmacol 589 (2008) 122–126. [DOI] [PubMed] [Google Scholar]
- [87].Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, Li XY, Aghajanian G, Duman RS, mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists, Science (80-.). 329 (2010) 959–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Li X, Jope RS, Is glycogen synthase kinase-3 a central modulator in mood regulation, Neuropsychopharmacology. 35 (2010) 2143–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Li X, Wolf ME, Multiple faces of BDNF in cocaine addiction, Behav. Brain Res 279 (2015) 240–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Li Y, Zhu ZR, Ou BC, Wang YQ, Tan ZB, Deng CM, Gao YY, Tang M, So JH, Mu YL, Zhang LQ, Dopamine D2/D3 but not dopamine D1 receptors are involved in the rapid antidepressant-like effects of ketamine in the forced swim test, Behav. Brain Res 279 (2015) 100–105. [DOI] [PubMed] [Google Scholar]
- [91].Lin J-C, Lee M-Y, Chan M-H, Chen Y-C, Chen H-H, Betaine enhances antidepressant-like, but blocks psychotomimetic effects of ketamine in mice, Psychopharmacology (Berl). 233 (2016) 3223–3235. [DOI] [PubMed] [Google Scholar]
- [92].Lin LH, Chen LL, Zirrolli JA, Harris RA, General anesthetics potentiate γ-aminobutyric acid actions on γ-aminobutyric acid(A) receptors expressed by Xenopus oocytes: Lack of involvement of intracellular calcium, J. Pharmacol. Exp. Ther 263 (1992) 569–578. [PubMed] [Google Scholar]
- [93].Lindholm JSO, Autio H, Vesa L, Antila H, Lindemann L, Hoener MC, Skolnick P, Rantamäki T, Castrén E, The antidepressant-like effects of glutamatergic drugs ketamine and AMPA receptor potentiator LY 451646 are preserved in bdnf +/− heterozygous null mice, Neuropharmacology. 62 (2012) 391–397. [DOI] [PubMed] [Google Scholar]
- [94].Liu JX, Zerbo E, Ross S, Intensive ketamine use for multiple years: A case report, Am. J. Addict 24 (2015) 7–9. [DOI] [PubMed] [Google Scholar]
- [95].Liu R-J, Fuchikami M, Dwyer JM, Lepack AE, Duman RS, Aghajanian GK, GSK3 Inhibition Potentiates the Synaptogenic and Antidepressant-Like Effects of Subthreshold Doses of Ketamine, Neuropsychopharmacology. 38128 (2013) 2268–2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Lodge D, Mercier MS, Ketamine and phencyclidine: the good, the bad and the unexpected., Br. J. Pharmacol 172 (2015) 4254–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].De Luca MT, Meringolo M, Spagnolo PA, Badiani A, The role of setting for ketamine abuse: clinical and preclinical evidence, Rev. Neurosci 23 (2012) 769–780. [DOI] [PubMed] [Google Scholar]
- [98].Lutz PE, Kieffer BL, Opioid receptors: Distinct roles in mood disorders, Trends Neurosci. 36 (2013) 195–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Lv Q, Yang L, Li G, Wang Z, Shen Z, Yu W, Jiang Q, Hou B, Pu J, Hu H, Wang Z, Large-Scale Persistent Network Reconfiguration Induced by Ketamine in Anesthetized Monkeys: Relevance to Mood Disorders, Biol. Psychiatry 79 (2016) 765–775. [DOI] [PubMed] [Google Scholar]
- [100].Ma XC, Dang YH, Jia M, Ma R, Wang F, Wu J, Gao CG, Hashimoto K, LongLasting Antidepressant Action of Ketamine, but Not Glycogen Synthase Kinase-3 Inhibitor SB216763, in the Chronic Mild Stress Model of Mice, PLoS One. 8 (2013) e56053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Maciel AL, Abelaira HM, de Moura AB, de Souza TG, Rosa T, Matos D, Tuon T, Garbossa L, Strassi AP, Fileti ME, Goldim MP, Mathias K, Petronilho F, Quevedo J, Réus GZ, Acute treatment with ketamine and chronic treatment with minocycline exert antidepressant-like effects and antioxidant properties in rats subjected different stressful events, Brain Res. Bull 137 (2018) 204–216. [DOI] [PubMed] [Google Scholar]
- [102].Maeng S, Zarate CA, Du J, Schloesser RJ, McCammon J, Chen G, Manji HK, Cellular Mechanisms Underlying the Antidepressant Effects of Ketamine: Role of αAmino-3-Hydroxy-5-Methylisoxazole-4-Propionic Acid Receptors, Biol. Psychiatry 63 (2008) 349–352. [DOI] [PubMed] [Google Scholar]
- [103].Maes M, Fišar Z, Medina M, Scapagnini G, Nowak Gk, Berk M, New drug targets in depression: Inflammatory, cell-mediate immune, oxidative and nitrosative stress, mitochondrial, antioxidant, and neuroprogressive pathways. and new drug candidatesNrf2 activators and GSK-3 inhibitors, Inflammopharmacology. 20 (2012) 127–150. [DOI] [PubMed] [Google Scholar]
- [104].Marland S, Ellerton J, Andolfatto G, Strapazzon G, Thomassen O, Brandner B, Weatherall A, Paal P, Ketamine: use in anesthesia., CNS Neurosci. Ther 19 (2013) 381–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Martin DC, Introna RP, Aronstam RS, Inhibition of neuronal 5-HT uptake by ketamine, but not halothane, involves disruption of substrate recognition by the transporter, Neurosci. Lett 112 (1990) 99–103. [DOI] [PubMed] [Google Scholar]
- [106].Martin LL, Bouchal RL, Smith DJ, Ketamine inhibits serotonin uptake in vivo, Neuropharmacology. 21 (1982) 113–118. [DOI] [PubMed] [Google Scholar]
- [107].Mathew SJ, Rivas-Grajales AM, Does the Opioid System Block or Enhance the Antidepressant Effects of Ketamine?, Chronic Stress. 3 (2019) 247054701985207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Mayberg HS, Modulating dysfunctional limbic-cortical circuits in depression: Towards development of brain-based algorithms for diagnosis and optimised treatment, Br. Med. Bull 65 (2003) 193–207. [DOI] [PubMed] [Google Scholar]
- [109].Mccarthy DA, Chen G, Kaump DH, Ensor C, General Anesthetic and Other Pharmacological Properties of 2-(O-Chlorophenyl)-2-Methylamino Cyclohexanone Hcl (Ci-58L), J. New Drugs 5 (1965) 21–33. [DOI] [PubMed] [Google Scholar]
- [110].McFarland K, Kalivas PW, The circuitry mediating cocaine-induced reinstatement of drug-seeking behavior., J. Neurosci 21 (2001) 8655–8663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].McShane R, Baldwin DS, Hamish McAllister-Williams R, Stone JM, Taylor D, Winstock AR, Young AH, Esketamine and the need for a new type of registry for drugs with abuse potential, Am. J. Psychiatry 176 (2019) 966. [DOI] [PubMed] [Google Scholar]
- [112].Miller OH, Yang L, Wang CC, Hargroder EA, Zhang Y, Delpire E, Hall BJ, GluN2B-containing NMDA receptors regulate depression-like behavior and are critical for the rapid antidepressant actions of ketamine, Elife. 2014 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Mion G, Villevieille T, Ketamine Pharmacology: An Update (Pharmacodynamics and Molecular Aspects, Recent Findings), CNS Neurosci. Ther 19 (2013) 370–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Monteggia LM, Gideons E, Kavalali ET, The role of eukaryotic elongation factor 2 kinase in rapid antidepressant action of ketamine., Biol. Psychiatry 73 (2013) 1199–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Moore K, Measham F, “It’s the most fun you can have for twenty quid”: Motivations, Consequences and Meanings of British Ketamine Use, Addict. Res. Theory 16 (2008) 231–244. [Google Scholar]
- [116].Moore NN, Bostwick JM, Ketamine dependence in anesthesia providers., Psychosomatics. 40 (1999) 356–9. [DOI] [PubMed] [Google Scholar]
- [117].Morgan CJ, Rees H, V Curran H, Attentional bias to incentive stimuli in frequent ketamine users., Psychol. Med 38 (2008) 1331–1340. [DOI] [PubMed] [Google Scholar]
- [118].Morgan CJA, Curran HV, Acute and chronic effects of ketamine upon human memory: a review., Psychopharmacology (Berl). 188 (2006) 408–24. [DOI] [PubMed] [Google Scholar]
- [119].Morgan CJA, Curran HV, Ketamine use: a review, Addiction. 107 (2012) 27–38. [DOI] [PubMed] [Google Scholar]
- [120].Morgan CJA, Mofeez A, Brandner B, Bromley L, Curran HV, Ketamine impairs response inhibition and is positively reinforcing in healthy volunteers: a dose-response study., Psychopharmacology (Berl). 172 (2004) 298–308. [DOI] [PubMed] [Google Scholar]
- [121].Morgan CJA, Muetzelfeldt L, Curran HV, Consequences of chronic ketamine self-administration upon neurocognitive function and psychological wellbeing: a 1-year longitudinal study., Addiction. 105 (2010) 121–33. [DOI] [PubMed] [Google Scholar]
- [122].Murrough JW, V Iosifescu D, Chang LC, Al Jurdi RK, Green CE, Perez AM, Iqbal S, Pillemer S, Foulkes A, Shah A, Charney DS, Mathew SJ, Antidepressant efficacy of ketamine in treatment-resistant major depression: A two-site randomized controlled trial, Am. J. Psychiatry 170 (2013) 1134–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Murrough JW, Perez AM, Pillemer S, Stern J, Parides MK, aan het Rot M, Collins KA, Mathew SJ, Charney DS, Iosifescu DV, Rapid and longer-term antidepressant effects of repeated ketamine infusions in treatment-resistant major depression., Biol. Psychiatry 74 (2013) 250–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Nagels A, Kirner-Veselinovic A, Wiese R, Paulus FM, Kircher T, Krach S, Effects of ketamine-induced psychopathological symptoms on continuous overt rhyme fluency, Eur. Arch. Psychiatry Clin. Neurosci 262 (2012) 403–414. [DOI] [PubMed] [Google Scholar]
- [125].Naranjo CA, Tremblay LK, Busto UE, The role of the brain reward system in depression, Prog. Neuro-Psychopharmacology Biol. Psychiatry 25 (2001) 781–823. [DOI] [PubMed] [Google Scholar]
- [126].Narendran R, Frankle WG, Keefe R, Gil R, Martinez D, Slifstein M, Kegeles LS, Talbot PS, Huang Y, Hwang D-R, Khenissi L, Cooper TB, Laruelle M, AbiDargham A, Altered prefrontal dopaminergic function in chronic recreational ketamine users., Am. J. Psychiatry 162 (2005) 2352–9. [DOI] [PubMed] [Google Scholar]
- [127].Neasta J, Barak S, Ben Hamida S, Ron D, MTOR complex 1: A key player in neuroadaptations induced by drugs of abuse, J. Neurochem 130 (2014) 172–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Nemeroff CB, Ketamine: Quo vadis?, Am. J. Psychiatry 175 (2018) 297–299. [DOI] [PubMed] [Google Scholar]
- [129].Nestler EJ, Carlezon WA, The Mesolimbic Dopamine Reward Circuit in Depression, Biol. Psychiatry 59 (2006) 1151–1159. [DOI] [PubMed] [Google Scholar]
- [130].Newman EL, Covington HE, Suh J, Bicakci MB, Ressler KJ, DeBold JF, Miczek KA, Fighting Females: Neural and Behavioral Consequences of Social Defeat Stress in Female Mice, Biol. Psychiatry 86 (2019) 657–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Newport DJ, Schatzberg AF, Nemeroff CB, WHITHER KETAMINE AS AN ANTIDEPRESSANT: PANACEA OR TOXIN?, Depress. Anxiety 33 (2016) 685–688. [DOI] [PubMed] [Google Scholar]
- [132].Noble F, Marie N, Management of opioid addiction with opioid substitution treatments: Beyond methadone and buprenorphine, Front. Psychiatry 10 (2019) 742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Noppers I, Niesters M, Swartjes M, Bauer M, Aarts L, Geleijnse N, Mooren R, Dahan A, Sarton E, Absence of long-term analgesic effect from a short-term S-ketamine infusion on fibromyalgia pain: A randomized, prospective, double blind, active placebocontrolled trial, Eur. J. Pain 15 (2011) 942–949. [DOI] [PubMed] [Google Scholar]
- [134].Olney JW, Newcomer JW, Farber NB, NMDA receptor hypofunction model of schizophrenia, J. Psychiatr. Res 33 (1999) 523–533. [DOI] [PubMed] [Google Scholar]
- [135].Pal HR, Berry N, Kumar R, Ray R, Ketamine dependence., Anaesth. Intensive Care 30 (2002) 382–4. [DOI] [PubMed] [Google Scholar]
- [136].Parise EM, Alcantara LF, Warren BL, Wright KN, Hadad R, Sial OK, Kroeck KG, Iñiguez SD, Bolaños-Guzmán CA, Repeated ketamine exposure induces an enduring resilient phenotype in adolescent and adult rats., Biol. Psychiatry 74 (2013) 750–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Park GR, Manara AR, Mendel L, Bateman PE, Ketamine infusion: Its use as a sedative, inotrope and bronchodilator in a critically ill patient, Anaesthesia. 42 (1987) 980–983. [DOI] [PubMed] [Google Scholar]
- [138].Pham TH, Gardier AM, Fast-acting antidepressant activity of ketamine: highlights on brain serotonin, glutamate, and GABA neurotransmission in preclinical studies, Pharmacol. Ther 199 (2019) 58–90. [DOI] [PubMed] [Google Scholar]
- [139].Polis AJ, Fitzgerald PJ, Hale PJ, Watson BO, Rodent ketamine depression-related research: Finding patterns in a literature of variability, Behav. Brain Res 376 (2019) 112153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Polter AM, Kauer JA, Stress and VTA synapses: Implications for addiction and depression, Eur. J. Neurosci 39 (2014) 1179–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Popik P, Hołuj M, Kos T, Nowak G, Librowski T, Sałat K, Comparison of the Psychopharmacological Effects of Tiletamine and Ketamine in Rodents, Neurotox. Res 32 (2017) 544–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Proescholdt M, Heimann A, Kempski O, Neuroprotection of S(+) ketamine isomer in global forebrain ischemia, Brain Res. 904 (2001) 245–251. [DOI] [PubMed] [Google Scholar]
- [143].Rafael DLT, Ketamine abuse: First medical evidence of harms we should confront, Addiction. 105 (2010) 134–135. [DOI] [PubMed] [Google Scholar]
- [144].Rasmussen KG, Has psychiatry tamed the “ketamine tiger?” Considerations on its use for depression and anxiety, Prog. Neuro-Psychopharmacology Biol. Psychiatry 64 (2016) 218–224. [DOI] [PubMed] [Google Scholar]
- [145].Réus GZ, Stringari RB, Ribeiro KF, Ferraro AK, Vitto MF, Cesconetto P, Souza CT, Quevedo J, Ketamine plus imipramine treatment induces antidepressant-like behavior and increases CREB and BDNF protein levels and PKA and PKC phosphorylation in rat brain, Behav. Brain Res 221 (2011) 166–171. [DOI] [PubMed] [Google Scholar]
- [146].Russo SJ, Nestler EJ, The brain reward circuitry in mood disorders, Nat Rev Neurosci. 14 (2013) 609–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].SAMHSA, No Title, Rockville, MD, 2008. [Google Scholar]
- [148].Sanacora G, Schatzberg AF, Ketamine: Promising Path or False Prophecy in the Development of Novel Therapeutics for Mood Disorders?, Neuropsychopharmacology. 40 (2015) 259–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Sarkar A, Kabbaj M, Sex Differences in Effects of Ketamine on Behavior, Spine Density, and Synaptic Proteins in Socially Isolated Rats, Biol. Psychiatry 80 (2016) 448–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Schak KM, Vande Voort JL, Johnson EK, Kung S, Leung JG, Rasmussen KG, Palmer BA, Frye MA, Potential Risks of Poorly Monitored Ketamine Use in Depression Treatment, Am. J. Psychiatry 173 (2016) 215–218. [DOI] [PubMed] [Google Scholar]
- [151].Schatzberg AF, A Word to the Wise About Intranasal Esketamine, Am. J. Psychiatry 176 (2019) 422–424. [DOI] [PubMed] [Google Scholar]
- [152].Schifano F, Corkery J, Oyefeso A, Tonia T, Ghodse AH, Trapped in the “K-hole”: Overview of deaths associated with ketamine misuse in the UK (1993–2006), J. Clin. Psychopharmacol 28 (2008) 114–116. [DOI] [PubMed] [Google Scholar]
- [153].Schoepfer KJ, Strong CE, Saland SK, Wright KN, Kabbaj M, Sex- and dose-dependent abuse liability of repeated subanesthetic ketamine in rats, Physiol. Behav 203 (2017) 60–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Segal A, Sisti D, Research Moratoria and Off-Label Use of Ketamine, Am. J. Bioeth 16 (2016) 60–61. [DOI] [PubMed] [Google Scholar]
- [155].Shimoyama M, Shimoyama N, Gorman AL, Elliott KJ, Inturrisi CE, Oral ketamine is antinociceptive in the rat formalin test: Role of the metabolite, norketamine, Pain. 81 (1999) 85–93. [DOI] [PubMed] [Google Scholar]
- [156].Siegel RK, Phencyclidine and ketamine intoxication: a study of four populations of recreational users., NIDA Res. Monogr (1978) 119–147. [PubMed] [Google Scholar]
- [157].Singh I, Morgan C, Curran V, Nutt D, Schlag A, McShane R, Ketamine treatment for depression: opportunities for clinical innovation and ethical foresight, The Lancet Psychiatry. 4 (2017) 419–426. [DOI] [PubMed] [Google Scholar]
- [158].Sisti D, Segal AG, Thase ME, Proceed with Caution: Off-label Ketamine Treatment for Major Depressive Disorder, Curr. Psychiatry Rep 16 (2014) 527. [DOI] [PubMed] [Google Scholar]
- [159].Strong CE, Schoepfer KJ, Dossat AM, Saland SK, Wright KN, Kabbaj M, Locomotor sensitization to intermittent ketamine administration is associated with nucleus accumbens plasticity in male and female rats, Neuropharmacology. 121 (2017) 195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Sun H-L, Zhou Z-Q, Zhang G-F, Yang C, Wang X-M, Shen J-C, Hashimoto K, Yang JJ, Role of hippocampal p11 in the sustained antidepressant effect of ketamine in the chronic unpredictable mild stress model, Transl. Psychiatry 6 (2016) e741–e741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Suzuki T, Kato H, Aoki T, Tsuda M, Narita M, Misawa M, Effects of the non-competitive NMDA receptor antagonist ketamine on morphine-induced place preference in mice., Life Sci. 67 (2000) 383–9. [DOI] [PubMed] [Google Scholar]
- [162].Talbot J, Phillips JL, Blier P, Ketamine for chronic depression: Two cautionary tales, J. Psychiatry Neurosci 44 (2019) 384–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Thelen C, Sens J, Mauch J, Pandit R, Pitychoutis PM, Repeated ketamine treatment induces sex-specific behavioral and neurochemical effects in mice, Behav. Brain Res 312 (2016) 305–312. [DOI] [PubMed] [Google Scholar]
- [164].Tizabi Y, Bhatti BH, Manaye KF, Das JR, Akinfiresoye L, Antidepressant-like effects of low ketamine dose is associated with increased hippocampal AMPA/NMDA receptor density ratio in female Wistar–Kyoto rats, Neuroscience. 213 (2012) 72–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Toro-Matos A, Rendon-Platas AM, Avila-Valdez E, Villarreal-Guzman RA, Physostigmine antagonizes ketamine, Anesth. Analg 59 (1980) 764–767. [PubMed] [Google Scholar]
- [166].Trujillo KA, Zamora JJ, Warmoth KP, Increased response to ketamine following treatment at long intervals: implications for intermittent use., Biol. Psychiatry 63 (2008) 178–83. [DOI] [PubMed] [Google Scholar]
- [167].Trullas R, Skolnick P, Functional antagonists at the NMDA receptor complex exhibit antidepressant actions, Eur. J. Pharmacol 185 (1990) 1–10. [DOI] [PubMed] [Google Scholar]
- [168].Turner EH, Esketamine for treatment-resistant depression: seven concerns about efficacy and FDA approval, The Lancet Psychiatry. 6 (2019) 977–979. [DOI] [PubMed] [Google Scholar]
- [169].Valentino RJ, Volkow ND, Untangling the complexity of opioid receptor function, Neuropsychopharmacology. 43 (2018) 2514–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Vargas-Perez H, Bahi A, Bufalino MR, Ting-A-Kee R, Maal-Bared G, Lam J, Fahmy A, Clarke L, Blanchard JK, Larsen BR, Steffensen S, Dreyer JL, van der Kooy D, BDNF signaling in the VTA links the drug-dependent state to drug withdrawal aversions, J. Neurosci 34 (2014) 7899–7909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Volkow ND, Morales M, The Brain on Drugs: From Reward to Addiction, Cell. 162 (2015) 712–725. [DOI] [PubMed] [Google Scholar]
- [172].Wakasugi M, Hirota K, Roth SH, Ito Y, The effects of general anesthetics on excitatory and inhibitory synaptic transmission in area CA1 of the rat hippocampus in vitro, Anesth. Analg 88 (1999) 676–680. [DOI] [PubMed] [Google Scholar]
- [173].Wang C, Zheng D, Xu J, Lam W, Yew DDT, Brain damages in ketamine addicts as revealed by magnetic resonance imaging, Front. Neuroanat 7 (2013) 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Wang DS, Penna A, Orser BA, Ketamine Increases the Function of γ-Aminobutyric Acid Type A Receptors in Hippocampal and Cortical Neurons, Anesthesiology. 126 (2017) 666–677. [DOI] [PubMed] [Google Scholar]
- [175].Wang X, Yang Y, Zhou X, Wu J, Li J, Jiang X, Qu Q, Ou C, Liu L, Zhou S, Propofol pretreatment increases antidepressant-like effects induced by acute administration of ketamine in rats receiving forced swimming test, Psychiatry Res. 185 (2011) 248–253. [DOI] [PubMed] [Google Scholar]
- [176].Wang XD, Yang G, Bai Y, Feng YP, Li H, The behavioral study on the interactive aggravation between pruritus and depression, Brain Behav. 8 (2018) e00964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].White PF, Way WL, anthony J Trevor, Ketamine - Its pharmacology and therapeutic uses, Anesthesiology. 56 (1982) 119–136. [DOI] [PubMed] [Google Scholar]
- [178].Whitton AE, Treadway MT, Pizzagalli DA, Reward processing dysfunction in major depression, bipolar disorder and schizophrenia., Curr. Opin. Psychiatry 28 (2015) 7–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Wiley JL, Evans RL, Grainger DB, Nicholson KL, Age-dependent differences in sensitivity and sensitization to cannabinoids and “club drugs” in male adolescent and adult rats, Addict. Biol 13 (2008) 277–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].Wiley JL, Evans RL, Grainger DB, Nicholson KL, Locomotor activity changes in female adolescent and adult rats during repeated treatment with a cannabinoid or club drug, Pharmacol. Reports 63 (2011) 1085–1092. [DOI] [PubMed] [Google Scholar]
- [181].Wilkinson ST, Howard DH, Busch SH, Psychiatric Practice Patterns and Barriers to the Adoption of Esketamine, JAMA. 322 (2019) 1039. [DOI] [PubMed] [Google Scholar]
- [182].Williams NR, Heifets BD, Bentzley BS, Blasey C, Sudheimer KD, Hawkins J, Lyons DM, Schatzberg AF, Attenuation of antidepressant and antisuicidal effects of ketamine by opioid receptor antagonism, Mol. Psychiatry 24 (2019) 1779–1786. [DOI] [PubMed] [Google Scholar]
- [183].Williams NR, Heifets BD, Blasey C, Sudheimer K, Pannu J, Pankow H, Hawkins J, Birnbaum J, Lyons DM, Rodriguez CI, Schatzberg AF, Attenuation of antidepressant effects of ketamine by opioid receptor antagonism, Am. J. Psychiatry 175 (2018) 1205–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Winger G, Hursh SR, Casey KL, Woods JH, Relative reinforcing strength of three N-methyl-D-aspartate antagonists with different onsets of action., J. Pharmacol. Exp. Ther 301 (2002) 690–7. [DOI] [PubMed] [Google Scholar]
- [185].Wise RA, Neurobiology of addiction, Curr. Opin. Neurobiol 6 (1996) 243–251. [DOI] [PubMed] [Google Scholar]
- [186].De Witte P, Littleton J, Parot P, Koob G, Neuroprotective and abstinence-promoting effects of acamprosate: Elucidating the mechanism of action, CNS Drugs. 19 (2005) 517–537. [DOI] [PubMed] [Google Scholar]
- [187].Wood DM, Nicolaou M, Dargan PI, Epidemiology of recreational drug toxicity in a nightclub environment., Subst. Use Misuse 44 (2009) 1495–502. [DOI] [PubMed] [Google Scholar]
- [188].Wright KN, Strong CE, Addonizio MN, Brownstein NC, Kabbaj M, Reinforcing properties of an intermittent, low dose of ketamine in rats: effects of sex and cycle, Psychopharmacology (Berl). 234 (2017) 393–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [189].Xu K, Lipsky RH, Repeated ketamine administration alters N-methyl-D-aspartic acid receptor subunit gene expression: implication of genetic vulnerability for ketamine abuse and ketamine psychosis in humans., Exp. Biol. Med. (Maywood) 240 (2015) 145–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [190].Yan J, Jiang H, Dual effects of ketamine: Neurotoxicity versus neuroprotection in anesthesia for the developing brain, J. Neurosurg. Anesthesiol 26 (2014) 155–160. [DOI] [PubMed] [Google Scholar]
- [191].Yang C, Hu Y-M, Zhou Z-Q, Zhang G-F, Yang J-J, Acute administration of ketamine in rats increases hippocampal BDNF and mTOR levels during forced swimming test., Ups. J. Med. Sci 118 (2013) 3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [192].Yang Y, Cui Y, Sang K, Dong Y, Ni Z, Ma S, Hu H, Ketamine blocks bursting in the lateral habenula to rapidly relieve depression, Nature. 554 (2018) 317–322. [DOI] [PubMed] [Google Scholar]
- [193].Yilmaz A, Schulz D, Aksoy A, Canbeyli R, Prolonged effect of an anesthetic dose of ketamine on behavioral despair, Pharmacol. Biochem. Behav 71 (2002) 341–344. [DOI] [PubMed] [Google Scholar]
- [194].Zanos P, Gould TD, Mechanisms of ketamine action as an antidepressant, Mol. Psychiatry 23 (2018) 801–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [195].Zanos P, Piantadosi SC, Wu H-Q, Pribut HJ, Dell MJ, Can A, Snodgrass HR, Zarate CA, Schwarcz R, Gould TD, The Prodrug 4-Chlorokynurenine Causes Ketamine-Like Antidepressant Effects, but Not Side Effects, by NMDA/GlycineB-Site Inhibition, J. Pharmacol. Exp. Ther 355 (2015) 76–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [196].Zarate CA, Singh J, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, Charney DS, Manji H, A Randomized Trial of an N-methyl-D-aspartate Antagonist in Treatment-Resistant Major Depression, Arch. Gen. Psychiatry 63 (2006) 856–864. [DOI] [PubMed] [Google Scholar]
- [197].Zhang K, Dong C, Fujita Y, Fujita A, Hashimoto K, 5-Hydroxytryptamine-Independent antidepressant actions of (R)-Ketamine in a chronic social defeat stress model, Int. J. Neuropsychopharmacol 21 (2018) 157–163. [DOI] [PMC free article] [PubMed] [Google Scholar]