ABSTRACT
Background
Retinal neovascularization, which is characterized by the increased proliferation, migration, and tube formation of retinal microvascular endothelial cells (RMECs), contributes to the progression of diabetic retinopathy (DR). MiR-409-5p has been reported to be upregulated in peripheral blood of DR patients and in vascular endothelial growth factor (VEGF)-induced RMECs. However, the role of miR-409-5p in retinal neovascularization of DR remains unelucidated.
Method
The expression of miR-409-5p was measured in retinal tissues of streptozocin-induced and db/db diabetic mice, in high glucose-induced mouse RMECs (mRMECs), and in vitreous fluid of proliferative DR patients. Antagomir of miR-409-5p was intravitreally injected into diabetic mice. Proliferation, migration, and tube formation were detected using cell counting kit-8 assay, transwell assay, and microscope observation, respectively. Luciferase reporter assay was used to detect the direct interaction between miR-409-5p and peroxisome proliferator-activated receptor-α (PPARα).
Result
MiR-409-5p was upregulated in retinal tissues of diabetic mice, in high glucose-induced mRMECs, and in vitreous fluid of proliferative DR patients. The knockdown of miR-409-5p attenuated retinal neovascularization in vivo. The overexpression of miR-409-5p promotes the proliferation, migration, and tube formation, and increased VEGF expression and secretion, while the knockdown of miR-409-5p suppressed the VEGF-induced retinal neovascularization in vitro. PPARα is a downstream target of miR-409-5p, and PPARα overexpression negated the promotion of miR-409-5p overexpression on the proliferation, migration, and tube formation of mRMECs.
Conclusion
Our findings demonstrated that miR-409-5p acted as a neovasculogenic factor in DR, and anti-miR-409-5p therapy may provide a novel strategy in treating DR.
KEYWORDS: Retinal neovascularization, miR-409-5p, PPARα, migration, tube formation
Introduction
Diabetic retinopathy (DR) is one of the microvascular complications of both type 1 and type 2 diabetes mellitus (DM). With the rising incidence of DM, DR is becoming more and more common and threatens patients’ sight. The pathologic basis of DR is complicated, involving retinal inflammation, elevated retinal vascular permeability, and breakdown of blood-retinal barrier [1]. These processes cause the uncontrolled proliferation and migration of retinal microvascular endothelial cells (RMECs) and the formation of capillary tubes, which finally results in the retinal neovascularization [2]. In the proliferative DR, retinal neovascularization is the main cause of retinal detachment, vitreous cavity bleeding, and vision loss [3]. Therefore, elucidating the mechanisms underlying retinal neovascularization may provide an ideal therapeutic strategy for DR. Vascular endothelial growth factor (VEGF) is a well-defined neovasculogenic factor in DR, and the medications targeting VEGF, such as ranibizumab and aflibercept, have achieved good effects [4]. However, the existence of other pro-inflammatory and pro-fibrotic factors, including tumor necrosis factor-α (TNF-α), monocyte chemotactic protein 1 (MCP-1), and intercellular adhesion molecule-1 (ICAM-1), contributes to the treatment failure of anti-VEGF therapy [5]. Thus, more molecular mechanisms are still needed to be explored behind the retinal neovascularization in DR.
MicroRNAs (miRNAs) are a subtype of non-coding RNAs that do not encode proteins. MiRNAs are small as they consist of about 18–25 nucleotides [6]. However, their “big” regulatory functions have been identified in a variety of diseases, including cancers, nervous system diseases, cardiovascular diseases, and so on [7]. By binding with the 3ʹ translational region (3ʹUTR) of the targeted mRNA, miRNAs promote the degradation or inhibit the translation of the mRNA, thus suppressing the gene expression [8]. For example, miR-21 aggravated DR via binding with the 3ʹUTR of peroxisome proliferator-activated receptor-α (PPARα) and suppressing its translation [9]. MiR-409-5p was reported to regulate epithelial-to-mesenchymal transition and cell invasion in gastric cancers and prostate cancers [10,11]. Recently, a study revealed that miR-409-5p was highly expressed in peripheral blood of the type 1 DM patients accompanied by DR [12]. Meanwhile, miR-409-5p was upregulated in the VEGF-induced human RMECs [13]. These data indicated that miR-409-5p may be involved in retinal neovascularization of DR, although the specific mechanisms remain unclear.
In the current study, we investigated the expression of miR-409-5p in diabetic retinal tissues, in a high concentration of glucose-induced mouse retinal microvascular endothelial cells (mRMECs), and in vitreous fluid of proliferative DR patients. We also explored the effect of miR-409-5p on retinal neovascularization both in vivo and in vitro, aiming to bring a novel insight into the regulation pattern of miR-409-5p on DR.
Methods
Clinical samples
A total of 46 patients who were admitted to Weihai Municipal Hospital from January of 2019 to January of 2020 were subjected to this study. Patients who were diagnosed with proliferative DR (n = 26) were assigned to the PDR group. Patients who were diagnosed with retinal detachment without proliferative DR (n = 20) were assigned to the Control group. Vitreous fluid was collected during the vitrectomy.
Animals
The 8-wk-old male C57BL/6 J mice, the male type 2 DM mice (db/db mice), and the control mice (db/m mice) were bought from the Shanghai Lab Animal Research Center (Shanghai, China). All the mice were kept in a 12 h light/dark cycle with free access to food and water. The average weight of mice was 23 ± 2.34 g. Care, use, and treatment of the animals were in strict agreement with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. Eighteen db/db mice and 18 db/m mice were sacrificed at the age of 2, 4, and 6 months, respectively (n = 6 in each group), and retinal tissues were collected.
Induction of diabetes in mice
Diabetes was induced in C57BL/6 J mice by the 5-d intraperitoneal injection of Streptozocin (STZ) at a dose of 50 mg/kg in 10 mM citrate buffer (pH 4.5) [14]. Blood glucose levels were detected after the induction. The mice whose blood glucose level was more than 250 mg/dL 2 wk after the final injection were considered the successful induction of diabetes. The control mice were injected with the same volume of saline. Eighteen STZ-induced mice and 18 control mice were sacrificed at 2, 4, and 6 months, respectively, after the induction (n = 6 in each group), and retinal tissues were collected.
mRMEC isolation and high glucose induction
mRMECs were isolated from healthy C57BL/6 J mice as previously described [15]. The cells in the third and fifth generations were included in this study. mRMECs were treated with the normal concentration of glucose (5 mM, the NG group), the mannitol (25 mM), or the high concentration of glucose (25 mM, the HG group). Cells were collected at 0, 24, 48, and 72 h after the treatment for the detection of the miR-409-5p expression.
Quantitative real-time PCR (qRT-PCR)
Total RNA was extracted from retinal tissues or cells using the TRIzol Reagent (Invitrogen, Waltham, MA, USA). The SYBR Green PCR kit (Takara, Dalian, China) was used to perform qRT-PCR. U6 was used as the internal control for the miR-409-5p quantification. GAPDH was used as the internal control for the mRNA quantification. The relative expression was calculated by 2−ΔΔCt. The primers used in this study are shown in Table 1.
Table 1.
The qRT-PCR primers used in this study.
| RNA names* | Forward or reverse | Sequences |
|---|---|---|
| Mouse | ||
| miR-409-5p | Forward | 5′-AGGTTACCCGAGCAACTTTG-3′ |
| Reverse | 5′-GTGTCGTGGAGTCGGCAA-3′ | |
| PPARα mRNA | Forward | 5′-AACGGCGTCGAAGACAAAGA-3′ |
| Reverse | 5′-GAACTCGCGTGTGATAAAGCC-3′ | |
| U6 | Forward | 5′-GCTTCGGCAGCACATATACTAAAAAT-3′ |
| Reverse | 5′-CGCTTCACGAATTTGCGTGTCAT-3′ | |
| GAPDH | Forward | 5′-AACTTTGGCATTGTGGAAGG-3′ |
| Reverse | 5′-GGATGCAGGGATGATGTTCT-3′ | |
| Human | ||
| miR-409-5p | Forward | 5′-TCCTTGGAGAGTGTGAGGCT-3′ |
| Reverse | 5′-GGCTAGTGGACCAGGTGAAG-3′ | |
| U6 | Forward | 5′-CTCGCTTCGGCAGCACA-3′ |
| Reverse | 5′-AACGCTTCACGAATTTGCGT-3′ |
*GAPDH was used as the internal control of PPARα mRNA; U6 was used as the internal control of miR-409-5p.
Intravitreal injection of miR-409-5p interfering vectors
The miR-409-5p interfering vector (miR-409-5p antagomir, anta-miR-409-5p) and its negative control vector (anta-NC) were constructed by the Genechem Corporation (Shanghai, China). Sixteen weeks after the STZ induction, mice were intravitreally injected with anta-miR-409-5p or anta-NC at a concentration of 25 μM (n = 6 in each group). Mice were sacrificed at 4 wk after the antagomir injection.
Observation of acellular capillaries
The periodic acid-Schiff (PAS) and hematoxylin staining were used to observe the acellular capillaries as previously described [16]. In brief, mouse eyes were fixed in 10% formalin to dissect out the retinas. Then, the retinas were subjected to 3% trypsin digestion at 37°C for 2 h. After removing the nonvascular retinal mass under a dissecting microscope, the vascular retinal mass was mounted on a silane-coated slide. The 0.5% periodic acid (Sigma-Aldrich, St Louis, MO, USA) was added for 10 min. Then, the slide was rinsed in dH2O, and exposed to Schiff’s reagent (Thermo Fisher Scientific, Waltham, MA, USA) for 45 min. The slide was stained with hematoxylin for 20 min after another dH2O rinse, and it was rinsed again with dH2O. After the dehydration with an ethanol gradient, the slide was cleared with xylene and mounted in mounting medium (Thermo Fisher Scientific, Waltham, MA, USA), followed by the observation under a microscope. Acellular capillaries are capillaries that lost both endothelial cells and pericytes, and appeared as a “thread-like” structure.
ELISA for VEGF, TNF-α, ICAM-1, and MCP-1
The retinal tissues were homogenized and centrifuged before the detection. The ELISA assay was used to detect the levels of VEGF, TNF-α, ICAM-1, and MCP-1 (Abcam, Cambridge, UK) in retinal tissues and supernatants of mRMECs according to the manufacturer’s instructions.
Cell transfection
The miR-409-5p mimic and its negative control (mimic-NC), the miR-409-5p inhibitor and its negative control (inhibitor-NC), the PPARα overexpressing plasmid (pcDNA-PPARα) and its negative control (pcDNA-NC) were constructed by the Genechem Corporation (Shanghai, China). The transfection of mRMECs was done by using lipofectamine 2000TM (Thermo Fisher Scientific, Waltham, MA, USA) for 48 h according to the manufacturer’s instructions. After the transfection, the mRMECs were treated with VEGF antibody (100 μg/mL) or recombinant VEGF (20 ng/mL) for 24 h. The control group was treated with the same volume of PBS.
Cell proliferation and migration
Cell proliferation of mRMECs was detected using the cell counting kit-8 (CCK-8) assay according to the manufacturer’s instructions. Cell migration of mRMECs was detected using the transwell migration assay. Briefly, the mRMECs were seeded to 1 × 105 cells in the upper chamber with a noncoated membrane which has 8-μm pores (Millipore, Temecula, CA, USA). The 20% FBS was added to the lower chamber. After 24 h, cells in the upper chamber were discarded, and the number of migration cells in the lower chamber were counted by a microscope.
Retinal endothelial cell tube formation
The mRMECs were planted in the 12-well plates and were incubated with 50 mL ice-cold Matrigel at 37°C for 30 min to solidify. The tube formation was examined at 6 h as previously described [14].
Western blot analysis
The Western blot analysis was performed as previously described [17]. The primary antibody used in this study included anti-VEGF (ab32152, 1/1000, Abcam, Cambridge, UK), anti-PPARα (ab24509, 6 µg/ml, Abcam), and anti-β-actin (ab8226, 1/500, Abcam). The secondary antibody was the horseradish peroxidase-conjugated anti-rabbit IgG (ab205718, 1/2000, Abcam).
Luciferase reporter assay
The direct interaction between miR-409-5p and PPARα was confirmed by using the luciferase reporter assay. According to the predicted binding sites in the 3ʹUTR of PPARα mRNA, the luciferase reporter vector was mutated (MUT) from GGUAACC into AACGGUU. The mutant or the wild-type (WT) sequences were cloned into a Dual-luciferase pmirGLO plasmid (Promega, Madison, WI, USA) by using the QuickChange site-directed mutagenesis system (Stratagene, La Jolla, CA, USA). The HEK293 T cells were transfected with the wild type (WT) or MUT vectors together with miR-409-5p mimic. The relative luciferase activity was detected after 48 h using the Dual-Glo Luciferase Assay System (Promega, Madison, WI, USA).
Statistical analysis
All the values in the results were shown as mean ± standard deviation (SD). Statistical analyses between two groups were performed using the Student’s t-test. Statistical analyses among multiple groups were performed using one‐way analysis of variance (one‐way ANOVA) followed by the LSD post hoc test. A P value less than 0.05 was considered statistically significant.
Results
miR-409-5p is upregulated in diabetic retinal tissues and HG-induced mRMECs
As the dysregulation of miR-409-5p was reported in peripheral blood of DR patients [12], the expression of miR-409-5p was compared in diabetic retinal tissues of mice and HG-induced mRMECs. Consistent with the previous study, miR-409-5p was markedly increased in retinal tissues of STZ-induced DM mice at 2, 4, and 6 months after the induction (Figure 1(a)) and db/db mice at the age of 2, 4, and 6 months (Figure 1(b)) in comparison with the control mice. Then, we investigated whether the expression of miR-409-5p was reduced in vitro. The result showed that the HG treatment significantly raised the expression of miR-409-5p at 24, 48, and 72 h (Figure 1(c)). In addition, the expression of miR-409-5p in vitreous fluid of proliferative DR patients was higher than that of control patients (Figure 1(d)). These data indicated that miR-409-5p may play an important role in DR; therefore, we then investigated the function of miR-409-5p knockdown in vivo.
Figure 1.
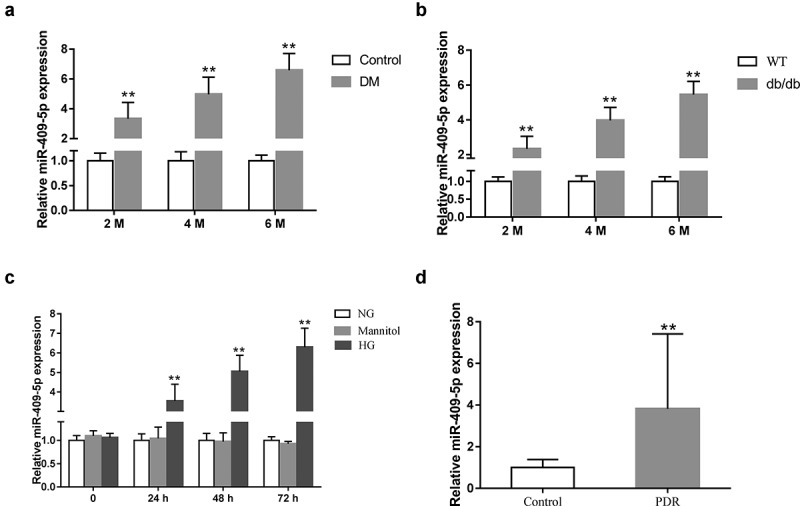
Expression of miR-409-5p in diabetic retinal tissues and HG-induced mRMECs. (a) C57BL/6 J mice were intraperitoneally injected with Streptozocin (STZ) at a dose of 50 mg/kg for 5 d. The expression of miR-409-5p was detected in retinal tissues of mice at 2, 4, and 6 months after STZ induction (n = 6 in each group). (b) The expression of miR-409-5p was detected in retinal tissues of db/db mice at the age of 2, 4, and 6 months (n = 6 in each group). The wild-type (WT) db/m mice were used as the control. (c) mRMECs were treated with a high concentration of glucose (HG, 25 mM). The normal concentration of glucose (NG, 5 mM) and mannitol (25 mM) was used as the control. The expression of miR-409-5p was detected at 0, 24, 48, and 72 h after the treatment. (d) The expression of miR-409-5p in vitreous fluid of proliferative DR patients (the PDR group, n = 26) and retinal detachment patients (the control group, n = 20). **p < 0.01 vs Control, WT, NG, or Mannitol.
miR-409-5p knockdown attenuates retinal neovascularization
Given that miR-409-5p was upregulated in diabetic retinal tissues and HG-induced mRMECs, we then investigated the effect of miR-409-5p knockdown on retinal neovascularization in vivo. After the intravitreal injection of miR-409-5p knockdown vectors (anta-miR-409-5p), mouse retinal tissues were collected, and the result of qRT-PCR confirmed the downregulation of miR-409-5p in retinal tissues after the injection (Figure 2(a)). The miR-409-5p knockdown reduced the number of acellular capillaries per mm2 compared with the control group (Figure 2(b)). Meanwhile, ELISA showed that the retinal levels of neovasculogenic factors, including VEGF, TNF-α, ICAM-1, and MCP-1, were markedly reduced by miR-409-5p (Figure 2(c-f)). All these findings indicated the anti-neovasculogenic effect of miR-409-5p knockdown on diabetic retina in vivo.
Figure 2.
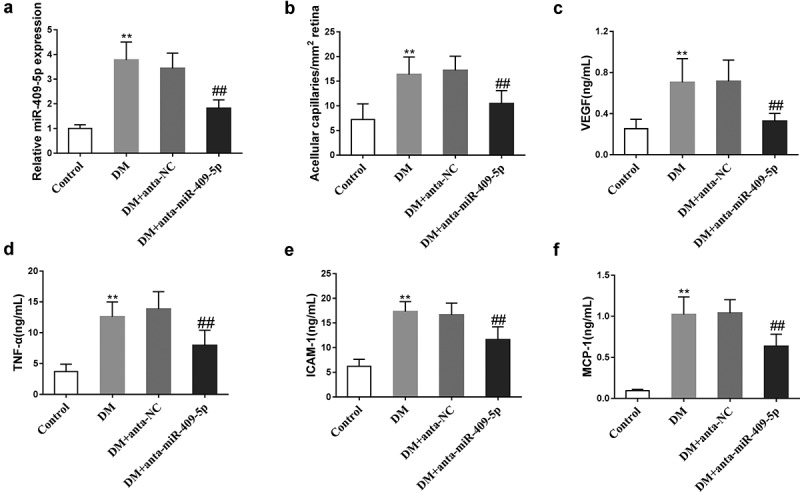
Effect of miR-409-5p knockdown on retinal neovascularization in vivo. C57BL/6 J mice were intraperitoneally injected with Streptozocin (STZ) at a dose of 50 mg/kg for 5 d. Sixteen weeks later, they were intravitreally injected with miR-409-5p knockdown vectors (anta-miR-409-5p) or negative control vectors (anta-NC) at a concentration of 25 μM. There were six mice in each group. Retinal tissues were collected at 4 wk after the intravitreal injection. (a) The expression of miR-409-5p was detected using qRT-PCR. (b) The number of acellular capillaries per mm2 was calculated. The retinal VEGF level was detected using immunohistochemistry. The retinal levels of neovasculogenic factors, including VEGF (c), TNF-α (d), ICAM-1 (e), and MCP-1 (f), were detected using ELISA. **p < 0.01 vs Control. ##p < 0.01 vs DM + anta-NC. Three samples in each bar graph.
Overexpression of miR-409-5p promotes neovascularization and increases VEGF expression and secretion
The effect of miR-409-5p on mRMEC migration and tube formation was investigated after overexpressing miR-409-5p (Figure 3(a)). The overexpression of miR-409-5p markedly increased mRMEC viability (Figure 3(b)) and the percentage of migrated mRMECs (Figure 3(c)). The tube formation was also promoted by miR-409-5p overexpression (Figure 3(d)). Meanwhile, the expression of VEGF in mRMECs, as well as in supernatants of culturing medium, was increased by miR-409-5p overexpression (Figure 3(e,f)). These data suggested that the overexpression of miR-409-5p promoted mRMEC migration and tube formation, and also increased the expression and release of VEGF. To further investigate the relationship between miR-409-5p and VEGF, mRMECs were treated with VEGF antibody after miR-409-5p mimic transfection. The inhibition of VEGF significantly reduced mRMEC viability (Figure 3(g)) and migration (Figure 3(h)), as well as reduced the tube formation (Figure 3(i)).
Figure 3.
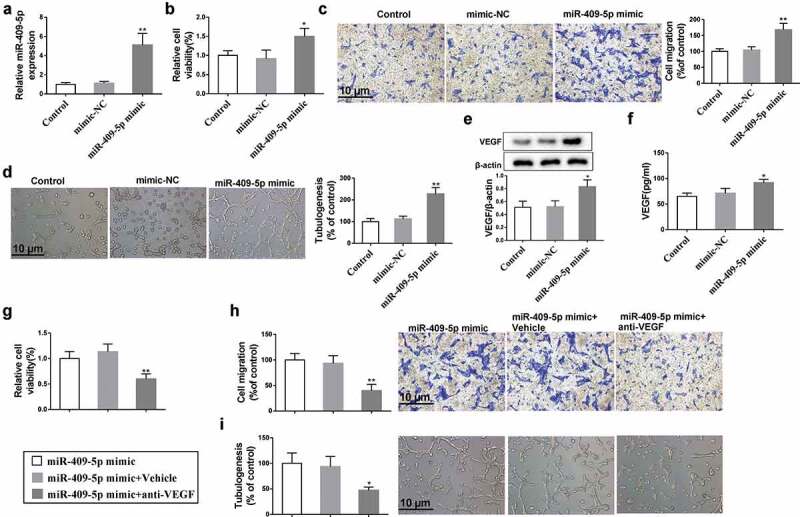
Effect of overexpressing miR-409-5p on neovascularization. mRMECs were transfected with miR-409-5p mimic or the negative control (mimic-NC) for 48 h. (a) The expression of miR-409-5p was detected using qRT-PCR. (b) Cell viability was detected using cell counting kit-8 (CCK-8) assay. (c) Cell migration was detected using transwell migration assay. (d) Tube formation was observed at 6 h under a microscope. (e) The expression of VEGF in mRMECs was detected using Western blot analysis. (f) The expression of VEGF in supernatants of culturing medium was detected using ELISA. mRMECs were transfected with miR-409-5p mimic for 48 h, followed by the treatment of VEGF antibody (100 μg/mL) or the control Vehicle (same volume of PBS) for 24 h. Cell viability (g), cell migration (h), and tube formation (i) were detected. *p < 0.05, **p < 0.01 vs mimic-NC or Vehicle. Three samples in each bar graph. Scale bar = 10 μm. Magnification = 200 ×.
Knockdown of miR-409-5p suppresses VEGF-induced neovascularization
The effect of miR-409-5p on VEGF-induced mRMEC migration and tube formation was also investigated after knocking down miR-409-5p (Figure 4(a)). After VEGF induction, the expression of miR-409-5p, mRMEC viability and migration, and tube formation were all elevated, while such promotions were negated by miR-409-5p knockdown (Figure 4(b-d)). These findings indicated that knockdown of miR-409-5p suppressed VEGF-induced mRMEC migration and tube formation.
Figure 4.
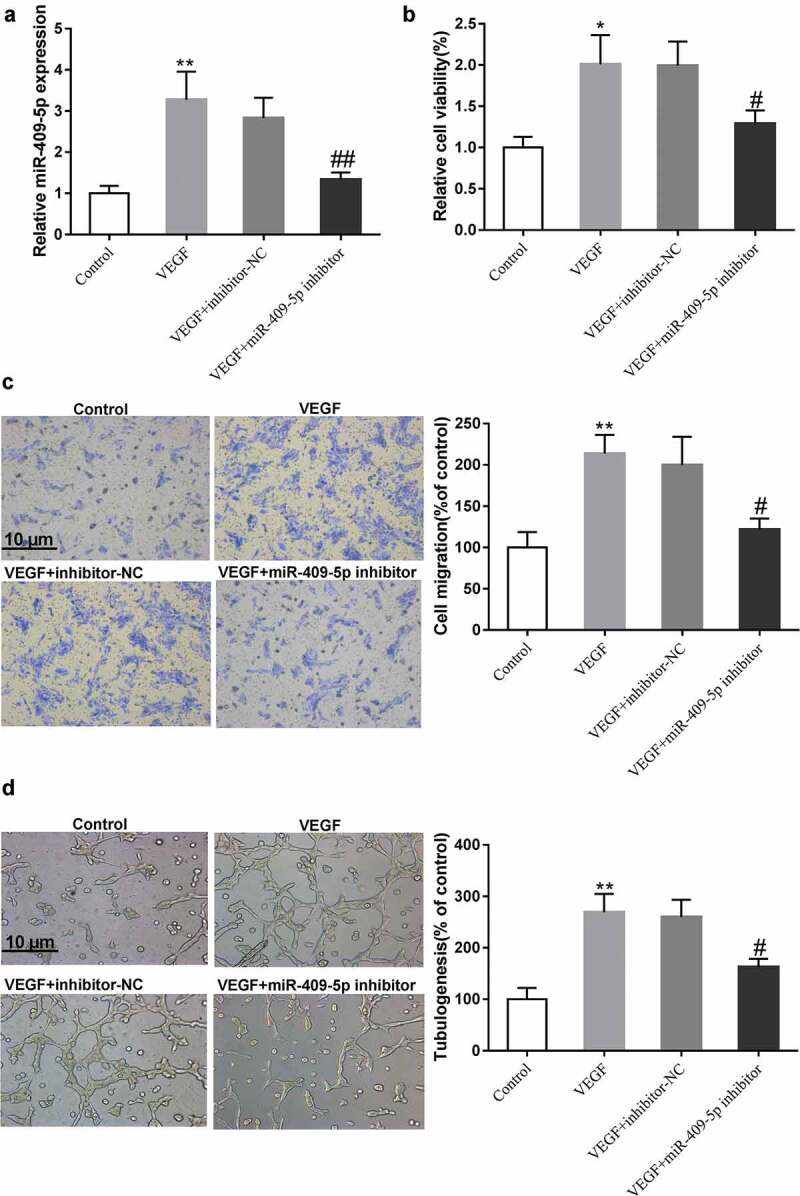
Effect of knocking down miR-409-5p on VEGF-induced neovascularization. mRMECs were treated with recombinant VEGF (20 ng/mL) for 24 h alone or after the transfection with miR-409-5p inhibitor or the negative control (inhibitor-NC) for 48 h. (a) The expression of miR-409-5p was detected using qRT-PCR. (b) Cell viability was detected using cell counting kit-8 (CCK-8) assay. (c) Cell migration was detected using transwell migration assay. (d) Tube formation was observed at 6 h under a microscope. *p < 0.05, **p < 0.01 vs Control. #p < 0.05, ##p < 0.01 vs VEGF + inhibitor-NC. Three samples in each bar graph. Scale bar = 10 μm. Magnification = 200 ×.
PPARα is a downstream target of miR-409-5p
To identify the potential target of miR-409-5p in DR regulation, we used the prediction database TargetScan and microRNA.org. As indicated, miR-409-5p was predicted to have binding sites with 3ʹUTR of PPARα in multiple types of species (Figure 5(a)). As PPARα has been identified to participate in the neovascularization of DR [14,18], we then explored whether miR-409-5p interacts with PPARα. The WT or the MUT PPARα 3ʹUTR luciferase reporter vectors were constructed, and were transfected into HEK293 T cells together with miR-409-5p mimic. The results showed that miR-409-5p overexpression reduced the luciferase activity in the WT group, while it did not change the luciferase activity in the MUT group, suggesting that the expression was inhibited via the binding between miR-409-5p and PPARα 3ʹUTR (Figure 5(b)). In addition, the overexpression of miR-409-5p suppressed the mRNA and the protein expressions of PPARα, while the knockdown of miR-409-5p enhanced the mRNA and the protein expressions of PPARα (Figure 5(c, d)). Together, our findings demonstrated that miR-409-5p inhibited PPARα expression via directly binding with its 3ʹUTR.
Figure 5.
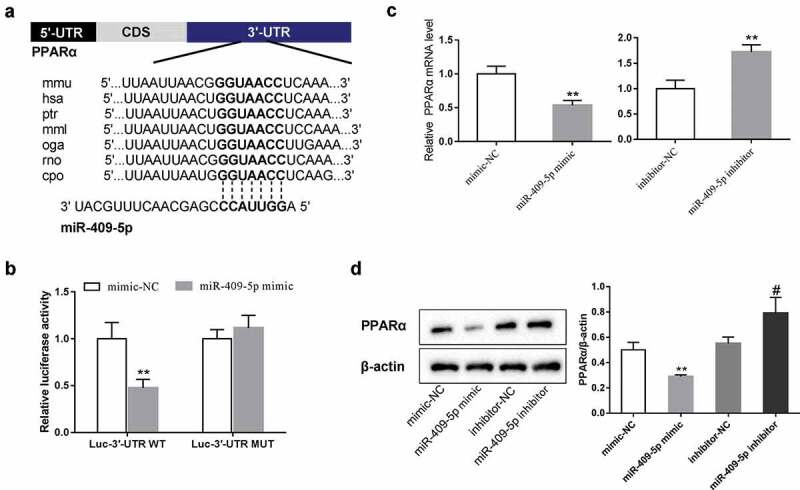
PPARα is a downstream target of miR-409-5p. (a) The predicted binding sites between miR-409-5p and 3ʹUTR of PPARα using the prediction database TargetScan and microRNA.org. (b) The wild type (WT) or the mutant (MUT) PPARα 3ʹUTR luciferase reporter vectors were constructed and were transfected into HEK293 T cells together with miR-409-5p mimic. The relative luciferase activity was detected using the luciferase reporter assay. mRMECs were transfected with miR-409-5p mimic or its negative control (mimic-NC), or transfected with miR-409-5p inhibitor or its negative control (inhibitor-NC) for 48 h. The mRNA expression of PPARα (c) and the protein expression of PPARα (d) were detected using qRT-PCR and Western blot analysis, respectively. **p < 0.01 vs mimic-NC or inhibitor-NC. #p < 0.05 vs inhibitor-NC. Three samples in each bar graph.
Overexpression of miR-409-5p promotes neovascularization via inhibiting PPARα expression
To identify whether miR-409-5p affects mRMEC migration and tube formation via regulating PPARα, mRMECs were transfected with miR-409-5p mimic or co-transfected with pcDNA-PPARα. The co-transfection rescued the reduction of PPARα protein expression which was defeated by miR-409-5p overexpression, and reduced the enhancement of VEGF protein expression which was raised by miR-409-5p overexpression (Figure 6(a)). Meanwhile, the promotion effect of miR-409-5p mimic on mRMEC viability and migration, as well as tube formation, was negated by the co-transfection with pcDNA-PPARα (Figure 6(b-d)). Taken together, these data demonstrated that overexpression of miR-409-5p promoted mRMEC migration and tube formation via inhibiting PPARα expression.
Figure 6.
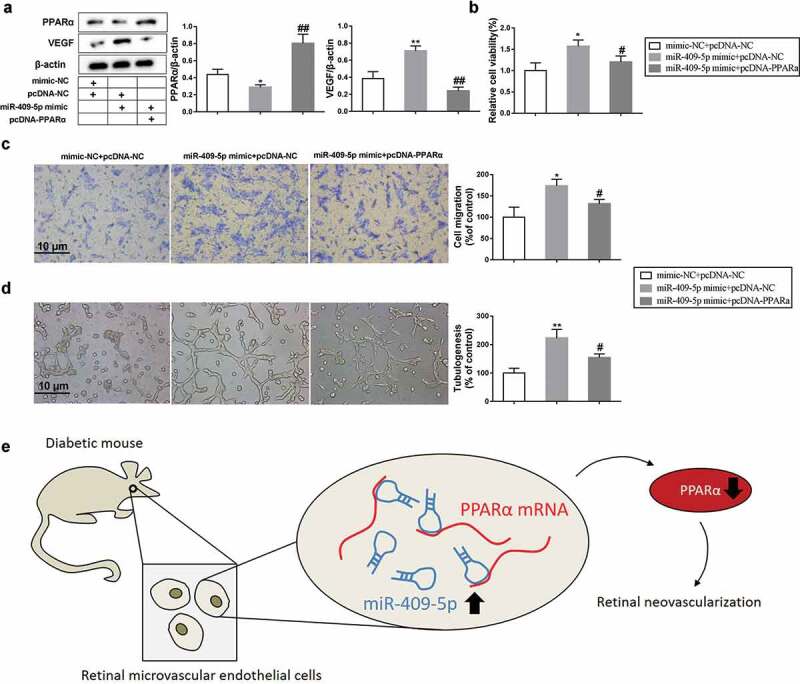
Overexpression of miR-409-5p promotes neovascularization via inhibiting PPARα expression. mRMECs were transfected with miR-409-5p mimic or co-transfected with pcDNA-PPARα for 48 h. The mimic-NC was used as the negative control of miR-409-5p mimic, and the pcDNA-NC was used as the negative control of pcDNA-PPARα. (a) The expression of PPARα protein and VEGF protein was detected using Western blot analysis. (b) Cell viability was detected using cell counting kit-8 (CCK-8) assay. (c) Cell migration was detected using transwell migration assay. (d) Tube formation was observed at 6 h under a microscope. (e) Schema depicting the mechanisms of miR-409-5p in DR. *p < 0.05, **p < 0.01 vs mimic-NC + pcDNA-NC. #p < 0.05, ##p < 0.01 vs miR-409-5p mimic + pcDNA-NC. Three samples in each bar graph. Scale bar = 10 μm. Magnification = 200 ×.
Discussion
MiRNAs play a vital role in the regulations of cell fate, including proliferation, migration, and differentiation, etc. They also provide novel targets in treating numbers of diseases. In the current study, the effect of miR-409-5p was investigated on retinal neovascularization, suggesting that miR-409-5p acts as a neovasculogenic factor in DR, and that anti-miR-409-5p therapy may suppress retinal neovascularization to treat DR. In addition, our results indicated that such response may be partly modulated by miR-409-5p/PPARα pathway (Figure 6(e)).
As reported, the dysregulated miRNAs in circulation can be used as biomarkers for DR. For instance, miR-3197 and miR-2116-5p were significantly increased in peripheral blood of DR patients and were identified as promising diagnostic biomarkers for DR [19]. Furthermore, the dysregulated miRNAs in RMECs are involved in the pathogenesis of DR. Gu et al. [20] revealed that miR-590-3p inhibited HG-induced pyroptosis in DR via targeting NLRP1 and NOX4. Thounaojam et al. [21] indicated that miR-34a induced the mitochondrial dysfunction and the loss of antioxidant activities in DR via targeting TrxR2. Except for the pyroptosis, mitochondrial function, and oxidative stress, it is well defined that retinal neovascularization, characterized by the increased proliferation, migration, and tube formation of RMECs, contributes to the uncontrolled vitreous bleeding in DR [2,13]. Therefore, in the current study, we focused on the mechanisms underlying retinal neovascularization in RMECs, and found that a novel miRNA, miR-409-5p, which was reported to be dysregulated in peripheral blood of DR patients, promoted retinal neovascularization via inhibiting the expression of PPARα. Our findings provide a novel target in preventing the progression of DR, although more evidence is still needed.
miR-409 can act as both oncogene and tumor suppressor in different kinds of cancers. For instance, miR-409 repressed the proliferation, invasion, and migration of glioma cells [22], while it promoted tumorigenesis, epithelial-to-mesenchymal transition, and bone metastasis of prostate cancer [10]. However, the effect of miR-409-5p in retinal neovascularization of DR has been rarely investigated. In our study, the expression of miR-409-5p was measured in retinal tissues of diabetic mice and in HG-induced mRMECs by using qRT-PCR, and the elevation of miR-409-5p in these samples suggested miR-409-5p as a critical regulator in DR development. Meanwhile, by using the prediction database and the luciferase reporter assay, we confirmed the direct interaction between miR-409-5p and PPARα. Notably, studies have shown that miRNAs can be sponged by another kind of non-coding RNAs, known as long non-coding RNAs (lncRNAs), thus negating the inhibition effects of miRNAs on downstream target mRNAs [23]. A study conducted by Yu et al. [24] showed that lncRNA MALAT1 participated in the pathological angiogenesis of DR by sponging miR-203a-3p. Whether miR-409-5p can be sponged by other lncRNAs to regulate retinal neovascularization deserves further investigations.
PPARs, which are a group of nuclear hormone receptors, function as transcription factors in regulating gene expressions in the pathogenesis of diabetes [25]. PPARα is an isotype of PPARs and is involved in the regulation of cell proliferation, differentiation, and lipid metabolism [26]. In addition, studies have shown that the inflammation and angiogenesis in kidney, liver, and other organs are related to PPARα expression [27,28]. Furthermore, the knock-out of PPARα in diabetic mice developed more severe nephropathy in comparison with the WT mice [29], suggesting PPARα may also play an important role in diabetes. Recently, researches have demonstrated that upregulating or activating PPARα may represent a novel therapeutic strategy for DR, as the administration of PPARα agonist achieved good therapeutic effects [14,30]. Consistent with their studies, we found that after overexpressing miR-409-5p, the expression of both mRNA and protein levels of PPARα was suppressed, and the proliferation, migration, and tube formation of mRMECs were increased, and such response could be negated by overexpressing PPARα. Our findings provided more evidence for the effect of PPARα on retinal neovascularization of DR.
For the preliminary in vivo experiments, we detected the retinal expression of miR-409-5p at 1, 2, 3, 4, and 5 wk after miR-409-5p antagomir injection. The results showed that 1 wk after the injection, the retinal expression of miR-409-5p was not significantly reduced compared with the control group (p > 0.05), while it was significantly reduced from 2 wk after injection (p < 0.05, Supplementary Figure 1). Many researches have shown that gene therapy mediated by lentiviral vector, adeno-associated virus vector, and miRNA antagomirs can effectively alleviate DR in animal models. In the current study, we collected retinal tissues at 4 wk after miR-409-5p antagomir injection, which was consistent with previous studies [30–32]. We believed that tissue collection at 2 wk after the injection was too short for the antagomirs to exert the therapeutic effects. The results showed that 4 wk after the miR-409-5p antagomir injection, mouse DR was markedly relieved. The long-term effects of miR-409-5p antagomir injection deserve further investigations in the future. miRNA antagomir is a specially chemically modified miRNA antagonist. It strongly competes with mature miRNAs in the body to prevent the complementary pairing of miRNAs and their target genes, thereby inhibiting miRNAs from functioning. Compared with ordinary inhibitors, miRNA antagomir has higher stability and inhibitory effect in animals, and the duration of the effect can be up to several weeks with less toxic and side effects. In this study, intravitreal injection of antagomir did not cause death or other significant side effects in mice. We believe that the miRNA antagomir therapy has large potentials in DR treatment.
In conclusion, our study indicated that miR-409-5p was upregulated in retinal tissues of diabetic mice and in HG-induced mRMECs and that miR-409-5p promoted retinal neovascularization via inhibiting PPARα expression. Anti-miR-409-5p therapy may represent a novel therapeutic strategy for DR in the future.
Supplementary Material
Availability of data and material
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Disclosure statement
All authors declare that they have no competing interests.
Ethics approval
This study, which included human samples and animal experiments, was approved by the Institute Research Medical Ethics Committee of Weihai Municipal Hospital. Written informed consent was obtained from all the patients involved. All animal experiments were complied with the ARRIVE guidelines and were carried out in accordance with the U.S. Public Health Service Policy on Humane Care and Use of Laboratory Animals.
Supplementary material
Supplemental data for this article can be accessed here.
References
- [1].Rask-Madsen C, King GL.. Vascular complications of diabetes: mechanisms of injury and protective factors. Cell Metab. 2013;17(1):20–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Gardner TW, Antonetti DA, Barber AJ, et al. Diabetic retinopathy: more than meets the eye. Surv Ophthalmol. 2002;47(Suppl 2):S253–62. [DOI] [PubMed] [Google Scholar]
- [3].Stitt AW, Bhaduri T, McMullen CB, et al. Advanced glycation end products induce blood-retinal barrier dysfunction in normoglycemic rats. Mol Cell Biol Res Commun. 2000;3(6):380–388. [DOI] [PubMed] [Google Scholar]
- [4].Ford JA, Elders A, Shyangdan D, et al. The relative clinical effectiveness of ranibizumab and bevacizumab in diabetic macular oedema: an indirect comparison in a systematic review. BMJ. 2012;345:e5182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Flyvbjerg A. Diabetic angiopathy, the complement system and the tumor necrosis factor superfamily, Nature reviews. Endocrinology. 2010;6(2):94–101. [DOI] [PubMed] [Google Scholar]
- [6].Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281–297. [DOI] [PubMed] [Google Scholar]
- [7].Wang J. A review of MicroRNAs related to the occurrence, diagnosis, and prognosis of non-small cell lung cancer. Clin Sur Res Commun. 2018;2(3):1–9. [Google Scholar]
- [8].Beermann J, Piccoli MT, Viereck J, et al. Non-coding RNAs in development and disease: background, mechanisms, and therapeutic approaches. Physiol Rev. 2016;96(4):1297–1325. [DOI] [PubMed] [Google Scholar]
- [9].Chen Q, Qiu F, Zhou K, et al. Pathogenic role of microRNA-21 in diabetic retinopathy through downregulation of PPARalpha. Diabetes. 2017;66(6):1671–1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Josson S, Gururajan M, Hu P, et al. miR-409-3p/-5p promotes tumorigenesis, epithelial-to-mesenchymal transition, and bone metastasis of human prostate cancer. Clin Cancer Res. 2014;20(17):4636–4646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Zheng B, Liang L, Huang S, et al. MicroRNA-409 suppresses tumour cell invasion and metastasis by directly targeting radixin in gastric cancers. Oncogene. 2012;31(42):4509–4516. [DOI] [PubMed] [Google Scholar]
- [12].Massaro JD, Polli CD, Costa ESM, et al. Post-transcriptional markers associated with clinical complications in Type 1 and Type 2 diabetes mellitus. Mol Cell Endocrinol. 2019;490:1–14. [DOI] [PubMed] [Google Scholar]
- [13].Walz JM, Wecker T, Zhang PP, et al. Impact of angiogenic activation and inhibition on miRNA profiles of human retinal endothelial cells. Exp Eye Res. 2019;181:98–104. [DOI] [PubMed] [Google Scholar]
- [14].Chen Y, Hu Y, Lin M, et al. Therapeutic effects of PPARalpha agonists on diabetic retinopathy in type 1 diabetes models. Diabetes. 2013;62(1):261–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Liu WL. MicroRNA-9 inhibits retinal neovascularization in rats with diabetic retinopathy by targeting vascular endothelial growth factor A. J Cell Biochem. 2019;120(5):8032–8043. [DOI] [PubMed] [Google Scholar]
- [16].Song B, Kim D, Nguyen N-H, et al. Inhibition of diabetes-induced lysyl oxidase overexpression prevents retinal vascular lesions associated with diabetic retinopathy. Invest Ophthalmol Vis Sci. 2018;59(15):5965–5972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Hu Y, Chen Y, Lin M, et al. Pathogenic role of the Wnt signaling pathway activation in laser-induced choroidal neovascularization. Invest Ophthalmol Vis Sci. 2013;54(1):141–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Platania CBM, Maisto R, Trotta MC, et al. Retinal and circulating miRNA expression patterns in diabetic retinopathy: an in silico and in vivo approach. Br J Pharmacol. 2019;176(13):2179–2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Ji H, Yi Q, Chen L, et al. Circulating miR-3197 and miR-2116-5p as novel biomarkers for diabetic retinopathy. Clin Chim Acta. 2020;501:147–153. [DOI] [PubMed] [Google Scholar]
- [20].Gu C, Draga D, Zhou C, et al. miR-590-3p inhibits pyroptosis in diabetic retinopathy by targeting NLRP1 and inactivating the NOX4 signaling pathway. Invest Ophthalmol Vis Sci. 2019;60(13):4215–4223. [DOI] [PubMed] [Google Scholar]
- [21].Thounaojam MC, Jadeja RN, Warren M, et al. MicroRNA-34a (miR-34a) mediates retinal endothelial cell premature senescence through mitochondrial dysfunction and loss of antioxidant activities. Antioxidants (Basel). 2019;8(9):328–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Cao Y, Zhang L, Wei M, et al. MicroRNA-409-3p represses glioma cell invasion and proliferation by targeting high-mobility group nucleosome-binding domain 5. Oncol Res. 2017;25(7):1097–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Thomson DW, Dinger ME. Endogenous microRNA sponges: evidence and controversy. Nat Rev Genet. 2016;17(5):272–283. [DOI] [PubMed] [Google Scholar]
- [24].Yu L, Fu J, Yu N, et al. Long non-coding RNA MALAT1 participates in the pathological angiogenesis of diabetic retinopathy in oxygen-induced retinopathy mouse model by sponging miR-203a-3p. Can J Physiol Pharmacol. 2019;98(4):219–227. [DOI] [PubMed] [Google Scholar]
- [25].Michalik L, Auwerx J, Berger JP, et al. International union of pharmacology. LXI. Peroxisome proliferator-activated receptors. Pharmacol Rev. 2006;58(4):726–741. [DOI] [PubMed] [Google Scholar]
- [26].Berger J, Moller DE. The mechanisms of action of PPARs. Annu Rev Med. 2002;53:409–435. [DOI] [PubMed] [Google Scholar]
- [27].Cuzzocrea S, Mazzon E, Di Paola R, et al. The role of the peroxisome proliferator-activated receptor-alpha (PPAR-alpha) in the regulation of acute inflammation. J Leukoc Biol. 2006;79(5):999–1010. [DOI] [PubMed] [Google Scholar]
- [28].Kasai T, Miyauchi K, Yokoyama T, et al. Efficacy of peroxisome proliferative activated receptor (PPAR)-alpha ligands, fenofibrate, on intimal hyperplasia and constrictive remodeling after coronary angioplasty in porcine models. Atherosclerosis. 2006;188(2):274–280. [DOI] [PubMed] [Google Scholar]
- [29].Park CW, Kim HW, Ko SH, et al. Accelerated diabetic nephropathy in mice lacking the peroxisome proliferator-activated receptor alpha. Diabetes. 2006;55(4):885–893. [DOI] [PubMed] [Google Scholar]
- [30].Hu Y, Chen Y, Ding L, et al. Pathogenic role of diabetes-induced PPAR-alpha down-regulation in microvascular dysfunction. Proc Natl Acad Sci U S A. 2013;110(38):15401–15406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Feng B, Chen S, McArthur K, et al. miR-146a-Mediated extracellular matrix protein production in chronic diabetes complications. Diabetes. 2011;60(11):2975–2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].McArthur K, Feng B, Wu Y, et al. MicroRNA-200b regulates vascular endothelial growth factor-mediated alterations in diabetic retinopathy. Diabetes. 2011;60(4):1314–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.


