Abstract
Aromatase inhibitor (AI)-induced arthralgia (AIA) is a common reason for AI noncompliance. Retrospective analysis of MA.27 study revealed no clear correlation between vitamin D levels and AIA. However, patients with a Fok-I vitamin D receptor polymorphism were more likely to have lower interleukin 1β, and less likely to develop AIA. Through this type of risk stratification, future AIA clinical trials might be able to focus on high-risk populations.
Background:
Approximately half of women taking aromatase inhibitor (AI) therapy develop AI-induced arthralgia (AIA), and many might discontinue AI therapy because of the pain. Using plasma samples from the MA.27 study, we assessed several factors potentially associated with AIA.
Patients and Methods:
MA.27 is a phase III adjuvant trial comparing 2 AIs, exemestane versus anastrozole. Within an 893-participant nested case-control AIA genome-wide association study, we nested a 72 AIA case-144 control assessment of vitamin D plasma concentrations, corrected for seasonal and geographic variation. We also examined 9 baseline inflammatory cytokines: interleukin (IL)-1β, IL-6, tumor necrosis factor-α, interferon (IFN)γ, IL-10, IL-12p70, IL-17, IL-23, and chemokine ligand (CCL)-20. Finally, we analyzed the multivariate effects of baseline factors: vitamin D level, previously identified musculoskeletal single nucleotide polymorphisms, age, body mass index, and vitamin D receptor (VDR) Fok-I variant genotype on AIA development.
Results:
Changes in vitamin D from baseline to 6 months were not significantly different between cases and controls. Elevated inflammatory cytokine levels were not associated with development of AIA. The multivariate model included no clinical factors associated with AIA. However, women with the VDR Fok-I variant genotype were more likely to have a lower IL-1β level (P = .0091) and less likely to develop AIA after 6 months of AI compared with those with the wild type VDR (P < .0001).
Conclusion:
In this nested case-control correlative study, vitamin D levels were not significantly associated with development of AIA; however, patients with the Fok-I VDR variant genotype were more likely to have a significant reduction in IL-1β level, and less likely to develop AIA.
Keywords: Aromatase inhibitor-induced arthralgias, IL-1 beta, Inflammatory cytokines, Vitamin D, Vitamin D receptor polymorphisms
Introduction
Aromatase inhibitors (AIs) are the most effective adjuvant anti-hormonal therapy for postmenopausal women with breast cancer,1 but approximately half of them will develop AI-induced arthralgias (AIAs).2 AIAs are detrimental to women’s quality of life, impairing functioning in household, recreational, and occupational activities.3,4 Furthermore, 13% to 20% of women discontinue AI therapy principally because of arthralgias.5,6 In larger studies, overall rates of nonadherence to AI therapy for any reason are 32% to 50% at 3 years.7 Nonetheless, neither the etiology nor the optimal management of AIA is clearly understood.
It is commonly thought that a low estrogen level causes AIA, similar to the phenomenon of “arthritis of menopause.” Estrogen is very important in vitamin D activation because it increases activity of 1α hydroxylase, which catalyzes conversion of 25(OH)D to its active form, 1,25(OH)2D.8 Estrogen also increases the activation of the vitamin D receptor (VDR).8 Therefore, a low estrogen state could potentially decrease the amount of active vitamin D available, supported by the fact that 75% to 90% of women receiving AI therapy are vitamin D insufficient.9,10 Vitamin D deficiency is known to cause a syndrome of muscle and joint aches, which is very similar to AIA,11 and some preliminary evidence has supported the role of vitamin D in preventing or treating AIA.12,13 Furthermore, vitamin D is known to inhibit release of inflammatory cytokines interleukin (IL)-1, IL-6, and tumor necrosis factor (TNF)-α from macrophages.14,15 Therefore, we hypothesized that AIs cause extremely low estrogen levels, which lead to lower vitamin D levels and, thus, cause disinhibition of inflammatory cytokines, ultimately resulting in AIA.
However, clinically, some women respond to vitamin D supplementation with complete resolution of AIA, whereas others reap almost no benefit.16 We suspect that individual host factors might play a role in patients’ differential response to vitamin D. Therefore, we examined plasma samples from 204 patients in the MA.27 study at baseline and at 6 months. The primary objective was to compare change in vitamin D level from baseline to 6 months between cases (women who developed AIA) and controls (those who did not develop AIA). The secondary objectives included evaluation of a multivariate model incorporating many clinical factors that might be associated with the development of AIA, as well as further investigation of the role of VDR polymorphisms in the development of AIA.
There are many described polymorphisms in the VDR gene, but the Fok-I variant is the only known alteration that results in 2 different protein products.17 Because of a different translation initiation site on the VDR, people with the Fok-I variant have a truncated VDR-FF variant with only 424 amino acids, as compared with the full-length VDR-ff, comprised of 427 amino acids.18 The Fok-I variant is not uncommon, with 35% homozygous FF, and 50% heterozygous for Ff in the European Caucasian population17 Previous work has shown that the FF variant is more responsive to lower levels of vitamin D, with higher vitamin D levels resulting from equivalent vitamin D supplementation, compared with the ff subtype.19
Patients and Methods
Patient Population
The Canadian Cancer Trials Group MA.27 study (ClinicalTrials.gov identifier: NCT00066573) is the largest adjuvant endocrine therapy trial conducted to date that has exclusively studied AIs and prospectively collected blood for DNA extraction and patient consent for its use in genetic studies.20 All samples banked in the North American patients were stored in strict accordance with National Institutes of Health processes. A total of 7576 women with early stage estrogen receptor-positive breast cancer were randomized, 1:1 to receive adjuvant exemestane or anastrozole. Stratification factors included trastuzumab therapy, lymph node status at diagnosis, and previous adjuvant chemotherapy. Patients were required to have discontinued hormone replacement therapy (HRT) and/or raloxifene at least 3 weeks before randomization. There was no significant difference in event-free survival between the treatment arms (exemestane vs. anastrozole) in the MA.27 trial.20
In the current study, we investigated a subset of the patients included in a GWAS that included a total of 878 cases and controls, examining AIA as the phenotype.21 Cases were defined as patients who had at least 1 of 6 Grade 3 to 4 musculoskeletal (MSK) adverse events (MS-AEs; joint pain, muscle pain, bone pain, arthritis, diminished joint function, or other MSK problems) within 1 to 2 years of AI treatment, or discontinued treatment for any Grade of MS-AE. Patients who fulfilled the case definition during celecoxib treatment, or within 3 months of stopping celecoxib, were excluded as cases. Controls did not experience any of the MS-AEs, were followed at least 2 years, and had at least 6 months longer follow-up than a case to which they were matched; thus, controls were not receiving celecoxib for at least 6 months.21 Of the 878 patients with GWAS information, we randomly selected 72 cases and 144 matched controls (Figure 1), which are all representative of the original 878 samples, with no statistically significant clinical differences.
Figure 1. Study Design: Consolidated Standards of Reporting Trials Flow Diagram.
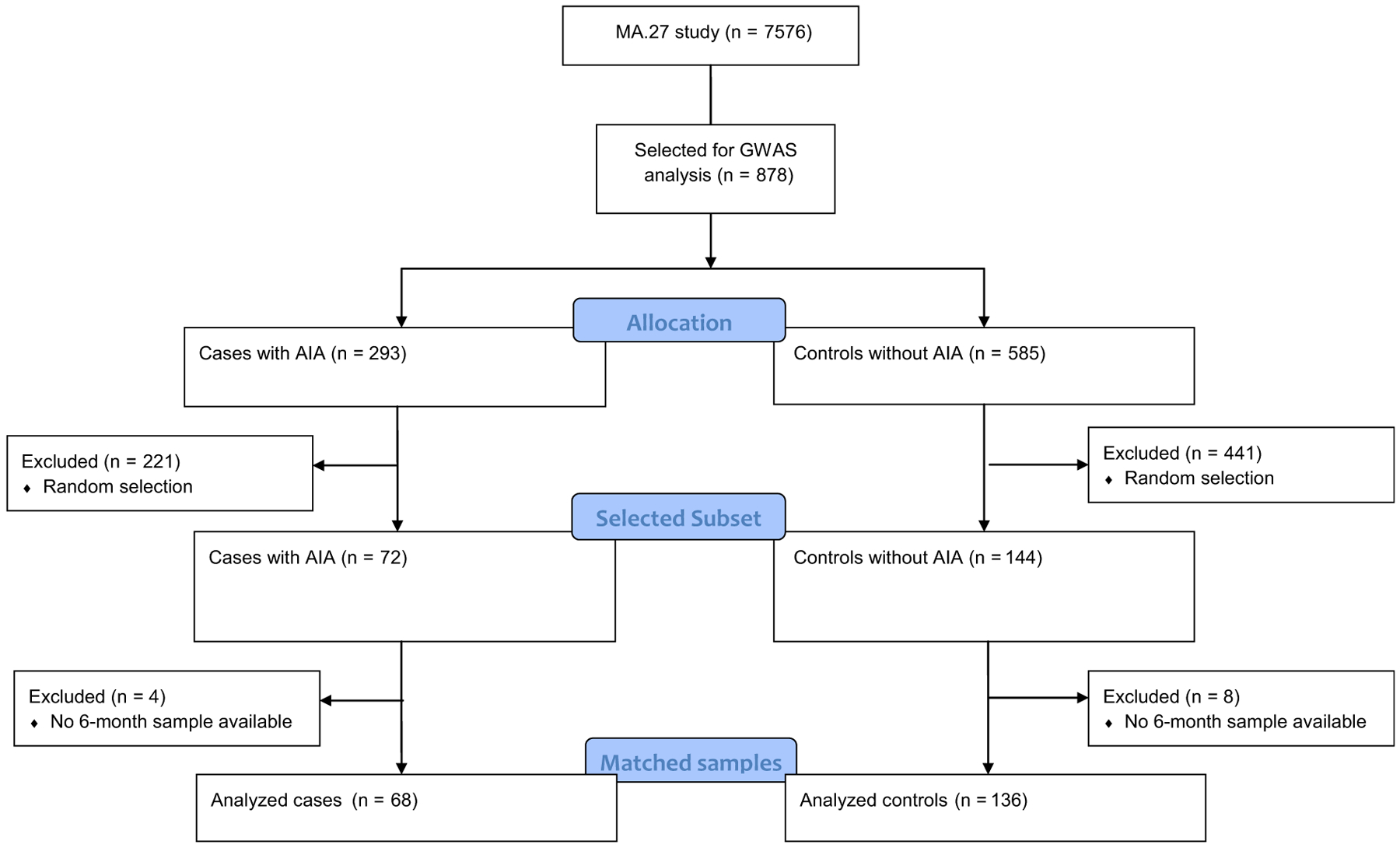
Abbreviations: AIA = aromatase inhibitor-induced arthralgia; GWAS = genome-wide association study.
Study Design and Methods
Plasma samples were drawn early in the morning during the MA.27 study period. Because inflammatory cytokines exhibit diurnal variation, this consistency in the timing of the blood draws limits potential error in cytokine quantitation. We tested each of the 204 patients’ baseline and 6-month plasma samples for 25-hydroxy vitamin D in a Clinical Laboratory Improvement Amendments (CLIA)-certified lab. We used the DiaSorin (Italy) Liaison chemi-luminescent immunoassay that has a range of 4 to 150 mg/mL. Using 200 μL of patient plasma for each assay, we followed the protocol recommended by the manufacturer instructions.
The inflammatory cytokines were assayed concurrently from the baseline and 6-month plasma samples. We determined plasma levels of pro- and anti-inflammatory cytokines TNF-α, IFNγ, IL-1β, IL-6, IL-10, IL-12p70, IL-17, IL-23, and CCL-20 using multiplex assays from EMD Millipore (Billerca, MA), via the manufacturer’s recommended protocol.
Finally we used the existing GWAS data21 including these patients. We assessed VDR polymorphisms in Fok-I gene rs2228570. The single nucleotide polymorphism (SNP) rs2228570 is located in chromosome 12 at base position 48272895 (GRCh37.p13). The variant allele in this report is allele A whereas the reference allele is reported as G.
Statistical Analysis
Cases and controls were matched on the basis of the following factors: treatment arm (anastrozole or exemestane), presence or absence of previous adjuvant chemotherapy, age at start of AI treatment (±5 years), and time enrolled in study. When possible, each case was matched with 2 controls.
A sample size of 216 (72 cases and 144 matched controls) was planned for this study. Assuming a laboratory assessment failure rate of 10%, there would be 192 assessments for analysis. We assumed the SD of change in vitamin D levels to be 15 ng/mL. With a 2-sided significant level α = 0.05 and power = 90%, the detectable effect size depends on the variance inflation factor (VIF) of the regression model: for a VIF = 1.5, the detectable effect size = 0.62 (9.3 ng/mL), for a VIF = 2, detectable effect size = 0.72 (10.8 ng/mL), for VIF = 3, the detectable effect size = 0.88 (13.2 ng/mL). Of the original 878 cases and controls,21 we examined 216 randomly selected subjects, comprised of 72 cases and 144 controls. Using exact Fisher tests, we confirmed that there are no significant imbalances in treatment and stratification factors for the selected cohorts of patients in this analysis, compared with the original 878 cases.21 Ultimately, 204 samples had matching baseline and 6-month plasma samples available and were included in the final analysis. A multivariate regression model was used for primary evaluation of the association between change in vitamin D level and AIA. The variables to be included in the model are listed in the paragraph plan below.
To assess the primary objective of change in vitamin D levels over 6 months between cases and controls, we developed a multivariate regression model. The model was adjusted for the effects of prespecified key factors (including AIA status, MA.27 treatment allocation, lymph node status, clinical stage, previous adjuvant chemotherapy, race, body mass index [BMI], previous use of taxane chemotherapy, recent use of HRT, osteoporosis therapy, fracture in past 1 year, raloxifene use, and inclusion in the MA.27B bone substudy22). Per Reporting Recommendations for Tumor Marker Prognostic Studies (REMARK) 2012, full fitting can overestimate significance, so for eventual application with fewer factors as well as sensitivity estimate of vitamin D effect, a backward stepwise model selection procedure was used to select factors that were significantly associated with change of vitamin D levels with an unadjusted significant level of 0.05 for including/excluding variables from the model. Because of geographic and seasonal variations in vitamin D, the Mayo Clinic formula23 was used to control for these differences and compare patients across various time points and geographic locations.
The secondary objective of modeling occurrence of AIA used several clinical factors, which were available at baseline, including initial vitamin D level, previously identified MSK SNPs, use of aspirin, use of trastuzumab, age, BMI, previous taxane chemotherapy, recent use of HRT, fracture in past 10 years, inclusion in MA.27B bone substudy, osteoporosis therapy, and VDR polymorphisms. These factors were taken into account using a multivariate linear regression analysis. A backward stepwise model selection procedure was used to select factors that were significantly associated with level of inflammatory cytokines, with an unadjusted significance level of 0.05 for including/excluding variables from the model.
To determine whether Fok-I VDR polymorphisms were associated with higher levels of inflammatory cytokines, we used a multivariate Fisher linear discriminant analysis to model the AIA outcome with the following variables: baseline vitamin D level, previously identified MSK SNPs, use of aspirin, use of herceptin, age, BMI, previous taxane chemotherapy, recent use of HRT, fracture in past 10 years, inclusion in MA.27B bone substudy, osteoporosis therapy, and VDR polymorphism. A backward stepwise model selection procedure was used to select factors that were significantly associated with level of inflammatory cytokines, with an unadjusted significance level of 0.05 for including/excluding variables from the model.
The χ2 goodness of fit test was used to compare the counts of cases and controls in the wild type VDR and Fok-I VDR groups to determine whether those patients with the Fok-I polymorphism have a higher incidence of AIA.
Results
Patient Characteristics
A total of 216 patients were initially included in this study (72 cases and 144 controls), and 204 were analyzed because they had baseline and 6-month samples available. Table 1 shows the patient characteristics according to AIA status. The median age was 64.33 (range, 47.97–86.89), and the median BMI was 27.7 (range, 18.44–61.01). There were no significant differences between cases and controls in the study patients included in this analysis.
Table 1.
Patient Characteristics According to AIA Status, and of Patients Who Were Included Versus Those Who Were Not Included in the Present Vitamin D Study
| Patient Characteristics According to AIA Status | AIA | No AIA | Total | P |
|---|---|---|---|---|
| Treatment Allocation | 1.000 | |||
| Anastrozole | 34 (47.2%) | 68 (47.2%) | 102 (47.2%) | |
| Exemestane | 38 (52.8%) | 76 (52.8%) | 114 (52.8)% | |
| Lymph Node Status | .524 | |||
| No | 49 (68.1%) | 105 (72.9%) | 154 (71.3%) | |
| All others | 23 (31.9%) | 39 (27.1%) | 62 (28.7%) | |
| Clinical Stage | 1.000 | |||
| T1 | 52 (72.2%) | 103 (71.5%) | 155 (71.8%) | |
| All others | 20 (27.8%) | 41 (28.5%) | 61 (28.2%) | |
| Previous Adjuvant Chemotherapy | 1.000 | |||
| No | 45 (62.5) | 90 (62.5%) | 135 (62.5%) | |
| Yes | 27 (37.5%) | 54 (37.5%) | 81 (37.5%) | |
| Age | .611 | |||
| <70 | 57 (79.2) | 108 (75.0) | 165 (76.4) | |
| ≥70 | 15 (20.8) | 36 (25.0) | 51 (23.6) | |
| Age | .749 | |||
| n | 72 | 144 | 216 | |
| Mean | 64.66 | 64.82 | 64.77 | |
| SD | 8.025 | 8.061 | 8.031 | |
| Median | 63.95 | 64.46 | 64.33 | |
| Minimum | 51.04 | 47.97 | 47.97 | |
| Maximum | 86.89 | 83.46 | 86.89 | |
| Race | N/A | |||
| White | 72 (100.0) | 144 (100.0) | 216 (100.0) | |
| BMI | .325 | |||
| Not recorded | 0 (0.0) | 2 (1.4) | 2 (0.9) | |
| <25 | 13 (18.1) | 38 (26.4) | 51 (23.6) | |
| 25 to <30 | 30 (41.7) | 57 (39.6) | 87 (40.3) | |
| ≥30 | 29 (40.3) | 47 (32.6) | 76 (35.2) | |
| Previous Use of Taxane Chemotherapy | .434 | |||
| No | 55 (76.4) | 114 (79.2) | 169 (78.2) | |
| Yes | 16 (22.2) | 30 (20.8) | 46 (21.3) | |
| Unknown/missing | 1 (1.4) | 0 (0.0) | 1 (0.5) | |
| Recent Use of Hormone Replacement Therapy | .246 | |||
| No | 30 (41.7) | 76 (52.8) | 106 (49.1) | |
| Yes | 40 (55.6) | 63 (43.8) | 103 (47.7) | |
| Unknown/missing | 2 (2.8) | 5 (3.5) | 7 (3.2) | |
| Osteoporosis Therapy | .911 | |||
| No | 64 (88.9) | 130 (90.3) | 194 (89.8) | |
| Yes | 7 (9.7) | 12 (8.3) | 19 (8.8) | |
| Unknown/missing | 1 (1.4) | 2 (1.4) | 3 (1.4) | |
| Fracture in Past 10 Years | .651 | |||
| No | 63 (87.5) | 129 (89.6) | 192 (88.9) | |
| Yes | 9 (12.5) | 15 (10.4) | 24 (11.1) | |
| Raloxifene | .403 | |||
| No | 72 (100.0) | 140 (97.2) | 212 (98.1) | |
| Yes | 0 (0.0) | 2 (1.4) | 2 (0.9) | |
| Unknown/missing | 0 (0.0) | 2 (1.4) | 2 (0.9) | |
| Characteristics of Patients Who Were Included in the Current Vitamin D Study, Versus Those Who Were Excluded | In Study | Excluded | Total | P |
| Treatment Allocation | .343 | |||
| Anastrozole | 101 (47.2) | 288 (43.4) | 389 (44.3) | |
| Exemestane | 113 (52.8) | 376 (56.6) | 489 (55.7) | |
| Lymph Node Status | .279 | |||
| No | 153 (71.5) | 501 (75.5) | 654 (74.5) | |
| All others | 61 (28.5) | 163 (24.5) | 224 (25.5) | |
| Clinical Stage | .368 | |||
| T1 | 154 (72.0) | 499 (75.2) | 653 (74.4) | |
| All others | 60 (28.0) | 165 (24.8) | 225 (25.6) | |
| Previous Adjuvant Chemotherapy | .034 | |||
| No | 134 (62.6) | 468 (70.5) | 602 (68.6) | |
| Yes | 80 (37.4) | 196 (29.5) | 276 (31.4) | |
| Age | .214 | |||
| <70 | 164 (76.6) | 479 (72.1) | 643 (73.2) | |
| ≥70 | 50 (23.4) | 185 (27.9) | 235 (26.8) | |
| Age | .641 | |||
| n | 214 | 664 | 878 | |
| Mean | 64.72 | 64.47 | 64.53 | |
| SD | 8.026 | 8.521 | 8.399 | |
| Median | 64.33 | 63.56 | 63.69 | |
| Minimum | 47.97 | 45.13 | 45.13 | |
| Maximum | 86.89 | 84.81 | 86.89 | |
| Q1 | 58.35 | 57.72 | 58.03 | |
| Q3 | 69.49 | 70.87 | 70.22 | |
| Race | 1.000 | |||
| White | 214 (100.0) | 661 (99.5) | 875 (99.7) | |
| All others | 0 (0.0) | 3 (0.5) | 3 (0.3) | |
| BMI | .093 | |||
| Not recorded | 2 (0.9) | 7 (1.1) | 9 (1.0) | |
| <25 | 50 (23.4) | 188 (28.3) | 238 (27.1) | |
| 25 to <30 | 87 (40.7) | 217 (32.7) | 304 (34.6) | |
| ≥30 | 75 (35.0) | 252 (38.0) | 327 (37.2) | |
| BMI | .857 | |||
| n | 212 | 657 | 869 | |
| Mean | 29.2 | 29.13 | 29.15 | |
| SD | 6.236 | 6.396 | 6.354 | |
| Median | 27.66 | 28.07 | 27.97 | |
| Minimum | 18.44 | 16.91 | 16.91 | |
| Maximum | 61.01 | 56.76 | 61.01 | |
| Q1 | 25.16 | 24.49 | 24.54 | |
| Q3 | 32.16 | 32.79 | 32.7 | |
| Previous Use of Taxane Chemotherapy | .019 | |||
| No | 167 (78.0) | 567 (85.4) | 734 (83.6) | |
| Yes | 46 (21.5) | 96 (14.5) | 142 (16.2) | |
| Unknown/missing | 1 (0.5) | 1 (0.2) | 2 (0.2) | |
| Recent Use of Hormone Replacement Therapy | .007 | |||
| No | 106 (49.5) | 274 (41.3) | 380 (43.3) | |
| Yes | 102 (47.7) | 337 (50.8) | 439 (50.0) | |
| Unknown/missing | 6 (2.8) | 53 (8.0) | 59 (6.7) | |
| Osteoporosis Therapy | .728 | |||
| No | 192 (89.7) | 604 (91.0) | 796 (90.7) | |
| Yes | 19 (8.9) | 49 (7.4) | 68 (7.7) | |
| Unknown/missing | 3 (1.4) | 11 (1.7) | 14 (1.6) | |
| Fracture in Past 10 Years | .609 | |||
| No | 190 (88.8) | 597 (89.9) | 787 (89.6) | |
| Yes | 24 (11.2) | 67 (10.1) | 91 (10.4) | |
| Raloxifene | .47 | |||
| No | 210 (98.1) | 653 (98.3) | 863 (98.3) | |
| Yes | 2 (0.9) | 9 (1.4) | 11 (1.3) | |
| Unknown/missing | 2 (0.9) | 2 (0.3) | 4 (0.5) |
Abbreviations: AIA = aromatase inhibitor-induced arthralgia; BMI = body mass index; Q1, Q3 = 1st and 3rd quartiles.
Table 1 shows a comparison of the patient characteristics of the GWAS patients who were included in the vitamin D study, and those who were not. They were balanced in all regards except for history of previous chemotherapy and HRT. A higher percentage of patients received previous adjuvant chemotherapy in the current analysis, compared with the original GWAS cohort study (37.4% versus 29.5%; Fisher exact test P = .034). Specifically, more patients in the current analysis had previously received taxane chemotherapy (21.5% vs. 14.5%; Fisher exact test P = .019). Additionally, in the current analysis, fewer women recently used HRT compared with those in the original GWAS cohort study (47.7% vs. 50.8%; P = .007).
Vitamin D
Although there was a statistically significant increase of 4.52 ng/mL in vitamin D level in the entire population studied (P = .049) during the initial 6 months of AI therapy, the cases and controls did not differ from each other in their increase in vitamin D level. In fact, there was no demonstration of statistically significant difference in vitamin D levels between cases and controls at any of the studied time points. The annual mean baseline vitamin D level for cases was 30.66 ng/mL, compared with 29.62 ng/mL for controls (P = .35). Similarly, there was no significant difference between cases and controls in the month 6 vitamin D level (P = .88), or in the change from baseline to month 6 vitamin D (P = .39). See Table 2 for complete details. The histograms in Figure 2 show the vitamin D level at baseline and month 6, in cases as well as in controls.
Table 2.
Change in Vitamin D Level in Cases Versus Controls
| Case | Control | P | |
|---|---|---|---|
| n | 68 | 136 | |
| Baseline Vitamin D | 30.7 | 29.6 | .351 |
| Month 6 Vitamin D | 34.2 | 34.7 | .877 |
| Mean Δ Vitamin D | 3.7 | 5.1 | .393 |
| Minimum Δ Vitamin D | −11.5 | −29.7 | |
| Maximum Δ Vitamin D | 28.9 | 62.57 |
Figure 2. (A) Histogram of Baseline Vitamin D Levels in Cases. (B) Histogram of Baseline Vitamin D Levels in Controls. (C) Histogram of 6-Month Vitamin D Levels in Cases. (D) Histogram of 6-Month Vitamin D Levels in Controls.
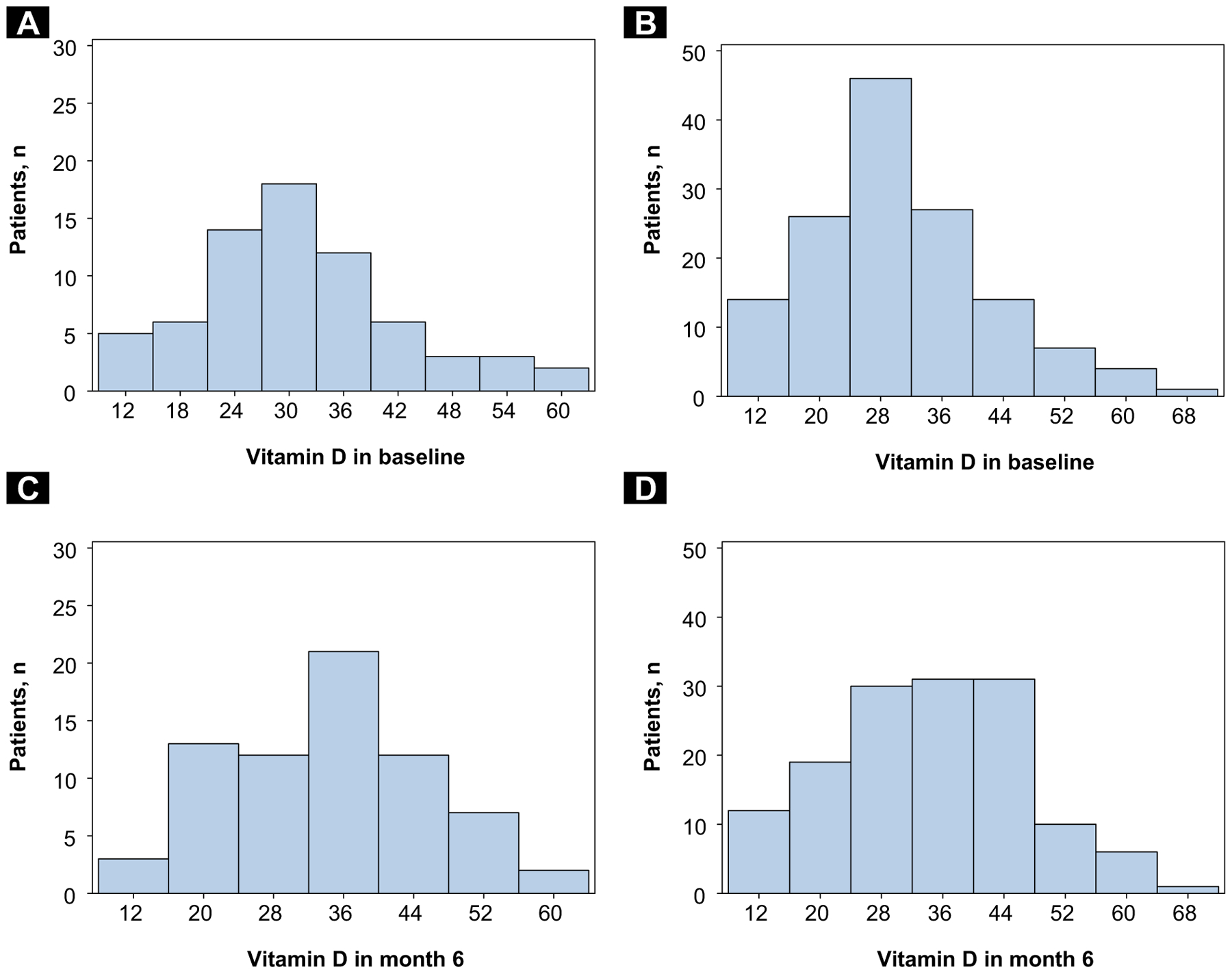
(A) Histogram of Baseline Vitamin D Levels in Cases. (B) Histogram of Baseline Vitamin D Levels in Controls. (C) Histogram of 6-Month Vitamin D Levels in Cases. (D) Histogram of 6-Month Vitamin D Levels in Controls
Although there was no difference in vitamin D levels in the overall study group, those who were overweight (BMI 25–30), were found to have an average of 4.2 ng/mL lower baseline vitamin D level than those with a healthy weight (BMI < 25; P = .038). Similarly, obese patients (BMI > 30) had an average 10.6 ng/mL lower baseline vitamin D level than patients with a healthy body weight (P ≤ .0001). Patients who had previously used HRT had higher baseline vitamin D levels (4.5 ng/mL higher, on average) compared with those who had not used HRT. The same pattern was observed in an examination of 6-month vitamin D levels in these groups.
After completing our analysis of one-third of the samples, we then performed another calculation to determine whether testing the remaining samples might yield a different result. The conditional power was calculated for baseline difference between cases and controls.24 The conditional power gives the probability of having a positive result when all samples are tested on the basis of results from current stage of study (which is the difference between controls and cases = −1.04; SD = 11.57). The conditional power is calculated to be 2.19%, meaning that testing all 848 samples from the GWAS analysis would yield < 5% chance of a positive outcome.
Multivariate AIA Model
We examined the subjects for associations between development of AIA and multiple clinical factors, including initial vitamin D level, previously identified MSK SNPs, use of aspirin, use of trastuzumab, age, BMI, previous taxane chemotherapy, recent use of HRT, fracture in past 10 years, inclusion in MA.27B bone substudy, osteoporosis therapy, and VDR polymorphisms. However, there was no statistically significant association between any of the predefined factors and development of AIA in our subset of 204 patients.
Inflammatory Cytokines and VDR Polymorphisms
In a comparison of the number of AIA cases versus controls in the VDR wild type group and VDR Fok-I variant group for SNP rs2228570, there was insufficient evidence (P = 1.0) to claim that there was a significantly less number of arthralgia patients with the Fok-I VDR variant compared with wild type patients. However, we observed sufficient evidence (P < 2.26e-16) within the Fok-I variant genotype group (minor allele frequency = 0.3) to state that there were significantly less AIA in these patients compared with patients with the wild type VDR (Table 3). In the univariate model, we compared the inflammatory cytokines between the patients who had the Fok-I VDR variant versus those with wild type VDR. In the multivariable model, we adjusted for treatment, lymph node status, stage, previous adjuvant chemotherapy, age, BMI, previous taxane chemotherapy, recent use of HRT, osteoporosis therapy, fracture in past 10 years, and raloxifene use. Patients with the Fok-I VDR variant genotype were found to have lower IL-1β levels at 6 months, compared with patients with wild type VDR. The average difference was 49.4 pg/mL (P = .0091). The other inflammatory cytokines that were tested (TNF-α, IFNγ, IL-6, IL-10, IL-12p70, IL-17, IL-23, and CCL-20) showed no clear association at baseline or 6-month levels with either VDR polymorphism or development of AIA (Table 4).
Table 3.
Distribution of AIA Cases and Controls, on the Basis of Vitamin D Receptor Polymorphism Status
| Polymorphism Status | Cases | Controls | P |
|---|---|---|---|
| Wild Type VDR | 24 | 49 | NS |
| Fok-I VDR | 269 | 536 | <.0001 |
Abbreviations: AIA = aromatase inhibitor-induced arthralgia; VDR = vitamin D receptor.
Table 4.
Difference in 6-Month Cytokine Level Between WT VDR and Fok-I Variant
| Cytokine | Univariate Model | Multivariate Model | ||||
|---|---|---|---|---|---|---|
| Fok-I | WT | Unadjusted Difference | Unadjusted P | Multivariate Adjusted Δ Fok-I vs. WT | Multivariate Model P | |
| IL-1β | 103.4 | 147.6 | −44.2 | .011 | −49.4 | .0091 |
| IL-6 | 136.4 | 133.9 | 2.5 | .91 | 3 | .9 |
| TNF-α | 99.1 | 107.3 | −8.2 | .52 | −10 | .48 |
| IFN-γ | 107.7 | 83.7 | 24 | .31 | 30.4 | .23 |
| IL-10 | 45 | 40.8 | 4.2 | .64 | 6 | .55 |
| IL-12p70 | 129.21 | 141.01 | −11.8 | .59 | −7.1 | .76 |
| IL-17 | 106.58 | 112.6 | −6.02 | .73 | −2.3 | .9 |
| IL-23 | 20.6 | 23.7 | −3.1 | .51 | −1.9 | .71 |
| CCL-20 | 156.9 | 167.9 | −11 | .63 | −6.9 | .78 |
Abbreviations: CCL = chemokine ligand; IFN = interferon; IL = interleukin; TNF = tumor necrosis factor; VDR = vitamin D receptor; WT = wild type.
Discussion
There were limitations of the study. This analysis of a subset of MA.27 patients did not show a significant association with decreasing vitamin D levels and the development of AIA. Because of this finding, clinical trials in this arena moving forward might benefit from focusing on factors other than vitamin D alone. This would also support the conflicting clinical trial results to date. Some studies have shown association between low baseline vitamin D levels and development of AIA,25–27 although other studies have not.28 The International Breast Cancer Intervention Study II (IBIS-II) trial28 was one of the larger analyses to ask this question in 416 patients, and it did not show a correlation with low baseline vitamin D level and AIA, similar to the results of this analysis.
In a study by Khan et al,12 60 women who were beginning adjuvant AI therapy were supplemented with vitamin D at 50,000 IU per week for 12 weeks if they had low baseline vitamin D levels (≤ 40 ng/mL). They reported that that the supplementation was effective in raising women’s vitamin D levels, and higher vitamin D level (25OHD > 66 ng/mL) correlated with less joint pain and disability from joint pain.12 In contrast, another prospective study with similar design only supplemented the women with vitamin D levels of < 40 ng/mL with 16,000 IU of oral vitamin D3 every 2 weeks. Half of the women failed to achieve vitamin D levels ≥ 40 ng/mL at 3 months, and there was no improvement in joint pains.29
The VITamin D and OmegA-3 TriaL (VITAL) trial13 then randomized 160 women starting adjuvant AI therapy with < 40 ng/ mL vitamin D level to receive either vitamin D3 at 30,000 IU weekly, or placebo. They reported that the women in the high-dose vitamin D arm had a lower incidence of MSK events (worsening pain or disability, or discontinuation of the AI), although the arthralgia was only significantly improved in the vitamin D arm when assessing joint pain with the Brief Pain Inventory, but not with the Health Assessment Questionnaire.13 These results were somewhat inconsistent because arthralgia was not consistently improved with high-dose vitamin D, and they did not investigate the physiology of the potential role for vitamin D in treating AIA.
Because we are faced with inconsistent results on the question of vitamin D and AIA, we must consider the possibility that AIA is not driven simply by vitamin D, but perhaps a more complex constellation of factors. For example, heterogeneity in VDR might play a role in AIA as well. When considering the entire subset of 878 patients,21 we found that presence of the Fok-I VDR variant genotype was associated with significantly lower probability of developing AIA (Table 4). Those with the Fok-I VDR variant also had significantly lower IL-1β levels at 6 months. This could be correlated to the fact that those with the variant SNP genotype are known to be more sensitive to vitamin D, with lower levels of exogenous vitamin D resulting in higher vitamin D levels compared with those of wild type.19 Thus, because the vitamin D level tended to increase in patients on this study, we hypothesize that those with the Fok-I VDR were more sensitive to this increase, resulting in more suppression of inflammatory cytokine IL-1β. Because IL-1β is one of the most important inflammatory cytokines implicated in arthritis,30 this might be associated with AIA if we were to study large numbers of patients.
We investigated only a small subset of patients from MA.27. Although the futility analysis showed that there was a low likelihood of finding a significant result if we examined additional patients, the study was only powered to find a difference in vitamin D levels between the arms of no smaller than 9 to 13 ng/mL. Furthermore, all of the patients included in the current analysis were Caucasian and living in the United States or Canada. Because vitamin D is dependent on skin color, sunlight exposure, and genetic variation, this lack of racial and geographic heterogeneity is a limitation.
The results of our study raise interesting clinical questions for future investigation. For example, the data do imply that individual host factors might play a role in a woman’s vitamin D metabolism and inflammatory cytokines. These factors, in turn, might influence a woman’s likelihood of developing AIA. Specifically, the lower 6-month IL-1β level and the lower incidence of AIA among patients with the Fok-I VDR variant genotype is thought-provoking. This truncated form of the VDR is known to be associated with a lower risk of breast cancer compared with the wild type.31,32 Perhaps the lower IL-1β level in the patients with the variant polymorphism confers an advantage in terms of breast cancer recurrence, as would be supported by studies that suggest an adverse effect of IL-1β in breast cancer.33,34 Also, IL-1β has been implicated in other joint pain syndromes, such as rheumatoid arthritis.35 Thus, the lower level of IL-1β might be associated with a decreased rate of AIA in patients with the Fok-I variant. However, this remains speculative, and should be studied in the future.
Conclusion
This retrospective analysis of 204 plasma samples from the MA.27 study indicated that there is likely no correlation between vitamin D levels and the development of AIA in the general population. However, in the subset of patients with the Fok-I VDR variant genotype, there is an association with lower IL-1β levels during AI therapy and a lower rate of AIA. Therefore, host heterogeneity might play a critical role in vitamin D metabolism and development of AIA.
Clinical Practice Points.
Although AIA is a common reason for noncompliance among women who are taking AI therapy, the cause and treatment of this syndrome remain unclear.
Many treatments, including vitamin D, have been tried with varying levels of success.
In our retrospective study of MA.27 serum samples, we found that changes in vitamin D level were not correlated with development of AIA.
However, those with a particular VDR polymorphism (Fok-I) do have lower levels of the inflammatory cytokine, IL-1β, and they are less likely to develop AIA.
This raises the question of whether IL-1β, which is known to be important in rheumatoid arthritis, could also play a role in the pathogenesis of AIA.
Finally, this work suggests that host heterogeneity might be a factor in determining who develops AIA, and it might help us to identify certain populations for future clinical trials, who would stand to benefit the most from AIA treatment.
Acknowledgments
Dr Mark Silberman completed vitamin D quantitation on all plasma samples.
The current analysis was supported by the National Institutes of Health/National Cancer Institute Spore Career Development Award at Baylor College of Medicine: P50CA186784. The research was also supported by Canadian Cancer Society Research Institute Grant 015469.
Footnotes
Declaration of Interest
Dr Bartlett receives research funding from NanoString Technologies, Stratifyer, MammaPrint, and Genoptix. He also is a consultant for Insight Genetics, BioNTech, Due North, and Biotheranostics. Dr Osborne is a consultant for AstraZeneca, Roche, and Roche/Ventana. Dr Pritchard has received speakers fees, honoraria, and consultant fees from Novartis, Esai, Pfizer, AstraZeneca, Roche, Eli Lilly, and Genomic Health. Dr Niravath has served as a consultant for Novartis.
References
- 1.Rimawi M, Osborne C. Adjuvant systemic therapy: endocrine therapy In: Harris J, Morrow M, Lippman M, Osborne C, eds. Diseases of the Breast. 4th ed Philadelphia, PA: Lippincoot Williams & Wilkins; 2010:610–30. [Google Scholar]
- 2.Crew K, Greenlee H, Capodice J. Prevalence of joint symptoms in postmenopausal women taking aromatase inhibitors for early stage breast cancer. J Clin Oncol 2007; 25:3877–83. [DOI] [PubMed] [Google Scholar]
- 3.Boonstra A, van Zadelhoff J, Timmer-Bonte A, Ottevanger P, Beurskens C, van Laarhoven H. Arthralgia during aromatase inhibitor treatment in early breast cancer patients: prevalence, impact, and recognition by healthcare providers. Cancer Nurs 2013; 36:52–9. [DOI] [PubMed] [Google Scholar]
- 4.Fenlon D, Addington-Hall J, O’Callaghan A, Clough J, Nicholls P, Simmonds P. A survey of joint and muscle aches, pain, and stiffness comparing women with and without breast cancer. J Pain Symptom Manage 2013; 46:523–35. [DOI] [PubMed] [Google Scholar]
- 5.Henry N, Giles J, Ang D, et al. Prospective characterization of musculoskeletal symptoms in early stage breast cancer patients treated with aromatase inhibitors. Breast Cancer Res Treat 2008; 111:365–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Presant C, Bosserman L, Young T, et al. Aromatase inhibitor-associated arthralgia and/or bone pain: frequency and characterization in non-clinical trial patients. Clin Breast Cancer 2007; 7:775–8. [DOI] [PubMed] [Google Scholar]
- 7.Partridge A, LaFountain A, Mayer E, et al. Adherence to initial adjuvant anastrazole therapy among women with early stage breast cancer. J Clin Oncol 2008; 26:556–62. [DOI] [PubMed] [Google Scholar]
- 8.Gallagher J, Riggs B, Deluca H. Effect of estrogen on calcium absorption and serum vitamin D metabolites in postmenopausal osteoporosis. J Clin Endocrinol Metab 1980; 51:1359–64. [DOI] [PubMed] [Google Scholar]
- 9.Imtiaz S, Siddiqui N, Raza S, Loya A, Muhammad A. Vitamin D deficiency in newly diagnosed breast cancer patients. Indian J Endocrinol Metab 2012; 16:409–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coleman R, Rathbone E, Marshall H, Wilson C, Brown J. Vitamin D, but not bone turnover markers, predict relapse in women with early breast cancer: an AZURE translational study. Cancer Res 2012; 72(24 Suppl), Abstract nr S6–4. [Google Scholar]
- 11.Chlebowski R, Johnson K, Lane D, et al. 25-hydroxyvitamin D concentration, vitamin D intake and joint symptoms in postmenopausal women. Maturitas 2011; 68:73–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khan QJ, Reddy PS, Kimler BF, et al. Effect of Vitamin D supplementation on serum 25 hydroxy vitamin D levels, joint pains, and fatigue in women starting adjuvant letrozole treatment for breast cancer. Breast Cancer Res Treat 2010; 119: 111–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khan Q, Kimler B, Reddy P, Sharma P, Klemp J, Fabian C. Randomized trial of vitamin D3 to prevent worsening of musculoskeletal symptoms and fatigue in women with breast cancer starting adjuvant letrozole: the VITAL trial Presented at American Society of Clinical Oncology (ASCO), Chicago, IL, June 1–5, 2012. [Google Scholar]
- 14.Villagio B, Soldano S, Cutolo M. 1,25-dihydroxyvitamin D3 downregulates aromatase expression and inflammatory cytokines in human macrophages. Clin Exp Rheumatol 2012; 30:934–8. [PubMed] [Google Scholar]
- 15.Grossmann R, Zughaier S, Liu S, Lyles R, Tangpricha V. Impact of vitamin D supplementation on markers of inflammation in adults with cystic fibrosis hospitalized for a pulmonary exacerbation. Eur J Clin Nutr 2012; 66:1072–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Servitja S, Martos T, Rodriguez S, et al. Skeletal adverse effects with aromatase inhibitors in early breast cancer: evidence to date and clinical guidance. Ther Adv Med Oncol 2015; 7:291–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferrari S, Rizzoli R, Manen D, Slosman D, Bonjour J. Vitamin D receptor gene start codon polymorphisms (FokI) and bone mineral density: interaction with age, dietary calcium, and 3′-end region polymorphisms. J Bone Miner Res 1998; 13: 925–30. [DOI] [PubMed] [Google Scholar]
- 18.Whitfield G, Remus L, Jurutka P, et al. Functionally relevant polymorphisms in the human nuclear vitamin D receptor gene. Mol Cell Endocrinol 2001; 177:145–59. [DOI] [PubMed] [Google Scholar]
- 19.Neyestani T, Djazayery A, Shab-Bidar S, et al. Vitamin D receptor Fok-I polymorphism modulates diabetic host response to vitamin D intake: need for a nutrigenetic approach. Diabetes Care 2013; 36:550–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goss P, Ingle J, Pritchard K, et al. Exemestane versus anastrozole in postmenopausal women with early breast cancer: NCIC CTG MA.27ea randomized controlled phase III trial. J Clin Oncol 2013; 31:1398–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ingle J, Schaid D, Goss P, et al. Genome-wide associations and functional genomic studies of musculoskeletal adverse events in women receiving aromatase inhibitors. J Clin Oncol 2010; 28:4674–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goss P, Hershman D, Cheung A, et al. Effects of adjuvant exemestane versus anastrozole on bone mineral density for women with early breast cancer (MA. 27B): a companion analysis of a randomised controlled trial. Lancet Oncol 2014; 15:474–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sachs M, Shoben A, Levin G, et al. Estimating mean annual 25-hydroxyvitamin D concentrations from single measurements: the Multi-Ethnic Study of Atherosclerosis. Am J Clin Nutr 2013; 97:1243–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jennison C, Turnbull B. Group Sequential Methods With Applications to Clinical Trials, Chapter 11: General Group Sequential Distribution Theory, pages 221–234, Boca Raton, FL: CRC Press; 1999. [Google Scholar]
- 25.Singer O, Cigler T, Moore A, Levine A, Do H, Mandl L. Hypovitaminosis D is a predictor of aromatase inhibitor musculokeletal symptoms. Breast J 2014; 20:174–9. [DOI] [PubMed] [Google Scholar]
- 26.Helzlsouer K, Gallicchio L, MacDonald R, Wood B, Rushovich E. A prospective study of aromatase inhibtor therapy, vitamin D, C-reactive protein and musculoskeletal symptoms. Breast Cancer Res Treat 2012; 131:277–85. [DOI] [PubMed] [Google Scholar]
- 27.Waltman N, Ott C, Twiss J, Gross G, Lindsey A. Vitamin D insufficiency and musculoskeletal symptoms in breast cancer survivors on aromatase inhibitor therapy. Cancer Nurs 2009; 32:143–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Singh S, Cuzick J, Mesher D, Richmond B, Howell A. Effect of baseline serum vitamin D levels on aromatase inhibitors induced musculoskeletal symptoms: results from the IBIS-II, chemoprevention study using anastrozole. Breast Cancer Res Treat 2012; 132:625–9. [DOI] [PubMed] [Google Scholar]
- 29.Prieto-Alhambra D, Kassim Javiad M, Servitja S, et al. Vitamin D threshold to prevent aromotase inhibitor induced arthrlagia: a prospective study. Breast Cancer Res Treat 2011; 125:869–78. [DOI] [PubMed] [Google Scholar]
- 30.Lawrence JT, Birmingham J, Toth AP. Emerging ideas: prevention of post-traumatic arthritis through interleukin-1 and tumor necrosis factor-alpha inihibition. Clin Orthop Relat Res 2011; 469:3522–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Raimondi S, Johansson H, Maisonneuve P, Gandini S. Review and meta-analysis on vitamin D receptor polymorphisms and cancer risk. Carcinogenesis 2009; 30: 1170–80. [DOI] [PubMed] [Google Scholar]
- 32.Tang C, Chen N, Wu M, Yuan H, Du Y. Fok1 polymorphism of vitamin D receptor gene contributes to breast cancer susceptibility: a meta-analysis. Breast Cancer Res Treat 2009; 117:391–9. [DOI] [PubMed] [Google Scholar]
- 33.Snoussi K, Strosberg AD, Bouaouina N, Ben Ahmed S, Chouchane L. Genetic variation in pro-inflammatory cytokines (interleukin-1β, interleukin-1α and interleukin-6) associated with the aggressive forms, survival, and relapse prediction of breast carcinoma. Eur Cytokine Netw 2005; 16:253–60. [PubMed] [Google Scholar]
- 34.Ben-Baruch A Host microenvironment in breast cancer development: inflammatory cells, cytokines and chemokines in breast cancer progression - reciprocal tumoremicroenvironment interactions. Breast Cancer Res 2002; 5:31–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kay J, Calabrese L. The role of interleukin-1 in the pathogenesis of rheumatoid arthritis. Rhematology 2004; 43(suppl 3):iii2–9. [DOI] [PubMed] [Google Scholar]


