Abstract
Purpose:
Enzyme replacement therapy (ERT) with recombinant human acid-α glucosidase (rhGAA) at standard dose of 20 mg/kg every other week is insufficient to halt the long-term progression of myopathy in Pompe disease.
Methods:
We conducted a retrospective study on infantile-onset Pompe disease (IPD) and late-onset Pompe disease (LOPD) patients with onset before age 5 years, ≥12 months of treatment with standard dose ERT, and rhGAA immunogenic tolerance prior to dose escalation. Long-term follow-up of up to 18 years was obtained. We obtained physical therapy, lingual strength, biochemical, and pulmonary assessments as dose was escalated.
Results:
Eleven patients with IPD (n = 7) or LOPD (n = 4) were treated with higher doses of up to 40 mg/kg weekly. There were improvements in gross motor function measure in 9/10 patients, in lingual strength in 6/6 patients, and in pulmonary function in 4/11. Significant reductions in urinary glucose tetrasaccharide, creatine kinase, aspartate aminotransferase, and alanine aminotransferase were observed at 40 mg/kg weekly compared with lower doses (p < 0.05). No safety or immunogenicity concerns were observed at higher doses.
Conclusion:
Higher rhGAA doses are safe, improve gross motor outcomes, lingual strength, pulmonary function measures, and biochemical markers in early-onset Pompe disease, and should be considered in patients with clinical and functional decline.
Keywords: alglucosidase alfa, Pompe disease, enzyme replacement therapy, high dose, recombinant human GAA
INTRODUCTION
Pompe disease or glycogen storage disease type II (OMIM 232300) is a lysosomal storage disorder caused by deficiency of the enzyme acid-α glucosidase (GAA).1 Classic infantile-onset Pompe disease (IPD) presents with hypertrophic cardiomyopathy and profound muscle weakness soon after birth. If untreated, rapid progression results in death by cardiopulmonary failure within the first two years of life.2 Patients with late-onset Pompe disease (LOPD) may present as early as the first year of life to as late as the sixth decade. However, patients with LOPD typically have a slower rate of progression and little to no cardiac involvement.3
Intravenous enzyme replacement therapy (ERT) with recombinant human GAA (rhGAA), also known as alglucosidase alfa, received FDA approval in 2006 to treat both IPD and LOPD. The pivotal clinical trial in IPD patients demonstrated increased ventilator-free survival and improved cardiac function in the first 18 months at a cumulative dose of 20 mg/kg every other week (eow).4–6 Clinical benefit in LOPD patients treated with ERT at the same dose was also demonstrated in a randomized, placebo-controlled trial with improved performance on a six-minute walk test (6MWT) and stabilization of upright forced vital capacity (FVC).7 Factors known to impact ERT efficacy include age, extent of disease burden at the start of therapy, cross-reactive immune material (CRIM) status, and IgG antibody titers.8,9
Over the past decade, it has become increasingly evident that IPD patients who initially respond well to treatment continue to have sustained cardiac benefits, but have a residual myopathy and respiratory decline that progresses despite therapy.9–13 These diminishing responses are observed even in ideal candidates for ERT: those identified as newborns and treated from an early age, producing little to no rhGAA antibodies and those who are CRIM positive.9,14 Progressive muscle weakness, gait abnormalities, contractures, deformities like scoliosis, ptosis, myopathic facies, hypernasal speech, sensorineural hearing loss, gastroesophageal reflux, dysphagia, urinary incontinence, and respiratory decline necessitating ventilation are noted in long-term survivors despite ERT.10 This clinical plateau and subsequent decline is typically noted after 20–24 months on ERT.9,10 It is accompanied by a rise in creatine kinase (CK), and urinary glucose tetrasaccharide (Glc4), the latter suggesting reduced glycogen clearance.10 Furthermore, rhGAA is primarily metabolized by the liver and only a fraction reaches skeletal muscle.15 In addition, as IPD survivors can now live through their second decade of life, increased growth rate and high metabolic demand at puberty results in more clinical decline (P.S.K., personal communication). This plateau and decline in function after approximately two years on ERT, even with early treatment initiation, suggests a need for more effective therapy including consideration of higher dosing regimens.10,16–18
In the pivotal clinical trial two doses were used in patients for 52 weeks: 40 mg/kg eow or 20 mg/kg eow. ERT increased skeletal muscle GAA activity in all patients, but to a greater degree in patients receiving 40 mg/kg eow.5 Although clinical benefit was not apparent with the higher dose, the treatment groups were not appropriately stratified: patients with the higher dose were CRIM negative or had developed antibody titers to the ERT. Thus, it is difficult to draw conclusions on dosing from this trial. As the standard dose can be inadequate in the longer term, clinicians and researchers have treated patients with higher doses and observed significant clinical improvement with minimal adverse events.16,19–21
Here we report the impact of higher rhGAA dosing regimens on clinical outcomes, efficacy, and safety profile in patients with IPD and early-onset LOPD, who showed clinical plateau or decline when treated with a standard dose of ERT. We show the impact of a higher dose through novel motor, lingual, pulmonary measures and through biomarkers. Additionally, we are the first to present higher ERT dose findings in LOPD patients. We also review the literature supporting higher ERT dosing in Pompe disease.
MATERIALS AND METHODS
Ethics approval
This retrospective study was part of the Duke Long-term Follow-up Pompe protocol (Pro00010830), approved by the Duke University School of Medicine Institutional Review Board. Written informed consent was obtained from patients and/or their parent/guardian.
Patient cohort
CRIM positive patients with IPD or early LOPD (onset within the first 5 years of life) were included in the study. The diagnosis was confirmed in all patients by demonstration of low acid ɑ-glucosidase activity and GAA gene sequencing. All patients were initially treated with the standard dose of ERT 20 mg/kg eow for ≥12 months and then with higher doses of up to 40 mg/kg weekly or eow for ≥ 12 months. Those with sustained intermediate (SIT ≥12,800) or high-sustained anti-rhGAA IgG antibody titers (HSAT ≥ 1:51,200) were excluded as these are known to negatively affect treatment outcomes.
Clinical evaluations
Clinical assessments and laboratory evaluations were performed at clinic visits every 6 to 12 months. Information collected included ages at onset, diagnosis, and ERT initiation; CRIM status; pathogenic GAA variants; clinical and physical examination findings; and progression of the clinical course up to the most recent assessment (MRA).
Physical therapy evaluations
Motor function was evaluated at regular intervals through clinical examination and physical therapy (PT) assessments by therapists experienced with patients with Pompe disease. PT assessments included qualitative and quantitative measures of posture, movement, and musculoskeletal status, and quantitative measures using a number of standardized assessments appropriate for the age of the patient at the time of testing. Qualitative assessments were made during clinic visits by asking patients and caregivers specific questions about functional and motor status. Quantitative standardized assessments of motor function included (1) the Stationary and Locomotion Subtests of the Peabody Developmental Motor Scales, 2nd edition (PDMS-2)22 for assessment of children aged 1 to 5 years; (2) the Strength and Agility Composite of the Bruininks–Oseretsky Test of Motor Proficiency, 2nd edition (BOT-2)23 for children at or above 4½ years of age; (3) the Gross Motor Function Measure 88 (GMFM-88)24 for patients of all ages; and (4) the six-minute walk test (6MWT) for patients independently ambulatory (with or without assistive devices), age five years or older and could follow directions.25
All assessments were administered and scored in accordance with standardized test procedures. For PDMS-2 and BOT-2, strength and gross motor function subscales were used. The 6MWT was administered in accordance with American Thoracic Society (ATS) guidelines and was reported as distance walked, with performance as a percentage of the predicted distance for the patient’s gender, age and height.25–30
Lingual muscle strength
An experienced speech–language pathologist quantitatively measured lingual muscle strength at baseline and regular intervals. The Iowa Oral Performance Instrument (IOPI) was used to quantify maximal anterior lingual strength. Following the manufacturer’s guidelines (IOPI Medical, LLC; Redmond, WA), patients were coached to push their tongue against an intraoral tongue bulb against their hard palate with maximal effort. Testing included repeated trials with minimal variability interspersed with rest periods of approximately 30–60 seconds. Lingual strength in kilopascals (kPa) was reported as the highest obtained value and compared with normative data.31
Pulmonary evaluation
Pulmonary function was assessed at baseline and follow-up visits using spirometry in accordance with ATS guidelines by a pediatric pulmonologist experienced with neuromuscular disorders. We measured FVC in an upright position and, when possible, while supine as the latter is a good indicator of diaphragmatic weakness.32
Laboratory evaluation
Laboratory monitoring of plasma CK, aspartate aminotransferase (AST), alanine aminotransferase (ALT), and urine Glc4 was performed at each clinical visit. Glc4 was analyzed by ultraperformance liquid chromatography–tandem mass spectrometry using a modification of a previously reported method and normalized to creatinine values.33 Anti-rhGAA IgG antibodies were assessed by Genzyme Corporation (Framingham, MA).
Statistical analysis
Statistical analyses were performed using GraphPad Prism 8 (GraphPad Software, San Diego, CA; 2018). Descriptive statistics including mean, median, standard deviation, and range were calculated. A comparison of biomarker means at different doses of ERT was performed using a Wilcoxon matched-pairs signed rank test; p values ≤ 0.05 were considered significant.
Review of literature
In addition to our clinical study, we conducted a review of previously published literature, which included patients with Pompe disease on alternate ERT dosing schedules.
RESULTS
Cohort description
Eleven patients with an early-onset form of Pompe disease (classic IPD = 7, early-onset LOPD = 4) were included in the study. Patients were classified as IPD based on the presence of cardiomyopathy in the first year of life. All patients were CRIM positive based on underlying GAA variants. Two of the patients with IPD (patient 4 and patient 5) have been previously discussed in detail in Case et al. as case 1 and case 2 respectively, with further updates on their clinical outcomes presented here.16 A summary of cohort characteristics is shown in Table 1.
Table 1.
Summary of cohort characteristics.
| Patient/sex | Phenotype | GAA genotype (allele 1 and allele 2) | Age at onset | Age at diagnosis | Age at ERT treatment initiation | Age at MRA (years) | Years on ERT | Range of antibody titers |
|---|---|---|---|---|---|---|---|---|
| 1/M | IPD | c.1933G>A and c.1933G>A | <1 month | 1 month | 3 months | 18.2 | 18.0 | 1:1600 to 1:6400 |
| 2/F | IPD | c.1293_1312del and c.1716C>G | 5 weeks | 2 months | 2 months | 10.2 | 10.0 | 1:200 to 1:1600 |
| 3/M | IPD | c.525delT and c.1642G>T, c.1880C>T | Prenatal | Prenatal | 2 weeks | 13.0 | 13.0 | Normal |
| 4/F | IPD | c.655G>A and c.655G>A | 7 months | 7 months | 7 months | 13.4 | 12.9 | ≤1:1600 |
| 5/M | IPD | c.1933G>A and c.1933G>A | 2 months | 2 months | 3 months | 15.1 | 14.8 | 1:200 to 1:3200 |
| 6/M | IPD | c.1438-1G>T and c.1655T>C | Prenatal | Prenatal | 2.4 months | 19.0 | 18.8 | Normal |
| 7/F | IPD | c.1802C>T, c.1726G>A, c.2065G>A and c.1099T>C | 3 months | 3 months | 5.4 months | 15.8 | 14.0 | 1:200 to 1:400 |
| 8/M | LOPD | c.525delT and c.−32-13T>G | 6 months | 10 months | 1.6 years | 15.2 | 13.6 | ≤1:1600 |
| 9/F | LOPD | c.1978C>T and c.1477C>T, c.2221G>A | 3 years | 3 years | 6 years | 15.7 | 9.7 | 1:3200 to 1:6400 |
| 10/F | LOPD | c.953T>C and c.−32-13T>G | 3 years | 12 years | 13 years | 18.2 | 5.2 | 1:400 to 1:800 |
| 11/M | LOPD | c.−32-13T>G and c.1447G>A | 5 months | 5 months | 1.3 years | 13.8 | 12.5 | ≤1:1600 |
ERT enzyme replacement therapy, IPD infantile-onset Pompe disease, LOPD late-onset Pompe disease, MRA most recent assessment, NA not available.
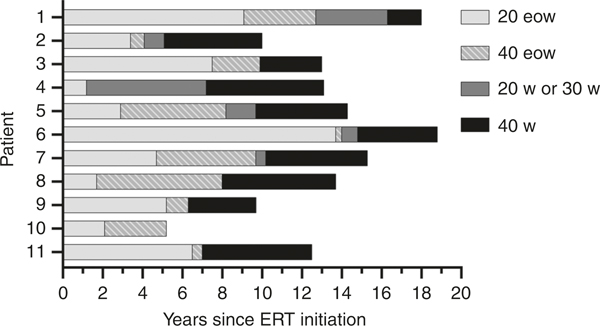
The age of onset for patients with early-onset LOPD ranged from 5 months to 3 years and overlapped with age of onset of the IPD cohort (≤7 months). The median age of the study cohort at the MRA was 15.2 years (range 10.2–18.2 years). By this time, patients had been receiving ERT for 10 to 18.8 years, with the exception of one LOPD patient (patient 10) who was treated for 5.2 years (Table 1). Patients were initially treated with a standard ERT dose of 20 mg/kg eow, which was later titrated to more frequent and/or higher doses of up to 40 mg/kg eow or weekly. The schedule of increasing the dose was customized for each patient individually. Reasons for a higher dose included a plateau or loss of motor function as determined by the treating physician (P.S.K.) and/or poor measurements on standardized physical therapy assessments. The median age at diagnosis, ERT initiation, and time on standard dose before increase are listed in Table S1. The timeline of dose increases is shown in Fig. 1. None of the patients included here developed SIT or HSAT to ERT at any point during treatment following an increase in dose.
Fig. 1. Timeline of dose increases in patients since initiation of enzyme replacement therapy (ERT).
Dose of 20 w or 30 w is an intermediate dose toward the final goal of 40 w. For patient 10, the dose was not increased from 40 eow to 40 w due to conflicts with academic schedules. 20 eow: 20 mg/kg every other week, 40 eow: 40mg/kg every other week; 20 w: 20mg/kg weekly; 30 w: 30mg/kg weekly; 40 w: 40mg/kg weekly.
Motor development history
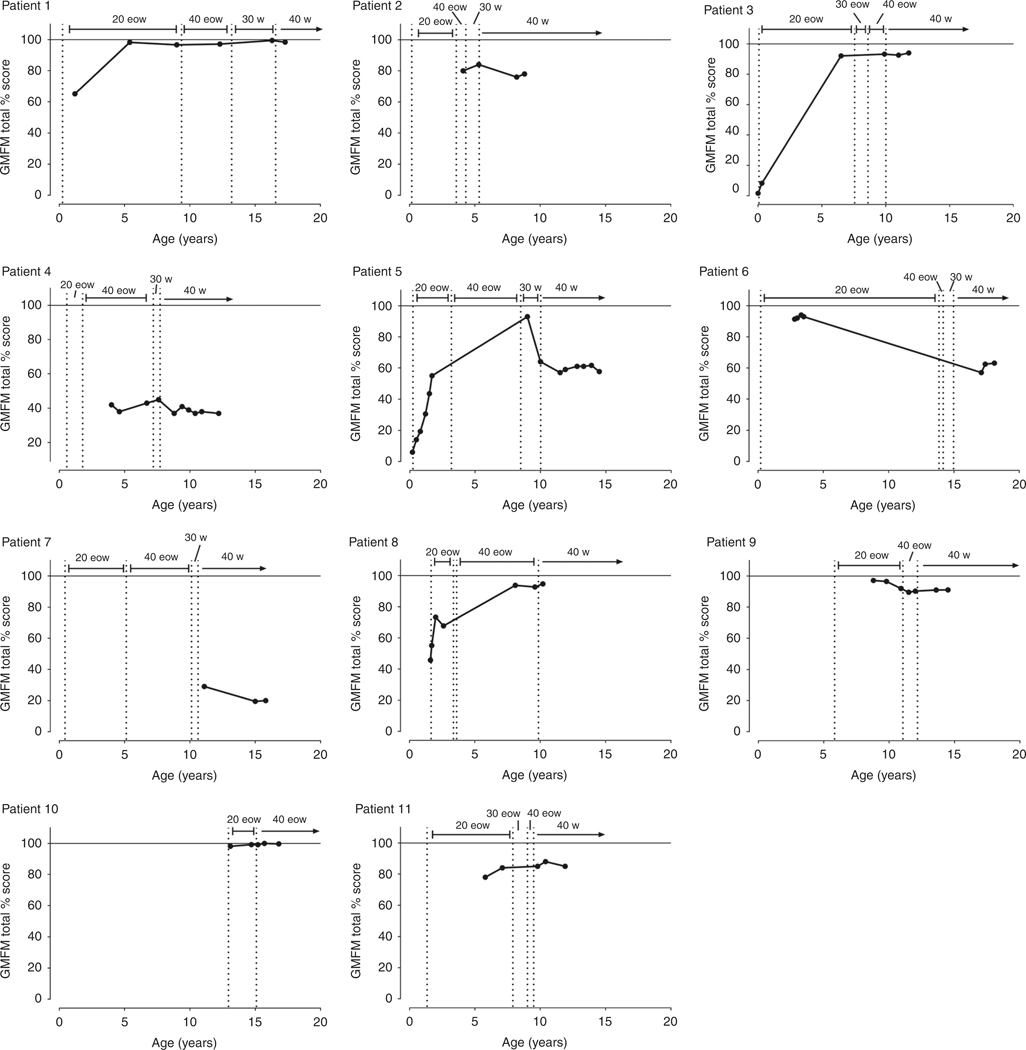
Here, we describe an IPD and LOPD patient (patients 1 and 9 respectively) to exemplify the motor development with an increasing ERT dose. PT assessments included GMFM (shown in Fig. 2) and PDMS-2, BOT-2, and 6MWT (shown in Fig. S1).
Fig. 2. Progression of gross motor function measure (GMFM) in each patient with increasing dose.
20 eow: 20 mg/kg every other week, 40 eow: 40 mg/kg every other week; 30 w: 30 mg/kg weekly; 40 w: 40 mg/kg weekly.
Patient 1 (IPD)
On standard dose:
Between 1.5 and 5 years of age, there was an overall decline in function as measured by the Peabody stationary and locomotion scales. GMFM increased between 2 and 6 years of age and then plateaued at 98%, demonstrating the achievement and relative maintenance of gross motor skills. BOT-2 assessment began at 8 years of age and initially demonstrated an extremely low score at the 1st percentile.
On higher doses:
At the age of 9 years, ERT dose was increased to 40 mg/kg eow. After the dose increase, a small but significant improvement of BOT-2 to the 7th percentile was observed, representing a change from well below average (2 SD) to below average (1 SD). However, at 40 mg/kg eow, the 6MWT declined from 75% to 58% predicted. Thus, the dose was further increased to 30 mg/kg weekly at 13 years of age, and then to 40 mg/kg weekly at 16 years. At this final dose, the patient was able to maintain a GMFM of 99.7%, demonstrating maintenance of motor function and improvement from baseline. In addition, at these higher doses, the 6MWT improved from 58% to 94%.
Patient 9 (LOPD)
On standard dose:
The patient was started on standard dose at the age of 8.8 years. Between the ages of 8.8 and 9.8 years, the patient’s GMFM score was high (97.1% and 96.5% respectively). This declined to 91.9% by age 10.9 years. BOT-2 remained low at the 1st percentile throughout the course of standard therapy, and 6MWT was low but increased from 64.2% to 68.9% at 10 years of age. The 6MWT dropped precipitously to 45.5% at age 10.9.
On higher doses:
Due to declining measures, ERT dose was increased to 40 mg/kg eow at 11 years of age. This stabilized the GMFM at ~90% for the next four years. The 6MWT continued to decline initially but went up to 46.3% by age 12 years. At 12 years, the dose was further increased to 40 mg/kg weekly. At the final dose, the patient was able to maintain steady GMFM (91%) and 6MWT (40%) scores until the end of the follow-up period.
A detailed, qualitative assessment of each patient is provided in Table S2. Based on the GMFM, 9/10 patients (patient 7 not included as she only had GMFM measured on highest dose) in our cohort demonstrated clinical improvement or motor performance stabilization at increased doses. Three of the six IPD patients (1, 3, and 5) demonstrated improvement in motor function, 2/6 (33%) IPD patients (2 and 4) remained stable, and only one showed clinical decline (patient 6). Three of four (75%) LOPD patients (8, 9, and 10) further showed an improvement in motor function as measured by GMFM.
Similarly, of patients with 6MWT percentile scores on low and high doses, there was marked improvement in 1/5 (patient 1), stabilization in 3/5 (patients 2, 3, and 9) and decline in 1/5 (patient 11). At present, all but three (patients 4, 6, and 7) can walk independently without assistive devices; the exceptions are wheelchair assisted. Individual patients demonstrated improvement or stabilization of scores on higher doses (shown in Fig. 2 and Fig S1, and described in Table S2).
Lingual strength
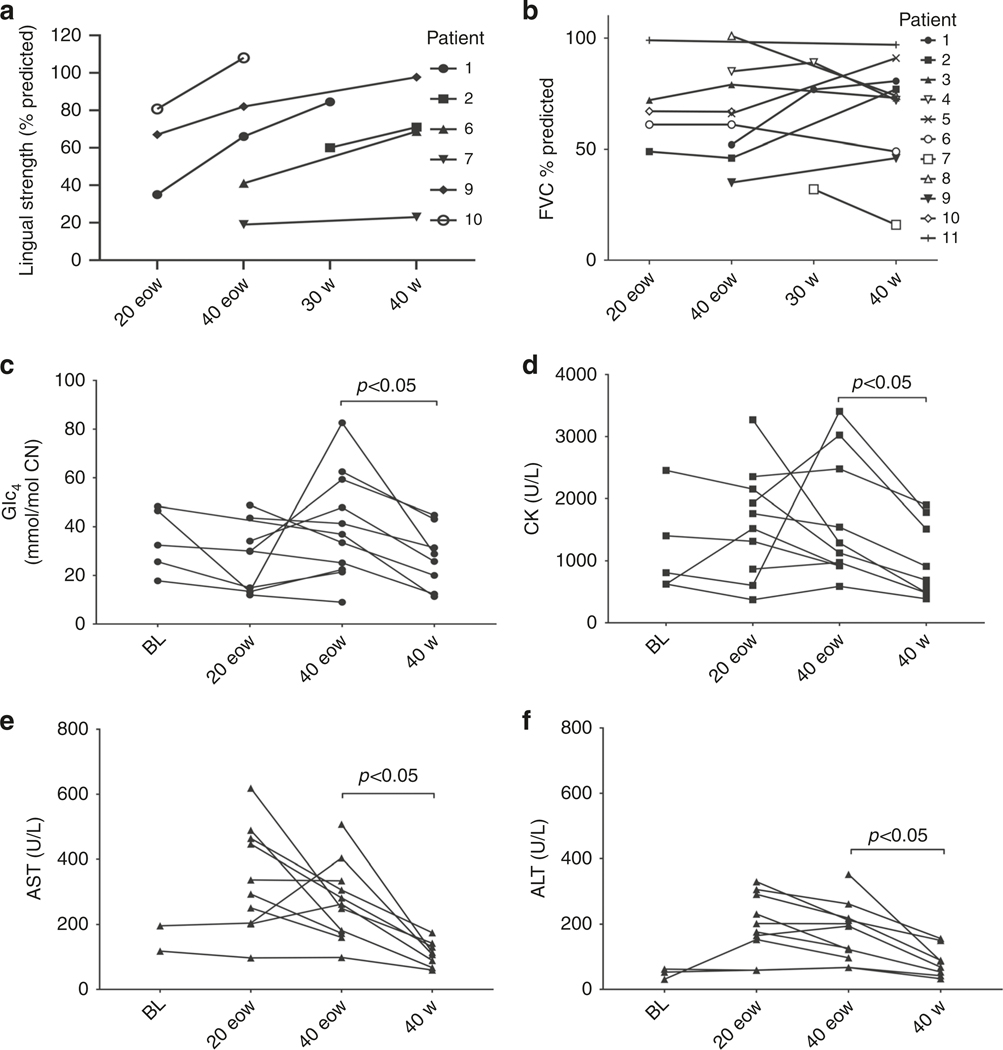
Lingual strength was assessed on a subset of patients including four with IPD (patients 1, 2, 6, and 7) and two with LOPD (patients 9 and 10). Mean scores were calculated for each patient at each dose and the trends are shown in Fig. 3a. An increase in lingual strength from 4% to 50% of predicted strength (median increase: 28%) was observed in response to a dose increase from 20 to 30–40 mg/kg weekly or eow.
Fig. 3. Changes in lingual strength, pulmonary function and biomarkers with dose increase.
Trends in (a) lingual strength, (b) upright FVC percent predicted, and biomarkers (c) Glc4, (d) CK, (e) AST, and (f) ALT with increasing dose. 20 eow: 20mg/kg every other week, 40 eow: 40mg/kg every other week; 30 w: 30mg/kg weekly; 40 w: 40mg/kg weekly. ALT alanine aminotransferase, AST aspartate aminotransferase, BL baseline, CK creatine kinase, FVC forced vital capacity, Glc4 glucose tetrasaccharide. Reference values: urine Glc4 values: <4mmol/mol Cr, CK values: 22–198 U/L, AST value: 10–40 U/L, ALT value: 7–56 U/L.
Pulmonary status
Pulmonary function was assessed at least annually as standard-of-care. Pulmonary function tests included upright and supine spirometry. Importantly, no patients had increasing ventilator needs or had become ventilator dependent at the time of this report. Two patients (7 and 9) required continued nocturnal bilevel positive pressure ventilation (BiPAP). Patient 9 had started using BiPAP while on standard dose, and patient 7 started using BiPAP just as her dose was increased to 40 mg/kg weekly. Five patients (4, 5, 7, 9, and 10) had a cough assist device at home. At higher ERT doses, the mean percent predicted upright forced vital capacity (FVC) generally remained stable and even improved for four patients (1, 2, 5, and 9). A nonsignificant improvement 7% in mean supine FVC was observed at 40 mg/kg weekly. The median FVC across patients increased with the higher dose: from 67% to 73% predicted upright, and from 53% to 68.5% predicted supine. Trends in variation of FVC with dose increases are shown in Fig. 3b.
Biochemical markers
Mean urinary Glc4 and serum CK, AST, and ALT at each dose were calculated per patient and the trends are shown in Fig. 3c–f. Biomarker concentrations were comparable for both IPD and LOPD cohorts over the course of the treatment. There was no significant difference in biomarkers between the standard dose (20 mg/kg eow) and intermediate dose (40 mg/kg eow). However, a significant (p < 0.05) decrease was observed in all four biomarkers when the dose was increased from 40 mg/kg eow to 40 mg/kg weekly. Thus, while values did not reach within the clinically normal range, the values of all four biochemical markers decreased on the highest ERT doses (Table S3).
Individual trends in Glc4 are shown in Fig. S2. Baseline Glc4 values were determined in five patients (1, 3, 8, 10, 11) and tended to decrease with standard ERT in all but two LOPD patients (8 and 11) who started ERT at 1.6 and 1.3 years, respectively. In four IPD patients (1, 3, 6, and 7) Glc4 initially remained within the normal range for the first two years on standard ERT but then increased. Comparably, IPD patients 2 and 5 had persistently elevated Glc4 while on the standard dose. Glc4 did not normalize in any of the patients in the LOPD cohort on the standard dose, and instead showed an upward trend (patients 8 and 9) or remained relatively constant (patients 10 and 11). The Glc4 response to the intermediate doses (30 and 40 mg/kg eow) was variable, with a decrease observed for patients 1, 5, 8, and 10 whereas patients 2, 3, 4, 7, 9, and 11 showed little or no improvement. A downward trend in Glc4 at the highest dose of 40 mg/kg weekly was observed for 9 of the 11 patients (1, 3, 4, 5, 6, 7, 9, 10, and 11). No decrease was observed for patients 2 and 8 after two years on this dose, although patient 8 maintained the lower concentrations achieved at the 40 mg/kg eow dose.
Safety outcomes
Based on inclusion criteria, none of the patients developed significant antibody titers to ERT at standard dose, and even as dose was increased, no development of titers was seen (Table 1). Antibody titers ranged from 1:6400 to 1:400 in our patient cohort throughout the course of treatment. Patients who developed infusion-associated reactions (IARs) did so with the initial standard dose of 20 mg/kg eow. No new IARs were reported at any of the higher doses.
Review of literature
A review of higher dosing in Pompe disease is elaborated in Table 2.16,19,20,34,35
Table 2.
Review of literature elaborating previous studies with higher ERT dosing in Pompe disease.
| Study | Study type, # of patients, doses | Results |
|---|---|---|
| Recombinant human acid [alpha]-glucosidase: major clinical benefits in infantile-onset Pompe disease. Kishnani PS, Corzo D, Nicolino M, et al. (2007)5 | Clinical trial, 18 patients total 9 patients: 20 mg/kg eow 9 patients: 40 mg/kg eow | 40 mg/kg eow group cleared glycogen more effectively in muscle; however, no clinically apparent difference seen between groups; higher dose group had CRIM negative patients and those who developed HSAT |
| Improvement of bilateral ptosis on higher dose enzyme replacement therapy in Pompe disease. Yanovitch TL, Casey R, Banugaria SG, Kishnani PS. (2010)35 | Case report Dose was increased from 20 mg/kg eow to 40 mg/kg eow | 17 year nonclassic IPD patient showed improvement in muscle function and partial resolution of ptosis when dose was increased |
| Safety and efficacy of alternative alglucosidase alfa regimens in Pompe disease. Case LE, Bjartmar C, Morgan C, et al. (2015)16 | Randomized open-label study 11 CRIM positive patients (4 LOPD, 7 IPD) 2 doses: 20 mg/kg weekly or 40 mg/kg eow | Improvement in GMFM, no difference in Pompe-PEDI score or manual muscle testing |
| Effects of a higher dose of alglucosidase alfa on ventilatorfree survival and motor outcome in classic infantile Pompe disease: an open-label single-center study. van Gelder CM, Poelman E, Plug I, et al. (2016)19 | 8 CRIM positive IPD patients 4 patients: 20 mg/kg eow 4 patients: 40 mg/kg weekly | 1 patient in low dose group was ventilation dependent at end of study (none in high dose group) 3/4 patients on low dose had repeated respiratory infections (no infections in high dose group) LVMI was normal in 3/4 of low dose group, all in high dose group had normal LVMI Walking at study end: 2/4 in low dose, all in high dose |
| Pompe disease treatment with twice a week high dose alglucoside alfa in a patient with severe dilated cardiomyopathy. Landis JL, Hyland H, Kindel SJ, Punnoose A, Geddes GC. (2018)20 | Case report Dose: 40 mg/kg twice a week | Cardiac status of CRIM positive IPD patient showed drastic improvements in left ventricular ejection fraction, LVMI, and left ventricular dilation |
| Recombinant human a-glucosidase from rabbit milk in Pompe patients Van den Hout, H Reuser, A Vulto A et al. (2000)34, a |
Open-label pilot study, 4 patients total 2 patients (over 6.5 kg): 15 mg/kg weekly 2 patients (under 6.5 kg): 20 mg/kg weekly Eventually all on 40 mg/kg weekly | 36 weeks after therapy: lysosomal glycogen content decreased, skeletal muscle glycogen did not significantly decrease, decline in LVMI in all patients, stabilization of clinical condition |
CHO Chinese hamster ovary, CRIM cross-reactive immune material, eow every other week, ERT enzyme replacement therapy, GMFM gross motor function measure, HSAT high and sustained antibody titers, IPD infantile-onset Pompe disease, LOPD late-onset Pompe disease, LVMI left ventricular mass index, PEDI Pediatric Evaluation of Disability Index.
This study used transgenic enzyme from rabbit milk (rest are from Chinese hamster ovary cell line).
DISCUSSION
Our study
We studied a cohort of 11 IPD and LOPD patients and followed them longitudinally for over 18 years. Most previous reports on higher dosing have followed patients for shorter durations and have not included LOPD patients. Our study demonstrated safety and efficacy of higher ERT dosing in IPD and LOPD patients. Infusions at higher doses were well tolerated and no IARs were observed. Anti-rhGAA antibody titers remained absent or unchanged at low levels. The clinical improvement in motor outcomes to dose escalation noted in our patients is consistent with previous observations in other patient cohorts.16,19,36
This study is the first to perform regular functional assessments to observe clinical outcomes as ERT dose was increased in a stepwise manner. In previous studies, cohorts of different dosing regimens were compared at treatment outset. In the current study, however, each patient can be compared with themselves on previous doses thus serving as internal controls. Patients in this study cohort are among the oldest survivors with IPD or early-onset LOPD and their clinical decline represents an unmet need for more effective therapy.
Our study is the first to look at functional measures such as 6MWT, PDMS-2, and BOT-2 for patients on a higher ERT dose. In addition, we are the first to report improvement in lingual strength and pulmonary function tests on an increasing ERT dose. All patients in the current study initially responded favorably to standard ERT with improvements in muscle function from baseline as demonstrated by improvement in their GMFM, PDMS-2, BOT-2, and 6MWT tests. As they advanced in age, however, they developed varying degrees of residual myopathy. Trends in standardized PT assessments showed improvement at the standard dose of 20 mg/kg eow followed by gradual decline and subsequent improvement at higher doses.
We are the first to report biochemical trends with an increasing ERT dose. A significant reduction was seen in biomarkers Glc4, CK, ALT and AST at the highest dose of ERT representing efficient clearance of glycogen from tissues. Pulmonary function testing improved in two of the LOPD patients and four of the IPD patients at higher rhGAA doses. We included both upright and supine assessments of FVC, as the latter is known to be an indicator of diaphragmatic function. It is known that despite standard dose of ERT, IPD patients can develop ventilator dependence in the second decade of life.12 In this study, we show improvement in pulmonary function but even more importantly, ventilator-free survival in our long-term follow-up of IPD patients.
Justification for higher dose regimens
Pediatric patients eliminate rhGAA faster than adults. This is evidenced by low area under the curve (AUC) values at standard doses of ERT. However, as the dose is increased to 40 mg/kg eow in children, AUC values approach closer to those in adults on standard doses.37 In short, pediatric patients require higher dosing regimens to match the pharmacokinetics of rhGAA seen in adults.
Early preclinical studies investigating efficacy of rhGAA showed that immune-tolerant mice on a 20 mg/kg dose had stabilization of disease, yet glycogen clearance in tissues was not complete. Mice on higher dosing regimens of 100 mg/kg weekly safely tolerated ERT and completely cleared glycogen from tissues.15
In previous studies, we have seen that a dose of 40 mg/kg weekly is safely tolerated in patients.19 Collective experience from earlier trials, evidence of greater clearance in children, and indication of safety from the murine model led to selecting a high dose of 40 mg/kg weekly in our patients.
An important concern among treating physicians is when to initiate or transition to a higher dose of rhGAA. Given the rapid progression of musculoskeletal involvement in IPD and patients with early-onset LOPD, initiation or early administration of a higher dose should strongly be considered before significant muscle damage develops. A difference of even a few days in ERT initiation affects treatment outcomes in IPD patients.38
Ideally, IPD patients should be started on 40 mg/kg weekly from the beginning. Where this may not be possible, patients should be followed closely and disease progression should be carefully examined. Similarly, LOPD patients should also have close monitoring of disease and progression.
For IPD and LOPD patients who are on a standard dose of 20 mg/kg eow, a combination of factors may be used to guide the clinical decision on dose escalation. Plateau or decline in motor function identified during clinical assessments during follow-up visits are indicative of poor response to treatment, and emphasize the importance of using repeated standardized measures across time. Worsening performance on various PT assessments such as PDMS-2, BOT-2, GMFM-88, and 6MWT distance indicate poor response to ERT. Individuals and caregivers should be asked about increased fatigue, decreased endurance, and decreased ability to keep up with peers. A decline in pulmonary function testing with a significant drop in FVC from upright to supine is also indicative of a deteriorating clinical course. Persistent elevation in biomarkers may warrant dose escalation; elevated levels of urinary Glc4 suggest an inadequate response, and have been shown to be closely correlated with skeletal muscle glycogen content and the clinical response in the early phase of treatment.39 Increasing trends in serum CK, ALT, and AST levels were found to closely reflect the extent of skeletal muscle damage.40 Higher ERT dose may also be required in times of high metabolic demand and growth such as puberty.
Limitations
Systematic studies and clinical trials involving dosing are difficult to perform once therapy is approved at a standard dose. This study was based on clinical experience, and to address an unmet need for more effective therapy. The patients reported were from a single site, and were selected as they are followed at our center; their complete clinical data (PT, pulmonary assessments and laboratory values) were available for analysis. There is variability in ERT initiation time and the dose increase schedule. Similarly, not all patients were able to undergo all assessments at set time-points. Motor assessments may also vary based on patient effort at different visits. For future studies, the impact of treatment over time needs to be collected via larger, multisite, postmarketing studies, and with the help of an international registry.
Conclusion and future directions
Higher dose of rhGAA is a safe and effective treatment option for patients with Pompe disease who demonstrate a suboptimal response, plateau, or clinical decline at the standard dose in the absence of IARs and clinically significant anti-rhGAA antibody titers. The plateauing of muscle response after the initial few years of treatment highlights the need for a higher dose.
Our cohort of IPD and LOPD patients were followed systematically by a group with clinical expertise. We were able to demonstrate the clear benefit of higher dosing regimens of rhGAA in Pompe disease.
Studies with superior skeletal muscle targeting with the development of next-generation therapies are underway. Even though ERT has changed the course of natural history in Pompe disease, it leaves much scope for improvement in terms of long-term outcomes and quality of life.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Mugdha Rairikar, Heidi Cope, and Katie Barrett for their support with the manuscript.
DISCLOSURE
P.S.K. has received grant support from Sanofi Genzyme, Valerion Therapeutics, Shire Pharmaceuticals, and Amicus Therapeutics. P.S.K. has received consulting fees and honoraria from Sanofi Genzyme, Shire Pharmaceuticals, Amicus Therapeutics, Vertex Pharmaceuticals, and Asklepios BioPharmaceutical, Inc. (AskBio). P.S.K. is a member of the Pompe and Gaucher Disease Registry Advisory Board for Sanofi Genzyme. P.S.K. has equity in AskBio, which is developing gene therapy for Pompe disease. H.J. has received research, grant support and honoraria from Sanofi Genzyme Corporation. H.J. has US patent applications for respiratory muscle training–related intellectual property licensed by Aspire LLC, and is a paid consultant for Aspire LLC. This work was also supported by NIH NICHD 1K08HD077040 (M.K.E.). The other authors declare no conflicts of interest.
Footnotes
SUPPLEMENTARY INFORMATION
The online version of this article (https://doi.org/10.1038/s41436-019-0738-0) contains supplementary material, which is available to authorized users.
REFERENCES
- 1.Hirschhorn R, Reuser A. Glycogen storage disease type II: acid alpha-glucosidase (acid maltase) deficiency In: Scriver C, Beaudet A, Sly W, editors. The metabolic and molecular bases of inherited disease. New York: McGraw-Hill; 2001. p. 3389–3420. [Google Scholar]
- 2.van den Hout HMP, Hop W, van Diggelen OP, et al. The natural course of infantile Pompe’s disease: 20 original cases compared with 133 cases from the literature. Pediatrics. 2003;112:332–340. [DOI] [PubMed] [Google Scholar]
- 3.Hagemans MLC, Winkel LPF, Van Doorn PA, et al. Clinical manifestation and natural course of late-onset Pompe’s disease in 54 Dutch patients. Brain. 2005;128:671–677. [DOI] [PubMed] [Google Scholar]
- 4.Amalfitano A, Bengur AR, Morse RP, et al. Recombinant human acid α-glucosidase enzyme therapy for infantile glycogen storage disease type II: results of a phase I/II clinical trial. Genet Med. 2001;3:132–138. [DOI] [PubMed] [Google Scholar]
- 5.Kishnani PS, Corzo D, Nicolino M, et al. Recombinant human acid [alpha]-glucosidase: major clinical benefits in infantile-onset Pompe disease. Neurology. 2007;68:99–109. [DOI] [PubMed] [Google Scholar]
- 6.Nicolino M, Byrne B, Wraith JE, et al. Clinical outcomes after long-term treatment with alglucosidase alfa in infants and children with advanced Pompe disease. Genet Med. 2009;11:210–219. [DOI] [PubMed] [Google Scholar]
- 7.van der Ploeg AT, Clemens PR, Corzo D, et al. A randomized study of alglucosidase alfa in late-onset Pompe’s disease. N Engl J Med. 2010;362:1396–1406. [DOI] [PubMed] [Google Scholar]
- 8.Kishnani PS, Goldenberg PC, DeArmey SL, et al. Cross-reactive immunologic material status affects treatment outcomes in Pompe disease infants. Mol Genet Metab. 2010;99:26–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chien Y-H, Lee N-C, Chen C-A, et al. Long-term prognosis of patients with infantile-onset Pompe disease diagnosed by newborn screening and treated since birth. J Pediatr. 2015;166:985–991.e1–2. [DOI] [PubMed] [Google Scholar]
- 10.Prater SN, Banugaria SG, DeArmey SM, et al. The emerging phenotype of long-term survivors with infantile Pompe disease. Genet Med. 2012;14: 800–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prater SN, Patel TT, Buckley AF, et al. Skeletal muscle pathology of infantile Pompe disease during long-term enzyme replacement therapy. Orphanet J Rare Dis. 2013;8:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chien Y-H, Lee N-C, Thurberg BL, et al. Pompe disease in infants: improving the prognosis by newborn screening and early treatment. Pediatrics. 2009;124:e1116–e1125. [DOI] [PubMed] [Google Scholar]
- 13.Kishnani PS, Hwu W-L, Mandel H, et al. A retrospective, multinational, multicenter study on the natural history of infantile-onset Pompe disease. J Pediatr. 2006;148:671–676.e2. [DOI] [PubMed] [Google Scholar]
- 14.Matsuoka T, Miwa Y, Tajika M, et al. Divergent clinical outcomes of alpha-glucosidase enzyme replacement therapy in two siblings with infantile-onset Pompe disease treated in the symptomatic or pre-symptomatic state. Mol Genet Metab Rep. 2016;9:98–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Raben N, Danon M, Gilbert AL. et al. Enzyme replacement therapy in the mouse model of Pompe disease. Mol Genet Metab. 2003;80:159–169. [DOI] [PubMed] [Google Scholar]
- 16.Case LE, Bjartmar C, Morgan C, et al. Safety and efficacy of alternative alglucosidase alfa regimens in Pompe disease. Neuromuscul Disord. 2015;25:321–332. [DOI] [PubMed] [Google Scholar]
- 17.Stepien KM, Hendriksz CJ, Roberts M, Sharma R. Observational clinical study of 22 adult-onset Pompe disease patients undergoing enzyme replacement therapy over 5 years. Mol Genet Metab. 2016;117:413–418. [DOI] [PubMed] [Google Scholar]
- 18.Parini R, De Lorenzo P, Dardis A, et al. Long term clinical history of an Italian cohort of infantile onset Pompe disease treated with enzyme replacement therapy. Orphanet J Rare Dis. 2018;13:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Gelder CM, Poelman E, Plug I, et al. Effects of a higher dose of alglucosidase alfa on ventilator-free survival and motor outcome in classic infantile Pompe disease: an open-label single-center study. J Inherit Metab Dis. 2016;39:383–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Landis JL, Hyland H, Kindel SJ, et al. Pompe disease treatment with twice a week high dose alglucoside alfa in a patient with severe dilated cardiomyopathy. Mol Genet Metab Rep. 2018;16:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kishnani PS, Corzo D, Leslie ND, et al. Early treatment with alglucosidase alfa prolongs long-term survival of infants with Pompe disease. Pediatr Res. 2009;66:329–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Folio M, Fewell R. The Peabody Developmental Motor Scales—Second Edition (PDMS-2): examiner’s manual. 2000. https://www.txautism.net/assets/uploads/docs/PDMS-2-ed-KS-AK.pdf. Accessed 22 July 2019.
- 23.Bruininks R, Bruininks B. Bruininks‐Oseretsky Test of Motor Proficiency (2nd edn). Minneapolis, MN: Pearson Assessment; 2005. [Google Scholar]
- 24.Russell DJ, Rosenbaum PL, Avery L, Lane M. Gross motor function measure (GMFM-66 and GMFM-88) user’s manual: clinics in developmental medicine. London, England: Mac Keith Press; 2002. [Google Scholar]
- 25.Henricson E, Abresch R, Han JJ, et al. Percent-predicted 6-minute walk distance in Duchenne muscular dystrophy to account for maturational influences. PLoS Curr. 2012;4:RRN1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166:111–117. [DOI] [PubMed] [Google Scholar]
- 27.Geiger R, Strasak A, Treml B, et al. Six-minute walk test in children and adolescents. J Pediatr. 2007;150:395–399. [DOI] [PubMed] [Google Scholar]
- 28.Lammers AE, Hislop AA, Flynn Y, Haworth SG. The 6-minute walk test: normal values for children of 4–11 years of age. Arch Dis Child. 2008; 93:464–468. [DOI] [PubMed] [Google Scholar]
- 29.Ulrich S, Hildenbrand FF, Treder U, et al. Reference values for the 6-minute walk test in healthy children and adolescents in Switzerland. BMC Pulm Med. 2013;13:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Holland AE, Spruit MA, Troosters T, et al. An official European Respiratory Society/American Thoracic Society technical standard: field walking tests in chronic respiratory disease. Eur Respir J. 2014;44:1428–1446. [DOI] [PubMed] [Google Scholar]
- 31.Potter NL, Short R. Maximal tongue strength in typically developing children and adolescents. Dysphagia. 2009;24:391–397. [DOI] [PubMed] [Google Scholar]
- 32.Culver BH, Graham BL, Coates AL, et al. Recommendations for a standardized pulmonary function report. An official American Thoracic Society technical statement. Am J Respir Crit Care Med. 2017;196: 1463–1472. [DOI] [PubMed] [Google Scholar]
- 33.Young SP, Stevens RD, An Y, et al. Analysis of a glucose tetrasaccharide elevated in Pompe disease by stable isotope dilution–electrospray ionization tandem mass spectrometry. Anal Biochem. 2003;316:175–180. [DOI] [PubMed] [Google Scholar]
- 34.Van den Hout H, Reuser AJ, Vulto AG, et al. Recombinant human α-glucosidase from rabbit milk in Pompe patients. Lancet. 2000;356:397–398. [DOI] [PubMed] [Google Scholar]
- 35.Yanovitch TL, Casey R, Banugaria SG, Kishnani PS. Improvement of bilateral ptosis on higher dose enzyme replacement therapy in Pompe disease. J Neuroophthalmol. 2010;30:165–166. [DOI] [PubMed] [Google Scholar]
- 36.Zeng Y-T, Hwu W-L, Torng P-C, et al. Longitudinal follow-up to evaluate speech disorders in early-treated patients with infantile-onset Pompe disease. Eur J Paediatr Neurol. 2017;21:485–493. [DOI] [PubMed] [Google Scholar]
- 37.Genzyme Sanofi, Mississauga ON. Product Monograph PrMYOZYME® alglucosidase alfa (Recombinant human acid alpha-glucosidase), 2016; 31–32. [Google Scholar]
- 38.Yang C-F, Yang CC, Liao H-C, et al. Very early treatment for infantile-onset Pompe disease contributes to better outcomes. J Pediatr. 2016; 169:174–180.e1. [DOI] [PubMed] [Google Scholar]
- 39.Young SP, Zhang H, Corzo D, et al. Long-term monitoring of patients with infantile-onset Pompe disease on enzyme replacement therapy using a urinary glucose tetrasaccharide biomarker. Genet Med. 2009; 11:536–541. [DOI] [PubMed] [Google Scholar]
- 40.Peng SS-F, Hwu W-L, Lee N-C, et al. Progressive myopathy in neonatally treated patients with infantile-onset Pompe disease: a muscle magnetic resonance imaging study. Orphanet J Rare Dis. 2016;11:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.