Abstract
Adeno-associated virus (AAV) vectors have been widely adopted for delivery of CRISPR-Cas components, especially for therapeutic gene editing. For a single vector system, both the Cas9 and guide RNA (gRNA) are encoded within a single transgene, usually from separate promoters. Careful design of this bi-cistronic construct is required due to the minimal packaging capacity of AAV. We investigated how placement of the U6 promoter expressing the gRNA on the reverse strand to SaCas9 driven by a cytomegalovirus promoter affected gene editing rates compared to placement on the forward strand. We show that orientation in the reverse direction reduces editing rates from an AAV vector due to reduced transcription of both SaCas9 and guide RNA. This effect was observed only following AAV transduction; it was not seen following plasmid transfection. These results have implications for the design of AAV-CRISPR vectors, and suggest that results from optimizing plasmid transgenes may not translate when delivered via AAV.
Introduction
Adeno-associated virus (AAV) vector delivery of CRISPR-Cas9 is a promising approach for gene editing of somatic tissues in vivo.1 AAV vectors have been used across a range of clinical gene therapy trials for efficient and safe transgene delivery.2–4 The CRISPR-Cas9 system can be packaged in AAV and has been used to edit genes in the liver,5–8 retina,1,9–12 brain,13 heart,14,15 and skeletal muscle16–18 in animal models.
For clinical translation, packaging both the Cas9 and guide RNA (gRNA) in a single AAV-CRISPR vector is ideal. This approach requires a bi-cistronic vector design, where both the Cas9 and gRNA components are packaged together with their respective promoters within the limited capacity of a single AAV (∼4.7 kb). Achieving optimal expression of Cas9 and gRNA is important to maximize on-target editing efficiency and minimize the required vector dose to be delivered. While the arrangement of expression cassettes within a bi-cistronic construct can affect expression of one or both of the transgenes,19–23 little is known about how vector design affects Cas9 and gRNA expression in a single AAV system.
Some authors have noted that the orientation of the U6–gRNA transcriptional unit within a vector can affect the efficiency of gene editing.5,24,25 To investigate this further, we compared the expression and gene editing efficiency from two single AAV-CRISPR vector designs where Cas9 is driven by the cytomegalovirus intermediate-early enhancer and promoter (CMV-IE) and the gRNA is expressed under the human U6 (hU6) promoter. Both these promoters are short and have strong ubiquitous expression, making them popular choices for all-in-one vectors. In this system, orientation of the gRNA relative to the Cas9 affects both Cas9 and gRNA expression and gene editing efficiency when delivered via AAV. This effect was observed for gRNAs targeting genes involved in the pathogenesis of the eye diseases age-related macular degeneration (vascular endothelial growth factor (VEGF)) and retinitis pigmentosa (rhodopsin (RHO)). This effect, however, was not observed when the Cas9 and gRNA were delivered to cells by transient transfection of plasmids.
Methods
Plasmid cloning
The pX601-AAV-CMV-SaCas9-U6-sgRNA plasmid was a gift from Feng Zhang (Addgene; plasmid #61591).5 The reverse-oriented U6–gRNA plasmid was generated by subcloning with NotI and KpnI (New England Biolabs). gRNAs were subcloned into the BsaI restriction site. gRNAs used in this study targeted VEGF26 and RHO, and sequences are listed in Supplementary Table 1. For transfection experiments, endotoxin-free plasmid preparations were created using the EndoFree Plasmid Maxi Kit (Qiagen).
Viral vector production and titration
AAV production and titration was performed, as described previously.27–29 AAV2/2 vectors were created by co-transfection of HEK293T cells seeded in HYPERflasks (Corning) with a polyethylenimine protocol to deliver a total of 500 μg of a pDG plasmid containing the required helper and packaging genes (PF421; PlasmidFactory) and the AAV-SaCas9 plasmid. Cells were harvested and lysed 3 days post transfection. AAV was subsequently isolated by ultracentrifugation with an iodixanol gradient and purified in Amicon Ultra-15 100K filter units (Merck Millipore). Sodium dodecyl sulfate polyacrylamide gel electrophoresis analysis was used to confirm the purity of each preparation. DNAse-resistant particle titering of all vectors was performed in parallel using primers to the bovine growth hormone poly-A tail present for all vectors and linearized vector plasmid as a known standard. Primer sets were confirmed to have 90–105% efficiency.
Cell culture
HEK293 cells (GenTarget, Inc.) were maintained in complete medium created from high-glucose Dulbecco's modified Eagle's medium (Gibco) with l-glutamine, supplemented with 10% fetal bovine serum (Gibco; South American origin) and 5% penicillin-streptomycin (Sigma–Aldrich). Cells were cultured at 37°C in a 5% CO2 humidified incubator (Thermo Electron). For plasmid transfections, cells were seeded on 24-well plates and were transfected 24 h after seeding, when they had reached approximately 80% confluence. Cells were transfected with 500 ng plasmid DNA together with TransIT-LT1 transfection reagent (Mirus Bio) mixed with OptiMEM serum-free media. At 48 h post transfection, cells were harvested, divided across two tubes, and frozen for analysis. For AAV transduction experiments, cells were seeded on 12-well plates and were transduced 24 h post seeding when approaching confluency at a multiplicity of infection (MOI) of 300 vg/cell. At 72 h post transduction, cells were harvested, divided across three tubes, and frozen for further analysis.
DNA analysis
Following genomic DNA extraction (QIAamp DNA mini kit; Qiagen), locus polymerase chain reaction (PCR) of the relevant target (VEGF, RHO) was performed (KOD Hotstart Mastermix; Merck Millipore). Products were analyzed on an agarose gel to confirm a clean amplification reaction before being sequenced with Sanger sequencing (Eurofins Genomics). DNA editing efficiency was calculated by uploading chromatograms to the Tracking of Indels by Decomposition (TIDE)30 online software to calculate indel percentage. Chromatograms from untransfected cells were used as a control. Decomposition windows and left boundaries were optimized to obtain the highest alignment possible. Indel sizes were kept constant at 10. The significance cutoff was maintained at p < 0.001 for all analyses.
Transcript analysis
RNA was extracted using the miRNAeasy mini kit (Qiagen) to ensure capture of the small gRNA fragments. RNA was reverse transcribed to cDNA using the Superscript III first strand synthesis system (Thermo Fisher Scientific). RNA (1 μg) was reverse transcribed using oligo dT primers and with gene-specific primers to the directed repeat region of the SaCas9-gRNA, and cDNA was cleaned in Qiagen spin columns. Semi-quantitative PCR (qPCR) was performed using Taqman Fast Universal Mastermix (Thermo Fisher Scientific). Taqman probes measuring expression of GAPDH (Hs9999905_m1) and ACTB (Hs01060665_g1) were used as endogenous controls, and measurement of SaCas9 and SaCas9-gRNA was performed with custom TaqMan probes (Thermo Fisher Scientific). All assays were validated using dilution series to ensure a reaction efficiency of 90–105%. For reactions amplifying the gRNA scaffold, betaine 1 × was added to the amplification reaction to reduce secondary structure formation. All qPCR experiments were performed in triplicate in 20 μL reactions on a real-time PCR machine (Bio-Rad). Results were normalized to endogenous controls and presented as a fold change relative to expression from the forward direction using the Livak (2–ΔΔCt) method.31 All primer and probe sequences are listed in the Supplementary Material.
Western blot
Samples were lysed in RIPA buffer (Merck Millipore) plus proteasome inhibitor (Roche) using a hand-held homogenizer prior to centrifugation. The Pierce bicinchoninic acid protein assay kit (Thermo Fisher Scientific) was used to measure total protein concentration. Samples of 50 μg protein were mixed with 5 × protein loading buffer (National Diagnostics) and denatured at room temperature for 15 min. Samples and a protein size ladder (BlueEYE; Sigma–Aldrich) were loaded onto a 10% Tris-glycine extended gel (Criterion TGX Precast Gel; Bio-Rad) and transferred to a polyvinylidene difluoride membrane using a TransBlotTurbo (Bio-Rad). Membranes were blocked in Odyssey blocking buffer in PBS (LI-COR Biosciences) for 1 h, incubated in primary antibody solution with rabbit anti-SaCas9 (1:10,000, ab203933; Abcam) and mouse anti-alpha-tubulin (1:5,000, ab7291; Abcam) for 2 h, and incubated in IRDye fluorescent secondary antibody solution (Donkey anti-rabbit 800W, donkey anti-mouse 680RD, both 1:10,000; LI-COR Biosciences) for 1 h. All steps were completed on an orbital shaker at room temperature, with washes between each step. Signals were recorded with the Odyssey imaging system, and band densities were assessed using Image Studio Lite software. SaCas9 levels were normalized to alpha-tubulin levels and presented as relative to expression from the forward-oriented U6.gRNA constructs.
Statistics
GraphPad Prism v8 (GraphPad Software, Inc.) was used for all statistical analysis. Statistics are presented as the mean [95% confidence interval]. Statistical analysis was performed using a two-way analysis of variance with Šídák's post hoc test for multiple comparisons.
Results
Reverse orientation of the gRNA reduces on-target editing in AAV-transduced cells
The smaller Cas9 orthologue of Staphyloccocus aureus (SaCas9, 3.2kb) is the most well-characterized Cas9 for single AAV delivery. The hU6 promoter is commonly used for high, ubiquitous RNA polymerase III-mediated expression of the gRNA. We cloned plasmid constructs that contained the hU6–gRNA construct in either the forward or reverse direction relative to an upstream SaCas9 driven by the ubiquitous RNA polymerase II-mediated CMV-IE promoter (Fig. 1). gRNAs targeting VEGF and RHO loci were cloned into both the forward and reverse constructs. AAV2 vectors were then created using these constructs.
FIG. 1.
(A) Vector diagram of the forward- and reverse-oriented U6–guide RNA (gRNA) promoter plasmids used for transient transfection. (B) Vector diagram of the forward- and reverse-oriented U6–gRNA promoter adeno-associated virus (AAV) vectors used for transduction. (C) Outline of transfection and transduction protocols.
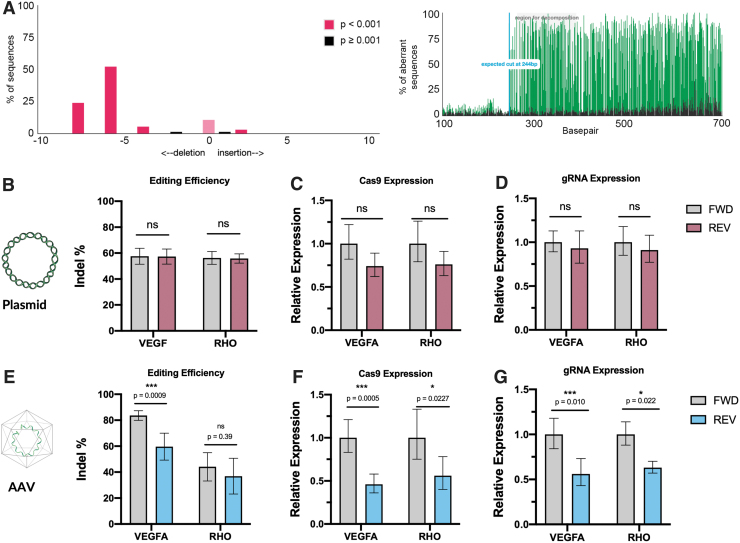
To investigate the effect of the orientation of the gRNA on SaCas9-mediated editing, HEK293 cells were either transduced with AAV or transiently transfected with plasmids. In cells transiently transfected with plasmids, no difference in editing rate between constructs with guides oriented in the forward or reverse direction was observed with either the VEGF (p = 0.98) or RHO (p = 0.98) guides. In cells transduced with AAV, however, editing rates with constructs containing the U6–gRNA in the reverse direction compared to constructs with the gRNA in the forward direction were reduced by 28.7% (p = 0.0009) for the VEGF guide (Fig. 2). A 16.3% (p = 0.39) reduction in editing observed for the reverse-oriented guide compared to the forward-oriented guide targeting the RHO locus was not statistically significant.
FIG. 2.
(A) Representative example of a Tracking of Indels by Decomposition (TIDE) analysis output analyzing indels in a sequencing chromatogram from samples transduced with vectors targeting vascular endothelial growth factor (VEGF). (B) On-target editing efficiency in plasmid-transfected samples for guides targeting VEGF (forward = 57.6% [95% confidence interval (CI) 51.4–63.8], reverse = 57.2% [95% CI 51.4–63.1], p = 0.98, n = 6) and rhodopsin (RHO; forward = 56.2% [95% CI 51.3–61.2], reverse = 56.7% [95% CI 52.2–59.3], p = 0.98, n = 6). (C) SaCas9 mRNA expression from plasmid transfected samples (VEGF-Rev = 0.74 [95% CI 0.62–0.89], p = 0.13, n = 5; RHO-Rev = 0.76 [95% CI 0.63–0.91], p = 0.12, n = 6). (D) gRNA expression from plasmid transfected samples (VEGF-Rev = 0.93 [95% CI 0.76–1.13], p = 0.80, n = 5; RHO-Rev = 0.91 [95% CI 0.77–1.08], p = 0.67, n = 6). (E) On-target editing efficiency in AAV-transduced cells as assessed by TIDE analysis for the VEGF gRNA (forward = 87.2% [95% CI 79.8–87.2], reverse = 59.6% [95% CI 49.2–70.0], p = 0.0009, n = 6) and RHO gRNA (forward = 44.0% [95% CI 33.2–54.9], reverse = 36.8% [95% CI 23.1–50.6], p = 0.39, n = 6). (F) SaCas9 expression in AAV-transduced cells (VEGF-Rev = 0.46 [95% CI 0.36–0.58], p = 0.0005, n = 6; RHO-Rev = 0.56 [95% CI 0.40–0.78], p = 0.023, n = 4). (G) gRNA expression assessed in AAV-transduced cells (VEGF-Rev = 0.56 [95% CI 0.43–0.73], p = 0.01, n = 6; RHO-Rev = 0.63 [95% CI 0.57–0.70], p = 0.02, n = 4). Two-way unpaired analysis of variance (ANOVA) with Šídák's multiple comparison testing. SaCas9 and gRNA expression calculated as fold change relative to the forward-oriented gRNA.
Reverse orientation of the gRNA reduces Cas9 mRNA and gRNA expression in AAV-transduced cells
We hypothesized that the reduced editing rates observed when using the reverse-oriented U6–gRNA promoter in the AAV vector may be due to promoter inhibition of either the CMV-IE or U6 promoter reducing SaCas9 or gRNA expression respectively. Thus, expression levels of SaCas9 mRNA and gRNA were measured using qPCR. We found that compared to the forward-oriented vector, the reverse-oriented U6 construct resulted in a significant reduction in SaCas9 expression in a guide-independent manner. A 54% reduction (p = 0.0005) in SaCas9 expression was observed in the VEGF-targeting constructs and a 44% reduction (p = 0.023) was observed in RHO-targeting constructs when the U6–gRNA complex was reversed compared to the forward direction. A similar significant reduction in gRNA expression was also found. A reduction of 44% (p = 0.01) and 37% (p = 0.02) of gRNA expression was seen for the VEGF and RHO guides, respectively, when expressed in the reverse relative to the forward direction. In cells transfected with the plasmid construct, no significant effect on SaCas9 or gRNA expression was observed across either gRNA, expressed in either the forward or reverse orientation.
Reverse orientation of the gRNA reduces Cas9 protein expression
To confirm that reduced SaCas9 mRNA resulted in lower SaCas9 protein expression, we analyzed Cas9 expression in the AAV-transduced samples with Western blot. For both the VEGF and RHO guides, SaCas9 protein expression appeared to be reduced in constructs with the gRNA in the reverse direction (Fig. 3). Using band densitometry, we confirmed that relative to the forward direction, there is a significant reduction in SaCas9 protein when the U6–gRNA cassette is in the reverse compared to forward orientation in both the VEGF construct (62% reduction, p = 0.0001) and the RHO construct (30% reduction, p = 0.001; Fig. 3). The greater reduction in expression observed in the reverse-oriented VEGF-targeting construct compared to the RHO-targeting construct is consistent with the greater loss of editing efficiency in the VEGF reverse construct.
FIG. 3.
(A) Western blot of AAV-transduced samples targeting VEGF. (B) Western blot of AAV-transduced samples targeting RHO. (C) Quantitative analysis of band densities normalized to alpha-tubulin, relative to the expression of SaCas9 with U6 in the forward orientation (VEGF-Rev = 0.38 [95% CI 0.23–0.52], p = 0.0001, n = 5; RHO-Rev = 0.70 [95% CI 0.54–0.86], p = 0.001, n = 5). Two-way unpaired ANOVA with Šídák's multiple comparison testing. NTC = non transfected control; rSaCas9 = 100 ng recombinant SaCas9 protein.
Discussion
As gene editing using AAV-delivered CRISPR-Cas9 moves toward the clinic,1 it is important to optimize the design of AAV transgenes to enable therapeutic efficacy. Here, we show that a small change to the transgene design, reversing the direction of the U6 promoter and gRNA complex, has a marked impact on Cas9 and gRNA expression and subsequent editing rates in a vector with SaCas9 driven by the CMV-IE promoter. This effect was only seen once the transgene was packaged and delivered via AAV; it was not seen through plasmid transfection. We demonstrate this finding independently across two different guides. As AAV creation is both time and resource intensive, transgenes are often tested and optimized in plasmid transfection experiments prior to creating AAV. These results indicate that data from experiments using plasmid transfection do not necessarily correlate with those found following AAV transduction.
While there is limited evidence as to how the position and orientation of the U6 promoter in an AAV-CRISPR vector affects gene editing, it is clear that orientation can impact gene editing rates. In the initial characterization of SaCas9, Friedland et al. packaged two U6–gRNA complexes with D10A SaCas9 nickase in the forward and reverse direction into AAV constructs.25 Friedland et al. found that lower Cas9 expression and editing efficiencies were seen when the U6–gRNA complexes were oriented in the reverse direction. The U6–gRNA cassette in Friedland et al. was located upstream of the Cas9 compared to the downstream location in the present study. Ran et al. evaluated both the in vitro and in vivo gene editing activity of SaCas9 using a vector expressing SaCas9 and gRNA.5 The plasmid used in the in vitro characterization (Px601; available from Addgene) has been widely used, including in the present paper, and contains the U6–gRNA in the forward direction.5 When Ran et al. developed an AAV for the in vivo characterization of SaCas9, however, the AAV transgene contained the U6–gRNA in the reverse direction. No data were presented to compare the activity of the forward and reverse constructs or validate why the U6–gRNA complex was reversed in the AAV vector.5 Most recently, Levy et al. engineered a dual-AAV split-intein SaCas9 base editor and found that moving the U6–gRNA complex from upstream of the SaCas9 in the forward orientation to downstream in the reverse orientation substantially improved editing rates.24
Our data build on work in other systems that have demonstrated that the arrangement of expression cassettes in a bi-cistronic transgene can affect transgene expression. When a second promoter is added, the activity of both the upstream and downstream promoter was shown to be affected in eukaryotes with lentiviral19 and transient transfection systems,20 and in prokaryotes with transient transfection systems.32 Although each of these studies examined two polymerase II promoters, the two studies that compared placement of the downstream promoter in reverse compared to a forward direction found that the reverse direction enhanced expression of both promoters.20,32
How bi-cistronic constructs are affected also appears to depend on the promoter used, in addition to how they are arranged. For example, an interphotoreceptor retinoid binding protein promoter suppressed the activity of a rhodopsin kinase promoter, but only when located in the downstream not the upstream position.21 Variable results have been found when the U6 promoter is used in combination with other promoters. Placed divergent to a ubiquitin C promoter, U6 promoter activity was inhibited compared to a tandem orientation in a retroviral system.22 Conversely, when the U6 promoter was paired with a phosphoglycerate kinase (PGK) promoter22 or another U6 promoter23 in any orientation, the activity of the U6 promoter was not affected. A lack of agreement between published studies prevents the extrapolation of general rules about the optimal arrangement of promoters for other vectors. This study considers only the combination of the CMV-IE and the hU6 promoter. However, it suggests that it is necessary to assess each system and set of promoters in the process of optimizing a transgene such as an all-in-one AAV-CRISPR vector.
The underlying mechanisms of our findings remain unknown. In the current study, we observe that the reversal of the RNA-pol III U6 promoter impairs expression from the U6 promoter itself and also a RNA-Pol II CMV-IE promoter upstream in the same construct, but only when expressed in an AAV system. Torsional effects from the unwinding of DNA during active transcription within the AAV episome may be implicated.33 Transcription from the U6 promoter in the forward direction would result in relaxed negatively supercoiled DNA upstream of the promoter. In contrast, orientation of the downstream U6 promoter in the reverse direction could result in an accumulation of tightly wound positively supercoiled DNA between the cistrons, downstream of both the CMV-IE and U6 promoter. This may stall transcription of both promoters.32,34
Transcriptional interference arising from one transcribing RNA polymerase directly impeding another transcribing RNA polymerase35 may also play a role. Following AAV transduction, AAV genomes form double-stranded episomal circular monomers and concatemers, where viral transgenes form linked circles connected by inverted terminal repeats (ITRs).36,37 Within the circular episome, the transgenes can be linked to one another in any direction, 5′-3′ or 3′-5′. For a given transgene, there are three possible arrangements (Fig. 4). In the vectors containing the U6–gRNA in the forward orientation relative to the Cas9, only one of these combinations places two promoters directly adjacent to one another, separated by ITRs. In the vectors containing the U6–gRNA in the reverse orientation relative to the Cas9, however, in all possible arrangements, the promoters are directly adjacent to one another. This is the case whether the U6–gRNA is located upstream25 or downstream of the Cas9 cassette. The closer physical proximity of both the polymerase II and III promoters in the concatemers when the gRNA is in the reverse direction may increase the susceptibility of expression from these promoters being affected by transcriptional interference and local torsional effects of transcription. ITRs are known to possess promoter activity,38 and the reverse orientation also places both promoters immediately adjacent to the ITRs in the concatemer. In plasmid transfection, the plasmid backbone is maintained and separates the ends of the transgene, and the formation of transgene monomers and concatemers does not occur. This model may explain the observed differences in expression between plasmid transfection and AAV transduction.
FIG. 4.
A model of the three possible arrangements of a dual-transgene containing Cas9 driven by a Pol-II promoter and a gRNA driven by a Pol-III promoter in a circular episomal monomer or concatemer. (A) With the gRNA and its promoter oriented in the forward direction, only one conformation of transgene concatemer results in promoter proximity. (B) In concatemers with the gRNA and its promoter oriented in the reverse direction, all conformations of the transgene concatemer result in proximity between the promoters.
Conclusions
Our study highlights two key points. First, for the design of all-in-one AAV-CRISPR vectors, it is important to consider the orientation of promoters within the transgene when optimizing transgene expression. This effect has been seen using the CMV-IE and hU6 promoter in vitro. Expression is likely to be promoter dependent. Further work is needed to test different vector designs across different tissue types, including in vivo. This includes further evaluation of other promoters such as a cell-specific promoter for each specific application. Second, this study suggests that this testing and optimization should be done with AAV vectors, as results found from plasmid transfection may not translate to those found with AAV vectors.
Supplementary Material
Author Disclosure Statement
The authors have no competing financial interests to declare. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the manuscript.
Funding Information
This research was funded by support from the NIHR Oxford Biomedical Research Centre, the Rhodes Trust, the North Harbour Club Charitable Trust, and the Amar-Franses and Foster-Jenkins Trust.
Supplementary Material
References
- 1. Maeder ML, Stefanidakis M, Wilson CJ, et al. Development of a gene-editing approach to restore vision loss in Leber congenital amaurosis type 10. Nat Med 2019;25:229–233. DOI: 10.1038/s41591-018-0327-9 [DOI] [PubMed] [Google Scholar]
- 2. Xue K, Jolly JK, Barnard AR, et al. Beneficial effects on vision in patients undergoing retinal gene therapy for choroideremia. Nat Med 2018;24:1507–1512. DOI: 10.1038/s41591-018-0185-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cehajic-Kapetanovic J, Xue K, Martinez-Fernandez de la Camara C, et al. Initial results from a first-in-human gene therapy trial on X-linked retinitis pigmentosa caused by mutations in RPGR. Nat Med 2020;26:354–359. DOI: 10.1038/s41591-020-0763-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Russell S, Bennett J, Wellman JA, et al. Efficacy and safety of voretigene neparvovec (AAV2-hRPE65v2) in patients with RPE65-mediated inherited retinal dystrophy: a randomised, controlled, open-label, Phase 3 trial. Lancet 2017;390:849–860. DOI: 10.1016/S0140-6736(17)31868-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ran FA, Cong L, Yan WX, et al. In vivo genome editing using Staphylococcus aureus Cas9. Nature 2015;520:186–191. DOI: 10.1038/nature14299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Li A, Lee CM, Hurley AE, et al. A self-deleting AAV-CRISPR system for in vivo genome editing. Mol Ther Methods Clin Dev 2019;12:111–122. DOI: 10.1016/j.omtm.2018.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yang Y, Wang L, Bell P, et al. A dual AAV system enables the Cas9-mediated correction of a metabolic liver disease in newborn mice. Nat Biotechnol 2016;34:334–338. DOI: 10.1038/nbt.3469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jarrett KE, Lee CM, Yeh Y-H, et al. Somatic genome editing with CRISPR/Cas9 generates and corrects a metabolic disease. Sci Rep 2017;7:44624 DOI: 10.1038/srep44624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yu W, Mookherjee S, Chaitankar V, et al. Nrl knockdown by AAV-delivered CRISPR/Cas9 prevents retinal degeneration in mice. Nat Commun 2017;8:14716 DOI: 10.1038/ncomms14716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kim E, Koo T, Park SW, et al. In vivo genome editing with a small Cas9 orthologue derived from Campylobacter jejuni. Nat Commun 2017;8:1–12. DOI: 10.1038/ncomms14500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hung SSC, Chrysostomou V, Li F, et al. AAV-mediated CRISPR/Cas gene editing of retinal cells in vivo. Invest Opthalmol Vis Sci 2016;57:3470–3476. DOI: 10.1167/iovs.16-19316 [DOI] [PubMed] [Google Scholar]
- 12. Tsai YT, Wu WH, Lee TT, et al. Clustered regularly interspaced short palindromic repeats–based genome surgery for the treatment of autosomal dominant retinitis pigmentosa. Ophthalmology 2018;125:1421–1430. DOI: 10.1016/j.ophtha.2018.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Murlidharan G, Sakamoto K, Rao L, et al. CNS-restricted transduction and CRISPR/Cas9-mediated gene deletion with an engineered AAV vector. Mol Ther Nucleic Acids 2016;5:e338 DOI: 10.1038/mtna.2016.49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Guo Y, VanDusen NJ, Zhang L, et al. Analysis of cardiac myocyte maturation using CASAAV, a platform for rapid dissection of cardiac myocyte gene function in vivo. Circ Res 2017;120:1874–1888. DOI: 10.1161/CIRCRESAHA.116.310283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ishizu T, Higo S, Masumura Y, et al. Targeted genome replacement via homology-directed repair in non-dividing cardiomyocytes. Sci Rep 2017;7:9363 DOI: 10.1038/s41598-017-09716-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tabebordbar M, Zhu K, Cheng JKW, et al. In vivo gene editing in dystrophic mouse muscle and muscle stem cells. Science 2016;351:407–411. DOI: 10.1126/science.aad5177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nelson CE, Hakim CH, Ousterout DG, et al. In vivo genome editing improves muscle function in a mouse model of Duchenne muscular dystrophy. Science 2016;351:403–407. DOI: 10.1126/science.aad5143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Long C, Amoasii L, Mireault AA, et al. Postnatal genome editing partially restores dystrophin expression in a mouse model of muscular dystrophy. Science 2016;351:400–403. DOI: 10.1126/science.aad5725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Curtin JA, Dane AP, Swanson A, et al. Bidirectional promoter interference between two widely used internal heterologous promoters in a late-generation lentiviral construct. Gene Ther 2008;15:384–390. DOI: 10.1038/sj.gt.3303105 [DOI] [PubMed] [Google Scholar]
- 20. Park SK, Hwang BJ, Kee Y. Promoter cross-talk affects the inducible expression of intronic shRNAs from the tetracycline response element. Genes Genomics 2019;41:483–490. DOI: 10.1007/s13258-019-00784-z [DOI] [PubMed] [Google Scholar]
- 21. Semple-Rowland SL, Coggin WE, Geesey M, et al. Expression characteristics of dual-promoter lentiviral vectors targeting retinal photoreceptors and Müller cells. Mol Vis 2010;16:916–934 [PMC free article] [PubMed] [Google Scholar]
- 22. Nie L, Thakur M Das, Wang Y, et al. Regulation of U6 promoter activity by transcriptional interference in viral vector-based RNAi. Genomics Proteomics Bioinformatics 2010;8:170–179. DOI: 10.1016/S1672-0229(10)60019-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lambeth LS, Van Hateren NJ, Wilson SA, et al. A direct comparison of strategies for combinatorial RNA interference. BMC Mol Biol 2010;11:77 DOI: 10.1186/1471-2199-11-77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Levy JM, Yeh W-H, Pendse N, et al. Cytosine and adenine base editing of the brain, liver, retina, heart and skeletal muscle of mice via adeno-associated viruses. Nat Biomed Eng 2020;4:97–110. DOI: 10.1038/s41551-019-0501-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Friedland AE, Baral R, Singhal P, et al. Characterization of Staphylococcus aureus Cas9: a smaller Cas9 for all-in-one adeno-associated virus delivery and paired nickase applications. Genome Biol 2015;16:257 DOI: 10.1186/s13059-015-0817-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kleinstiver BP, Prew MS, Tsai SQ, et al. Engineered CRISPR-Cas9 nucleases with altered PAM specificities. Nature 2015;523:481–485. DOI: 10.1038/nature14592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. McClements ME, Barnard AR, Singh MS, et al. An AAV dual vector strategy ameliorates the Stargardt phenotype in adult Abca4−/− mice. Hum Gene Ther 2019;30:590–600. DOI: 10.1089/hum.2018.156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lipinski DM, Barnard AR, Singh MS, et al. CNTF gene therapy confers lifelong neuroprotection in a mouse model of human retinitis pigmentosa. Mol Ther 2015;23:1308–1319. DOI: 10.1038/mt.2015.68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fischer MD, McClements ME, Martinez-Fernandez de la Camara C, et al. Codon-optimized RPGR improves stability and efficacy of AAV8 gene therapy in two mouse models of X-linked retinitis pigmentosa. Mol Ther 2017;25:1854–1865. DOI: 10.1016/j.ymthe.2017.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Brinkman EK, Chen T, Amendola M, et al. Easy quantitative assessment of genome editing by sequence trace decomposition. Nucleic Acids Res 2014;42:e168 DOI: 10.1093/nar/gku936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001;25:402–408. DOI: 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- 32. Yeung E, Dy AJ, Martin KB, et al. Biophysical constraints arising from compositional context in synthetic gene networks. Cell Syst 2017;5:11–24.e12. DOI: 10.1016/j.cels.2017.06.001 [DOI] [PubMed] [Google Scholar]
- 33. Ma J, Bai L, Wang MD. Transcription under torsion. Science 2013;340:1580–1583. DOI: 10.1126/science.1235441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kouzine F, Liu J, Sanford S, et al. The dynamic response of upstream DNA to transcription-generated torsional stress. Nat Struct Mol Biol 2004;11:1092–1100. DOI: 10.1038/nsmb848 [DOI] [PubMed] [Google Scholar]
- 35. Shearwin KE, Callen BP, Egan JB. Transcriptional interference—a crash course. Trends Genet 2005;21:339–345. DOI: 10.1016/j.tig.2005.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Penaud-Budloo M, Le Guiner C, Nowrouzi A, et al. Adeno-associated virus vector genomes persist as episomal chromatin in primate muscle. J Virol 2008;82:7875–7885. DOI: 10.1128/JVI.00649-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Nakai H, Yant SR, Storm TA, et al. Extrachromosomal recombinant adeno-associated virus vector genomes are primarily responsible for stable liver transduction in vivo. J Virol 2001;75:6969–6976. DOI: 10.1128/jvi.75.15.6969-6976.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yan Z, Sun X, Feng Z, et al. Optimization of recombinant adeno-associated virus-mediated expression for large transgenes, using a synthetic promoter and tandem array enhancers. Hum Gene Ther 2015;26:334–346. DOI: 10.1089/hum.2015.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.