Abstract
Background
This analysis characterizes the immunogenicity profile of galcanezumab, a humanized monoclonal antibody that selectively binds calcitonin gene-related peptide and inhibits its activity, in phase 3 migraine trials.
Methods
Immunogenicity data were analyzed from baseline and double-blind, placebo-controlled phases of the 3-month chronic migraine study REGAIN, the 6-month episodic migraine studies EVOLVE-1 and EVOLVE-2, and from baseline and open-label phases of the 12-month chronic and episodic migraine Study CGAJ. The incidence of baseline antidrug antibodies, treatment-emergent antidrug antibodies, neutralizing antidrug antibodies, and the effect of antidrug antibody titer on pharmacokinetics and pharmacodynamics were assessed. The relationship between antidrug antibody status and efficacy was explored using average change in monthly migraine headache days. Safety analyses assessed the potential relationship between treatment-emergent antidrug antibodies and hypersensitivity events or adverse events related to injection sites.
Findings
Across studies, 5.9–11.2% of patients had baseline antidrug antibodies. The incidence of treatment-emergent antidrug antibodies was 2.6–12.4% in the galcanezumab group and 0.5–1.7% in the placebo group. The majority of treatment-emergent antidrug antibodies were detected approximately 3–6 months after first study drug dose. Overall, the observed antidrug antibody titer did not impact galcanezumab concentrations, calcitonin gene-related peptide concentrations, or galcanezumab efficacy. There was no evidence that hypersensitivity events or adverse events related to injection sites were mediated by treatment-emergent antidrug antibodies.
Interpretation
These data showed that immunogenicity did not impact galcanezumab concentrations, calcitonin gene-related peptide concentrations, or the efficacy and hypersensitivity profile of galcanezumab in patients with migraine.
Keywords: Calcitonin gene-related peptide, anti-drug antibodies, immunogenicity, monoclonal antibodies, galcanezumab
Introduction
Therapeutic monoclonal antibodies (mAb) have several characteristics that make them useful and attractive options in the treatment of multiple conditions. First, their binding is highly specific for their target antigen, reducing the risk for off-target effects (1–3). Also, the half-life of a therapeutic mAb is generally days to weeks, allowing for less frequent dosing intervals (weekly or monthly) relative to traditional small-molecule medicines that are usually dosed daily (3,4). Furthermore, there is a lower risk for interactions with other drugs since mAb catabolism does not involve the cytochrome P450 system (3,4).
As with virtually all therapeutic proteins, mAb may result in anti-drug antibody (ADA) responses (5,6). Many factors influence the likelihood of ADA formation, including the manufacturing process, antibody structure, dose level, dosing frequency, route of administration, concomitant immunomodulatory therapies, and patient-related factors such as immune status or genetic characteristics not yet fully understood (6).
ADAs can be classified as neutralizing antibodies (NAb), those that target the antigen binding site of the therapeutic mAb, or as non-neutralizing antibodies (non-NAb), those that target different regions of the therapeutic mAb. Several studies have revealed that the majority of ADA against different therapeutic mAb are Nab (7–9). NAb may directly interfere with the ligand’s binding to the mAb, and depending on their affinity, titer and persistency may potentially affect target engagement and the pharmacodynamics (PD) of the therapeutic mAb. Both NAb and non-NAb can form immune complexes with the therapeutic mAb and influence its elimination by either decreasing or increasing the half-life, affecting the pharmacokinetic (PK) profile of the therapeutic mAb. The clinical consequences of these changes to PK and PD parameters may range from no clinically important effects to reduced drug efficacy and/or increased risk of adverse events (AEs) (10–12).
Galcanezumab is a humanized mAb that selectively binds calcitonin gene-related peptide (CGRP) and inhibits its activity. CGRP plays a central role in the pathophysiology of migraine (13). To date, galcanezumab has demonstrated efficacy in phase 2 and phase 3 clinical trials for the prevention of migraine (14–16), and in one phase 3 trial for the prevention of episodic cluster headache (17).
The aim of this manuscript is to characterize the immunogenicity profile of galcanezumab across four phase 3 clinical trials in which patients with episodic or chronic migraine were treated for up to 52 weeks.
Methods
Study designs
The migraine phase 3 clinical development program consisted of four clinical trials: REGAIN (NCT02614261) (18), EVOLVE-1 (NCT02614183) (16), EVOLVE-2 (NCT02614196) (15), and Study CGAJ (NCT02614287) (19). In all these studies, two galcanezumab dose regimens were evaluated: 240 mg/month and 120 mg/month after an initial loading dose of 240 mg. Table 1 presents a brief description of each study design. The analyses presented herein are restricted to the 3-month, double-blind treatment phase of REGAIN, the 6-month double-blind treatment phase of EVOLVE-1 and EVOLVE-2, and the 12-month, open-label treatment phase of Study CGAJ.
Table 1.
Study designs for phase 3 galcanezumab studies in patients with episodic and chronic migraine.
| Study name | Migraine population | Active treatment period* | Number randomized |
|
|---|---|---|---|---|
| Placebo | Galcanezumab120 mg+/240 mg | |||
| REGAIN | Chronic | 3-month double-blind, placebo-controlled, followed by optional 9-month open-label period† | 558 | 278/277 |
| EVOLVE-1 | Episodic | 6-month double-blind, placebo-controlled | 433 | 213/212 |
| EVOLVE-2 | Episodic | 6-month double-blind, placebo-controlled | 461 | 231/223 |
| Study CGAJ | Episodic or chronic | 12-month open-label | – | 135/135 |
Notes:
The active treatment period of all four studies was followed by a 4-month post-treatment follow-up (washout) period.
Patients randomized to the 120 mg arm received an initial loading dose of 240 mg.
The open-label extension and follow-up periods of REGAIN were ongoing at the time of these analyses.
Assessment of anti-drug antibodies
Immunogenicity sampling times for the four studies are shown in Supplementary Table 1. Immunogenicity samples were collected at baseline (before the first drug administration) and at regular intervals ranging from every 2 weeks to every 6 months over the study period.
Immunogenicity was assessed by a validated assay designed to detect ADA in the presence of the investigational product. Antibodies were further evaluated for their ability to produce NAb. The ADA assay used an “affinity-capture elution” (ACE) format with upfront acid dissociation of ADA–drug complexes, enhancing the detection of both free ADA and ADA in complex with the drug (20,21). The ACE format was based on a published method (20,21). An important feature of the ACE format is that it allows a minor modification of the assay to detect neutralizing ADA with the same sensitivity and drug tolerance as the screening ADA assay. Therefore, both assays were designed to have similar and very high sensitivity (able to detect 7.5 ng/mL of ADA by using affinity-purified, hyper-immunized monkey serum (APHIMS) as positive control) and high drug tolerance (able to detect ADA in the presence of the observed serum galcanezumab concentrations). The assay was validated at Pacific Biomarkers Inc. (Seattle, Washington) using a minimum required dilution of 1:10 with an experimentally determined sensitivity of 7.5 ng/mL of APHIMS. Inter-assay precisions were all below the 20% coefficient of variation for the screening, confirmatory, and neutralizing ADA assays tested for low, mid, and high positive controls. Drug tolerance limits were established as 606.3, 266.5, and 108.0 µg/mL drug in the presence of 500, 250, and 125 ng/mL of APHIMS, respectively.
Assessment of serum galcanezumab and plasma CGRP concentrations
Blood samples were collected for determination of serum concentrations of galcanezumab and plasma concentrations of CGRP according to the schedule outlined in Supplemental Table 1. Blood samples for ADA, PK, and PD measurements were collected pre-dose at the visit specified in Supplemental Table 1. Concentrations of galcanezumab and CGRP were determined using validated bioanalytical methods (14).
Efficacy and safety assessments
The efficacy of galcanezumab was evaluated by assessing the average change in monthly migraine headache days (MHD) for galcanezumab-treated patients through the 3-month double-blind treatment phase of REGAIN, the 6-month double-blind treatment phase in EVOLVE-1 and EVOLVE-2, and the 12-month open-label treatment phase of Study CGAJ.
Safety assessments for these analyses included the identification of hypersensitivity events and adverse events (AEs) related to injection sites. These AEs were selected based on the biologic plausibility of a potential association with immunogenicity and were flagged using a Standardized MedDRA Query (SMQ) search strategy that identified all potential hypersensitivity events through the assessment of three SMQs of MedDRA v20: Anaphylactic reactions, angioedema, and hypersensitivity. A broad search (all narrow and broad preferred terms) within each of these SMQs was performed for highest query sensitivity, as well as a narrow search (only narrow preferred terms) for specificity. To identify the AEs related to injection sites, multiple preferred terms within the Injection Site Reaction High Level Term of MedDRA were used.
Statistical analysis
The treatment-emergent ADA (TE ADA)-evaluable population consists of patients with both a baseline assessment and at least one postbaseline assessment of ADA. TE ADA positive (TE ADA+) patients were defined as patients with: a) ADA not present at baseline and at least one postbaseline sample with ADA detected at a titer ≥1:20; or b) ADA present at baseline and at least one postbaseline sample with ADA detected at a titer ≥4-fold higher than the baseline titer. A patient was classified as NAb present at baseline if the baseline ADA sample had NAb detected, and as NAb present during the postbaseline period if the patient was TE ADA+, and at least one postbaseline sample had NAb detected.
Descriptive statistics (counts and percentages) were used to assess incidence of baseline ADA, TE ADA, and NAb. ADA kinetics was examined through the time to first TE ADA, titer distribution of maximum postbaseline titers, and TE ADA titer evolution over time. Time to first TE ADA+ observation was summarized by cumulative incidence in 3-month intervals. Titer evolution over time was evaluated in EVOLVE-1 and EVOLVE-2 through 6 months of treatment, and in Study CGAJ through 12 months of treatment. The 3-month duration of the double-blind, placebo-controlled period of the REGAIN study was considered too short to specifically evaluate titer evolution over time.
To evaluate the potential impact of immunogenicity on galcanezumab PK, a graphical analysis of the time course of serum galcanezumab concentrations categorized by ADA titer during the specified treatment period of the four studies was conducted. A similar graphical analysis was conducted using concentrations of plasma CGRP to assess the potential impact of ADA titer on CGRP binding to galcanezumab.
To evaluate the impact of TE ADA (and specifically of the highest titers) on galcanezumab efficacy, the distribution of change in overall average monthly MHD was plotted, for three nested groups of TE ADA-evaluable patients who received galcanezumab: a) All patients, regardless of TE ADA status; b) those who were TE ADA+; and c) those who were TE ADA+ with maximum titer > 1:160.
To evaluate the impact of TE ADA on safety, Cochran–Mantel–Haenszel statistics (p-value and odds ratio, stratified by study) were used to compare the proportions of patients with ≥1 hypersensitivity event preferred term or ≥1 preferred term related to injection sites between TE ADA+ and TE ADA− patients, in TE ADA-evaluable patients who received galcanezumab. For the preferred terms reported more often by TE ADA+ patients (p ≤ 0.05) or with an odds ratio > 2, a subsequent evaluation of the temporal relationship between the occurrence of the AE and the presence of TE ADA was conducted. For the purposes of this assessment, the patient’s titer values were reviewed to identify the time point at which the TE ADA+ result was detected, and the time point at which the AEs of interest occurred. In cases where an ADA sample was collected at the same time as the AE of interest occurred, the occurrences were considered temporally related if TE ADA were present at that time point. In cases where an ADA sample was not collected at the same time the AE of interest occurred, the occurrences were considered temporally related if TE ADA were present in the closest titer measurement prior to or subsequent to the AE. For those cases where a temporal relationship between the AE and TE ADA was established, a potential relationship between the TE ADA titer and the severity of the AE was also evaluated.
Results
ADA incidence
The proportion of patients with ADA present at baseline, TE ADA during treatment, and NAb are shown in Table 2. Baseline ADA were detected in 5.9 to 11.2% of patients. During the double-blind, placebo-controlled periods of the REGAIN, EVOLVE-1, and EVOLVE-2 studies, up to 9.4% of patients treated with galcanezumab 120 mg/month, up to 5.2% of patients treated with galcanezumab 240 mg/month, and up to 1.7% of patients treated with placebo were TE ADA+. In the open-label Study CGAJ, where patients received up to 12 months of continuous galcanezumab treatment, 12.4% of patients receiving galcanezumab 120 mg/month and 7.3% of patients receiving galcanezumab 240 mg/month were TE ADA+. Most TE ADA+ patients had NAb present. Of the TE ADA+ patients across studies, 76.1% were treatment-induced TE ADA (i.e. ADA was not detected at baseline). Across all studies, there were no patients with TE ADA inconclusive status; hence all patients without TE ADA were confirmed to be TE ADA−.
Table 2.
Anti-drug and neutralizing antibody findings across the phase 3 galcanezumab studies.
| Study | Study phase | Placebo n (%) |
Galcanezumab |
|||
|---|---|---|---|---|---|---|
| 120 mg n (%) |
240 mg n (%) |
Pooled n (%) |
||||
| REGAIN | Evaluable subjects DB phase | 535 | 264 | 272 | 536 | |
| Baseline | ADA present | 33 (6.2) | 22 (8.3) | 27 (9.9) | 49 (9.1) | |
| NAb present | 26 (4.9) | 15 (5.7) | 18 (6.6) | 33 (6.2) | ||
| 3-month DB treatment | TE ADA+ | 8 (1.5) | 7 (2.7) | 7 (2.6) | 14 (2.6) | |
| NAb present | 3 (0.6) | 6 (2.3) | 4 (1.5)a | 10 (1.9) | ||
| EVOLVE-1 | Evaluable subjects DB phase | 422 | 202 | 213 | 415 | |
| Baseline | ADA present | 25 (5.9) | 18 (8.9) | 23 (10.8) | 41 (9.9) | |
| NAb present | 11 (2.6) | 10 (5.0) | 17 (8.0) | 27 (6.5) | ||
| 6-month DB treatment | TE ADA+ | 7 (1.7) | 9 (4.5) | 11 (5.2)a | 20 (4.8)a | |
| NAb present | 6 (1.4) | 9 (4.5) | 11 (5.2)a | 20 (4.8)a | ||
| EVOLVE-2 | Evaluable subjects DB phase | 443 | 223 | 214 | 437 | |
| Baseline | ADA present | 37 (8.4) | 18 (8.1) | 24 (11.2) | 42 (9.6) | |
| NAb present | 19 (4.3) | 10 (4.5) | 13 (6.1) | 23 (5.3) | ||
| 6-month DB treatment | TE ADA+ | 2 (0.5) | 21 (9.4)b | 11 (5.1)b | 32 (7.3)b | |
| NAb present | 1 (0.2) | 21 (9.4)b | 9 (4.2)b | 30 (6.9)b | ||
| Study CGAJ | Evaluable subjects OL phase | – | 129 | 137 | 266 | |
| Baseline | ADA present | – | 8 (6.2) | 12 (8.8) | 20 (7.5) | |
| NAb present | – | 8 (6.2) | 6 (4.4) | 14 (5.3) | ||
| 12-month OL treatment | TE ADA+ | – | 16 (12.4) | 10 (7.3) | 26 (9.8) | |
| NAb present | – | 16 (12.4) | 10 (7.3) | 26 (9.8) | ||
ADA: anti-drug antibody; DB: double blind; n: number of patients within each specific category; Nab: neutralizing antibody; OL: open label; TE ADA: treatment-emergent ADA.
p < 0.05 versus placebo.
p < 0.001 versus placebo.
ADA kinetics
A summary of time to first TE ADA+ titer during galcanezumab treatment is shown in Table 3. Most TE ADA+ titers were first detected 3 − 6 months after initiation of treatment. The majority (98.1%) of patients had maximum TE ADA titers ≤1:160. The most commonly observed maximum TE ADA titers were 1:20 and 1:40. Titer distributions were similar regardless of study or galcanezumab dosage (data not shown).
Table 3.
Summary of time to first TE ADA+ titer during treatment phase.
| Study | N | Time to first TE ADA+ titer, n (%) |
||||
|---|---|---|---|---|---|---|
| ≤1 month | ≤2 months | ≤3 months | ≤6 months | ≤12 months | ||
| REGAIN (3-month DB phase) | 536 | 7 (1.3) | 9 (1.7) | 14 (2.6) | – | – |
| EVOLVE-1 (6-month DB phase) | 415 | 4 (1.0) | 6 (1.5) | 12 (2.9) | 20 (4.8) | – |
| EVOLVE-2 (6-month DB phase) | 437 | 2 (0.5) | 3 (0.7) | 10 (2.3) | 32 (7.3) | – |
| Study CGAJ (12-month OL phase) | 266 | 4 (1.5) | 6 (2.3) | 8 (3.0) | 21 (7.9) | 26 (9.8) |
DB: double blind; N: number of patients in the analysis population; n: number of patients within each specific category; OL: open label; TE ADA: treatment emergent anti-drug antibody.
Table 4 shows titer evolution over time in TE ADA+ patients from EVOLVE-1, EVOLVE-2 and Study CGAJ. A similar TE ADA titer evolution pattern was observed across the three studies during up to 6 months of treatment. The majority of TE ADA+ patients exhibited their maximum TE ADA titer at 6 months. Within the 6-month period, 20.5% of patients (15 of 73) who were TE ADA+ earlier in the study no longer had TE ADA titers at the final assessment. Declines in titers were more common in the longer-term Study CGAJ, where there was up to 12 months of continuous galcanezumab treatment. In CGAJ, a greater proportion (relative to 6-month data from the two shorter studies) of TE ADA+ patients (12 of 26, 46.2%) had titers that decreased over time and 38.5% (10 of 26) no longer had TE ADA titers at the final assessment. Of the 26 TE ADA+ patients (30.8%), eight exhibited their first maximum TE ADA titer at their final assessment in the 12-month period; however, six of them first became TE ADA+ at that same time point. Across all three studies, the majority of TE ADA+ patients (61.5–84.4%) had a TE ADA+ titer detected at only one time point during the treatment period.
Table 4.
ADA titer evolution over time in TE ADA+ patients from EVOLVE-1, EVOLVE-2, and Study CGAJ.
| EVOLVE-1 | EVOLVE-2 | CGAJ |
||
|---|---|---|---|---|
| (6 mo) | (6 mo) | (6 mo) | (12 mo) | |
| TE ADA evaluable | 415 (100) | 437 (100) | 266 (100) | 266 (100) |
| TE ADA+ during treatment | 20 (4.8) [100] | 32 (7.3) [100] | 21 (7.9) [100] | 26 (9.8) [100] |
| TE ADA+ exactly once | 13 (3.1) [65.0] | 27 (6.2) [84.4] | 17 (6.4) [81.0] | 16 (6.0) [61.5] |
| TE ADA+ *earlier* than last visit | 11 (2.7) [55.0] | 9 (2.1) [28.1] | 8 (3.0) [38.1] | 20 (7.5) [76.9] |
| Last titer is maximum titer | 14 (3.4) [70.0] | 27 (6.2) [84.4] | 17 (6.4) [81.0] | 14 (5.3) [53.8] |
| Maximum titer first occurs at last visit | 12 (2.9) [60.0] | 27 (6.2) [84.4] | 15 (5.6) [71.4] | 8 (3.0) [30.8] |
| Last titer is less than maximum titer | 6 (1.4) [30.0] | 5 (1.1) [15.6] | 4 (1.5) [19.0] | 12 (4.5) [46.2] |
| Last titer is not TE ADA+ | 6 (1.4) [30.0] | 5 (1.1) [15.6] | 4 (1.5) [19.0] | 10 (3.8) [38.5] |
Note: Data are shown as number (percentage of TE ADA evaluable) [percentage of TE ADA+].
mo: month; TE ADA: treatment emergent anti-drug antibody; TE ADA+: TE ADA positive.
Impact of ADA titer on galcanezumab pharmacokinetics and pharmacodynamics
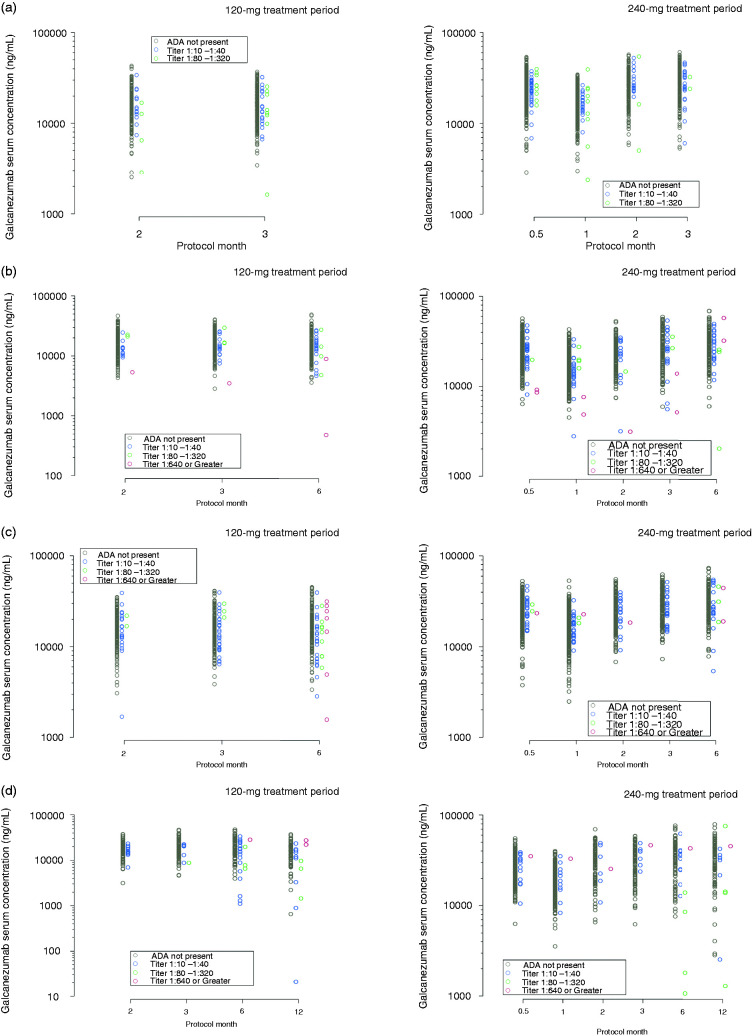
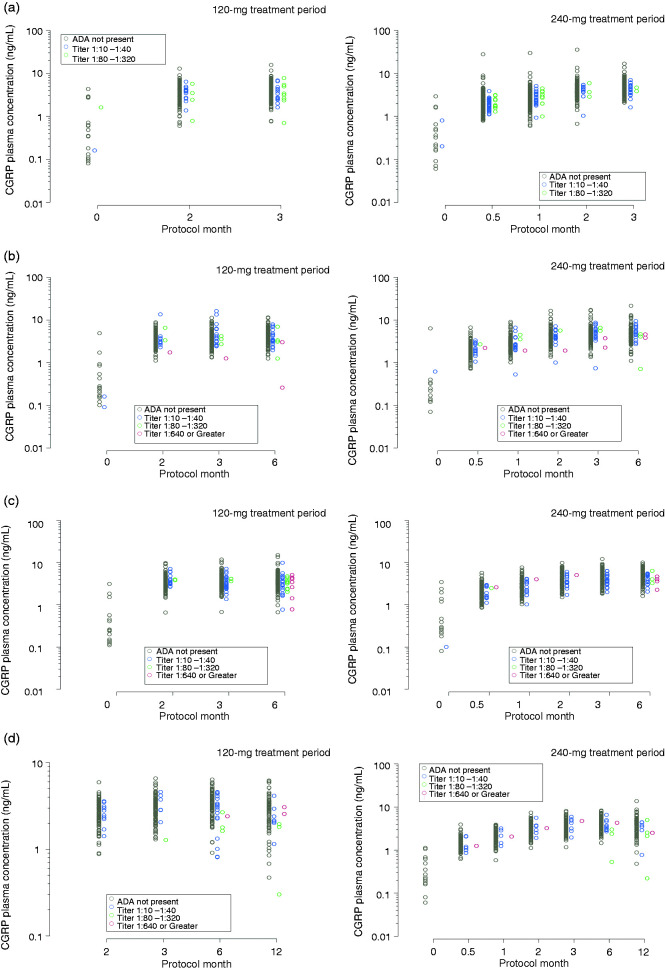
Galcanezumab serum and CGRP plasma concentrations categorized by ADA titer are shown in Figure 1 and Figure 2, respectively, for REGAIN, EVOLVE-1, EVOLVE-2, and Study CGAJ. Galcanezumab and CGRP concentrations at higher ADA titers during the treatment phases were generally within the range of ADA not present, or the lowest ADA titers, when compared to the same time point within a dose level.
Figure 1.
Galcanezumab serum concentrations categorized by anti-drug antibody (ADA) titer during the treatment period of (a) REGAIN, (b) EVOLVE-1, (c) EVOLVE-2, and (d) Study CGAJ. Concentrations of galcanezumab following a dose of 120 mg and 240 mg are shown separately.
Figure 2.
Total CGRP plasma concentrations categorized by anti-drug antibody (ADA) titer during the treatment period of (a) REGAIN, (b) EVOLVE-1, (c) EVOLVE-2, and (d) Study CGAJ. Concentrations of total calcitonin gene-related peptide (CGRP) at baseline and following a dose of 120 mg and 240 mg galcanezumab are shown separately.
Impact of TE ADA on efficacy
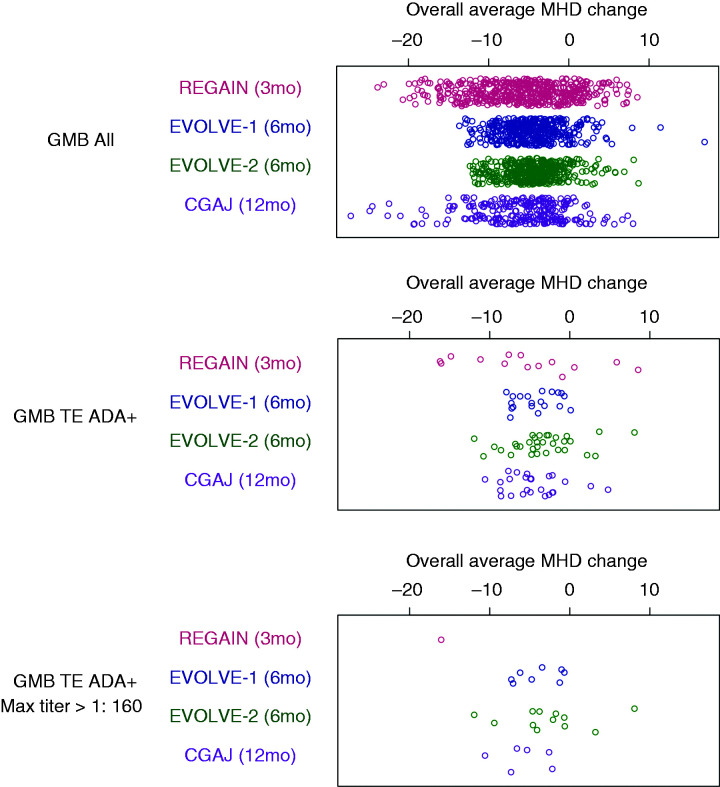
The average change from baseline in MHD was plotted for individual study participants during the 3-month double-blind treatment phase of REGAIN, the 6-month double-blind treatment phase in EVOLVE-1 and EVOLVE-2, and the 12-month open-label treatment phase of Study CGAJ (Figure 3). The range of mean monthly MHD changes observed in TE ADA+ patients (regardless of TE ADA titers) was within the range observed for all galcanezumab-treated patients (shown in Figure 3) and that of TE ADA− patients (not shown).
Figure 3.
Average change from baseline in monthly migraine headache days (MHD) for individual study participants receiving galcanezumab (GMB).
Impact of TE ADA on safety
No anaphylactic reactions were confirmed in any galcanezumab-treated patients regardless of TE ADA status. Additionally, no particular type of hypersensitivity events or AEs related to the injection site were reported exclusively in TE ADA+ patients. The events that met the flagging criteria for further review are shown in Table 5. A case-level review of the seven TE ADA+ patients who reported those events revealed that three patients reported pruritus and one reported injection site inflammation before the development of TE ADA, which did not recur after the detection or titer increase of such antibodies. One patient reported asthma shortly before the detection of TE ADA; however, the event resolved and did not recur despite the persistency of such antibodies. Two patients had already detectable TE ADA before reporting injection site rash. However, despite the persistence of TE ADA and monthly galcanezumab administrations, those events were only reported once or twice, respectively.
Table 5.
Treatment-emergent hypersensitivity events and adverse events related to injection sites in galcanezumab-treated patients that met flagging criteria for further review.
| Preferred term | TE ADA+ status | N | n (%) |
|---|---|---|---|
| Hypersensitivity events | |||
| Pruritus | Yes | 92 | 3 (3.3%) |
| No | 1562 | 17 (1.1%) | |
| Asthma | Yes | 92 | 1 (1.1%) |
| No | 1562 | 4 (0.3%) | |
| Adverse events related to injection sites | |||
| Injection site rash | Yes | 92 | 2 (2.2%) |
| No | 1562 | 10 (0.6%) | |
| Injection site inflammation | Yes | 92 | 1 (1.1%) |
| No | 1562 | 1 (0.1%) | |
N: number of patients in the analysis population; n: number of patients within each specific category; TE ADA: treatment emergent anti-drug antibody.
Discussion
Treatment-emergent ADA were observed in up to 9.4% of patients treated with galcanezumab 120 mg/month, up to 5.2% of patients treated with galcanezumab 240 mg/month, and up to 1.7% of patients treated with placebo during the double-blind, placebo-controlled treatment periods of the REGAIN, EVOLVE-1, and EVOLVE-2 studies. In the open-label Study CGAJ, where patients received up to 12 months of continuous galcanezumab treatment, 12.4% of patients receiving galcanezumab 120 mg/month and 7.3% of patients receiving galcanezumab 240 mg/month were TE ADA+.
Galcanezumab and CGRP concentrations were generally similar in patients with and without detectable ADA. These findings for galcanezumab concentrations are consistent with those of a population PK analysis demonstrating that galcanezumab apparent clearance was unaffected by ADAs (22). The range of mean monthly MHD changes observed in TE ADA+ patients was within the range observed for all galcanezumab-treated patients (regardless of TE ADA status), supporting a lack of clinically meaningful impact of the observed immunogenicity on galcanezumab efficacy. Further, immunogenicity was not shown to be a mediator of hypersensitivity events or AEs related to injection sites.
Before treatment (during the baseline phase), 5.9% to 11.2% of patients had low but detectable baseline ADA titers and 2.6% to 8.0% had NAb. Pre-existing ADA to biotherapeutic drugs has been commonly detected in drug-naïve subjects for different biotherapeutic modalities with a variable frequency that can range from 1% to 42% (23). The frequency of pre-existing ADA to galcanezumab reported herein is consistent with these rates. Detection of pre-existing ADA is greatly influenced by the selection of the bioanalytical ADA assay cut point. The immunogenicity assays used during the galcanezumab development program were validated in compliance with the US Food and Drug Administration and the European Medicines Agency Guidance for Industry, and other published recommendations (24–26). The most commonly reported sources of pre-existing reactivities are serum proteins or immunoglobulins present in the serum able to cross-react with the biotherapeutic drug (27). This cross-reactivity can occur to different parts of the biotherapeutic product, including the idiotype region containing the target-binding site. This may explain why some pre-existing ADA can have neutralizing capacity.
Increasing evidence supports the notion that the majority of ADA against therapeutic mAb are anti-idiotypic antibodies; that is, antibodies that target the antigen-binding site of the therapeutic mAb, and thus are neutralizing antibodies (5). In a study evaluating ADA to different tumor necrosis factor inhibitors (TNFi), more than 94% of ADA to adalimumab or golimumab (both fully human antibodies) and to certolizumab (humanized Fab fragment, pegylated) were revealed to be neutralizing. Even for infliximab, a chimeric mouse/human TNFi, more than 90% of ADA had neutralizing capacity (28). This immune-dominant role of the antigen-binding site of the drug has been observed for other biotherapeutics with different mechanisms of action (8).
As with all types of ADA, the detection of NAb is also greatly influenced by the performance characteristics of the assay adopted. Different formats of NAb assays exist and, most commonly, they have lower sensitivity and drug tolerance than the screening or confirmatory ADA assays (29,30). These technical limitations provide the main explanation for why, in many cases, a relatively low percentage of ADA+ patients have detectable NAb. The relatively low drug tolerance often seen in NAb assays limits the assay’s ability to detect NAb in samples that contain drug. That induces a detection bias towards the highest titers, as NAb present at lower titers may easily be hidden by drug interference in the NAb assay. This bias towards the highest titers also explains why generally only NAb have been associated with clinical impact. In the galcanezumab trials, both ADA screening and NAb assays had the same sensitivity and drug tolerance. That fact allowed us to confirm that, similar to other therapeutic antibodies, the immunogenic response to galcanezumab is highly restricted to the idiotype, and that the presence of NAb is not necessarily associated with deleterious clinical consequences. The clinical impact of TE ADA, including NAb, is essentially dependent on the magnitude, affinity, and persistence of ADA responses. Across the galcanezumab phase 3 randomized controlled trials, the majority of patients had detectable TE ADA only at a single time point during the treatment period, and the majority achieved maximum postbaseline titers ≤1:160. Additionally, a significant proportion of TE ADA+ patients had lower titers or even negative TE ADA titers by their last visit of the treatment period. Together, these findings indicate that the ADA responses to galcanezumab are of low magnitude and transient in nature and, thus, did not cause a clinically meaningful impact.
Across the presented studies, galcanezumab concentrations and CGRP concentrations in patients with ADA (regardless of titer) were within the range observed in patients without ADA. These findings indicate that ADA did not significantly interfere with the binding of CGRP to galcanezumab. Free CGRP has a faster elimination than CGRP bound to galcanezumab. The binding of CGRP to galcanezumab prevents rapid CGRP clearance, resulting in a slower CGRP elimination and an increase in CGRP concentrations after galcanezumab administration. Had ADA inhibited the binding of CGRP to galcanezumab, lower CGRP concentrations would be expected with increasing ADA titer. The similar CGRP concentrations observed in patients with or without ADA suggests that although the majority of ADA detected in the phase 3 studies demonstrated neutralizing activity in vitro, they had no appreciable effect of inhibiting the binding of the CGRP ligand to galcanezumab. These findings are consistent with similar efficacy of galcanezumab in patients with and without TE ADA (regardless of titer). In support of this, a recently published European Headache Federation guideline on the use of anti-CGRP monoclonal antibodies in migraine prevention found that the presence of binding and/or neutralizing antibodies has not been associated with poor response to treatment or adverse events (31). Authors of this guideline concluded that there is no evidence to suggest that testing for antibodies is warranted in routine clinical practice at this time, though they acknowledged that further study is needed.
By forming immune complexes with the drug, ADA can potentially increase the risk of AEs, most typically hypersensitivity events. In these analyses, hypersensitivity events and AEs related to injection sites were examined in detail, and there was no evidence that such events were TE ADA mediated. There were no specific types of hypersensitivity events or AEs related to injection site reported exclusively in TE ADA+ patients, meaning that the same type of AEs also occurred in patients without TE ADA. There were few types of events reported at an apparent higher frequency in TE ADA+ patients, namely pruritus (three patients), asthma (one patient), injection site rash (two patients) and injection site inflammation (one patient). However, a more detailed case-level review found that the majority of AEs occurred during periods of time when TE ADA were not yet present and did not recur when TE ADA became detectable. Even in the three cases where the AEs occurred after the detection of TE ADA, they were reported transiently (only once or twice), despite the persistence of TE ADA. Moreover, no relationship was observed between the TE ADA titer and the occurrence or severity of the AE, as patients with the highest TE ADA titers across the galcanezumab studies did not report such AEs.
In conclusion, this analysis found that although galcanezumab (like all therapeutic proteins) can elicit the production of ADA in some patients, the characteristics of the immune response observed in the phase 3 migraine clinical program were not found to have clinically meaningful consequences on galcanezumab PK, PD, efficacy, or safety.
Clinical implications
As with virtually all therapeutic proteins, therapeutic monoclonal antibodies may elicit an anti-drug antibody (ADA) response in some patients.
Analyses reported here found that although galcanezumab (a humanized monoclonal antibody that selectively binds calcitonin gene-related peptide and inhibits its activity) can elicit the production of ADA in some patients, the characteristics of the immune response observed in the phase 3 episodic and chronic migraine clinical program were not found to have clinically meaningful consequences on galcanezumab concentrations, calcitonin gene-related peptide concentrations, or the efficacy and hypersensitivity profile of galcanezumab.
Supplemental Material
Supplemental material, sj-pdf-1-cep-10.1177_0333102420920642 for Assessment of immunogenicity from galcanezumab phase 3 trials in patients with episodic or chronic migraine by James M Martinez, Nada Hindiyeh, Greg Anglin, Kavita Kalidas, Michael E Hodsdon, William Kielbasa, Brian A Moser, Eric M Pearlman and Sandra Garces in Cephalalgia
Acknowledgements
The authors would like to acknowledge Bret Fulton, Caroline Spencer and Sue Williamson (Rx Communications, Mold, UK) for medical writing assistance with the preparation of this manuscript, funded by Eli Lilly and Company.
Author contributions
JMM was involved with the conception of the work and the interpretation of the data. NH and BAM were involved with the analysis and interpretation of the data. GA was involved with the conception of the work, and the analysis and interpretation of the data. KK and EP were involved with the interpretation of the data. MEH was involved with acquisition, analysis and interpretation of the data. WK was involved with the conception and design of the work, and the analysis and interpretation of the data. SG was involved with the conception and design of the work, and the acquisition, analysis and interpretation of the data. All authors contributed sufficiently to the work and provided critical revision of the manuscript for important intellectual content. All authors give their approval of the manuscript to be submitted and published in Cephalalgia, and agree to be accountable for all aspects of the work.
Declaration of conflicting interests
The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: JMM, GA, MH, WK, BAM, and EP are full-time employees and minor shareholders of Eli Lilly and Company. SG was a full-time employee of Eli Lilly and Company at the inception of the work. NH is a member of the speakers’ bureau for Amgen, Electrocor, and Eli Lilly; advisory boards for Alder, Amgen, Eli Lilly and Zosano. KK received grants from Allergan, personal fees from Allergan, Assertio Therapeutics, Amgen, Eli Lilly and Company, Teva Pharmaceuticals, Promius Pharma, and Electrocore, outside the submitted work.
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This article was funded by Eli Lilly and Company.
Ethics or Institutional Review Board approval
This was an integrated assessment of data from the REGAIN (NCT02614261), EVOLVE-1 (NCT02614183), EVOLVE-2 (NCT02614196), and Study CGAJ (NCT02614287) studies. The protocols of the REGAIN, EVOLVE-1, EVOLVE-2, and Study CGAJ studies were reviewed and approved by the appropriate institutional review board for each site, and each study was conducted according to Good Clinical Practice and Declaration of Helsinki guidelines. All patients provided written informed consent prior to initiating study procedures.
Data accessibility
Lilly provides access to all individual participant data collected during the trial, after anonymization, with the exception of pharmacokinetic or genetic data. Data are available to request 6 months after the indication studied has been approved in the US and EU and after primary publication acceptance, whichever is later. No expiration date of data requests is currently set once data are made available. Access is provided after a proposal has been approved by an independent review committee identified for this purpose and after receipt of a signed data sharing agreement. Data and documents, including the study protocol, statistical analysis plan, clinical study report, blank or annotated case report forms, will be provided in a secure data sharing environment. For details on submitting a request, see the instructions provided at www.vivli.org.
References
- 1.Elgundi Z, Reslan M, Cruz E, et al. The state-of-play and future of antibody therapeutics. Adv Drug Deliv Rev 2017; 122: 2–19. [DOI] [PubMed] [Google Scholar]
- 2.Brekke OH, Sandlie I. Therapeutic antibodies for human diseases at the dawn of the twenty-first century. Nat Rev Drug Discov 2003; 2: 52–62 (updated 2: 240). [DOI] [PubMed] [Google Scholar]
- 3.Silberstein S, Lenz R, Xu C. Therapeutic monoclonal antibodies: What headache specialists need to know. Headache 2015; 55: 1171–1182. [DOI] [PubMed] [Google Scholar]
- 4.Wan H. An overall comparison of small molecules and large biologics in ADME testing. ADMET & DMPK 2016; 4: 1–22. [Google Scholar]
- 5.Garcês S, Demengeot J. The immunogenicity of biologic therapies. Curr Probl Dermatol 2018; 53: 37–48. [DOI] [PubMed] [Google Scholar]
- 6.Deehan M, Garcês S, Kramer D, et al. Managing unwanted immunogenicity of biologicals. Autoimmun Rev 2015; 147: 569–574. [DOI] [PubMed] [Google Scholar]
- 7.Harding FA, Stickler MM, Razo J, et al. The immunogenicity of humanized and fully human antibodies: Residual immunogenicity resides in the CDR regions. MAbs 2010; 2: 256–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Schie KA, Kruithof S, van Schouwenburg PA, et al. Neutralizing capacity of monoclonal and polyclonal anti-natalizumab antibodies: The immune response to antibody therapeutics preferentially targets the antigen-binding site. J Allergy Clin Immunol 2017; 139: 1035–1037. [DOI] [PubMed] [Google Scholar]
- 9.van Schouwenburg PA, van de Stadt LA, de Jong RN, et al. Adalimumab elicits a restricted anti-idiotypic antibody response in autoimmune patients resulting in functional neutralisation. Ann Rheum Dis 2013; 72: 104–109. [DOI] [PubMed] [Google Scholar]
- 10.Garcês S, Demengeot J, Benito-Garcia E. The immunogenicity of anti-TNF therapy in immune-mediated inflammatory diseases: A systematic review of the literature with a meta-analysis. Ann Rheum Dis 2013; 72: 1947–1955. [DOI] [PubMed] [Google Scholar]
- 11.Garcês S, Antunes M, Benito-Garcia E, et al. A preliminary algorithm introducing immunogenicity assessment in the management of patients with RA receiving tumour necrosis factor inhibitor therapies. Ann Rheum Dis 2014; 73: 1138–1143. [DOI] [PubMed] [Google Scholar]
- 12.Reich K, Jackson K, Ball S, et al. Ixekizumab pharmacokinetics, anti-drug antibodies, and efficacy through 60 weeks of treatment of moderate to severe plaque psoriasis. J Invest Dermatol 2018; 138: 2168–2173. [DOI] [PubMed] [Google Scholar]
- 13.Raddant AC, Russo AF. Calcitonin gene-related peptide in migraine: Intersection of peripheral inflammation and central modulation. Expert Rev Mol Med 2011; 13: e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oakes TMM, Skljarevski V, Zhang Q, et al. Safety of galcanezumab in patients with episodic migraine: A randomized placebo-controlled dose-ranging Phase 2b study. Cephalalgia 2018; 38: 1015–1025. [DOI] [PubMed] [Google Scholar]
- 15.Skljarevski V, Matharu M, Millen BA, et al. Efficacy and safety of galcanezumab for the prevention of episodic migraine: Results of the EVOLVE-2 Phase 3 randomized controlled clinical trial. Cephalalgia 2018; 38: 1442–1454. [DOI] [PubMed] [Google Scholar]
- 16.Stauffer VL, Dodick DW, Zhang Q, et al. Evaluation of galcanezumab for the prevention of episodic migraine: The EVOLVE-1 randomized clinical trial. JAMA Neurol 2018; 75: 1080–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martinez JM, Goadsby P, Dodick D, et al. Study CGAL: A phase 3 placebo-controlled study of galcanezumab in patients with episodic cluster headache: Results from the 8-week double-blind treatment phase. Headache 2018; 58: 1289–1290 (Abstract IOR-03LB). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Detke HC, Goadsby PJ, Wang S, et al. Galcanezumab in chronic migraine: The randomized, double-blind, placebo-controlled REGAIN study. Neurology 2018; 91: e2211–e2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Camporeale A, Kudrow D, Sides R, et al. A phase 3, long-term, open-label safety study of galcanezumab in patients with migraine. BMC Neurol 2018; 18: 188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bourdage JS, Cook CA, Farrington DL, et al. An affinity capture elution (ACE) assay for detection of anti-drug antibody to monoclonal antibody therapeutics in the presence of high levels of drug. J Immunol Methods 2007; 327: 10–17. [DOI] [PubMed] [Google Scholar]
- 21.Butterfield AM, Chain JS, Ackermann BL, et al. Comparison of assay formats for drug-tolerant immunogenicity testing. Bioanalysis 2010; 2: 1961–1969. [DOI] [PubMed] [Google Scholar]
- 22.Kielbasa W, Quinlan T. Population pharmacokinetics of galcanezumab, an anti-CGRP antibody, following subcutaneous dosing to healthy individuals and patients with migraine. J Clin Pharmacol 2020; 60: 229--239. [DOI] [PMC free article] [PubMed]
- 23.Xue L, Rup B. Evaluation of pre-existing antibody presence as a risk factor for posttreatment anti-drug antibody induction: Analysis of human clinical study data for multiple biotherapeutics. AAPS J 2013; 15: 893–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Food and Drug Administration. Assay development and validation for immunogenicity testing of therapeutic protein products: Guidance for industry, https://www.fda.gov/downloads/Drugs/Guidances/UCM192750.pdf (2016, accessed 27 June 2018).
- 25.European Medicines Agency. Guideline on immunogenicity assessment of therapeutic proteins, http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2017/06/WC500228861.pdf (2017, accessed 27 June 2018).
- 26.Shankar G, Devanarayan V, Amaravadi L, et al. Recommendations for the validation of immunoassays used for detection of host antibodies against biotechnology products. J Pharm Biomed Anal 2008; 48: 1267–1281. [DOI] [PubMed] [Google Scholar]
- 27.Xue L, Fiscella M, Rajadhyaksha M, et al. Pre-existing biotherapeutic-reactive antibodies: Survey results within the American Association of Pharmaceutical Scientists. AAPS J 2013; 15: 853–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Schie KA, Hart MH, de Groot ER, et al. The antibody response against human and chimeric anti-TNF therapeutic antibodies primarily targets the TNF binding region. Ann Rheum Dis 2015; 74: 311–314. [DOI] [PubMed] [Google Scholar]
- 29.Hu J, Wala I, Han H, et al. Comparison of cell-based and non-cell-based assay platforms for the detection of clinically relevant anti-drug neutralizing antibodies for immunogenicity assessment of therapeutic proteins. J Immunol Methods 2015; 419: 1–8. [DOI] [PubMed] [Google Scholar]
- 30.Zhong ZD, Clements-Egan A, Gorovits B, et al. Drug target interference in immunogenicity assays: Recommendations and mitigation strategies. AAPS J 2017; 19: 1564–1575. [DOI] [PubMed] [Google Scholar]
- 31.Sacco S, Bendtsen L, Ashina M, et al. European Headache Federation guideline on the use of monoclonal antibodies acting on the calcitonin gene related peptide or its receptor for migraine prevention. J Headache Pain 2019; 20: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-cep-10.1177_0333102420920642 for Assessment of immunogenicity from galcanezumab phase 3 trials in patients with episodic or chronic migraine by James M Martinez, Nada Hindiyeh, Greg Anglin, Kavita Kalidas, Michael E Hodsdon, William Kielbasa, Brian A Moser, Eric M Pearlman and Sandra Garces in Cephalalgia
Data Availability Statement
Lilly provides access to all individual participant data collected during the trial, after anonymization, with the exception of pharmacokinetic or genetic data. Data are available to request 6 months after the indication studied has been approved in the US and EU and after primary publication acceptance, whichever is later. No expiration date of data requests is currently set once data are made available. Access is provided after a proposal has been approved by an independent review committee identified for this purpose and after receipt of a signed data sharing agreement. Data and documents, including the study protocol, statistical analysis plan, clinical study report, blank or annotated case report forms, will be provided in a secure data sharing environment. For details on submitting a request, see the instructions provided at www.vivli.org.