Abstract
Objective
Structured electrocardiography (ECG) analysis is used to screen athletes for high-risk cardiovascular conditions (HRCC) to prevent sudden cardiac death. ECG criteria have been specified and recommended for use in young athletes ≤ 35 years. However, it is unclear whether these ECG criteria can also be applied to master athletes >35 years.
Aim
The purpose of this study was to test whether the existing ECG criteria for detecting HRCC in young athletes can be applied to master athletes.
Methods
We conducted a cross-sectional study among athletes >35 years screened for HRCC between 2006 and 2010. We performed a blinded retrospective analysis of master athletes’ ECGs, separately applying European Society of Cardiology (ESC)-2005, Seattle, and International criteria. HRCC were defined using recommendations from the international cardiac societies American Heart Association and American College of Cardiology, and ESC, based on ECG screening and cardiovascular evaluation (CVE).
Results
We included 2578 master athletes in the study, of whom 494 had initial screening abnormalities mandating CVE. Atrial enlargement (109, 4.1%) and left ventricular hypertrophy (98, 3.8%) were the most common ECG abnormalities found using the ESC-2005 or Seattle criteria. Applying the International criteria, ST-segment deviation (66, 2.6%), and T-wave inversion (58, 2.2%) were most frequent. The ESC-2005 criteria detected more HRCC (46, 1.8%) compared with the Seattle (36, 1.4%) and International criteria (33, 1.3%). The most frequently detected HRCC was coronary artery disease (24, 0.9%).
Conclusion
ECG criteria recommended for use in young athletes can be applied to master athletes’ ECGs to detect HRCC. The ESC-2005 criteria had the highest sensitivity for detecting HRCC among master athletes.
Keywords: ECG, prevention, screening, sudden cardiac death, athlete
What is already known about this subject?
Electrocardiography (ECG)-criteria sets to detect high-risk cardiovascular conditions (HRCC) have been developed on the basis of the ECG interpretation of asymptomatic athletes aged 12–35 years who exercise weekly >4–8 hours. No similar detection criteria are available for master athletes aged >35 years, and it is unclear whether the ECG criteria recommended for young athletes can be applied to master athletes.
What does this study add?
The three ECG criteria sets (European Society of Cardiology (ESC)-2005, Seattle, International) recommended for use in young athletes can also be applied to master athletes to detect HRCC. The ESC-2005 criteria performed better than the Seattle and the International criteria in detecting HRCC in master athletes.
How might this impact on clinical practice?
All three sets of ECG criteria can be used to detect HRCC in master athletes >35 years. The ESC-2005 criteria had the highest sensitivity when detecting HRCC among master athletes.
Introduction
Athletes are screened for cardiovascular conditions to prevent exercise-related sudden cardiac arrest (SCA) and/or sudden cardiac death (SCD).1–3 The most common high-risk cardiovascular conditions (HRCC) among young athletes ≤35 years are cardiomyopathies and ion channelopathies.1–7 Screening asymptomatic athletes with electrocardiography (ECG) increases our ability to detect these potentially fatal HRCC in time.8–12 A 12-lead ECG at rest is, therefore, usually included in the preventive screening program.1,4,5
Several consensus recommendations have been published describing ECG criteria that raise the suspicion of HRCC and for which cardiovascular evaluation (CVE) should be requested. In 2005, the European Society of Cardiology (ESC) endorsed an ECG criteria set to interpret athletes’ ECGs.1,4 The ESC-2005 criteria were updated in 2010 to distinguish between training-related and training-unrelated ECG changes.13 They were further updated and revised to increase the sensitivity of the screening process to detect HRCC and reduce the number of false positives – the ‘Seattle criteria’.14–16 The most recent consensus-based statement of the ESC and the American Heart Association and American College of Cardiology (AHA/ACC) are the ‘International criteria’, which includes criteria for athletes from different ethnicities.17 All ECG criteria sets were developed on the basis of the ECG interpretation of asymptomatic athletes aged 12–35 years who exercise >4–8 hours weekly.
However, an increasing number of master athletes aged >35 years participate in organized and competitive sports events, and actively seek advice regarding their fitness to participate. Screening master athletes for HRCC is complex, and largely focusses on the detection of coronary artery disease (CAD). The International criteria are currently recommended for young athletes.17 For master athletes, no such detection criteria are available and it is unclear whether the ECG criteria recommended for young athletes can be applied to master athletes.18
The aim of our study was to test whether the existing ECG criteria for young athletes can be applied to master athletes to detect HRCC. To determine which of the three ECG criteria sets is most suitable, we compared the outcome of the interpretation of the same 12-lead ECG at rest with clinically detected HRCC (after CVE) in a large sample of master athletes.
Methods
We conducted a cross-sectional study in athletes aged > 35 undergoing cardiovascular screening (Lausanne protocol) for HRCC by the sports physicians at the Sports Medical Centre Papendal in Arnhem (liaised to the Netherlands Olympic Committee), the Netherlands, between 2006 and 2010. We included individuals with screening abnormalities referred for further specialized CVE. All tested athletes regularly participated in competitive or recreational sports or other forms of physical training aiming to improve their performance. Initial screening for HRCC included personal history, physical examination, and 12-lead ECG at rest. CVE requested for athletes with screening abnormalities consisted of the methods recommended by the ESC and AHA/ACC: exercise testing, Holter monitoring, cardiac imaging, and invasive diagnostics.4,19 Cardiovascular conditions were assessed after CVE was completed.
To reduce the risk of false negative interpretations of the screening results, we reviewed all cases with doubtful initial screening results at the multidisciplinary sports cardiology meeting. These athletes’ ECGs were then reviewed by an expert sports cardiologist (NP), and, if classified as abnormal, the athlete was referred for CVE.
We included all referred athletes for the analyses, even if further CVE revealed a history of a cardiovascular condition that was not documented during the initial sports physicians’ screening. Initial ECG assessment was based on the ESC-2005 criteria set, as this was the only available set of ECG criteria during the inclusion period. The primary outcome of our study was ECG-detected HRCC.
ECG screening
A 12-lead ECG (Cardiosoft 6.7 Diagnostic System, GE Health Care) in the supine position was recorded for all athletes referred for CVE. The ECGs were retrospectively reviewed by an experienced sports cardiologist (NP), blinded for all other data, using the three ECG criteria sets: ESC-2005, Seattle, and International recommendations. The ECGs were categorized as either ‘normal’ or ‘abnormal’ according to the ECG criteria set used.
The ECG criteria sets have been extensively described elsewhere.1,14–17 In short, all three ECG criteria sets classify ECG abnormalities as the presence of Mobitz II or complete AV-block (AVB), atrial or ventricular tachyarrhythmias, left bundle branch block (LBBB), pathologic Q-waves, ST-segment deviation (STD), T-wave inversion (TWI), prolonged corrected QT (QTc) interval (females >480 ms, males >470 ms), ventricular pre-excitation, Brugada type 1, or ≥ 2 ventricular extrasystoles/10 s. The ESC-2005 and Seattle criteria classify left ventricular hypertrophy (LVH), right and/or left atrial enlargement (RAE/LAE), right bundle branch block (RBBB), and axis deviation as abnormal; the ESC-2005 criteria classify PR-interval >200 ms as abnormal.
Outcomes and definitions
Cardiovascular conditions were defined as congenital or inherited, hypertension, cardiac arrhythmias, myocardial disease, valvular heart disease, CAD, and aorta or peripheral artery disease. We classified cardiovascular conditions either as high risk (HRCC) or low risk (LRCC) for SCA/SCD.
The primary outcome of our analysis was HRCC detected by any of the three ECG criteria sets (ESC-2005, Seattle, International). The presence of these HRCC was based on all available data from CVE. We defined HRCC as:
inherited cardiovascular conditions: hypertrophic cardiomyopathy (HCM), arrhythmogenic cardiomyopathy (ACM), dilated cardiomyopathy, long QT syndrome (QTc >470 ms in symptomatic males, >480 ms in females, or ≥ 500 ms in asymptomatic individuals), short QT syndrome (QTc ≤320 ms), Brugada syndrome, catecholaminergic polymorphic ventricular tachycardia (VT), or idiopathic VT;
congenital cardiovascular conditions: coronary artery anomalies, bicuspid aortic valve, or ventricular pre-excitation with malignant properties over the accessory atrioventricular connection;
cardiac valve conditions: mitral valve prolapse, or aortic valve stenosis (AVS; moderate or severe);
other cardiovascular conditions: aortic disease (including Marfan syndrome), atherosclerotic CAD, conduction system abnormalities (Mobitz II AVB, complete AVB, Lev Lenègre disease), and myocarditis; or the sequalae of commotio cordis.
Atherosclerotic CAD was defined as a history of coronary artery revascularization (percutaneous coronary artery intervention, coronary artery bypass grafting) and/or myocardial infarction (>six months before inclusion, which is the time period of athletes’ rehabilitation to restrict exercise), or other CAD (as demonstrated with invasive or non-invasive investigations), symptomatic or asymptomatic.
We defined LRCC as all cardiovascular conditions not defined as HRCC.
Screening abnormalities were defined as: (a) exercise-related complaints (i.e. dyspnoea, chest discomfort, palpitations, dizziness or fainting, syncope, abnormal fatigue), history of any cardiovascular condition; (b) family history of SCA/SCD, inherited or congenital cardiovascular conditions; (c) hypertension, cardiac murmur, peripheral artery disease; and (d) ECG abnormalities. See the supplementary material for other definitions.
Statistical methods
To assess the applicability of the ECG criteria, we evaluated HRCC in the entire screened population (n = 2578). Continuous variables were described as means and standard deviations, and dichotomous variables as absolute numbers and percentages. If HRCC was detected with the ECG, it was considered to be present in all comparisons in the analysis. The ECGs of all athletes >35 years with normal screening results (2084) were classified as normal in all analyses.
We calculated sensitivity, specificity, false positive rate (FPR), and false negative rate (FNR) for each of the three ECG criteria sets for the initially screened group (2578) using 2 × 2 contingency tables. Due to the nature of our study, athletes with normal initial screening results (2084) were not referred for CVE; consequently, data on cardiovascular findings/outcomes were not available. Therefore, depending on the detection method, we were unable to differentiate between true negative and false negative results. We conducted statistical analyses using SPSS version 21.0 (SPSS® Inc, Chicago, IL, USA). In accordance with Dutch law (1998), the requirement for medical ethical approval was waived as the study only included case record reviews (Dutch Committee of Human Research number 2017-3928).
Results
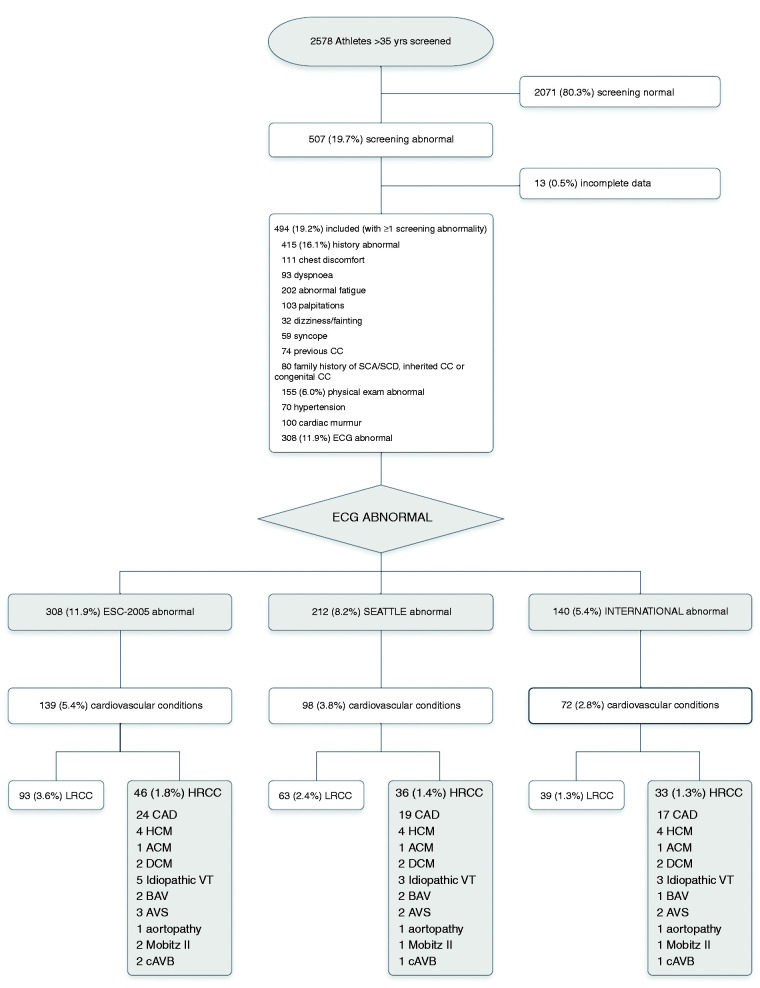
Of all 2578 athletes >35 years screened, 494 fulfilled the screening abnormality inclusion criteria, of whom 308 had ECG abnormalities using the ESC-2005 criteria (Figure 1).
Figure 1.
Study flow chart and outcome of 2578 athletes >35 years screened for HRCC.
A total of 2578 athletes > 35 years had ECG-inclusive screening for HRCC. Of the 507 with screening abnormalities, 13 were excluded (incomplete data). Of the included 494 athletes, the ECG at rest was abnormal applying the ESC-2005 criteria in 308, applying the Seattle criteria in 212, and International criteria in 140. The corresponding ECG-detected HRCC were 46, 36, and 33, respectively.
ACM: arrhythmogenic cardiomyopathy; AV: aortic valve; AVS: moderate or severe aortic valve stenosis; BAV: bicuspid aortic valve; CAD: coronary artery disease; cAVB: complete AV-block; DCM: dilated cardiomyopathy; HCM: hypertrophic cardiomyopathy; HRCC: high-risk cardiovascular conditions; LRCC: low-risk cardiovascular conditions VT: ventricular tachyarrhythmia.
The majority of the study cohort (494) were male (396, 80.2%) and Caucasian (488, 98.8%); 51.8% participated in competitive sports (256).
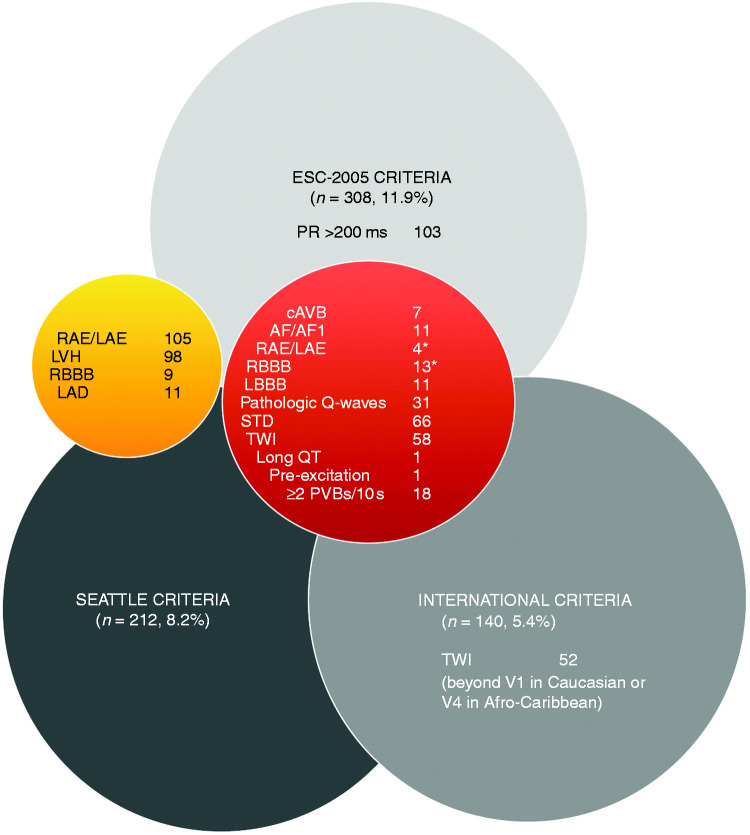
The number of ECG abnormalities varied dependent on the ECG criteria set. The ESC-2005 criteria identified more ECG abnormalities (308, 11.9%) compared with the Seattle (212, 8.2%) and the International criteria (140, 5.4%) (Figures 1 and 2). RAE/LAE (109) and LVH (98) were the most common ECG abnormalities applying ESC-2005 or Seattle criteria, and STD (66) and TWI (58) when applying the International criteria (Figure 2).
Figure 2.
ECG abnormalities at rest in 2578 master athletes > 35 years assessed applying the ECG criteria of the ESC-2005, Seattle, and International recommendations.
Red sphere: ECG findings assessed abnormal applying the three ECG sets (ESC-2005, Seattle, International); orange sphere: ECG findings assessed abnormal applying the ESC-2005 and Seattle criteria.
*The International criteria considers two or more borderline normal ECG criteria as abnormal.
AF/AFl: atrial fibrillation or flutter; AV: aortic valve; cAVB: complete AV block; LAD: left axis deviation; LBBB: complete left bundle branch block; long QT: QT-interval > 470 ms males and > 480 ms females; LVH: voltage criteria for left ventricular hypertrophy; PVB: premature ventricular beat; RAE/LAE: right and/or left atrial enlargement; RBBB: complete right bundle branch block; STD: ST-segment deviation; TWI: T-wave inversion.
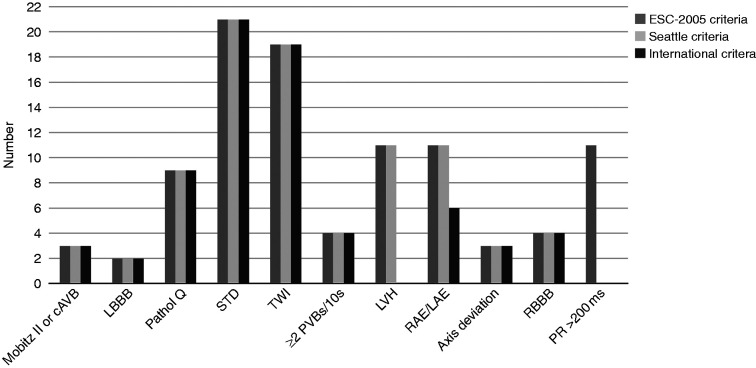
The ECG abnormalities associated with HRCC detection are shown in Figure 3. Less HRCC were detected using the International criteria, as borderline criteria for LVH and RAE/LAE did not always classify as indicating pathology.
Figure 3.
ECG abnormalities in each of the three ECG criteria sets associated with detecting HRCC.
AV: aortic valve; cAVB: complete AV-block; LBBB: left bundle branch block; LVH: QRS voltage criteria for left ventricular hypertrophy; pathol Q: pathologic Q-waves; PR: PR-interval; PVB: premature ventricular beats; RAE/LAE: right and/or left atrial enlargement; RBBB: complete right bundle branch block; RCC: high-risk cardiovascular conditions; STD: ST-segment deviation; TWI: T-wave inversion.
The number of ECG-detected cardiovascular conditions varied among the athletes (Figure 1). The most common ECG-detected HRCC was CAD (24, 0.9%) followed by HCM (4, 0.15%). Applying the ESC-2005 criteria, more ECG-detected HRCC were found (46, 1.8%) than with the Seattle (36, 1.4%) or International criteria (33, 1.3%). Compared with the ESC-2005 criteria, the Seattle criteria missed 10 HRCC: CAD (5), idiopathic VT (2), AVS (1), Mobitz II AVB (1), and complete AVB (1). Compared with the Seattle criteria, the International criteria CAD remained undetected in two athletes. Of the ECG findings assessed as normal at rest by the three ECG sets, J-point elevation (JPE) was found among 112 athletes, of which 13 were identified with HRCC, including six CAD.
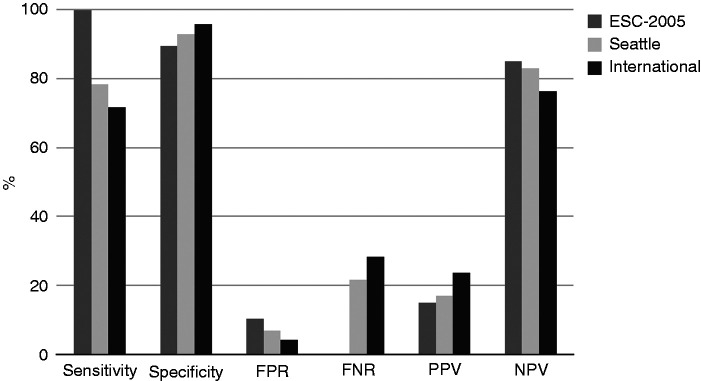
Of the three ECG criteria sets for the detection of HRCC, the ESC-2005 criteria had the highest sensitivity (100%) and lowest specificity (89.6%), but the highest FPR (10.3%) and lowest PPV (14.9%) (Figure 4).
Figure 4.
Test performance indices of the three ECG criteria sets: ESC-2005, Seattle, and International.
Sensitivity: the total number of athletes with ECG abnormalities and high-risk cardiovascular conditions (HRCC) present (true positives) divided by the total number of athletes with HRCC present; specificity: the total number of athletes with normal ECG results and HRCC absent (true negatives) divided by the total number of athletes with HRCC absent; FPR: false positive rate, the total number of athletes with abnormal ECG results and HRCC absent (false positives) divided by the total number of athletes with HRCC absent; FNR: false negative rate, the total number of athletes with normal ECG results and HRCC present (false negatives) divided by the total number of athletes with HRCC present; PPV: positive predictive value, the number of true positives divided by total number of athletes with ECG abnormalities; NPV: negative predictive value, one minus PPV.
Discussion
In our single-centre study, we evaluated whether the ESC-2005, Seattle, and International ECG criteria sets developed for HRCC in athletes ≤35 years could be applied to athletes >35 years. We found that the ESC-2005 criteria identified the largest number of HRCC in master athletes, suggesting that these criteria are most preferable for interpreting a 12-lead ECG at rest in this group.
The most common HRCC missed by the Seattle and International criteria was CAD, with the associated STD, TWI, voltage criteria for LVH, and RAE/LAE. This is consistent with the fact that the ESC-2005 and Seattle criteria classify LVH and RAE/LAE as abnormal, while the International criteria classify these as normal or borderline. Whereas LVH and RAE/LAE are assumed to be a sign of physiologic cardiac adaptation in young athletes, among master athletes these ECG findings may be secondary, for instance to hypertension.
When interpreting our findings and translating them to clinical practice, a number of issues should be considered. There are limited data on the predictive value of ECG-findings in master athletes. Although our study gives a number of indications about which ECG findings should be viewed as abnormal, the retrospective study design and the lack of follow-up or validation of the clinical decisions limit the generalizability of these findings. Ideally, a large prospective study of master athletes including ECGs and follow-up data should be performed to inform clinicians how to differentiate between physiologic cardiac adaptation and pathophysiology, as reflected in the ECG.
The variable outcome of ECG-detected HRCC can be explained by the methods – that is, using an ECG at rest and assessing these ECGs at rest by applying the three different ECG criteria sets. ECG findings considered abnormal at rest, such as AVB, LBBB, pathologic Q-waves, STD, and TWI, were not always related to the detection of HRCC (Figure 2). Therefore, athletes could have been mistakenly restricted from sports participation. Conversely, the ECG at rest using the three ECG criteria sets did not always detect HRCC, like VT and AVB, placing the athlete at risk of exercise-related cardiovascular events. In addition, the interpretation (normal, abnormal) of the ECG findings for LVH, RAE/LAE, axis deviation, RBBB, and prolonged PR-interval at rest explain the variable outcome of ECG-detected HRCC further. Therefore, our results suggest that findings deemed as ‘normal’ among young athletes may not automatically be interpreted as normal in master athletes.
An unintended consequence of screening athletes for HRCC was the detection of LRCC, resulting in unnecessary additional CVE being requested by the screening physicians.
Considerably more LRCC were identified when applying the ESC-2005 criteria (93, 3.6%) compared with the Seattle (63, 2.4%) and the International criteria (39, 1.5%), increasing FPR. Physicians performing screenings in master athletes should be aware of the potential consequences of identifying LRCC, such as athletes’ concerns about eligibility-to-play and the medical costs of additional CVE.
With increasing awareness that physical exercise reduces the risk of cardiovascular events, aging athletes are a rapidly growing group whose activities also include high-intensity exercise training, such as marathon running, speed-skating, cycling tours, and trail running, amongst others. The most common cause of SCA/SCD among master athletes is CAD (incidence 2.1/100,000 athletes per year).5,9,10 The mechanism of this SCA/SCD was non-atherosclerotic CAD with myocardial oxygen supply–demand mismatch, in contrast to atherosclerotic CAD with plaque rupture.5,6
Other causes of SCA/SCD in this specific group of master athletes include HCM, ACM, myocarditis, and valvular heart disease.6,7,20 The relative contribution of these different causes of SCA/SCD are not uniform. For example, in the Race Associated Cardiac Arrest Event registry of long-distance running races, HCM was a more frequent cause of SCA/SCD (26%) than CAD (16%) at a mean victim age of 42 years.6 Moreover, SCA/SCD was predominantly seen in male athletes (86%), individuals who do not exercise regularly, and those with known cardiovascular conditions and/or cardiovascular risk factors.6,7,21
As HCM and CAD are the most common HRCC leading to SCA/SCD in aging athletes, ideally ECG-inclusive screening should assist physicians in detecting these two entities and recognizing associated ECG abnormalities. In HCM, ECG abnormalities can include pathologic Q-waves, LVH, STD, TWI in the anterolateral and/or inferior leads, LBBB, and frequent PVBs.1,13,17 However, TWI in the right precordial leads (V1–4) may be a normal ECG finding in teen-aged and African/Afro-Caribbean athletes.22 In CAD, pathologic Q-waves, STD, TWI, and LBBB can be found.23 Although young athletes may suffer from (premature) CAD, the ECG abnormalities typical for suspected CAD and the consequent CVE are briefly mentioned in the ECG criteria sets used in our study.1,13,17 The ESC-2005 criteria classify all the ECG findings associated with both entities as abnormal.1 In contrast, when found in isolation, the International criteria assess LVH and RAE/LAE as normal or training-related.17
Table 1.
Baseline characteristics and screening abnormalities of athletes > 35 years screened for HRCC (n = 2578).
| Athletes with screening abnormalities (n = 494) | |
|---|---|
| n (%) | |
| Baseline characteristics | |
| Male | 396 (80.2) |
| Age, mean (range) in years | 47.9 (36–79) |
| Ethnicity | |
| Caucasian | 488 (98.8) |
| African/Afro-Caribbean | 3 (0.6) |
| Other | 3 (0.6) |
| Level of competition | |
| Elite (national/international competition) | 7 (1.4) |
| Competitive | 249 (50.4) |
| Recreational | 238 (48.2) |
| >6 weekly training hours | 283 (57.3) |
| Screening abnormalities (Lausanne protocol) | |
| History | 415 (84.0) |
| Exercise-related complaintsa | 381 (77.1) |
| Chest discomfort | 111 (22.5) |
| Dyspnoea | 93 (18.8) |
| Palpitations | 103 (20.8) |
| Dizziness or fainting | 32 (6.5) |
| Syncope | 59 (11.9) |
| Abnormal fatigue | 202 (40.9) |
| History of any CC | 74 (15.0) |
| Family history SCA/SCD, inherited or congenital CC | 165 (33.4) |
| Physical examination | 155 (31.4) |
| Hypertension (>140/90 mm Hg) | 70 (14.2) |
| Cardiac murmur | 96 (19.4) |
| Peripheral vascular disease | 3 (0.6) |
| Pectus excavatum | 9 (1.8) |
| Resting 12-lead ECG | |
| ESC-2005 criteria | 308 (62.3) |
CC: cardiovascular conditions; ECG: electrocardiogram; ESC: European Society of Cardiology; HRCC: high-risk cardiovascular conditions; SCA: sudden cardia arrest; SCD: sudden cardiac death.
Athletes had one or more exercise-related complaint.
JPE is a common ECG finding among young athletes and is generally considered to be benign.1,13,17,24,25 However, in master athletes, JPE was more prevalent in SCA victims due to idiopathic ventricular fibrillation, challenging the benign nature of JPE in athletes.25,26 We found JPE in 13 HRCC, including six CAD. This highlights the complexity of interpreting master athletes’ ECGs, with the potential ECG findings associated with cardiac adaptation, (early-stage) pathology, or a mix of both. Consequently, screening physicians should be aware that JPE may be considered a borderline or abnormal ECG finding in the aging athlete, thereby raising suspicion for HRCC.
Clearly, a resting ECG alone is not an ideal screening tool for detecting either CAD or a high risk of CAD. Screening for CAD should include symptoms, family history, risk SCORE assessment (age, hypertension, hypercholesterolemia, smoking), and exercise testing in suspected cases to simulate an athlete’s physiology.21
With imaging techniques being increasingly implemented to identify coronary calcifications and plaques, risk assessment and therapeutic consequences in master athletes can be challenging, as several studies have demonstrated a higher prevalence of calcified coronary plaques in master athletes.21,27,28
Coronary CT angiography (CCTA) is a promising diagnostic tool for identifying individuals with CAD. However, due to the lack of dedicated studies in athletes with long-term clinical outcomes, we currently recommend adding CCTA on an individual basis only.29,30
Our findings suggest that the three ECG criteria sets designed for the detection of HRCC in young athletes can be used for the interpretation of master athletes’ ECGs. The ESC-2005 criteria, no longer used to interpret young athletes’ ECGs, appear to be the best for detecting HRCC among master athletes.
Limitations
Some aspects of our study warrant consideration. First, we conducted a retrospective cross-sectional analysis of the ECGs in master athletes. To assess the ECGs of young athletes, we used the ECG criteria available at that time – that is, the ESC-2005 criteria set, originally described for the general population. However, the ESC-2005 classified prolonged PR >200 ms and voltage criteria for LVH as abnormal, which current ECG criteria sets classify as normal or as training-related ECG changes.
As a result, more athletes with screening abnormalities were referred for CVE, increasing both FPR and specificity.
Second, we present a cross-sectional analysis without follow-up or validation of the physicians’ decision-making for eligibility-to-exercise and participate in sports. Third, the sports physicians interpreted the initial screening results, including the ECG interpretation. They requested CVE for those with screening abnormalities and discussed doubtful cases at the sports cardiology meeting. The ECGs were reviewed by the sports cardiology expert. Therefore, the risk of a false negative interpretation of the ECG was considered low.
Fourth, the gold standard of our study was the presence of ECG-detected HRCC based on CVE, including the ECG. Although including the ECG in the gold standard may be methodologically questionable, it would be unrealistic to exclude the ECG from the definition because potential lethal cardiovascular conditions, such as ion channelopathy and cardiomyopathy, would remain undetected in asymptomatic athletes. Fifth, the key issue is the long-term predictive value of the ECG assessment in the screening process of athletes. Although there is increasing knowledge about physiological ECG findings in young athletes, the long-term significance of numerous ECG abnormalities remains unknown. Furthermore, it is uncertain whether ECG findings observed in master athletes have the same predictive value as in young athletes. The age limit of 35 years is, however, questionable.
Conclusion
The three ECG criteria sets recommended for use in young athletes can be applied to master athletes for the detection of HRCC. Of the three sets, the ESC-2005 criteria performed slightly better for the detection of HRCC in master athletes. Future studies are required to determine the long-term significance of the ECG abnormalities found in master athletes.
Supplemental Material
Supplemental material, CPR901060 Supplemental material for ECG criteria for the detection of high-risk cardiovascular conditions in master athletes by Nicole M Panhuyzen-Goedkoop, Hein J Wellens, André LM Verbeek, Harald T Jørstad, Joep RLM Smeets and Ron JG Peters in European Journal of Preventive Cardiology
Acknowledgement
We would like to express our gratitude to Roger Staats, Radboudumc ‘in’to Languages’, for his assistance with the language.
Author contribution
NP, AV, HJ, JS, and RP contributed to the conception and/or design of the work; NP, HW, AV, and HJ contributed to the acquisition, analysis, and/or interpretation of data for the work; NP drafted the manuscript; all authors critically revised the manuscript, gave final approval, and agreed to be accountable for all aspects of the work ensuring integrity and accuracy.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Supplemental Material
Supplemental material for this article is available online.
References
- 1.Corrado D, Pelliccia A, Bjornstad HH, et al. Cardiac pre-participation screening of young competitive athletes for prevention of sudden death: proposal for a common European protocol. Consensus Statement of the Study Group of Sport Cardiology of the Working Group of Cardiac Rehabilitation and Exercise Physiology and the Working Group of Myocardial and Pericardial Diseases of the European Society of Cardiology. Eur Heart J 2005; 26: 516–524. [DOI] [PubMed] [Google Scholar]
- 2.Maron BJ, Levine BD, Washington RL, et al. Eligibility and disqualification recommendations for competitive athletes with cardiac abnormalities: Task Force 2: pre-participation screening for cardiac disease in competitive athletes. Circulation 2015; 132: e267–e272. [DOI] [PubMed] [Google Scholar]
- 3.Drezner JA, O’Connor FG, Harmon KG, et al. AMSSM Position statement on cardiac preparticipation screening in athletes: current evidence, knowledge gaps, recommendations and future directions. Br J Sports Med 2016; 51: 153–167. [DOI] [PubMed] [Google Scholar]
- 4.Pelliccia A, Fagard R, Bjørnstad H, et al. Recommendations for competitive sports participation in athletes with cardiac disease: a consensus document from the Study Group of Sports Cardiology of the Working Group of Cardiac Rehabilitation and Exercise Physiology, and the Working Group of Myocardial and Pericardial diseases of the European Society of Cardiology. Eur Heart J 2005; 26: 1422–1445. [DOI] [PubMed] [Google Scholar]
- 5.Mohlenkamp S, Lehmann N, Breuckmann F, et al. Prevalence and prognostic relevance of coronary atherosclerosis in marathon runners. Eur Heart J 2008; 15: 1903–1910. [DOI] [PubMed] [Google Scholar]
- 6.Kim JH, Malhotra R, Chiampas G, et al. Cardiac arrest during long-distance running races. N Engl J Med 2012; 366: 130–140. [DOI] [PubMed] [Google Scholar]
- 7.Marijon E, Uy-Evanado A, Reinier K, et al. Sudden cardiac arrest during sports activity in middle age. Circulation 2015; 131: 1384–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baggish AL. A decade of athlete ECG criteria: where we’ve come and where we’re going. J Electrocardiol 2015; 48: 324–328. [DOI] [PubMed] [Google Scholar]
- 9.Magalski A, McCoy M, Zabel M, et al. Cardiovascular screening with electrocardiography and echocardiography in collegiate athletes. Am J Med 2011; 124: 511–518. [DOI] [PubMed] [Google Scholar]
- 10.Hevia AC, Fernández MM, Palacio JMA, et al. ECG as a part of the preparticipation screening programme: an old and still present international dilemma. Br J Sports Med 2011; 45: 776–779. [DOI] [PubMed] [Google Scholar]
- 11.Riding NR, Sheikh N, Adamuz C, et al. Comparison of three current sets of electrocardiographic interpretation criteria for use in screening athletes. Heart 2015; 101: 384–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fuller C, Scott C, Hug-English C, et al. Five-year experience with screening electrocardiograms in National Collegiate Athletic Association Division I Athletes. Clin J Sport Med 2016; 26: 369–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Corrado D, Pelliccia A, Heidbuchel H, et al. Recommendations for interpretation of 12-lead electrocardiogram in the athlete. Eur Heart J 2010; 31: 243–259. [DOI] [PubMed] [Google Scholar]
- 14.Drezner JA, Ashley E, Baggish AL, et al. Abnormal electrocardiographic findings in athletes: recognizing changes suggestive of cardiomyopathy. Br J Sports Med 2013; 47: 137–152. [DOI] [PubMed] [Google Scholar]
- 15.Drezner JA, Ackerman MJ, Cannon BC, et al. Abnormal electrocardiographic findings in athletes: recognizing changes suggestive of primary electrical disease. Br J Sports Med 2013; 47: 153–167. [DOI] [PubMed] [Google Scholar]
- 16.Drezner JA, Ackerman MJ, Anderson J, et al. Electrocardiographic interpretation in athletes: the “Seattle criteria”. Br J Sports Med 2013; 47: 122–124. [DOI] [PubMed] [Google Scholar]
- 17.Sharma S, Drezner JA, Baggish A, et al. International recommendations for electrocardiographic interpretation in athletes. Eur Heart J 2018; 39: 1466–1480. [DOI] [PubMed] [Google Scholar]
- 18.Panhuyzen-Goedkoop NM, Jorstad HT, Smeets JLRM. A new consensus document on electrocardiographic interpretation in athletes: does it help to prevent sudden cardiac death in athletes? Neth Heart J 2018; 26: 127–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maron BJ, Zipes DP, Kovacs RJ. Eligibility and disqualification recommendations for competitive athletes with cardiovascular abnormalities: preamble, principles, and general considerations: a scientific statement from the American Heart Association and American College of Cardiology. J Am Coll Cardiol 2015; 66: 2343–2349. [DOI] [PubMed] [Google Scholar]
- 20.Chugh SS, Weiss JB. Sudden cardiac death in the older athlete. J Am Coll Cardiol 2015; 65: 493–502. [DOI] [PubMed] [Google Scholar]
- 21.Morrison BN, McKinney J, Isserow S, et al. Assessment of cardiovascular risk and preparticipation screening protocols in masters athletes: the Masters Athlete Screening Study (MASS): a cross-sectional study. BMJ Open Sport & Exerc Med 2018; 4: e000370–e000370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McClean G, Riding NR, Pieles G, et al. Prevalence and significance of T-wave inversion in Arab and Black paediatric athletes: should anterior T-wave inversion interpretation be governed by biological or chronological age? Eur J Prev Cardiol 2019; 26: 641–652. [DOI] [PubMed] [Google Scholar]
- 23.Steg PG, James SK, Atar D, et al. ESC guidelines for the management of acute myocardial infarction in patients presented with ST-segment elevation. The Task Force on the management of ST-segment elevation acute myocardial infarction of the European Society of Cardiology (ESC). Eur Heart J 2012; 33: 2569–2619. [DOI] [PubMed] [Google Scholar]
- 24.Junttila MJ, Sager SJ, Tikkanen JT, et al. Clinical significance of variants of J-points and J-waves: early repolarization patterns and risk. Eur Heart J 2012; 33: 2639–2644. [DOI] [PubMed] [Google Scholar]
- 25.Haissaguerre M, Derval N, Sacher F, et al. Sudden cardiac arrest associated with early repolarization. N Engl J Med 2008; 358: 2016–2023. [DOI] [PubMed] [Google Scholar]
- 26.Rosso R, Kogan E, Belhassen B, et al. J-point elevation in survivors of primary ventricular fibrillation and matched control subjects: incidence and clinical significance. J Am Coll Cardiol 2008; 52: 1231–1238. [DOI] [PubMed] [Google Scholar]
- 27.Merghani A, Maestrini V, Rosmini S. Prevalence of subclinical coronary artery disease in masters endurance athletes with a low atherosclerotic risk profile. Circulation 2017; 136: 126–137. [DOI] [PubMed] [Google Scholar]
- 28.Aengevaeren VL, Mosterd A, Braber TL, et al. Relationship between lifelong exercise volume and coronary atherosclerosis in athletes. Circulation 2017; 136: 138–148. [DOI] [PubMed] [Google Scholar]
- 29.Baggish AL, Levine BD. Coronary artery calcification among endurance athletes – “Hearts of stone”. Circulation 2017; 136: 149–151. [DOI] [PubMed] [Google Scholar]
- 30.Gervasi SF, Palumbo L, Cammarano M, et al. Coronary atherosclerosis in apparently healthy master athletes discovered during pre-participation screening. Role of coronary CT angiography (CCTA). Int J Cardiol 2019; 282: 99–107. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, CPR901060 Supplemental material for ECG criteria for the detection of high-risk cardiovascular conditions in master athletes by Nicole M Panhuyzen-Goedkoop, Hein J Wellens, André LM Verbeek, Harald T Jørstad, Joep RLM Smeets and Ron JG Peters in European Journal of Preventive Cardiology