Abstract
This phase 3, prospective, open-label, multicenter, continuation study (NCT01286779) investigated the use of a recombinant factor IX (FIX), nonacog gamma (BAX 326, RIXUBIS®) in patients with severe or moderately severe hemophilia B. The study population included 85 patients transitioning from a phase 1/3 pivotal study (NCT01174446), a pediatric study (NCT01488994), and 30 newly recruited patients, naïve to nonacog gamma. Patients received nonacog gamma as prophylaxis treatment (standard, modified or PK-tailored) or on-demand, as determined by the investigator. Treatment was assessed for safety, immunogenicity, hemostatic efficacy and consumption. In this study, after ≥100 exposure days, nonacog gamma resulted in no treatment-related serious adverse events, and no patients developed inhibitory antibodies to FIX. Nonacog gamma was efficacious at controlling bleeding episodes, with an 89.1% overall hemostatic efficacy rating of excellent or good, and 56% of bleeds resolved with one infusion. The annualized bleeding rate was considerably lower during prophylactic treatment (median ABR of 1.3 in 108 patients) than during on-demand treatment (median ABR of 16.5 in 13 patients). These results show that in previously treated patients and nonacog gamma-naïve patients, long-term use of nonacog gamma had acceptable safety and tolerability, and was efficacious as a prophylactic treatment for the management of bleeding episodes.
NCT01286779, EudraCT: 2010-022726-33
Keywords: hemophilia B, factor IX, nonacog gamma, hemostatic efficacy, safety, continuation study
Introduction
Hemophilia B is an inherited X-linked disorder caused by a deficiency of coagulation factor IX (FIX). Patients with severe or moderately severe hemophilia B experience various types of bleeds, with spontaneous joint bleeding being the hallmark of severe hemophilia and one of the most serious complications. Frequent spontaneous and trauma-related bleeding into joints can cause debilitating pain and arthropathy, significantly impacting quality of life.1 Management of bleeding episodes requires either on-demand or prophylactic FIX replacement therapy. In contrast with factor VIII treatment for hemophilia A, formation of neutralizing antibodies to FIX treatment for hemophilia B is rare (occurring in <5% of patients receiving FIX).2
The recombinant human FIX nonacog gamma (BAX 326, RIXUBIS®; Baxalta US Inc., a Takeda company, Lexington, MA, USA), is approved for use in the United States and the European Union as both on-demand and prophylactic treatment of hemophilia B, including surgical prophylaxis, in patients of all ages.3,4 Previous clinical studies have shown nonacog gamma to have an acceptable safety and tolerability profile and to be efficacious in adult and pediatric patients with hemophilia in a variety of clinical settings.5–7 In a phase 1/3 pivotal study (NCT01174446) of 73 patients (12-65 years of age) with severe or moderately severe hemophilia B, treatment with nonacog gamma resolved 84.7% of bleeding episodes with 1 to 2 infusions. Hemostatic efficacy was rated as excellent or good in 96% of bleeds, and only 2.7% of adverse events (AEs) were considered related to treatment, none of which were serious.5 Similarly, in a phase 2/3 pediatric study (NCT01488994) of 23 children (<12 years of age) with severe or moderately severe hemophilia B, there were no AEs considered related to treatment with nonacog gamma. Hemostatic efficacy was rated as excellent or good in 96% (25/26) of bleeds, and 88.5% of bleeding episodes were resolved with 1 to 2 infusions of nonacog gamma.6 In a phase 3 study (NCT01507896) of 30 patients (12-65 years of age) with moderate to severe hemophilia B undergoing surgery, a preoperative loading dose and perioperative bolus infusion of nonacog gamma provided effective hemostatic control.7
The aim of this continuation study was to further investigate the safety, hemostatic efficacy, and immunogenicity of nonacog gamma in adults and children with severe (FIX level <1 IU/dL) or moderately severe (FIX level 1-2 IU/dL) hemophilia B for up to 100 exposure days.
Materials and Methods
Patient Participation
This phase 3, prospective, open-label, multicenter, continuation study (NCT01286779) comprised patients transitioning from the nonacog gamma phase 1/3 pivotal study or phase 2/3 pediatric study, and newly recruited patients naïve to nonacog gamma. The study protocol, informed consent form, and all amendments were reviewed and approved by the relevant ethics committees prior to implementation.
Inclusion criteria required transitioning patients to have completed the pivotal or pediatric study without developing inhibitory FIX antibodies. At screening, newly recruited patients had to have been diagnosed with severe or moderately severe hemophilia B, be aged between 2 and 70 years, and have no evidence of a history of FIX inhibitors. Patients in this group were naïve to treatment with nonacog gamma, but could have received prior treatment with plasma-derived and/or recombinant FIX concentrate(s). Patients <6 years of age had a minimum of 50 exposure days, whereas those ≥6 years of age had a minimum of 150 exposure days. All patients were HIV negative or HIV positive with a viral load of <200 particles/μL, and were immunocompetent (CD4 count ≥200 cells/mm3) at screening.
As with the pivotal and pediatric studies, patients were excluded if they had been diagnosed with a hemostatic defect other than hemophilia B, or were scheduled to receive an immunomodulating drug or to take part in another clinical trial (with the exception of the nonacog gamma surgery study).7 Additional exclusion criteria included a history of FIX inhibitors or allergic reaction to FIX concentrate therapy; low or high body weight (<35 kg or >120 kg, pivotal study patients); low platelet count (<100 000/mL); evidence of thrombotic disease, hyperfibrinolysis, or disseminated intravascular coagulation; abnormal renal function; active hepatic disease; a clinically significant medical, psychiatric, or cognitive illness; or recreational drug/alcohol use.
Patients were asked to maintain diaries to record information regarding infusions, bleeding episodes, and AEs. The diaries were analyzed by the investigator every 3 months (± 1 week) until study completion.
Study Visits
For eligible patients from other studies, screening visits for this study were performed on the same day as the end-of-study visit of the pivotal or pediatric study, to ensure continuity of nonacog gamma therapy. Patients returned to the study site 4 ± 1 weeks after screening, and thereafter every 3 months ± 1 week until study completion. Study duration varied (up to a maximum of 68 months) depending on the completion date of the previous pivotal or pediatric study and date of licensure in the patient’s country. Participation continued until patients had each accumulated a total of ≥100 exposure days to nonacog gamma. For newly recruited patients naïve to nonacog gamma, participation continued until patients had each accumulated up to approximately 100 exposure days, or until nonacog gamma was licensed in the patient’s country.
Treatment with Nonacog Gamma
All patients naïve to nonacog gamma received prophylactic treatment; patients transferring from previous studies had the option of prophylaxis or on-demand treatment. Prophylactic treatment regimens consisted of standard prophylaxis, modified prophylaxis (determined by the investigator), or pharmacokinetically tailored (PK-tailored) prophylaxis, based on the patient’s individual pharmacokinetics (PK) (Table 1). On-demand treatment was dependent on the severity of the bleed (Table 1). The selection of treatment regimen remained at the discretion of the investigator. Patients could switch between treatment regimens during the study and may therefore be included in more than one treatment group. When a patient changed regimen, data were analyzed under the initial regimen until a change of regimen was recorded. Data recorded thereafter were analyzed under the new regimen. Patients may have switched treatment regimens more than once.
Table 1.
Dosing Regimens.
| Prophylaxis regimen | Dose and frequency |
|---|---|
| Standard | |
| Patients ≥12 years of age | 50 IU/kg (range 40-60 IU/kg; max dose 75 IU/kg), twice weekly; max 75 IU/kg |
| Patients <12 years of age | Range 40-80 IU/kg, twice weekly |
| Modified | Dose and frequency determined by investigator; max dose 100 IU/kg |
| PK-tailored | Dose and frequency based on patient’s individual PK; max dose 120 IU/kg |
| On-Demand regimen by degree of hemorrhage | Required FIX Level and frequency of infusion dose (determined by severity of the bleeding episode) |
| Early hemarthrosis, muscle bleeding, or oral bleeding | 20-40 IU/dL infusion every 24 hours until the bleeding episode, as indicated by pain, was resolved or healing was achieved |
| More extensive hemarthrosis, muscle bleeding, or hematoma | 30-60 IU/dL infusion every 24 hours for 3-4 days or more until pain and acute disability were resolved |
| Life-threatening hemorrhages | 60-100 IU/dL infusion every 8-24 hours with close laboratory monitoring of FIX plasma activity until threat was resolved |
Abbreviations: FIX, factor IX; max, maximum; PK, pharmacokinetics.
Dose and treatment frequency could be adjusted according to the individual patient’s age, the number of breakthrough bleeds, and/or the patient’s physical activity, with prophylactic infusions administrated prior to weekdays with increased activity. In patients with severe arthropathy and/or target joints who continued to experience recurrent bleeding episodes despite adjustments of the prophylactic dose and/or frequency of administration, an ultrasound of the affected joint(s) was recommended to verify the presence of a bleed. If a bleed occurred, patients resumed their prophylaxis regimen the day after the last therapeutic infusion for the treatment of the bleeding episode. A target joint was defined as a joint in which there had been ≥4 bleeds during the 6 months prior to study entry or during the last 6-month period within the study. At each study visit (every 3 months ± 1 week) patients received 75 ± 5 IU/kg nonacog gamma to assess incremental recovery (peak level of nonacog gamma in the first hour after infusion).
Safety End Point Assessments
Safety end points included the assessment of serious and nonserious AEs possibly or probably related to nonacog gamma, based on patient diary entries; development of inhibitory and total binding antibodies to FIX; development of antibodies to Chinese hamster ovary (CHO) proteins and rFurin; occurrence of severe allergic reactions and thrombotic events; and clinically significant changes in routine laboratory parameters (hematology and clinical chemistry) and vital signs.
Efficacy End Point Assessments
Efficacy end points were recorded in patient diaries by the patient, their representative, or qualified personnel at the participating site, and included categorization of bleeding episodes in terms of cause (spontaneous or injury) and severity (minor, moderate, major, or life/limb-threatening) as assessed by patients and/or investigators), hemostatic efficacy rating at resolution of bleeding episodes (see Supplemental Table 1; rating scale for hemostatic efficacy), and annualized bleeding rate (ABR). The ABR was calculated by treatment regimen for patients who had received nonacog gamma on the specified regimen for ≥3 months. The number of infusions of nonacog gamma required to resolve each bleeding episode was monitored, and the weight-adjusted consumption of nonacog gamma recorded.
Pharmacokinetics
A PK study was conducted for patients who had not participated in the previous nonacog gamma pivotal5 or surgery7 studies, and were receiving the PK-tailored prophylaxis regimen. One preinfusion sample and 5 postinfusion samples were collected up to 72 hours following the PK infusion of 75 ± 5 IU/kg of nonacog gamma. PK outcomes included incremental recovery over time, area under the plasma concentration versus time curve, half-life, mean residence time, clearance, and volume of distribution at steady state.
Health-Related Quality of Life
Health-related quality of life was determined using the following instruments: 36-item Short Form Survey (SF-36), Pediatric Quality of Life Inventory (Peds-QL), Haemophilia Quality of Life Questionnaire for Adults (Haem-A-QoL) total score, Haemophilia Quality of Life Questionnaire for Children (Haemo-QoL) total score, EuroQol 5 Dimension (EQ-5D) total index, EQ-5D visual analog scale (VAS), and pain score, plus Health Resource Use. Changes from baseline were only available for newly recruited patients.
Statistical Analyses
The sample size for the study was determined by the number of patients treated in the pivotal and pediatric studies who were willing to participate in this study and who met the eligibility criteria and regulatory requirements. The full analysis set (FAS) comprised all patients who were exposed to nonacog gamma. Baseline characteristics, safety, and efficacy analyses were based on the FAS. All patients in the FAS met the criteria for the per protocol analysis set. For patients who discontinued before reaching 100 exposure days, data were included up to the day of discontinuation. AEs and health-related quality-of-life scores were descriptively summarized by treatment regimen.
Results
Patient Participation
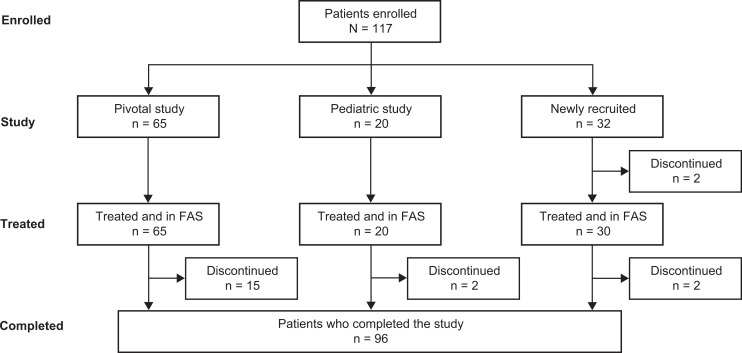
Overall, 45 sites participated in this study; 29 had been involved with previous nonacog gamma studies, and 16 were new study sites. Of the participating study sites, 40 enrolled patients into the study. Enrollment began in April 2011 and completed in June 2017. A total of 117 patients were enrolled, of which 115 received treatment with nonacog gamma (severe hemophilia B, n = 74; moderately severe hemophilia B, n = 41): 65 patients transferred from the phase 1/3 pivotal study, 20 patients transferred from the phase 2/3 pediatric study, and 30 patients naïve to nonacog gamma were newly recruited (Figure 1). The 85 patients who transitioned from the pivotal and pediatric studies had a mean (SD) of 49.7 (15.47) prior exposure days to nonacog gamma when they entered this continuation study (median 52.0, range 5.0-83.0). Of 110 patients who received a prophylaxis regimen, 108 received standard prophylaxis, 26 received modified prophylaxis, and 3 received PK-tailored prophylaxis. Patients may have been counted for more than one treatment regimen if they switched regimens during the study. Thirteen patients received nonacog gamma on demand only. The number of patients who switched from one treatment regimen to another are shown in Table 2. Overall, 21 patients made 1 switch of treatment, 5 patients made 2 switches and 7 made ≥3 switches. Overall, 97.5% of patients were compliant with the recommended dose and 90% were compliant with the frequency of dosing.
Figure 1.
Patient participation flowchart. After enrollment, but prior to treatment, 2 patients discontinued: 1 patient, patient decision; 1 patient, owing to screen failure. After treatment, 19 patients discontinued: 9 patients, withdrawal by patient; 5 patients, protocol violation; 2 patients, physician decision; 1 patient, scheduled surgery; 1 patient, emigrated; 1 patient, discontinued by sponsor. Abbreviation: FAS, full analysis set.
Table 2.
Number of Patients in Each Treatment Regimen.
| Original treatment regimen | Treatment regimen following switch | |||||
|---|---|---|---|---|---|---|
| First assigned treatment | No switch | Standard prophylaxis | Modified prophylaxis | PK-tailored prophylaxis | On-demand treatment | |
| Standard prophylaxis | 104 | 75 | – | 26 | 0 | 5 |
| Modified prophylaxis | 0 | 0 | 12 | – | 0 | 0 |
| PK-tailored prophylaxis | 3 | 2 | 1 | 0 | – | 0 |
| On-demand treatment | 8 | 5 | 3 | 0 | 0 | – |
Abbreviation: PK, pharmacokinetics.
All patients were male, with a mean (SD) age of 29.6 (16.39) years, ranging from 2 to 70 years (Table 3); 21 patients were <12 years of age and 94 patients were ≥12 years of age. Of the 30 newly recruited patients, 23 patients (76.7%) had severe hemophilia B. Three patients who underwent PK-tailored prophylaxis were ≥18 years of age.
Table 3.
Demographics and Baseline Characteristics of Patients Who Received ≥1 Dose of Nonacog Gamma.a
| Parameter | Standard prophylaxis n = 108 |
Modified prophylaxis n = 26 |
PK-tailored prophylaxis n = 3 |
Overall prophylaxis n = 110 |
On-demand treatment n = 13 |
Overall N = 115 |
|---|---|---|---|---|---|---|
| Male, n | 108 | 26 | 3 | 110 | 13 | 115 |
| Age, years | ||||||
| Mean | 28.9 | 32.3 | 43.0 | 29.1 | 36.6 | 29.6 |
| SD | 16.19 | 17.76 | 18.52 | 16.28 | 11.64 | 16.39 |
| Median | 27.5 | 33.5 | 50.0 | 27.5 | 33.0 | 28.0 |
| Range | 2-70 | 3-70 | 22-57 | 2-70 | 20-56 | 2-70 |
| Race, n (%) | ||||||
| White | 95 (88.0) | 23 (88.5) | 0 | 95 (86.4) | 12 (92.3) | 99 (86.1) |
| Asian | 7 (6.5) | 1 (3.8) | 3 (100) | 9 (8.2) | 1 (7.7) | 10 (8.7) |
| Black | 1 (0.9) | 0 | 0 | 1 (0.9) | 0 | 1 (0.9) |
| Other | 5 (4.6) | 2 (7.7) | 0 | 5 (4.5) | 0 | 5 (4.3) |
| Number of target joints at screening, n (%) | ||||||
| 0 | 65 (60.2) | 16 (61.5) | 2 (66.7) | 67 (60.9) | 6 (46.2) | 71 (61.7) |
| 1-2 | 24 (22.2) | 4 (15.4) | 1 (33.3) | 24 (21.8) | 4 (30.8) | 25 (21.7) |
| 3-4 | 12 (11.1) | 3 (11.5) | 0 | 12 (0.9) | 2 (15.4) | 12 (10.4) |
| >4 | 7 (6.5) | 3 (11.5) | 0 | 7 (6.4) | 1 (7.7) | 7 (6.1) |
Abbreviation: PK, pharmacokinetics.
a Patients may have been counted for ≥1 treatment regimen if they switched regimens during the study.
Safety Outcomes
All serious and nonserious AEs, development of antibodies, allergic reactions, and thrombotic events are recorded in Table 4. A total of 459 AEs were reported in 85 (73.9%) patients. Of these, 443 were nonserious AEs reported in 85 patients, and 16 serious AEs were reported in 9 patients. Two nonserious AEs were considered related to nonacog gamma, both of which were antibodies to rFurin, which increased 2-fold from the detection titer, and occurred in patients ≥18 years of age (1 patient with 2 positive results after 6 months’ treatment; 1 patient with 5 positive results after 21 months’ treatment). Two other patients had antibodies to rFurin, but these were considered unrelated to nonacog gamma as there was no increase in titer. All abovementioned tests were negative by study completion and considered transient. The most frequently reported AEs (≥15 AEs) were nasopharyngitis (55 AEs in 25 patients), arthralgia (48 AEs in 15 patients), pyrexia (23 AEs in 14 patients), headache (20 AEs in 8 patients), upper respiratory tract infection (17 AEs in 11 patients), cough (15 AEs in 11 patients), and rhinitis (15 AEs in 8 patients).
Table 4.
Number of AEs and Immunogenicity During Treatment With Nonacog Gamma (Full Analysis Set).
| Seriousness of AE | Severity of AE | Number of AEs (number of patients) | |
|---|---|---|---|
| Unrelated to treatment | Related to treatment | ||
| Serious | Mild | 1 (1) | 0 |
| Moderate | 8 (2) | 0 | |
| Severe | 7 (6) | 0 | |
| Total | 16 (9) | 0 | |
| Nonserious | Mild | 317 (40) | 1 (1) |
| Moderate | 119 (41) | 1 (1) | |
| Severe | 5 (4) | 0 | |
| Total | 441 (85) | 2 (2) | |
| All AEs | 459 (85) | ||
| Type of AE developed during sudy, patients, n | Unrelated to treatment | Related to treatment | |
| Inhibitory antibodies to FIX | 0 | 0 | |
| Binding antibodies to FIX | 0 | 0 | |
| Antibodies to CHO | 0 | 0 | |
| Antibodies to rFurin | 2 | 2 | |
| Severe allergic reaction | 0 | 0 | |
| Thrombotic event | 0 | 0 | |
Abbreviations: AE, adverse event; CHO, Chinese hamster ovary; FIX, factor IX.
There were no cases of development of FIX inhibitory antibodies, binding antibodies to FIX (with confirmed specificity at any time point), or antibodies to CHO proteins at screening or after treatment. There were also no severe allergic reactions or thrombotic events during or after treatment, and no significant treatment-related changes in clinical laboratory parameters or vital signs.
Efficacy Outcomes
Bleeding episodes
Of 1149 bleeding episodes overall, 617 (53.7%) were spontaneous and 387 (33.7%) resulted from injury (Table 5). Patients receiving prophylaxis had a higher proportion of injury-related bleeding episodes (304/693 bleeding episodes, 43.9%) than patients receiving on-demand treatment (83/456 bleeding episodes, 18.2%). In contrast, patients receiving on-demand treatment reported a higher proportion of spontaneous bleeds (311/456 bleeding episodes, 68.2%) than patients receiving prophylaxis (306/693 bleeding episodes, 44.2%).
Table 5.
Cause and Severity of Bleeding Episodes.
| Standard prophylaxis n = 80 |
Modified prophylaxis n = 18 |
PK-tailored prophylaxis n = 3 |
Overall prophylaxis n = 88 |
On-demand treatment n = 12 |
Overall N = 97 |
|
|---|---|---|---|---|---|---|
| Number of bleeding episodes | 574 | 111 | 8 | 693 | 456 | 1149 |
| Cause of bleeding episode, n (%) | ||||||
| Spontaneous | 247 (43.0) | 53 (47.7) | 6 (75.0) | 306 (44.2) | 311 (68.2) | 617 (53.7) |
| Injury | 257 (44.8) | 45 (40.5) | 2 (25.0) | 304 (43.9) | 83 (18.2) | 387 (33.7) |
| Unknown | 70 (12.2) | 13 (11.7) | 0 | 83 (12.0) | 62 (13.6) | 145 (12.6) |
| Standard prophylaxis n# = 78 |
Modified prophylaxis n# = 18 |
PK-tailored prophylaxis n# = 3 |
Overall prophylaxis n# = 86 |
On-demand treatment n# = 12 |
Overall n# = 95 |
|
| Number of treated bleeding episodes with available dose information | 542 | 109 | 8 | 659 | 453 | 1112 |
| Number of treated bleeding episodes with available dose information by severity (%) | ||||||
| Minor | 110 (20.3) | 9 (8.3) | 5 (62.5) | 124 (18.8) | 47 (10.4) | 171 (15.4) |
| Moderate | 355 (65.5) | 83 (76.1) | 2 (25.0) | 440 (66.8) | 363 (80.1) | 803 (72.2) |
| Major | 76 (14.0) | 16 (14.7) | 1 (12.5) | 93 (14.1) | 43 (9.5) | 136 (12.2) |
| Life/limb-threatening | 1 (0.2) | 1 (0.9) | 0 | 2 (0.3) | 0 | 2 (0.2) |
Abbreviation: PK, pharmacokinetics.
n, number of patients with ≥1 bleeding episode within the respective treatment regimen.
n#, number of patients with available dose information.
Of 1147 bleeding episodes rated for severity, most (813 bleeding episodes, 70.9%) were considered moderate. Of the remainder, 196 bleeds (17.1%) were rated as minor, 136 bleeds (11.9%) were rated as major, and only 2 bleeding episodes (0.2%) were regarded as limb- or life-threatening. Anatomically, 80% of bleeding episodes (n = 924/1149) occurred in joints. Of 1114 bleeding episodes treated with nonacog gamma, dosing information was available for 1112 bleeding episodes. For these, most (803 bleeding episodes, 72.2%) were considered moderate, 171 (15.4%) were rated as minor and 136 bleeds (12.2%) were rated as major (Table 5).
Hemostatic efficacy
Overall, during the study, patients experienced a total of 1112 bleeding episodes that were treated with nonacog gamma and rated for hemostatic efficacy. Response to treatment was rated as excellent or good for 991 bleeding episodes (89.1%) at resolution of the bleed (Figure 2).
Figure 2.
Hemostatic efficacy rating. The rating scale for hemostatic efficacy is described in Supplemental Table 1.
ABRs
For patients receiving prophylactic treatment with an observation period of ≥3 months (n = 108), the median ABR was 1.3, compared with a median ABR of 16.5 for patients receiving on-demand treatment (n = 13). ABR was higher for joint bleeds than for other bleeds. Median ABRs of joint bleeds during overall prophylaxis and on-demand treatment were 0.6 and 14.4, respectively, compared with median ABRs of 0.2 and 0.7, respectively, for other bleeds (Table 6).
Table 6.
Annualized Bleeding Rates.
| Bleeding site | Standard prophylaxisa
n = 106 |
Modified prophylaxisa
n = 22 |
PK-tailored prophylaxisa
n = 2 |
Overall prophylaxisa
n = 108 |
On-demand treatment n = 13 |
|---|---|---|---|---|---|
| All bleeds | |||||
| Mean | 3.6 | 5.9 | 1.9 | 3.3 | 18.2 |
| SD | 8.72 | 9.79 | 1.96 | 6.67 | 11.17 |
| Median | 1.3 | 1.4 | 1.9 | 1.3 | 16.5 |
| Range | 0.0-78.7 | 0.0-34.6 | 0.5-3.3 | 0.0-52.2 | 0.0-31.1 |
| Joint bleeds | |||||
| Mean | 2.7 | 4.2 | 1.9 | 2.5 | 16.6 |
| SD | 7.25 | 8.65 | 1.96 | 5.68 | 10.57 |
| Median | 0.5 | 0.6 | 1.9 | 0.6 | 14.4 |
| Range | 0.0-61.8 | 0.0-34.6 | 0.5-3.3 | 0.0-39.5 | 0.0-30.4 |
| Non-joint bleeds | |||||
| Mean | 0.8 | 1.6 | 0.0 | 0.8 | 1.6 |
| SD | 1.94 | 2.91 | 0.00 | 1.55 | 2.03 |
| Median | 0.2 | 0.3 | 0.0 | 0.2 | 0.7 |
| Range | 0.0-16.9 | 0.0-10.7 | 0.0-0.0 | 0.0-12.7 | 0.0-5.7 |
Abbreviation: PK, pharmacokinetics.
a Calculated for patients receiving nonacog gamma with an observation period of at least 3 months on the specified regimen.
Infusions of nonacog gamma required to resolve a bleed
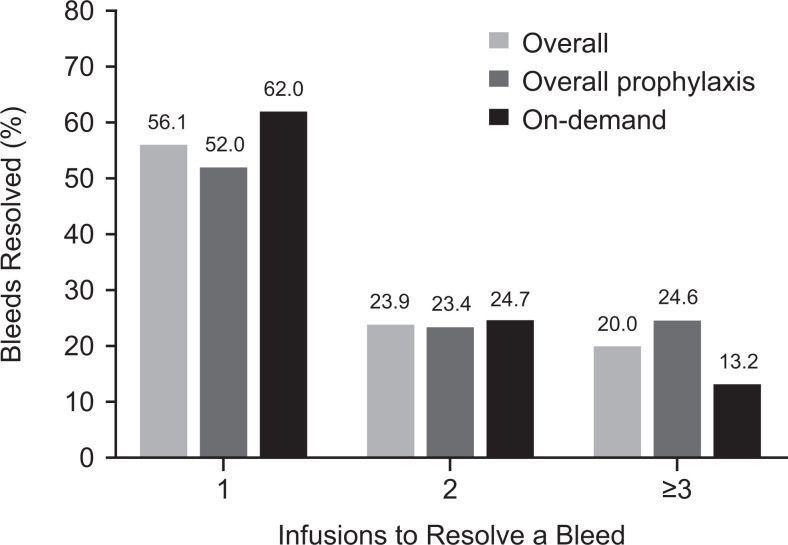
Nonacog gamma successfully resolved 889 (79.9%) bleeding episodes using only 1 or 2 infusions. For overall prophylaxis, 496 bleeding episodes (75.4%) were controlled with 1 or 2 infusions of nonacog gamma, compared with 393 bleeding episodes (86.6%) for patients receiving nonacog gamma on demand (Figure 3). Overall, a mean (SD) number of 1.8 (1.65) infusions were required until bleed resolution.
Figure 3.
Number of infusions required to resolve a bleed.
Consumption
Patients receiving prophylaxis received a mean (SD) of 8.4 (1.38) infusions per month, whereas those receiving on-demand treatment received 3.6 (2.44) infusions per month (Table 7). The mean (SD) weight-adjusted consumption per month for the 110 patients receiving prophylaxis was 464.2 (111.46) IU/kg, compared with 199.8 (124.18) IU/kg for the 13 patients receiving on-demand treatment.
Table 7.
Accumulated Number of Infusions and Weight-Adjusted Consumption of Nonacog Gamma Per Patient.
| Assessment | Standard prophylaxis n = 108 |
Modified prophylaxis n = 26 |
PK-tailored prophylaxis n = 3 |
Overall prophylaxis n = 110 |
On-demand treatment n = 13 |
|---|---|---|---|---|---|
| Number of infusions per month | |||||
| Mean | 8.5 | 10.8 | 4.0 | 8.4 | 3.6 |
| SD | 1.25 | 4.34 | 0.6 | 1.38 | 2.44 |
| Median | 8.4 | 9.4 | 4.3 | 8.4 | 3.3 |
| Range | 4.2-17.6 | 4.4-22.8 | 3.3-4.4 | 3.3-16.6 | 0.8-9.3 |
| Number of infusions per year | |||||
| Mean | 101.8 | 130.2 | 48.3 | 101.1 | 43.1 |
| SD | 15.03 | 52.13 | 7.23 | 16.50 | 29.28 |
| Median | 101.3 | 113.2 | 51.7 | 101.2 | 39.4 |
| Range | 50.9-211.1 | 52.7-273.9 | 39.9-53.1 | 39.9-198.9 | 9.9-111.7 |
| Weight-adjusted consumption per month, IU/kg | |||||
| Mean | 462.3 | 684.4 | 250.9 | 464.2 | 199.8 |
| SD | 102.05 | 337.7 | 41.37 | 111.46 | 124.18 |
| Median | 451.3 | 583.3 | 252.2 | 449.7 | 174.3 |
| Range | 294.6-1239.9 | 314.1-1674.2 | 208.8-291.5 | 208.8-1243.3 | 45.4-506.1 |
| Weight-adjusted consumption per year, IU/kg | |||||
| Mean | 5547.8 | 8212.4 | 3010.3 | 5570.7 | 2397.4 |
| SD | 1224.65 | 4052.36 | 496.44 | 1337.53 | 1490.22 |
| Median | 5415.8 | 6999.1 | 3026.7 | 5396.9 | 2091.4 |
| Range | 3535.3-14 879.1 | 3769.1-20 090.9 | 2505.8-3498.3 | 2505.8-14 919.7 | 544.7-6073.4 |
Abbreviation: PK, pharmacokinetics.
When a patient changed regimen, data were analyzed under the initial regimen until a change of regimen was recorded. Data recorded thereafter were analyzed under the updated regimen.
For overall prophylaxis, a mean (SD) dose of 122.0 (134.02) IU/kg of nonacog gamma was administered per bleeding episode (n = 659), whereas on-demand patients received 82.6 (48.21) IU/kg per bleeding episode (n = 453) (Table 8).
Table 8.
Consumption of Nonacog Gamma per Bleeding Episode.
| Assessment | Standard prophylaxis n = 78 |
Modified prophylaxis n = 18 |
PK-tailored prophylaxis n = 3 |
Overall prophylaxis n = 86 |
On-demand treatment n = 12 |
|---|---|---|---|---|---|
| Number of bleeding episodes | 542 | 109 | 8 | 659 | 453 |
| Consumption, IU/kga | |||||
| Mean | 124.2 | 114.8 | 67.4 | 122.0 | 82.6 |
| SD | 140.70 | 99.41 | 34.39 | 134.02 | 48.21 |
| Median | 90.4 | 62.8 | 53.8 | 81.3 | 67.5 |
| Range | 13.9-2278.2 | 14.7-481.6 | 38.5-128.2 | 13.9-2278.2 | 23.4-335.5 |
Abbreviation: PK, pharmacokinetics.
n indicates the number of patients.
a The only infusions considered were those required until the bleed was resolved.
Pharmacokinetics
The PK parameters measured during this study (Supplemental Tables 2 and 3) are consistent with data from the previous pivotal and pediatric studies evaluating nonacog gamma. For example, the mean half-life in the current study was 28.52 hours, compared with 26.70 hours (initial crossover study) and 25.36 hours (repeated evaluation) in the pivotal study.
Health-Related Quality of Life
The results of EQ-5D (VAS score), pain score, SF-36, Haemo-QoL, and Haem-A-QoL parameters all demonstrated a numerical improvement from baseline assessments to end of study (Supplemental Table 4).
Discussion and Conclusion
In this continuation study, nonacog gamma was well tolerated after ≥100 exposure days in 85 previously treated and 30 newly recruited patients naïve to nonacog gamma with severe or moderately severe hemophilia B. Only 2 nonserious AEs were considered related to nonacog gamma, both of which were positive tests for antibodies to rFurin. The clinical significance of these antibodies is unknown. These tests were negative by study completion and, therefore, considered transient.
No patients developed inhibitory antibodies to FIX, which is consistent with results from previous studies of recombinant FIX or plasma-derived FIX treatment.8,9 Similarly, no antibodies to CHO were detected, in contrast to other studies that reported increases in antibodies to CHO cell-derived components in patients treated with CHO-derived antihemophilic factors.10 This study confirmed the safety profile of nonacog gamma, with no new safety signals identified.
Nonacog gamma was effective at controlling bleeding episodes across all age groups, with an 89.1% overall hemostatic efficacy rating of excellent or good, and 56% of bleeds resolved with 1 infusion. On the basis of ≥3 months’ treatment, the ABR was considerably lower during prophylactic treatment (108 patients) than during on-demand treatment (13 patients). A lower ABR with prophylaxis is consistent with that observed in the phase 2/3 study of nonacog gamma by Windyga et al. (prophylaxis twice-weekly, mean 4.2 vs on-demand, 20.0)5 and in a phase 4 multicenter, randomized, open-label study of nonacog alfa (prophylaxis twice-weekly, mean 2.6 vs on-demand, 35.1).11
Although improvements in some health-related quality-of-life parameters were observed during the study, because the majority of patients transitioned from a prophylaxis regimen in a previous study, marked changes were not anticipated. Overall, the results suggest an improved health status and quality of life with nonacog gamma and complement previously published data.12
An interesting aspect of this study is the comparison between different prophylaxis regimens. Standard prophylaxis was effective for the majority of patients; however, a significant proportion of patients required more intensive treatment regimens (modified prophylaxis). This reflects the need for an optimal therapy to be sought for each individual on the basis of clinical phenotype of hemophilia, which is not the same in every patient. Prophylaxis based on individual PK analysis seems to be an attractive treatment option. Unfortunately, only 3 patients in the current study were treated in this way.
The limitations of this continuation study are those known to be associated with extension studies. Primarily, as hemophilia B is rare, direct comparison of nonacog gamma with an alternative treatment is not possible. In this study, participation of patients who transitioned from previous studies was more likely to involve those who had responded well to nonacog gamma. Furthermore, patients were not assigned randomly to the treatment regimen options. Inherent limitations of continuation studies such as this include variation in group size across different treatment cohorts, patients switching between treatment regimens, and variations in the duration of observations and time spent on different treatment modalities. These serve to reduce the robustness of comparisons between treatment regimens and can skew values for parameters such as ABR, which are derived by extrapolation. For example, wide ranges reported for ABR would be more likely to occur if single events occurring within a short time period are then extrapolated over 1 year. Subjective self-assessment could have introduced inconsistency, and the calculation of ABR on the basis of 3 months of treatment and observation can only be an estimation and therefore is a limitation of this study.
These results show that in previously treated patients and patients naïve to nonacog gamma with severe or moderately severe hemophilia B, where 106 of 115 treated patients reached ≥100 exposure days, long-term use of nonacog gamma displayed acceptable safety and tolerability, and was efficacious for prophylactic treatment and control of bleeding episodes across all age groups.
Acknowledgments
The authors would like to thank all patients and their caregivers who participated in the study, and all principal investigators and personnel at the study sites.
Authors’ Note: Jerzy Windyga, Oleksandra Stasyshyn, and Srilatha Tangada were involved in the study conception and design. All authors were involved in study conduct, data acquisition, and analysis and interpretation of the data. All authors reviewed and approved the final version of the manuscript. The datasets, including the redacted study protocol, redacted statistical analysis plan, and individual participant’s data supporting the results reported in this article, will be made available within 3 months from initial request, to researchers who provide a methodologically sound proposal. The data will be provided after its de-identification, in compliance with applicable privacy laws, data protection and requirements for consent and anonymization.
Data Sharing statement: Data requests should follow the process outlined in the Data Sharing section on: http://www.takeda.com/what-we-do/research-and-development/takeda-clinical-trialtransparency/
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Jerzy Windyga has received research funding from Alnylam, Baxalta,* Bayer, Novo Nordisk, Octapharma, Rigel, Roche, Sanofi, Shire,* and SOBI; and honoraria from Alexion, Baxalta,* Bayer, CSL Behring, Ferring, Novo Nordisk, Octapharma, Rigel, Roche, Sanofi, Shire,* Siemens, SOBI, and Werfen. Oleksandra Stasyshyn has received honoraria from CSL Behring, Novo Nordisk, and Shire*; and has served on speaker bureaus for Novo Nordisk, Pfizer, and Shire.* Toshko Lissitchkov has consulted for Bayer, Roche, Shire,* and Sobi; and has served on speaker bureaus for Bayer, Octapharma, Roche, Shire,* and Sobi. Margit Serban has received research funding from Baxalta,* Bayer, Biotest, Novo Nordisk, Octapharma, Roche, Sanofi, and Shire*; and honoraria from Baxalta,* Bayer, CSL Behring, Novo Nordisk, Octapharma, Roche, Sanofi, and Shire.* Bettina Ploder is an employee of Baxalta Innovations GmbH, a Takeda company. Srilatha Tangada is an employee of Baxalta US Inc., a Takeda company, and a Takeda stock owner. This study was funded by Baxalta US Inc., a Takeda company, Lexington, MA, USA, and Baxalta Innovations GmbH, a Takeda company, Vienna, Austria. Writing and editorial support for this manuscript was provided by Paul Lidbury, PhD, employee of Excel Medical Affairs (Fairfield, CT, USA), and was funded by Baxalta US Inc., a Takeda company, Lexington, MA, USA.
*A Takeda company.
ORCID iD: Srilatha Tangada  https://orcid.org/0000-0001-9721-4993
https://orcid.org/0000-0001-9721-4993
References
- 1. Srivastava A, Brewer A, Mauser-Bunschoten E, et al. Treatment Guidelines Working Group on Behalf of the World Federation of Hemophilia. Guidelines for the management of hemophilia. Haemophilia. 2013;19(1):e1–e47. [DOI] [PubMed] [Google Scholar]
- 2. Key NS. Inhibitors in congenital coagulation disorders. Br J Haematol. 2004;127(4):379–391. [DOI] [PubMed] [Google Scholar]
- 3. US Food and Drug Administration. RIXUBIS. Product information. Published 2014 Accessed October 1, 2019 http://www.fda.gov/vaccines-blood-biologics/approved-blood-products/rixubis.
- 4. European Medicines Agency. RIXUBIS. Summary of product characteristics. Accessed October 1, 2019 https://www.ema.europa.eu/documents/product-information/rixubis-epar-product-information_en.pdf.
- 5. Windyga J, Lissitchkov T, Stasyshyn O, et al. Pharmacokinetics, efficacy and safety of BAX326, a novel recombinant factor IX: a prospective, controlled, multicenter phase I/III study in previously treated patients with severe (FIX level <1%) or moderately severe (FIX level ≤2%) haemophilia B. Haemophilia. 2014;20(1):15–24. [DOI] [PubMed] [Google Scholar]
- 6. Urasinski T, Stasyshyn O, Andreeva T, et al. Recombinant factor IX (BAX326) in previously treated paediatric patients with haemophilia B: a prospective clinical trial. Haemophilia. 2015;21(2):196–203. [DOI] [PubMed] [Google Scholar]
- 7. Windyga J, Lissitchkov T, Stasyshyn O, et al. Efficacy and safety of a recombinant factor IX (BAX326) in previously treated patients with severe or moderately severe haemophilia B undergoing surgery or other invasive procedures: a prospective, open-label, uncontrolled, multicenter, phase III study. Haemophilia. 2014;20(5):651–658. [DOI] [PubMed] [Google Scholar]
- 8. Recht M, Pollmann H, Tagliaferri A, Musso R, Janco R, Neuman WR. A retrospective study to describe the incidence of moderate to severe allergic reactions to factor IX in subjects with haemophilia B. Haemophilia. 2011;17(3):494–499. [DOI] [PubMed] [Google Scholar]
- 9. Poon MC, Lillicrap D, Hensman C, Card R, Scully MF. Recombinant factor IX recovery and inhibitor safety: a Canadian post-licensure surveillance study. Thromb Haemost. 2002;87(3):431–435. [PubMed] [Google Scholar]
- 10. Fukui H, Yoshioka A, Shima M, et al. Clinical evaluation of recombinant human factor VIII (BAY w 6240) in the treatment of hemophilia A. Int J Hematol. 1991;54(5):419–427. [PubMed] [Google Scholar]
- 11. Valentino LA, Rusen L, Elezovic I, Smith LM, Korth-Bradley JM, Rendo P. Multicentre, randomized, open-label study of on-demand treatment with two prophylaxis regimens of recombinant coagulation factor IX in haemophilia B subjects. Haemophilia. 2014;20(3):398–406. [DOI] [PubMed] [Google Scholar]
- 12. Windyga J, Lin VW, Epstein JD, et al. Improvement in health-related quality of life with recombinant factor IX prophylaxis in severe or moderately severe haemophilia B patients: results from the BAX326 Pivotal Study. Haemophilia. 2014;20(3):362–368. [DOI] [PubMed] [Google Scholar]