Abstract
Objective
Our objective was to investigate the association between anastomotic leakage (AL) and hyponatremia after colorectal cancer surgery.
Methods
All anastomoses in colorectal cancer surgery performed in our hospital between January 2015 and December 2017 were retrospectively identified. According to the diagnostic criteria of AL, the patients were divided into an AL group and a no anastomotic leakage (NAL) group.
Results
We reviewed records of 498 consecutive colorectal cancer patients. The total incidence of AL was 5.4%. Postoperative serum sodium levels differed significantly: 137.63 ± 4.29 and 139.81 ± 3.41 mmol/L in the AL and NAL groups, respectively. By using area under the receiver-operating characteristic (auROC) curves, we determined the optimum postoperative serum sodium cut-off to be 139.5 mmol/L and redefined hyponatremia as postoperative serum sodium <139.5 mmol/L. Redefined hyponatremia had an auROC of 0.65, corresponding to a 97.2% negative predictive value. The negative predictive value reached 99.1% when serum sodium level was combined with leukocytosis. Multivariable analysis found that redefined hyponatremia (odds ratio, 1.176) was an independent predictive factor for AL.
Conclusions
Redefined hyponatremia has good negative predictive value for AL diagnosis after colorectal cancer surgery and could be used as a marker to exclude the diagnosis.
Keywords: Hyponatremia, colorectal cancer, anastomotic leakage, leukocytosis, biomarker, postoperative serum sodium level
Introduction
Colorectal cancer is among the most common carcinomas in the world, with more than 1.2 million new cases and 600,000 deaths reported worldwide in 2012.1 The disease ranks third in morbidity in men and second in women. Anastomotic leakage (AL) is one of the most serious complications following colorectal surgery, with an incidence of 3.9% to 11.4%.2–4 AL can lead to an inflammatory reaction, and fever and abdominal pain are common nonspecific manifestations. Laboratory examination shows increases of inflammatory markers such as leukocyte count, C-reactive protein (CRP), and erythrocyte sedimentation rate, among others, which seriously affect prognosis of the patients.5
Hyponatremia is the most frequent electrolyte disorder in the clinical setting.6 Illness often causes a disturbance in water and electrolyte balance, which leads to changes in electrolyte concentrations in blood. A few studies have reported that systemic inflammatory diseases may cause hyponatremia, including pulmonary infection,7 urinary tract infection,8 peritonitis caused by perforation of infectious intestinal disease,9 and spontaneous bacterial peritonitis of liver cirrhosis.10 Based on the relationship between hyponatremia and inflammatory diseases, we aimed to investigate correlations between hyponatremia and AL after colorectal cancer surgery through a retrospective analysis of colorectal cancer patients treated in our hospital in the last 3 years and to provide a reference for clinical diagnosis.
Methods
Ethics
The Institutional Review Board and Research Ethics committee of China-Japan Friendship Hospital approved this study. Because this was a retrospective study and all data were anonymous and collected routinely in clinical practice, the need for patient informed consent was waived.
Patient data
We retrospectively analyzed a consecutive series of patients who underwent colorectal surgery for colorectal cancer between January 2015 and December 2017. The inclusion criteria were patients with colorectal cancer confirmed by pathology and who underwent one-stage anastomosis during the operation. The exclusion criteria were patients with abnormal serum sodium levels before surgery, patients with perforation during or before surgery, patients with serum creatinine and urea nitrogen abnormalities, and patients with preoperative or postoperative pulmonary infections (because it has been reported that pneumonia may lead to hyponatremia).7
Demographic and clinical data, including age, sex, type of surgery, state of the preventive stoma, tumor diameter, American Society of Anesthesiologists (ASA) score, nutritional state according to Nutrition Risk Score (NRS) 2002, anastomotic technique, localization and histology of the disease, and stage of the disease according to the American Joint Committee on Cancer (AJCC) staging system (7th edition), were recorded. Blood test results such as preoperative and postoperative serum sodium level, leukocyte count, neutrophil count (NEUT), and neutrophil ratio (NEUT%) were recorded. For patients with AL after surgery, the time of AL diagnosis was also recorded.
In patients with AL, the most recent laboratory values before diagnosis were assessed. In patients without AL, serum sodium level and leukocyte count were assessed (preferably) on postoperative day 4 because the incidence of AL was highest on that day in this study. Because laboratory values were not assessed daily, we defined a ranking of preferred days for laboratory assessments (postoperative days 3, 5, 6, and 2) to be as close as possible to postoperative day 4.
The diagnostic criteria of postoperative AL were (1) fecal drainage in the drainage tube, (2) anastomotic fistula verified by reoperation, or (3) pelvic abscess around the anastomotic site found by computed tomography (CT) examination.11 According to whether AL occurred after surgery, all patients were divided into two groups: the AL group and the no anastomotic leakage (NAL) group.
Definition of hyponatremia and leukocytosis
According to the reference ranges of our laboratory, a serum sodium level <135 mmol/L was defined as hyponatremia, and a leukocyte count >10,000/mm3 was defined as leukocytosis.
Data availability
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
Statistical analysis
SPSS for Windows version 18.0 (SPSS Inc., Chicago, IL, USA) was used to compare variables. Independent samples t-test and chi-square tests for univariate analysis were performed, and descriptive statistics were expressed as mean ± standard deviation (SD). Correlation analysis was carried out using the Pearson test to determine the relationship between two variables. Optimum cut-off laboratory values for the prediction of AL were determined by receiver-operating characteristic (ROC) curve analysis. Multivariate analyses were performed with logistic regression analysis. All differences were considered significant at p < 0.05.
Results
A total of 498 patients were identified; AL after colorectal surgery occurred in 27 patients (5.4%). Of these, 20 cases were rectal cancer surgery (incidence rate of 8%; i.e., as a percent of all rectal cancer cases), and 7 cases were colon cancer surgery (incidence rate of 2.8%; i.e., as a percent of all colon cancer cases). There was a significant difference between groups (p = 0.011; Table 1).
Table 1.
Characteristics of the analyzed patients with or without anastomotic leakage (AL) after colorectal cancer surgery.
| Anastomotic leakage(n = 27) | No anastomotic leakage(n = 471) | p-value | |
|---|---|---|---|
| Age (years) | 65.8 ± 10.7 | 64.2 ± 12.7 | 0.282 |
| Sex | 0.138 | ||
| Male | 19 (70.4%) | 263 (55.8%) | |
| Female | 8 (29.6%) | 208 (44.2%) | |
| Tumor location | 0.011 | ||
| Rectum | 20 (74.1%) | 231 (49.0%) | |
| Colon | 7 (25.9%) | 240 (51.0%) | |
| Operation type | 0.741 | ||
| Open | 19 (70.4%) | 317 (67.3%) | |
| Laparoscope | 8 (29.6%) | 154 (32.7%) | |
| Defunctioning stoma | 0.332 | ||
| No | 24 (88.9%) | 448 (95.1%) | |
| Yes | 3 (11.1%) | 23 (4.9%) | |
| Tumor grade | 0.722 | ||
| G1 | 4 (14.8%) | 71 (15.1%) | |
| G2 | 17 (63.0%) | 313 (66.5%) | |
| G3 | 6 (22.2%) | 87 (18.5%) | |
| AJCC stage | 0.593 | ||
| I | 2 (7.4%) | 42 (8.9%) | |
| II | 9 (33.3%) | 195 (41.4%) | |
| III | 12 (44.4%) | 146 (31.0%) | |
| IV | 4 (14.8%) | 88 (18.7%) | |
| Tumor diameter (cm) | 5.3 ± 3.5 | 6.2 ± 2.5 | 0.322 |
| Tumor distance from AV(rectal tumors, cm) | 6.9 ± 2.3 | 7.1 ± 2.9 | 0.817 |
| Anastomotic technique | 0.541 | ||
| Hand sewn | 1 (3.7%) | 8 (1.7%) | |
| Stapled | 12 (44.4%) | 194 (41.2%) | |
| Stapled and hand sewn | 14 (51.9%) | 269 (57.1%) | |
| Preoperative NRS | 2.4 ± 1.2 | 2.6 ± 1.1 | 0.516 |
| ASA | 2.2 ± 0.8 | 2.2 ± 0.9 | 0.936 |
AJCC, American Joint Committee on Cancer; ASA, American Society of Anesthesiology; AV anal verge, NRS, Nutrition Risk Score; SSI, surgical site infection.
AL occurred, on average, on postoperative day 5.3 ± 2.8. Twenty cases (74.1%) occurred between days 3 and 6 after surgery, and the highest incidence was on postoperative day 4 (6 cases; 22.2%). The distribution is shown in Figure 1.
Figure 1.
The number of cases of anastomotic leakage occurring on each day following colorectal cancer surgery.
In the AL group, the most recent laboratory values before AL were assessed on postoperative day 4.6 ± 2.6. In the NAL group, the time was postoperative day 4.4 ± 2.1. There was no significant difference between the two groups.
Preoperative and postoperative serum sodium level, leukocyte count, NEUT, and NEUT% were analyzed. There were no significant differences between the AL and NAL groups before surgery. After surgery, serum sodium level was significantly lower in patients with AL compared with those without (137.63 ± 4.29 vs. 139.81 ± 3.41 mmol/L, p = 0.002), and the leukocyte count was significantly higher in the AL group than in the NAL group (89,937 ± 36,045/mm3 vs. 74,591 ± 23,069/mm3, p = 0.038). There were no significant differences in postoperative NEUT or NEUT% between the two groups (Table 2).
Table 2.
Comparison of preoperative and postoperative laboratory values in patients with or without anastomotic leakage (AL) after colorectal cancer surgery.
| Anastomotic leakage(n = 27) | No anastomotic leakage(n = 471) | p-value | |
|---|---|---|---|
| Serum sodium level (mmol/L) | |||
| Preoperative | 141.26 ± 2.61 | 140.66 ± 2.90 | 0.291 |
| Postoperative | 137.63 ± 4.29 | 139.81 ± 3.41 | 0.002 |
| Leukocyte count (/mm3) | |||
| Preoperative | 60,389 ± 16,868 | 61,822 ± 13,956 | 0.608 |
| Postoperative | 89,937 ± 36,045 | 74,591 ± 23,069 | 0.038 |
| Neutrophil count (/mm3) | |||
| Preoperative | 36,600 ± 12,525 | 38,256 ± 11,733 | 0.478 |
| Postoperative | 69,571 ± 34,915 | 55,837 ± 21,522 | 0.053 |
| Neutrophil (%) | |||
| Preoperative | 59.88 ± 7.86 | 61.28 ± 9.36 | 0.446 |
| Postoperative | 75.71 ± 12.24 | 73.57 ± 9.67 | 0.271 |
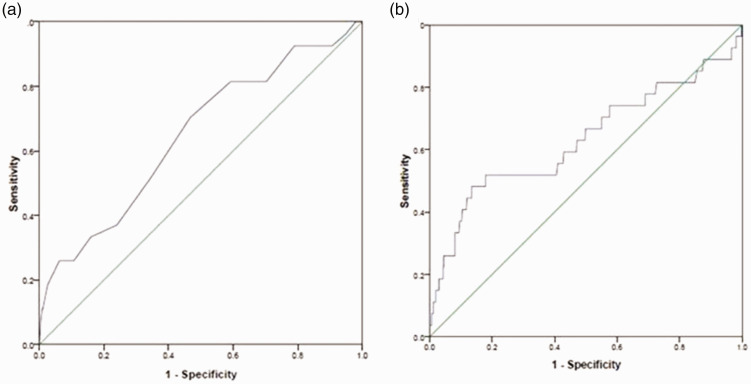
The optimum cut-off value of postoperative serum sodium was 139.5 mmol/L, which was higher than the normal reference value of 135 mmol/L. We redefined hyponatremia as postoperative serum sodium <139.5 mmol/L. The area under the receiver-operating characteristic (auROC) curve was 0.65. The best diagnostic value of postoperative leukocyte count was 9985/mm3, which had an auROC of 0.63. The threshold was slightly lower than the normal leukocyte reference value of 10,000/mm3. We redefined leukocytosis as postoperative leukocyte count >9985/mm3. These results are graphically displayed in Figure 2.
Figure 2.
Area under the receiver-operator characteristic (auROC) curve analysis for prediction of anastomotic leakage based on (a) postoperative serum sodium level and (b) postoperative leukocyte count.
According to the above optimum cut-off values, there were 19 patients with redefined hyponatremia in the AL group, with an incidence of 70.4% (19 out of 27 total cases with AL), and 130 patients with redefined hyponatremia in the NAL group, with an incidence of 27.6% (130 out of 471 total cases of NAL) (p < 0.001). There were 13 patients with redefined leukocytosis in the AL group (of 27 total patients), with an incidence of 48.1%, and 63 patients with redefined leukocytosis in the NAL group (of 471 total patients), with an incidence of 13.4% (p < 0.001). The sensitivities, specificities, positive predictive values, and negative predictive values of redefined hyponatremia, redefined leukocytosis, and combinations for the diagnosis of AL are shown in Table 3.
Table 3.
Sensitivities, specificities, and positive and negative predictive values of hyponatremia, leukocytosis, and their combination.
| Sensitivity | Specificity | Positive predictive value | Negative predictive value | |
|---|---|---|---|---|
| Hyponatremia (<139.5 mmol/L) | 70.4% | 72.4% | 12.8% | 97.2% |
| Leukocytosis (>9985/mm3) | 48.1% | 86.6% | 17.1% | 96.7% |
| Leukocytosis and hyponatremia | 29.6% | 91.5% | 16.7% | 95.8% |
| Leukocytosis or hyponatremia | 88.9% | 67.5% | 13.6% | 99.1% |
Postoperative serum sodium level was inversely correlated with postoperative leukocyte counts, as shown in Figure 3 (r = −0.106, p = 0.018). Compared with preoperative values, postoperative serum sodium level decreased by 3.63 ± 4.97 mmol/L in the AL group and by 0.84 ± 3.80 mmol/L in the NAL group. This reduction in postoperative serum sodium level was associated with development of AL (p < 0.001).
Figure 3.
Correlation between postoperative serum sodium level and postoperative leukocyte count in patients with colorectal cancer.
Multiple logistic regression analyses showed that postoperative serum sodium level (odds ratio, 1.176, p = 0.006) and tumor location (odds ratio, 3.396, p = 0.012) were independent predictive factors for AL (Table 4).
Table 4.
Multivariate analysis of laboratory values, and patients’ characteristics associated with anastomotic leakage.
| Odds ratio | 95%CI | p-value | |
|---|---|---|---|
| Postoperative serum sodium level | 1.176 | 1.049–1.318 | 0.006 |
| Tumor location | 3.396 | 0.114–0.762 | 0.012 |
| Sex | 1.422 | 0.288–1.719 | 0.440 |
| Age | 0.987 | 0.951–1.023 | 0.468 |
| Postoperative leukocyte count | 0.815 | 0.357–1.861 | 0.627 |
| Postoperative neutrophil count | 0.993 | 0.363–2.721 | 0.990 |
| Postoperative neutrophil % | 1.003 | 0.930–1.082 | 0.932 |
| Type of operation | 1.238 | 0.332–1.964 | 0.637 |
| Defunctioning stoma | 0.815 | 0.318–4.725 | 0.767 |
Twenty-five patients developed surgical site infections after the operation, with an incidence of 5.1%, of which 8 patients (8/149; 5.4%) had redefined hyponatremia and 17 patients (17/349; 4.9%) did not. This difference was not significant.
Discussion
In this study, we found that postoperative serum sodium level represented a potential negative predictive marker for the diagnosis of AL and was significantly associated with AL after colorectal cancer surgery. Patients with postoperative AL are more likely to have hyponatremia. As early as 1966, Clowes et al. analyzed 25 cases and found that secondary peritonitis may lead to hyponatremia.12 In 2014, Käser et al. found that patients with AL after colorectal surgery were likely to develop hyponatremia,13 but a limitation of that study was that it did not focus on colorectal cancer patients, so the results might be biased by heterogeneity among the study patients. All of our patients had colorectal cancer, and the redefined hyponatremia in these patients had high negative predictive value for AL, meaning that patients without hyponatremia most likely do not have AL. We confirmed the results of the study of Käser et al.13 (performed in a Caucasian population) in an Asian population, which makes our findings more generalizable.
The clinical manifestations of AL are atypical. At present, the diagnosis of AL mainly depends on clinical symptoms and signs, and subjectivity is strong. Objective tests such as CT imaging are expensive, repeat examinations are difficult, and CT thus has limited clinical application. Other studies have shown that CRP and procalcitonin can be used to help to diagnose postoperative AL.14,15 However, these laboratory tests, which are not regularly reviewed after surgery, are expensive. In contrast, serum sodium level is monitored frequently after surgery. Therefore, we explored the use of postoperative serum sodium levels in the diagnosis of AL after colorectal cancer surgery. According to the optimum cut-off value of postoperative serum sodium (139.5 mmol/L), we found that redefined hyponatremia had good negative predictive value for the diagnosis of AL after colorectal cancer surgery and thus may be a marker to exclude the diagnosis of AL. Because of its low sensitivity (70.4%) and positive predictive value (12.8%), the presence of redefined hyponatremia could not be used to diagnose AL. However, the negative predictive value was as high as 97.2%; thus, the absence of redefined hyponatremia could be used to exclude AL. Considering that 94.6% patients will not have anastomotic leakage after surgery (5.4% rate of AL), the negative predictive value of redefined hyponatremia is not too high. Therefore, it is better to use it in combination with redefined leukocytosis. When combined with the absence of redefined leukocytosis, the negative predictive value increased to 99.1%.
Therefore, the postoperative serum sodium is mainly used to exclude the diagnosis of AL. The study of Käser et al. used normal reference values to determine hyponatremia and leukocytosis,9 and the resulting negative predictive values were lower than the values obtained in this study, considering the difference in judging criteria. According to our results, if a patient does not have redefined hyponatremia or leukocytosis, the negative predictive value for the diagnosis of AL reaches 99.1%. Therefore, for these patients, there may be no need to perform CT to confirm or exclude AL.
Although inflammation may lead to hyponatremia, as reported in 1966,12 the underlying mechanisms have not been established. Possible mechanisms include the following. First, in normal physiological conditions, humans can regulate release of antidiuretic hormone (ADH) through the nervous endocrine system and achieve stability between the volume of extracellular fluid and osmotic pressure. Inflammatory cytokines released by infection, such as interleukin-1, interleukin-6, and tumor necrosis factor, can lead to increased release of ADH, resulting in hyponatremia.16,17 Second, the area of the peritoneum is large, approximately 1.3 to 1.4 m2. Peritonitis due to AL causes a large amount of fluid loss into the peritoneal cavity, which increases ADH release, resulting in hyponatremia.12 We also compared the incidence of surgical site infection between patients with redefined hyponatremia and those without but found no significant difference. This may be related to the fact that most surgical site infections are local inflammation and have little effect systemically.
This study had some limitations. Because it was a retrospective study, the frequency and time of laboratory sample collection and testing after surgery differed among patients, which may have biased the results. We did set specific criteria for collection of laboratory samples to maintain consistency as much as possible. There was no uniform regulation of the amount of sodium supplemented after surgery. Therefore, the results may be biased, but the preoperative sodium levels of patients in the study were normal, and the amount of sodium supplemented postoperatively was based on physiological needs. Only when tests showed hyponatremia was the amount of supplemental sodium adjusted. The preferred day for laboratory assessment was postoperative day 3, which was mostly the time of the first reexamination, especially in the NAL group. In the AL group, two cases of hyponatremia appeared before the results were available, and the amount of supplementation was increased. Compared with the NAL group, the level of sodium in the AL group was still low, which further supported the study conclusions.
In conclusion, measurement of serum sodium is a useful test in the postoperative period after colorectal cancer surgery to exclude a diagnosis of AL. It has a high negative predictive value, meaning that patients with the absence of redefined hyponatremia are unlikely to have an AL, especially when combined with the absence of redefined leukocytosis. In this situation, CT examination to determine whether there is an AL may not be necessary. However, further prospective trials are needed to confirm these results.
Supplemental Material
Supplemental material, sj-pdf-1-imr-10.1177_0300060520950565 for Redefined hyponatremia as a marker to exclude the diagnosis of anastomotic leakage after colorectal cancer surgery by Guochao Zhang, Rui Lian, Lichao Sun, Haibin Liu, Yan Wang and Lei Zhou in Journal of International Medical Research
Author contributions
GZ, YW, and LZ contributed to the conception and design of the study; RL collected the data, designed the figures and tables, and revised the manuscript; GZ and RL wrote the original manuscript; LS analyzed the data; HL revised the manuscript; and all authors approved the final manuscript.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This study was funded by the National Key R&D Program of China (2019YFF0216303).
ORCID iD
Guochao Zhang https://orcid.org/0000-0002-9531-8374
References
- 1.Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015; 65: 87–108. DOI: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Parthasarathy M, Greensmith M, Bowers D, et al. Risk factors for anastomotic leakage after colorectal resection: a retrospective analysis of 17518 patients. Colorectal Dis 2017; 19: 288–298. DOI: 10.1111/codi.13476. [DOI] [PubMed] [Google Scholar]
- 3.Marinello FG, Baguena G, Lucas E, et al. Anastomotic leakage after colon cancer resection: does the individual surgeon matter? Colorectal Dis 2016; 18: 562–569. DOI: 10.1111/codi.13212. [DOI] [PubMed] [Google Scholar]
- 4.Vermeer TA, Orsini RG, Daams F, et al. Anastomotic leakage and presacral abscess formation after locally advanced rectal cancer surgery: Incidence, risk factors and treatment. Eur J Surg Oncol 2014; 40: 1502–1509. DOI: 10.1016/j.ejso.2014.03.019. [DOI] [PubMed] [Google Scholar]
- 5.Mantzoros I. Oncologic impact of anastomotic leakage after low anterior resection for rectal cancer. Tech Coloproctol 2010; 4: S39–S41. DOI: 10.1007/s10151-010-0633-9. [DOI] [PubMed] [Google Scholar]
- 6.Thompson C, Hoorn EJ. Hyponatraemia: an overview of frequency, clinical presentation and complications. Best Pract Res Clin Endocrinol Metab 2012; 26: S1–S6. DOI: 10.1016/S1521-690X(12)00019-X. [DOI] [PubMed] [Google Scholar]
- 7.Don M, Valerio G, Korppi M, et al. Hyponatremia in pediatric community-acquired pneumonia. Pediatr Nephrol 2008; 23: 2247–2253. DOI: 10.1007/s00467-008-0910-2. [DOI] [PubMed] [Google Scholar]
- 8.Park SJ, Oh YS, Choi MJ, et al. Hyponatremia may reflect severe inflammation in children with febrile urinary tract infection. Pediatr Nephrol 2012; 27: 2261–2267. DOI: 10.1007/s00467-012-2267-9. [DOI] [PubMed] [Google Scholar]
- 9.Käser SA, Furler R, Evequoz DC, et al. Hyponatremia is a specific marker of perforation in sigmoid diverticulitis or appendicitis in patients older than 50 years. Gastroenterol Res Pract 2013; 2013: 462891. DOI: 10.1155/2013/462891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sigal SH. Hyponatremia in cirrhosis. J Hosp Med 2012; 7: S14–S17. DOI: 10.1002/jhm.1915. [DOI] [PubMed] [Google Scholar]
- 11.Rahbari NN, Weitz J, Hohenberger W, et al. Definition and grading of anastomotic leakage following anterior resection of the rectum: a proposal by the International Study Group of Rectal Cancer. Surgery 2010; 147: 339–351. DOI: 10.1016/j.surg.2009.10.012. [DOI] [PubMed] [Google Scholar]
- 12.Clowes GH, Jr, Vucinic M, Weidner MG. Circulatory and metabolic alterations associated with survival or death in peritonitis: clinical analysis of 25 cases. Ann Surg 1966; 163: 866–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Käser SA, Nitsche U, Maak M, et al. Could hyponatremia be a marker of anastomotic leakage after colorectal surgery? A single center analysis of 1,106 patients over 5 years. Langenbecks Arch Surg 2014; 399: 783–788. DOI: 10.1007/s00423-014-1213-7. [DOI] [PubMed] [Google Scholar]
- 14.Garcia-Granero A, Frasson M, Flor-Lorente B, et al. Procalcitonin and C-reactive protein as early predictors of anastomotic leak in colorectal surgery: a prospective observational study. Dis Colon Rectum 2013; 56: 475–483. DOI: 10.1097/DCR.0b013e31826ce825. [DOI] [PubMed] [Google Scholar]
- 15.Reynolds IS, Boland MR, Reilly F, et al. C-reactive protein as a predictor of anastomotic leak in the first week after anterior resection for rectal cancer. Colorectal Dis 2017; 19: 812–818. DOI: 10.1111/codi.13649. [DOI] [PubMed] [Google Scholar]
- 16.Watanabe T, Abe Y, Sato S, et al. Hyponatremia in Kawasaki disease. Pediatr Nephrol 2006; 21: 778–781. DOI: 10.1007/s00467-006-0086-6. [DOI] [PubMed] [Google Scholar]
- 17.Weber KT. Aldosterone in congestive heart failure. N Engl J Med 2001; 345: 1689–1697. DOI: 10.1056/NEJMra000050. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-imr-10.1177_0300060520950565 for Redefined hyponatremia as a marker to exclude the diagnosis of anastomotic leakage after colorectal cancer surgery by Guochao Zhang, Rui Lian, Lichao Sun, Haibin Liu, Yan Wang and Lei Zhou in Journal of International Medical Research
Data Availability Statement
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.