Abstract
Objective
Cisplatin (CDDP) toxicity is a dose-limiting clinical problem in clinical practice, mainly because of nephrotoxicity or ototoxicity. However, the mechanism of CDDP-induced cardiotoxicity is poorly understood. Acetyl-l-carnitine (ALCAR) is an antioxidant agent with protective effects against the side effects of various chemotherapeutics. CDDP-induced cardiotoxicity and the protective role of ALCAR were evaluated in this study.
Methods
Morphological changes were evaluated in hematoxylin and eosin-stained sections, and immunohistochemistry for caspase-3, superoxide dismutase-2 (SOD-2), inducible nitrite oxide synthase (iNOS), cyclooxygenase-2, and Bcl-2 was performed using the hearts of athymic nude mice carrying xenograft neuroblastoma tumors. Mice were randomized (six/group) to the control, CDDP (16 mg/kg), and ALCAR (200 mg/kg)+CDDP (16 mg/kg) groups. Results were analyzed using nonparametric tests.
Results
No difference was observed in the rates of cardiac necrosis, dilated/congested blood vessels, hemorrhage, polymorphonuclear leukocyte infiltration, edema, and pyknotic nuclei among the groups. SOD-2 expression was increased in the CDDP group but not in the ALCAR+CDDP group. iNOS, Bcl-2, and caspase-3 levels were not significantly different among the groups.
Conclusions
ALCAR might be a candidate protective agent for CDDP-induced cardiotoxicity. SOD-2, as a member of the oxidant system, should be evaluated in further studies as a biomarker of cardiotoxicity.
Keywords: Cisplatin, cardiotoxicity, acetyl-L-carnitine, superoxide dismutase-2, immunohistochemistry, xenograft, mice, antioxidant
Introduction
Cisplatin, also called cis-diamminedichloroplatinum (CDDP), is a widely used antineoplastic agent in oncology clinics. It is used either alone or in combination regimens.1,2 The molecular structure of CDDP consists of a platinum atom in the center surrounded by two chloride and two ammonium ions.3,4 The molecular weight of CDDP is 300.1 g/mol. When this complex is in solid form, it has a yellow color, whereas it is clear in liquid form. In vivo, CDDP exists in a neutral form in the extracellular matrix. The presence of CDDP in the neutral state is caused by the high chlorine level in the extracellular space. This neutral form prevents hydrolysis.5 CDDP binds to DNA inside the cell and exerts antineoplastic effects. This activity of CDDP prevents DNA replication and mitotic activity in cells, resulting in programmed cell death.6 In the cell, chlorine ions separate from CDDP and displace water molecules because of the low chlorine ion levels. Then, the nucleophilic groups in DNA replace the water molecules; thus, the binding process of CDDP occurs.7–10 CDDP is a highly nephrotoxic drug because of its excretion via the urinary system. In addition to its nephrotoxicity, its other common side effects, such as neurotoxicity and ototoxicity, have limited its usage. In addition to these side effects, CDDP also has cardiotoxic effects.11–14 Recent studies revealed that the cardiotoxicity of CDDP might be related to an increase in reactive oxygen species (ROS) levels.14 Furthermore, CDDP disrupts mitochondrial permeability and induces cardiomyopathy, myocarditis, coronary heart disease, arrhythmia, and Takotsubo syndrome.15
Superoxide dismutase 2 (SOD-2) is a member of the iron/manganese SOD family.16 Inactivating mutations in the SOD-2 gene have been associated with idiopathic cardiomyopathy, premature aging, sporadic motor neuron disease, and cancer. Inducible nitrite oxide synthase (iNOS) is produced in response to oxidative stress via the conversion of NOS in different tissues.16 Cyclooxygenase-2 (COX-2) is an enzyme that plays a role in pathological conditions such as inflammation. Bcl-2 has anti-apoptotic effects against different toxic conditions. Caspase-3 is a mediator of programmed cell death (apoptosis).17
CDDP mainly has nephrotoxic, ototoxic, and neurotoxic effects. However, CDDP-induced cardiotoxicity and the mechanism of this toxicity are not well understood.
Acetyl-l-carnitine (ALCAR) is an ester of l-carnitine. It is a cofactor in mitochondrial fatty acid β-oxidation. Its functions include membrane stabilization and repair through the reduction of oxidative stress. ALCAR is an important compound for various physiological and pathophysiological conditions because of its antioxidant activity.20–24 ALCAR exhibited a protective role against CDDP-induced neurotoxicity, nephrotoxicity, and ototoxicity in different in vivo and in vitro studies.25–27 Ultrasensitive cardiac troponin I (cTnI-Ultra) is a good diagnostic marker for myocardial infarction in cardiology clinics. Furthermore, serum TnI-ultra measurement has also been a useful test for monitoring chemotherapeutic drug-induced cardiotoxicity in experimental and clinical studies.24,25
One of the most important mechanisms underlying chemotherapy-induced cardiotoxicity is increased Ca2+ levels in cardiomyocytes. Increased Ca2+ levels stimulate ROS production, and there are bidirectional interactions between these parameters.
Studies are needed to identify methods for the early detection of cardiotoxicity and discover antioxidants for preventing the side effects of chemotherapeutic agents. This study evaluated CDDP-induced cardiotoxicity and the protective role of ALCAR from the viewpoint of oxidative stress.
Methods
The Local Animal Care and Use Ethics Committee of Dokuz Eylul University approved the protocol of this study. The studies adhered to the National Institutes of Health guidelines for the experimental use of animals. Archival formalin-fixed, paraffin-embedded cardiac tissues were used in this study. Heart sections were obtained along the horizontal axis after 2 days of fixation with 10% formalin. In all cases, heart tissues were weighed, and ventricular wall thickness was measured. The study consisted of three groups, each containing six animals. Paraffin blocks from animals in all three groups were sectioned to a thickness of 5 µm, and immunohistochemical analysis was conducted using the avidin–biotin peroxidase method.
Animals
This study was conducted using 18 young adult (8 weeks old) athymic male nude mice weighing 25 to 30 g. The neuroblastoma mouse model was developed via the subcutaneous xenograft transplantation of 106 mouse-derived C1300 neuroblastoma (MycN amplification-positive) cells. Animals were housed in HEPA-filtered cages in a clean, ventilated room at 55% relative humidity, 22°C, and a 12-hour/12-hour light/dark cycle. Animals were fed sterile standard mouse pellet food, and sterile tap water was provided ad libitum.
Chemical agents
Cisplatin (CDDP-Ebewe®, EBEWE Pharma, Österreich, Austria) was purchased in vials (50 mg/100 mL) from Liba (İstanbul, Turkey).
ALCAR hydrochloride was purchased from Sigma-Aldrich (Taufkirchen, Germany) and freshly dissolved in normal saline at a concentration of 10 mg/mL prior to injection.
The doses were selected according to our previous studies.20,22,26,27
Experimental groups
Nude mice received oral saline (0.75 mL/day) for 10 days for hydration. On days 8, 9, and 10, mice in the CDDP+ALCAR group were intraperitoneally injected with ALCAR (200 mg/kg), whereas mice in the other groups were injected with the same doses of normal saline on these days. On the 11th day, a single dose of 16 mg/kg CDDP in 0.2 mL was infused intraperitoneally into mice in the CDDP and CDDP+ALCAR groups, and in control mice, 0.2 mL of saline were infused intraperitoneally. No arrhythmia was observed in any group.
Experimental procedure
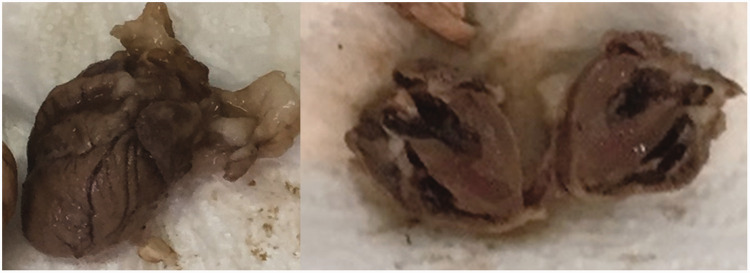
On the 18th day, 7 days after CDDP infusion, mice were sacrificed using diethyl ether anesthesia by whole blood aspiration. The heart tissues were weighed and measured, and macroscopic heart examination was performed (Figure 1). After 2 days of formalin fixation, 3-mm sections were paraffin=embedded after routine tissue processing.
Figure 1.
Normal macroscopic appearance of the heart after sacrifice.
Biochemistry evaluation
Serum levels of cTnI-Ultra were measured using a sandwich immunoassay method in an ADVIA Centaur autoanalyzer (Bayer HealthCare LLC, Leverkusen, Germany). The sensitivity of the cTnI-Ultra test was 0.006 to 50 ng/mL. This analysis was conducted in the control and CDDP groups to check the effect of CDDP as a preliminary study.
Histopathological evaluation
Heart samples were fixed in 10% buffered neutral formalin for 48 hours, and after routine tissue processing (dehydration using alcohol and clearing using xylol), they were embedded in paraffin. Paraffin blocks were sectioned at a thickness of 5 µm, deparaffinized in xylene, rehydrated through decreasing concentrations of alcohol, and stained with hematoxylin and eosin (H&E) to evaluate morphological changes such as edema, dilated/congested blood vessels, cardiac necrosis, hemorrhage, polymorphonuclear leukocyte infiltration, cells with pyknotic nuclei, eosinophilic cytoplasm, and cytoplasmic vacuolization as evidence of degeneration. The findings were marked as positive (+) or negative (−).
Immunohistochemical procedures
Anti-caspase-3 (ab13847, Abcam, Cambridge, UK), anti–SOD-2/MnSOD (ab13534, Abcam), iNOS antibody (PA3-030A, Thermo Fisher Scientific, Waltham, MA, USA), anti–COX-2 (ab15191, Abcam), and anti-Bcl-2 antibodies [E17] (ab32124, Abcam) were applied at a 1:200 dilution. Heat-mediated antigen retrieval was performed using Tris/EDTA buffer pH 9 for Bcl-2. Cytoplasmic staining was considered positive for all markers.
The obtained sections were stored at 60°C overnight. Then, they were incubated in three different xylol solutions for 30 minutes each, passed through a descending alcohol series, and rinsed with distilled water. Dehydrated tissues were drawn with PapPen and then incubated in sodium citrate solution (Bio-Optica, Milan, Italy) in the microwave for 5 minutes at 600 W. Then, sections were cooled for 20 to 30 minutes and washed three times with PBS. Then, they were exposed to H2O2 for 10 minutes at room temperature. Sections were then washed again with PBS and incubated with blocking solution (TA-125-UB, Invitrogen, Fremont, CA, USA) for 1 hour. The primary antibody was applied overnight at 4°C. The next morning, sections were washed with PBS and incubated for 30 minutes with anti-mouse biotin–streptavidin hydrogen peroxidase secondary antibody (Histostain-Plus Broad Spectrum 85-9043, Invitrogen). Then, the sections were washed with PBS and incubated in 3, 3′-diaminobenzidine tetrahydrochloride (Roche, Basel, Germany) to determine the visibility of the immunohistochemical reaction. Then, the sections were washed with distilled water, stained with Mayer’s hematoxylin dye, rinsed with alcohol and a xylol series for transparency, and sealed with Entellan (UN 1866, Merck, Darmstadt, Germany). The cytoplasmic expression of SOD-2, COX-2, and iNOS, evaluated to determine oxidative stress, was measured using a BX50 light microscope (Olympus, Tokyo, Japan) according to the staining intensity (negative, mild, moderate, high) and the percentage positivity in heart tissue sections.
Statistical analysis
All statistical analyses were performed using IBM SPSS Statistics Version 22.00 (IBM, Armonk, NY, USA), and P < 0.05 was considered significant. For comparisons between two groups, the chi-squared test or Fisher’s exact test was performed. For biochemistry results, the Mann–Whitney U test was used.
Results
Biochemistry results
In the CDDP group, serum cTnI-Ultra levels were 8.06 ± 13.55 ng/mL (0.035–35.71), compared with 0.023 ± 0.15 ng/mL (0.016–4.94) in the control group (Mann–Whitney U test, P = 0.045). These results indicated cardiotoxicity despite the absence of obvious histopathological changes.
Histopathology results
There were no differences in heart measurements and weights among the groups. Tumor regression was observed in the CDDP and ALCAR+CDDP groups compared with the control group findings when the tumor diameter, necrosis, and apoptosis ratio were evaluated in the tumor sections. Tumor necrosis was less common in the ALCAR+CDDP group than in the CDDP group, albeit without significance.
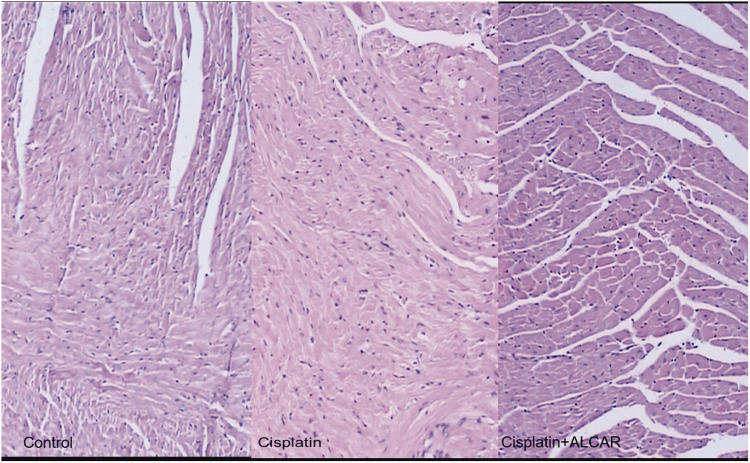
In microscopic evaluations of H&E-stained sections from the three groups, there were no statistically significant morphological differences among the groups. In all mice in the control group, the myofibril morphology was normal, and edema and congestion were detected in two and five mice, respectively. However, in addition to edema and congestion, microscopic examination revealed cytoplasmic vacuolization in CDDP-administrated mice and eosinophilic cytoplasmic change in the ALCAR+CDDP group compared with sections from the control group. Although the rate of cytoplasmic vacuolization was statistically significant among the three groups via the Kruskal–Wallis test (P = 0.006), the Mann–Whitney U test revealed no significant differences among the groups (P = 0.067, Figure 2).
Figure 2.
Microscopic appearance of the myocardium within normal limits (hematoxylin and eosin, ×40).
Immunohistochemical results
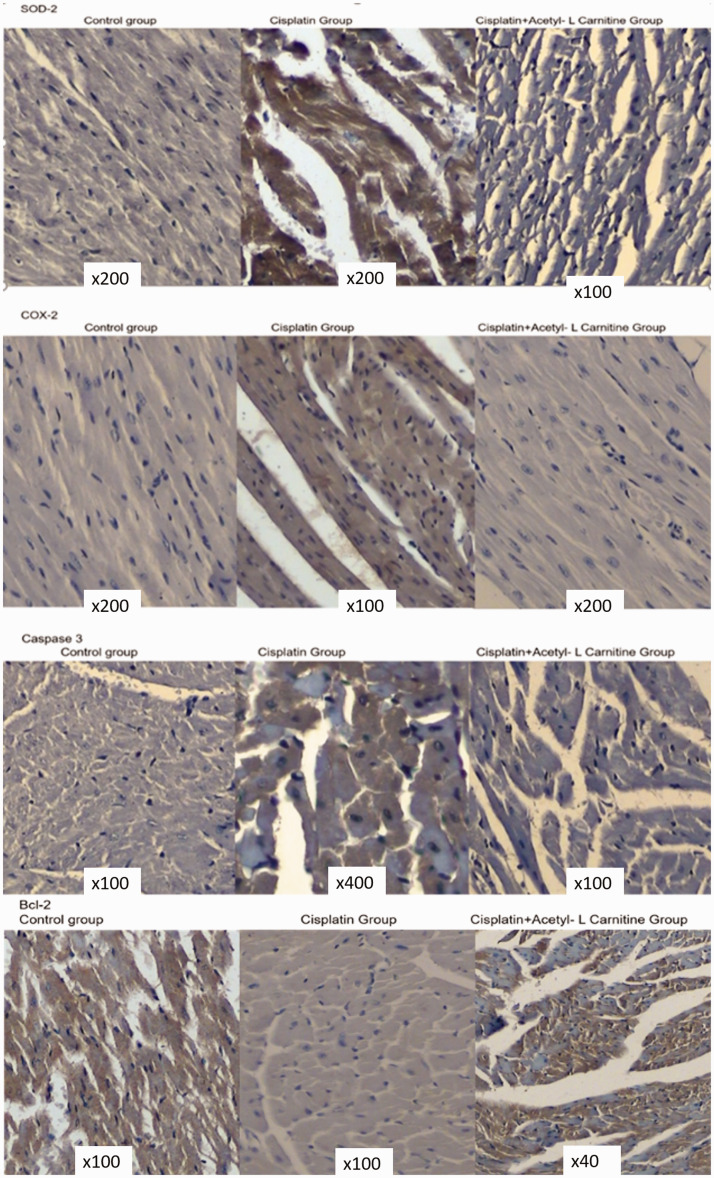
The immunohistochemical results are presented in Table 1. SOD-2 cytoplasmic expression was negative in all sections in the control and CDDP+ALCAR groups. In the CDDP group, SOD-2 expression was diffuse and mild in five sections and diffuse and strongly positive in one section compared with the findings in the control and combination treatment groups (P = 0.001). In all groups, iNOS cytoplasmic expression was observed as mildly positive. COX-2 cytoplasmic expression was negative in all sections in the control and CDDP+ALCAR groups. In the CDDP group, COX-2 was expressed in four sections (P = 0.03). Regarding apoptosis-related proteins, expression of the anti-apoptotic protein Bcl-2 was observed in three control sections and one CDDP+ALCAR section, but no expression was observed in the CDDP group. However, no statistical significance was noted for Bcl-2 expression. For the pro-apoptotic protein caspase-3, only one section in the CDDP group displayed expression in some of the cardiomyocytes. The level of cardiotoxicity for a single high dose of CDDP was not sufficient to cause prominent cell death, but oxidative stress was observed (Figure 3).
Table 1.
Immunohistochemical results for heart tissue from each mouse.
| Group/mouse No. | SOD2 | iNOS | COX-2 | Bcl-2 | Casp-3 |
|---|---|---|---|---|---|
| Control 1 | negative | positive | negative | negative | negative |
| Control 2 | negative | positive | negative | negative | negative |
| Control 3 | negative | positive | negative | positive | negative |
| Control 4 | negative | positive | negative | positive | negative |
| Control 5 | negative | positive | negative | positive | negative |
| Control 6 | negative | positive | negative | negative | negative |
| Cisplatin 1 | positive | positive | negative | negative | negative |
| Cisplatin 2 | positive | positive | positive | negative | negative |
| Cisplatin 3 | positive | positive | positive | negative | negative |
| Cisplatin 4 | positive | positive | negative | negative | negative |
| Cisplatin 5 | positive | positive | positive | negative | negative |
| Cisplatin 6 | highly positive | positive | positive | negative | positive |
| Cisplatin+ALCAR 1 | negative | positive | negative | negative | negative |
| Cisplatin+ALCAR 2 | negative | positive | negative | negative | negative |
| Cisplatin+ALCAR 3 | negative | positive | negative | negative | negative |
| Cisplatin+ALCAR 4 | negative | positive | negative | positive | negative |
| Cisplatin+ALCAR 5 | negative | positive | negative | negative | negative |
| Cisplatin+ALCAR 6 | negative | positive | negative | negative | negative |
| P (Fisher’s exact test) | 0.001 | 0.1 | 0.03 | 0.091 | 0.5 |
SOD, superoxide dismutase; iNOS, inducible nitric oxide synthase; COX, cyclooxygenase; Casp-3, caspase-3; ALCAR, acetyl-l-carnitine.
Figure 3.
Immunohistochemical expression of superoxide dismutase 2 (SOD-2), cyclooxygenase (COX)-2, caspase-3 and Bcl-2 in all groups. For SOD-2, COX-2, and caspase-3, cytoplasmic expression was observed in the cisplatin group, whereas these markers were negative in the control and cisplatin + acetyl-l-carnitine groups (3, 3′-diaminobenzidine tetrahydrochloride, ×200). Bcl-2 expression was positive in three control sections and one section in the combination group, whereas its expression was negative in the cisplatin group.
Discussion
In this study, paraffin blocks of heart tissue from nude mice were evaluated for CDDP-induced cardiotoxicity to determine the protective role of ALCAR. In our previous study, we used a neuroblastoma xenograft model of nude to assess the effects of ALCAR and an ALCAR–CDDP combination. We demonstrated that ALCAR did not cause tumor progression. Minimal interference with the cytotoxic effect of CDDP was observed. In the ALCAR+CDDP group, tumors exhibited less necrosis than those in CDDP-treated mice, albeit without significance (unpublished data, poster presentation).28 In this study, we demonstrated that ALCAR might be a candidate protective agent for CDDP-induced cardiotoxicity, and SOD-2 might be useful as a biomarker for oxidative stress to evaluate CDDP-induced cardiotoxicity. We also examined whether there was a significant difference in heart histopathology among the groups. Our data revealed that CDDP caused histopathological changes such as edema, congestion, and eosinophilic cytoplasm in the myocardium, but the differences among the groups were not significant.
Gianfranco et al.18 revealed that ALCAR increases mitochondrial metabolism by increasing the metabolism of oxygen. In another rat study of ALCAR, the agent reduced cardiac interfiber bleeding and cardiac fiber atrophy.19 Altun et al.20 observed that combined treatment with ALCAR and CDDP decreased autotoxic effects by approximately 22%. Handzlik et al.21 found that ALCAR decreased these effects by 59% under acute hypoxic conditions.
ALCAR was found to be protective against doxorubicin-induced cardiotoxicity in our previous study using a Wistar albino rat animal model.22 We demonstrated that ALCAR is protective against CDDP-induced nephrotoxicity and ototoxicity. In this study, we confirmed that cTnI-Ultra is a good diagnostic indicator for myocardial injury induced by chemotherapeutic drugs or hypoxia. In this study, CDDP-induced cardiotoxicity was detected using cTnI-Ultra levels in concordance with another study of CDDP.25 In that study, they evaluated the mechanism of CDDP-induced cardiotoxicity using malondialdehyde (MDA) levels, SOD activity, and reduced glutathione (GSH) content of levels in cardiac tissue.25 Cardiac MDA levels were increased and SOD activity and GSH content were significantly decreased in the aforementioned animal study. SOD levels in cardiac tissue in the treatment group were significantly lower than those in the control group, and these results were compatible with those reported by El-Awady et al. Furthermore, ALCAR decreased SOD expression compared with the findings in the CDDP group.
In an in vivo study by Singh et al.,16 decreases in SOD1 and SOD-2 expression were observed in relation to the nephrotoxic effect of CDDP caused by an increase of ROS levels. In addition, this study revealed significant increases in COX-2 and iNOS expression. In this study, SOD-2 expression was significantly increased in heart tissues from CDDP-administrated mice. Additionally, COX-2 expression was also detected in most sections. However, the inclusion of only six animals in each group might explain this result.
Chowdhury et al. found that the cardiotoxic effect of CDDP is related to oxidative stress, endoplasmic reticulum stress, and increased inflammation-induced apoptosis. They reported that CDDP administration increased caspase-3 activation and inhibited Bcl-2 expression.17 CDDP also induced pyknotic nuclei, edema, fibrillary hypertrophy, irregulation, and signs of dilatation in vascular structures in the heart muscle, as observed using histopathological sections.17
Some chemotherapeutic drugs have cardiotoxic effects, such as doxorubicin, ifosfamide, and cetuximab.22–24 These cardiotoxic effects are induced via different mechanisms. CDDP is also the main chemotherapeutic drug used to treat neuroblastoma, lung cancer, and testicular cancer. The acute and cumulative cardiovascular complications of CDDP include arrhythmia, myocardial ischemia, cardiac insufficiency, and ventricular hypertrophy. These complications affect the quality of life of patients after treatment. CDDP-induced cardiotoxicity is caused by the generation of ROS. ROS cause lipid peroxidation of the cell membrane and damage to proteins and DNA. In our study, cardiotoxicity in the animal model was not of sufficient severity to cause arrhythmia or ischemia, but oxidative stress was prominent.
Previous studies claimed that CDDP is accumulated by cells via diffusion. However, recent studies revealed that CDDP is actively transferred into cells by Cooper transfer protein 1.8 Oxidative stress directly affects the formation of damage caused by CDDP.9,10 After CDDP treatment, ROS disrupt the structure of intracellular macromolecules by changing intracellular components.11,12
Zhau concluded that the metabolic modulator trimetazidine and coenzyme Q10, a component of the electron transport chain, exerted protective effects against CDDP-induced cardiotoxicity by attenuating oxidative stress. This protection occurred in a synergistic manner when used in combination with other agents. Zhau studied rat cardiomyocyte cell cultures in vitro, finding that ROS and MDA levels were decreased by these agents whereas SOD-2 expression was increased compared with the effects of CDDP administration.29 His data contract the literature and our in vivo data. A candidate protective agent such as an antioxidant is expected to reverse the increases in protein expression caused by CDDP. Xing et al.30 found that saponins from the leaves of Panax quinquefolius decreased CDDP-induced cardiotoxicity by inhibiting oxidative stress-associated inflammation and apoptosis in mice. As a member of the iron/manganese SOD family, SOD-2 transforms toxic superoxide into H2O2 and diatomic oxygen. Under oxidative stress caused by CDDP, SOD-2 levels should be elevated, as observed in our study. CDDP increases the total oxidant capacity in tissues.31,32
One weakness of our study was that we could not perform electrocardiography or echocardiography in mice because of the unavailability of equipment. Another disadvantage might be that we did not use an ALCAR monotherapy group. This group was not included to minimize the number of mice sacrificed. The lack of statistical differences in histopathology is also a weakness of our study, but we believe that earlier symptoms for cardiotoxicity should also be evaluated. One strength of our study was that we used tumor-bearing mice with similarity to the oncology patient model.
We conclude that ALCAR is a candidate cardioprotective agent against CDDP-induced cardiotoxicity at the oxidative stress level. The importance of SOD-2 should be studied in detail. Our next step will be to evaluate SOD2 levels in the serum of patients with cancer under CDDP treatment in comparison with a control group to determine its possible role as a biomarker for the early detection of cardiotoxicity. The protective effects of ALCAR against CDDP-induced toxicity could be evaluated in clinical trials after animal toxicity and high-dose safety studies.
Supplemental Material
Supplemental material, sj-jpg-1-imr-10.1177_0300060520951393 for Antioxidant effect of acetyl-l-carnitine against cisplatin-induced cardiotoxicity by Serdar Bayrak, Safiye Aktaş, Zekiye Altun, Yasemin Çakir, Merve Tütüncü, Selen Kum Özşengezer, Osman Yilmaz and Nur Olgun in Journal of International Medical Research
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This work was financially supported by Dokuz Eylul University, Scientific Research Projects Council (2019.KB.SAG.031).
ORCID iD
Serdar Bayrak https://orcid.org/0000-0003-1573-9572
References
- 1.Tsuruya K, Ninomiya T, Tokumot M, et al. Direct involvement of the receptor-mediated apoptotic pathways in CDDP induced renal tubular cell death. Kidney Int 2003; 63: 72–82. [DOI] [PubMed] [Google Scholar]
- 2.Rosenberg B, VanCamp L, Trosko JE, et al. Platinum compounds: a new class of potent antitumour agents. Nature 1969; 222: 385–386. [DOI] [PubMed] [Google Scholar]
- 3.Bertram G. Basic Clinical Pharmacology. 9th ed New York, USA: McGraw Hill, 2001, p.1285–1300. [Google Scholar]
- 4.AHFS Drug Information. In: McEvoy GK (ed.) Pharmacists Bethesda, Maryland: American Society of Health-System, 2004, pp.929–952.
- 5.Gonzalez VM, Fuertes MA, Alonso C, et al. Is CDDP-induced cell death always produced by apoptosis? Mol Pharmacol 2001; 59: 657–663. [DOI] [PubMed] [Google Scholar]
- 6.Jamieson ER, Lippard SJ. Structure, Recognition, and Processing of CDDP- DNA Adducts. Chem Rev 1999; 99: 2467–2498. [DOI] [PubMed] [Google Scholar]
- 7.Gong JG. The tyrosine kinase c-Abl regulates p73 in apoptotic response to CDDP induced DNA damage. Nature 1999; 399: 806–809. [DOI] [PubMed] [Google Scholar]
- 8.Fuertes MA, Alonso C, Perez JM. Biochemicalmodulation of CDDP mechanisms of action: enhancement of antitumor activity and circumvention of drug resistance. Chem Rev 2003; 103: 645–662. [DOI] [PubMed] [Google Scholar]
- 9.White AC, Sousa AM, Blumberg J, et al. Plasma antioxidants in subjects before hematopoietic stem cell transplantation. Bone Marrow Transplant 2006; 38: 513–520. [DOI] [PubMed] [Google Scholar]
- 10.Crohns M, Liippo K, Erhola M, et al. Concurrent decline of several antioxidants and markers of oxidative stress during combination chemotherapy for small cell lung cancer. Clin Biochem 2009; 42: 1236–1245. [DOI] [PubMed] [Google Scholar]
- 11.Brea-Calvo G, Rodríguez-Hernández A, Fernández-Ayala DJ, et al. Chemotherapy induces an increase in coenzyme Q10 levels in cancer cell lines. Free Radic Biol Med 2006; 40: 1293–1302. [DOI] [PubMed] [Google Scholar]
- 12.Giaccone G. Clinical perspectives on platinum resistance. Drugs 2000; 59: 9–17, 37-38. [DOI] [PubMed] [Google Scholar]
- 13.Kuo MT, Chen HH, Song IS, et al. The roles of copper transporters in CDDP resistance. Cancer Metastasis Rev 2007; 26: 71–83. [DOI] [PubMed] [Google Scholar]
- 14.Topal İ, Bilgin A, Çimen FK, et al. The effect of rutin on CDDP-induced oxidative cardiac damage in rats. Anatol J Cardiol 2018; 20: 136–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Finsterer J, Ohnsorge P. Influence of mitochondrion-toxic agents on the cardiovascular system. Regul Toxicol Pharmacol 2013; 67: 434–445. [DOI] [PubMed] [Google Scholar]
- 16.Singh MP, Chauhan AK, Kang SC. Morin hydrate ameliorates cisplatin-induced ER stress, inflammation and autophagy in HEK-293 cells and mice kidney via PARP-1 regulation. Int Immunopharmacol 2018; 56: 156–167. [DOI] [PubMed] [Google Scholar]
- 17.Chowdhury S, Sinhai K, Banerjee S, et al. Taurine protects cisplatin induced cardiotoxicity by modulating inflammatory and endoplasmic reticulum stress responses. Biofactors 2016; 42: 647–664. [DOI] [PubMed] [Google Scholar]
- 18.Gianfranco P, Raffaella N, Emilia R, et al. Cancer and anticancer-induced modifications on metabolism mediated by ALCAR system. J Cell Physiol 2000; 182: 339–350. [DOI] [PubMed] [Google Scholar]
- 19.Huwait EA. Combination of vitamin E and L-ALCAR is superior in protection against Isoproterenol-induced cardiac affection: a histopathological evidence. Folia Morphol (Warsz) 2019; 78: 274–282. [DOI] [PubMed] [Google Scholar]
- 20.Altun Z, Olgun Y, Ercetin P, et al. Protective effect of acetyl-l-ALCAR against CDDP ototoxicity: role of apoptosis-related genes and pro-inflammatory cytokines. Cell Prolif 2014; 47: 72–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Handzlik MK, Constantin-Teodosiu D, Greenhaff PL, et al . Increasing cardiac pyruvate dehydrogenase flux during chronic hypoxia improves acute hypoxic tolerance. J Physiol 2018; 596: 3357–3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dundar HA, Kiray M, Kir M, et al. Protective Effect of Acetyl-L-ALCAR Against Doxorubicin-induced cardiotoxicity in Wistar Albino Rats. Arch Med Res 2016; 47: 506–514. [DOI] [PubMed] [Google Scholar]
- 23.Sayed-Ahmed MM, Aldelemy ML, Al-Shabanah OA, et al. Inhibition of gene expression of ALCAR palmitoyltransferase I and heart fatty acid binding protein in cyclophosphamide and ifosfamide-induced acute cardiotoxic rat models. Cardiovasc Toxicol 2014; 14: 232–242. [DOI] [PubMed] [Google Scholar]
- 24.Tang XM, Chen H, Liu Y, et al. The cardiotoxicity of cetuximab as single therapy in Chinese chemotherapy-refractory metastatic colorectal cancer patients. Medicine (Baltimore) 2017; 96: e5946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.El-Awady el-SE, Moustafa YM, Abo-Elmatty DM, et al. CDDP-induced cardiotoxicity: Mechanisms and cardioprotective strategies. Eur J Pharmacol 2011; 650: 335–341. [DOI] [PubMed] [Google Scholar]
- 26.Tufekci O, Gunes D, Ozoğul C, et al. Evaluation of the effect of acetyl L-ALCAR on experimental CDDP nephrotoxicity. Chemotherapy 2009; 55: 451–459. [DOI] [PubMed] [Google Scholar]
- 27.Gunes D, Kirkim G, Kolatan E, et al. Evaluation of the effect of acetyl L-ALCAR on experimental CDDP ototoxicity and neurotoxicity. Chemotherapy 2011; 57: 186–194. [DOI] [PubMed] [Google Scholar]
- 28.Aktas S, Altun ZS, Pamukoglu A, et al. Acetyl L Carnitine Interferes With Antitumor Effect Of Cisplatin. Ped Blood and Cancer 2014: 61/S2/S327-S328 (Abstract). [Google Scholar]
- 29.Zhao L. Protective effects of trimetazidine and coenzyme Q10 on cisplatin-induced cardiotoxicity by alleviating oxidative stress and mitochondrial dysfunction. Anatol J Cardiol 2019; 22: 232–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xing JJ, Hou JG, Liu Y, et al. Supplementation of Saponins from Leaves of Panax quinquefolius Mitigates Cisplatin-Evoked Cardiotoxicity via Inhibiting Oxidative Stress-Associated Inflammation and Apoptosis in Mice. Antioxidants (Basel) 2019; 8: pii: E347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gunturk EE, Yucel B, Gunturk I, et al. The effects of N-acetylcysteine on cisplatin induced cardiotoxicity. Bratisl Lek Listy 2019; 120: 423–428. [DOI] [PubMed] [Google Scholar]
- 32.Qi L, Luo Q, Zhang Y, et al. Advances in Toxicological Research of the Anticancer Drug Cisplatin. Chem Res Toxicol 2019; 32: 1469–1486. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-jpg-1-imr-10.1177_0300060520951393 for Antioxidant effect of acetyl-l-carnitine against cisplatin-induced cardiotoxicity by Serdar Bayrak, Safiye Aktaş, Zekiye Altun, Yasemin Çakir, Merve Tütüncü, Selen Kum Özşengezer, Osman Yilmaz and Nur Olgun in Journal of International Medical Research