Abstract
Objective
To identify the risk factors for early death and determine the predictive value of the sequential organ failure assessment (SOFA) score for prognosis of severe acute ischemic stroke (AIS).
Methods
A total of 110 patients with severe AIS were enrolled and divided into the non-survivor (n = 34) and survivor groups (n = 76). Logistic regression analysis was conducted to identify risk factors for early death, while the receiver operator characteristic (ROC) curve was used to determine the predictive effect of the SOFA score on prognosis.
Results
Logistic regression analysis showed that urinary tract infection (odds ratio [OR] = 17.364, 95% confidence interval [CI]: 1.903–158.427), mechanical ventilation (OR = 1.754, 95% CI: 1.648–2.219), and osmotic therapy (OR = 2.835, 95% CI: 1.871–5.102) were significantly correlated with early death of severe AIS. ROC curve analysis of the area under the curve after hospitalization showed that the maximum SOFA and ΔSOFA scores exceeded 0.7.
Conclusion
Our study shows that urinary tract infection, mechanical ventilation, and osmotic therapy are risk factors for early death of severe AIS. The SOFA score has good predictive value for prognosis of severe AIS. These findings may provide a guideline for improving clinical outcome.
Keywords: Risk factor, early death, predictive value, sequential organ failure assessment score, severe acute ischemic stroke, National Institutes of Health Stroke Scale
Introduction
Stroke is a local cerebrovascular disease with a rapid onset. The symptoms of a stroke last for more than 24 hours or are interrupted by death within 24 hours.1 Stroke is a common and frequently occurring disease of the nervous system, as well as the main cause of death in China.2 At present, the incidence of stroke worldwide is nearly 0.16% annually, and ischemic stroke accounts for 70% to 80% of all strokes.3 Severe acute ischemic stroke is characterized by various complications, high mortality, a prolonged hospital stay, and concomitant severe neurological deficits. All of these not only affect the quality of life of patients, but also impose a heavy burden on friends, family, and society.4 Current clinical studies are focusing on the clinical features of patients with severe acute ischemic stroke (AIS). Some studies have reported that opium addiction increases the risk of stroke,5 and could be a risk factor for ischemic stroke.6 However, there have been few studies on the risk factors for early death in these patients.
The sequential organ failure assessment (SOFA) score originates from a score of sepsis-related organ failure assessment created by the Working Group on Sepsis-related Problems of the European Society of Intensive Care Medicine (ESICM) in Paris (December 1994). This score was named because the original score has been applied beyond sepsis.7 The SOFA score is a commonly used scoring system for describing the occurrence, development, and evaluation of multiple organ dysfunction among critically ill patients worldwide.8–10 SOFA scoring has the advantages of being objective, simple, and easy for data collection.11 While current research mainly focuses on the application of SOFA scores in patients with sepsis, it is also useful in aluminum phosphide-poisoned patients12 and patients with cirrhosis.13 However, few studies have examined the predictive value of the SOFA score in prognosis of patients with severe AIS.
In this study, we aimed to identify the risk factors for early death and determine the predictive value of the SOFA score for prognosis of severe AIS. The risk factors for early death in these patients were identified by using logistic regression analysis and the predictive effect of the SOFA score on prognosis was analyzed by the receiver operator characteristic (ROC) curve.
Materials and methods
Patients and grouping
Patients who were hospitalized in the neurological intensive care unit of Beijing Chao-Yang Hospital, Capital Medical University from February 2011 to June 2015 and had been pathologically diagnosed with severe AIS were enrolled. Patients who conformed to the following inclusion and exclusion criteria were recruited and divided into the non-survivor group or the survivor group according to the outcome within 14 days of onset. The inclusion criteria were as follows: 1) age ≥ 18 years old; 2) admission to hospital within 48 hours after the onset of symptoms; 3) the diagnostic criteria were met for acute ischemic stroke;14 4) magnetic resonance imaging and/or computed tomographic examinations were performed, and there were new infarction lesions consistent with neurological deficits; and 5) a National Institutes of Health Stroke Scale (NIHSS) score of ≥15 and NIHSS 1a ≥1 at admission. The exclusion criteria were as follows: 1) presence of non-acute cerebral infarction diseases, such as cerebral hemorrhage, viral encephalitis, and Guillain–Barré syndrome; 2) a brain computed tomography or magnetic resonance imaging examination showed intracranial hemorrhage, a mass, infection, or other phenomena; 3) concomitant acute coronary syndrome, severe congestive heart failure, pulmonary embolism, renal failure, rhabdomyolysis or septic conditions (e.g., sepsis, endocarditis, myocarditis); 4) the patient visited hospital more than 48 hours after onset or died within 48 hours of onset; and 5) the patient gave up treatment. This study conformed to the ICMJE Recommendations for the Conduct, Reporting, Editing, and Publication of Scholarly Work in Medical Journals. The study was approved by the Ethics Committee of Beijing Chao-Yang Hospital, Capital Medical University and all patients provided written informed consent before enrollment.
Demographic and clinical assessment
Demographic and clinical data of each patient were obtained by medical chart review, as well as by face-to-face interviews. Demographic data included age, sex, and stroke-related risk factors (hypertension, diabetes, previous stroke, chronic obstructive pulmonary disease, coronary artery disease, atrial fibrillation, smoking, and drinking). The clinical data of patients who were admitted to hospital were collected and included body temperature, respiratory rate, heart rate, blood pressure, white blood cell count, and levels of hemoglobin, high-density lipoprotein, low-density lipoprotein, thyroglobulin, albumin, hemoglobin A1c, creatinine, and brain natriuretic peptide, all of which were tested at admission.
In this study, of the following assessments were performed on the patients: 1) the severity of stroke was determined at admission by the NIHSS, 2) the severity of illness was determined at admission on the basis of the Acute Physiology and Chronic Health Evaluation (APACHE) II score, 3) consciousness levels of patients at admission were determined using the Glasgow Coma Scale (GCS), 4) comorbid conditions were determined by the Charlson comorbidity index, and 5) the prognostic risk was determined on the basis of preadmission comorbidities, level of consciousness, age, and neurologic deficit (PLAN).
Neither magnetic resonance angiography nor cardiac ultrasonography was performed on many patients who were enrolled in the study. Therefore, the Trial of ORG 10172 in Acute Stroke Treatment classification criteria were not suitable for determining the etiology of patients.15 Consequently, we used the Oxfordshire Community Stroke Project (OCSP) for classification of patients on the basis of clinical manifestations of the largest neurological deficit caused by ischemic stroke. Patients were then divided into the following four categories: total anterior circulation infarcts, partial anterior circulation infarcts, posterior circulation infarcts, and lacunar infarcts.16
Cerebral imaging parameters
The imaging data of all patients were analyzed independently by two experienced neurologists who were not aware of the patient's clinical information or SOFA score. Discussion was performed for reaching a consensus once an inconsistent opinion occurred. According to the location, lesions were divided into the following four categories: infarction of the anterior cerebral artery territory, middle cerebral artery territory, posterior cerebral artery territory, and vertebrobasilar artery territory. Cerebral imaging parameters were used to determine the location of the lesion and to then identify infarction in either a single vascular territory or multi-vascular territories (vascular territories ≥2).
Medical complications and clinical outcome
An experienced neurologist assessed the patients’ complications after admission (≤14 days) without knowing the patients’ clinical information or SOFA score. We documented seven complications, including pulmonary infection, stress ulcer, urethral infection, myocardial infarction, hemorrhagic transformation, venous thrombus, and symptomatic epilepsy. We also recorded the number of patients who received mechanical ventilation (non-invasive and invasive) and/or acute phase osmotic therapy (e.g., mannitol, glycerol fructose).
SOFA score calculation
The original SOFA score (Table 1) was composed of parameters from six organ systems (respiratory, cardiovascular, neurological, hepatic, renal, and coagulation). Each organ system was graded from 0 to 4 according to the extent of failure, and the total score ranged from 0 to 24.17 Each organ system was scored on the basis of its worst case of the particular day, and the total score was calculated by summing the scores for each of the six organ systems. This study assessed the total SOFA scores on days 1, 4, 7, 10, and 14 after admission, the total maximum SOFA score, and the change in SOFA (ΔSOFA) score. The highest SOFA score at these time points was the total maximum SOFA score, and the ΔSOFA was defined as the difference between the total maximum SOFA score and the SOFA score on day 1.18
Table 1.
The SOFA score.
| SOFA score | 0 | 1 | 2 | 3 | 4 |
|---|---|---|---|---|---|
| Respiration: PaO2/FiO2 (mm Hg) | ≥400 or no artificial ventilation and no additional O2 | ≤400 | ≤300 | ≤200 | ≤100 |
| Coagulation: platelets × 103/mm3 | >150 | ≤150 | ≤100 | ≤50 | ≤20 |
| Liver: bilirubin (mg/dL) | <1.2 | 1.20–1.90 | 2.0–5.9 | 6.0–11.9 | >12 |
| Cardiovascular: hypotension | No hypotension | MAP< 70 mm Hg | Dopamine ≤5 µg/kg/minute or dobutamine (any dose) | Dopamine ≥5 µg/kg/minute or norepinephrine ≤0.1 µg/kg/minute | Dopamine ≥15 µg/kg/minute or norepinephrine ≥0.1 µg/kg/minute |
| Central nervous system: Glasgow Coma Scale score | 15 | 14–13 | 12–10 | 9–6 | <6 |
| Renal: creatinine in mg/dL or urine output in mL/day | <1.2 | 1.2–1.9 | 2.0–3.4 | 3.5–4.9 or <500 mL/day | >5 or < 200 mL/day |
SOFA, sequential organ failure assessment; PaO2, arterial partial pressure of oxygen; FiO2, fraction of inspired oxygen; MAP, mean arterial pressure.
Statistical analysis
SPSS (version 20.0; IBM Corp., Armonk, NY, USA) was used for data analysis. All data are presented as mean ± standard deviation for continuous variables with a normal distribution, as median and interquartile range for continuous variables with a non-normal distribution, or as frequency and percentage for categorical variables. Univariate analysis was used to compare the non-survivor group and survivor group. Continuous variables with a normal distribution were compared by the Student’s t test, while the Mann–Whitney U test was used to compare continuous variables with a non-normal distribution. Categorical variables were compared by the χ2 test or Fisher’s exact test. Independent risk factors for early death were analyzed by the method of logistic regression analysis. The ROC curve was performed to compare the area under the curve (AUC) of each SOFA score and to examine the predictive value of the SOFA score for in-hospital death. Statistical significance was set at P < 0.05.
Results
Patients’ characteristics
A total of 110 patients with severe AIS were included in this study. Of the 110 patients, 34 and 76 were allocated into the non-survivor group and the survivor group, respectively, on the basis of their clinical outcome within 14 days. Creatinine levels, brain natriuretic peptide levels, the NIHSS score, APACHE II score, and PLAN score were significantly higher, but the GSC score was significantly lower in the non-survivor group than in the survivor group (all P < 0.05). Additionally, there were significant differences in infarction in a single territory or multi-vascular territories, complications, including urinary tract infection and myocardial infarction, and number of patients who had received mechanical ventilation or osmotic therapy, between the two groups (all P < 0.05). OCSP classification tended to be different between the groups (P = 0.05) (Tables 2 and 3).
Table 2.
Comparison of demographic data in patients with severe acute ischemic stroke between the non-survivor and survivor groups.
| Variable | All patients (n = 110) | Non-survivor group (n = 34) | Survivor group (n = 76) | P value |
|---|---|---|---|---|
| Age, years | 74.25 ± 12.10 | 77.09 ± 10.05 | 72.99 ± 12.77 | 0.101 |
| Sex, female | 51 (46.4) | 16 (47.1) | 35 (46.1) | 0.922 |
| Hypertension | 84 (76.4) | 28 (82.4) | 56 (73.7) | 0.323 |
| Diabetes | 39 (35.5) | 11 (32.4) | 28 (36.8) | 0.649 |
| Stroke/TIA (previous) | 42 (38.2) | 11 (32.4) | 31 (40.8) | 0.400 |
| COPD | 8 (7.3) | 3 (8.8) | 5 (6.6) | 0.983 |
| Coronary artery disease | 40 (36.4) | 13 (38.2) | 27 (35.5) | 0.785 |
| Atrial fibrillation | 16 (14.5) | 7 (20.6) | 9 (11.8) | 0.229 |
| Smoking | 41 (37.3) | 14 (41.2) | 27 (35.5) | 0.571 |
| Drinking | 26 (23.6) | 7 (20.6) | 19 (25.0) | 0.615 |
Values are mean ± standard deviation or n (%). TIA, transient ischemic attack; COPD, chronic obstructive pulmonary disease.
Table 3.
Comparison of clinical data in patients with severe acute ischemic stroke between the non-survivor and survivor groups.
| Variable | All patients (n = 110) | Non-survivor group (n = 34) | Survivor group (n = 76) | P value |
|---|---|---|---|---|
| Body temperature at admission, °C | 37.06 ± 0.87 | 37.23 ± 1.07 | 36.99 ± 0.76 | 0.244 |
| Respiratory rate at admission, breaths/minute | 22.79 ± 10.25 | 23.38 ± 13.38 | 22.53 ± 8.58 | 0.688 |
| Heart rate at admission, beats/minute | 88.76 ± 24.80 | 94.27 ± 27.24 | 86.30 ± 23.40 | 0.120 |
| Systolic BP at admission, mmHg | 160.74 ± 28.69 | 165.03 ± 37.18 | 158.82 ± 24.00 | 0.376 |
| Diastolic BP at admission, mmHg | 84.80 ± 20.56 | 84.41 ± 21.54 | 84.97 ± 20.25 | 0.895 |
| WBC count, 109/L | 11.33 ± 3.49 | 11.19 ± 3.75 | 11.39 ± 3.39 | 0.784 |
| HGB, g/L | 133.65 ± 20.37 | 131.77 ± 20.00 | 134.49 ± 20.61 | 0.520 |
| HDL, mmol/L | 1.32 ± 0.36 | 1.34 ± 0.36 | 1.31 ± 0.37 | 0.698 |
| LDL, mmol/L | 2.46 ± 0.86 | 2.34 ± 0.79 | 2.51 ± 0.88 | 0.341 |
| TG, mmol/L | 1.73 ± 1.43 | 1.62 ± 1.19 | 1.78 ± 1.53 | 0.586 |
| ALB, g/L | 32.29 ± 5.82 | 31.39 ± 7.08 | 32.70 ± 5.16 | 0.335 |
| HbA1c, % | 6.20 (5.80, 7.53) | 6.20 (5.78, 7.50) | 6.25 (5.80, 7.68) | 0.511 |
| CREA, µmol/L | 100.42 ± 48.29 | 121.85 ± 67.90 | 90.83 ± 32.54 | 0.015 |
| BNP, pg/mL | 1445 (436, 3416) | 2644 (652, 9708) | 1109 (371, 2383) | 0.008 |
| NIHSS score at admission | 21.28 ± 5.50 | 23.35 ± 5.91 | 20.36 ± 5.08 | 0.008 |
| APACHE II score at admission | 17.02 ± 4.95 | 19.21 ± 5.38 | 16.04 ± 4.43 | 0.002 |
| GCS score at admission | 7.36 ± 2.73 | 6.35 ± 2.27 | 7.82 ± 2.80 | 0.009 |
| Charlson comorbidity score at admission | 1.51 ±1.27 | 1.53 ±1.11 | 1.50 ±1.35 | 0.912 |
| PLAN score at admission | 17.33 ± 2.53 | 18.25 ± 1.91 | 16.92 ± 2.67 | 0.010 |
| OCSP | 0.050 | |||
| TACI | 53 (48.2) | 21 (61.8) | 32 (42.1) | |
| PACI | 20 (18.2) | 2 (5.9) | 18 (23.7) | |
| POCI | 37 (33.6) | 11 (32.4) | 26 (34.2) | |
| LACI | 0 (0) | 0 (0) | 0 (0) | |
| Radiological parameters (CT/MRI) | 0.005 | |||
| Single vascular territory | 91 (82.7) | 23 (67.6) | 68 (89.5) | |
| multi vascular territories (≥2) | 19 (17.3) | 11 (32.4) | 8 (10.5) | |
| complications | ||||
| Pulmonary infection | 109 (99.1) | 34 (100) | 75 (98.7) | 0.502 |
| Stress ulcer | 56 (50.9) | 19 (55.9) | 39 (48.7) | 0.485 |
| Urinary tract infection | 28 (25.5) | 4 (11.8) | 24 (31.6) | 0.049 |
| Myocardial infarction | 4 (3.6) | 4 (11.8) | 0 (0) | 0.008 |
| Hemorrhagic transformation | 6 (5.5) | 1 (2.9) | 5 (6.6) | 0.747 |
| Deep vein thrombosis | 7 (6.4) | 3 (8.8) | 4 (5.3) | 0.776 |
| Symptomatic epilepsy | 5 (4.5) | 2 (5.9) | 3 (3.9) | 1.000 |
| Mechanical ventilation | 30 (27.3) | 17 (50.0) | 13 (17.1) | 0.000 |
| Osmotic therapy | 79 (71.8) | 29 (85.3) | 50 (65.8) | 0.036 |
Values are mean ± standard deviation, n (%), or median (interquartile range). BP, blood pressure; WBC, white blood cell; HGB, hemoglobin; HDL, high-density lipoprotein; LDL, low-density lipoprotein; TG, thyroglobulin; ALB, albumin; HbA1c, hemoglobin A1c; CREA, creatinine; BNP, brain natriuretic peptide; NIHSS, National Institute of Health Stroke Scale; APACHE, Acute Physiology and Chronic Health Evaluation; GCS, Glasgow Coma Scale; PLAN, preadmission comorbidities, level of consciousness, age, and neurologic deficit; OCSP, Oxfordshire Community Stroke Project; TACI, total anterior circulation infarcts; PACI, partial anterior circulation infarcts; POCI, posterior circulation infarcts; LACI, lacunar infarcts; CT, computed tomography; MRI, magnetic resonance imaging.
Risk factors for early death
To further determine the risk factors for early death of patients with severe AIS, we performed logistic regression analysis. Multivariate analysis included the APACHE score, GCS score, PLAN score, OCSP, urinary tract infection, mechanical ventilation, and osmotic therapy in the logistic regression model. We found that urinary tract infection (OR = 17.364, 95% confidence interval [CI]: 1.903–158.427), mechanical ventilation (OR = 1.754, 95% CI: 1.648–2.219), and osmotic therapy (OR = 2.835, 95% CI: 1.871–5.102) were significantly correlated with early death of severe AIS (all P < 0.05) (Table 4).
Table 4.
Risk factors for early death in patients with severe acute ischemic stroke.
| Baseline variables | n |
Death |
|||
|---|---|---|---|---|---|
| β | P value | OR | 95% CI | ||
| APACHE | 110 | −0.063 | 0.565 | 0.939 | 0.758–1.163 |
| GCS | 110 | −0.100 | 0.644 | 0.905 | 0.592–1.383 |
| PLAN | 110 | −0.249 | 0.145 | 0.780 | 0.558–1.089 |
| OCSP | 110 | 0.578 | 0.466 | 1.783 | 0.376–8.458 |
| Urinary tract infection | 110 | 2.854 | 0.011 | 17.364 | 1.903–158.427 |
| Mechanical ventilation | 110 | 0.065 | 0.012 | 1.754 | 1.648–2.219 |
| Osmotic therapy | 110 | 0.726 | 0.021 | 2.835 | 1.871–5.102 |
OR, odds ratio; CI, confidence interval; APACHE, Acute Physiology and Chronic Health Evaluation; GCS, Glasgow Coma Scale; PLAN, preadmission comorbidities, level of consciousness, age, and neurologic deficit; OCSP, Oxfordshire Community Stroke Project.
SOFA score
To analyze the difference in SOFA scores between the non-survivor and survivor groups, we recorded the total SOFA scores on days 1, 4, 7, 10, and 14 after admission, the maximum SOFA score, and ΔSOFA score for each group. The total SOFA scores at all different time points were significantly higher in the non-survivor than in the survivor group (all P < 0.01) (Table 5). Notably, we observed that the difference in the maximum SOFA score and ΔSOFA score between the two groups was the same as that for the total SOFA scores. Collectively, these results suggest that the SOFA score has a predictive effect on the patient’s prognosis.
Table 5.
SOFA scores of patients with severe acute ischemic stroke in the non-survivor group and survivor group.
| All patients (n = 110) | Non-survivor group (n = 34) | Survivor group (n = 76) | P value | |
|---|---|---|---|---|
| Total SOFA score on day 1 | 5.02 ± 2.14 | 5.94 ± 2.70 | 4.61 ± 1.71 | 0.002 |
| Total SOFA score on day 4 | 5.25 ± 2.32 | 7.25 ± 2.54 | 4.62 ± 1.85 | <0.001 |
| Total SOFA score on day 7 | 4.97 ± 2.38 | 7.77 ± 3.25 | 4.32 ± 1.56 | <0.001 |
| Total SOFA score on day 10 | 4.68 ± 2.29 | 7.21 ± 3.83 | 4.16± 1.39 | <0.001 |
| Total SOFA score on day 14 | 4.47 ± 1.99 | 6.91 ± 2.74 | 4.02 ± 1.44 | <0.001 |
| Max SOFA | 6.54 ± 2.84 | 8.85± 3.22 | 5.50± 1.90 | <0.001 |
| ΔSOFA | 1 (0, 2.5) | 3 (0, 4) | 0 (0, 1) | <0.001 |
Values are mean ± standard deviation or median (interquartile range). SOFA, sequential organ failure assessment; max, maximum.
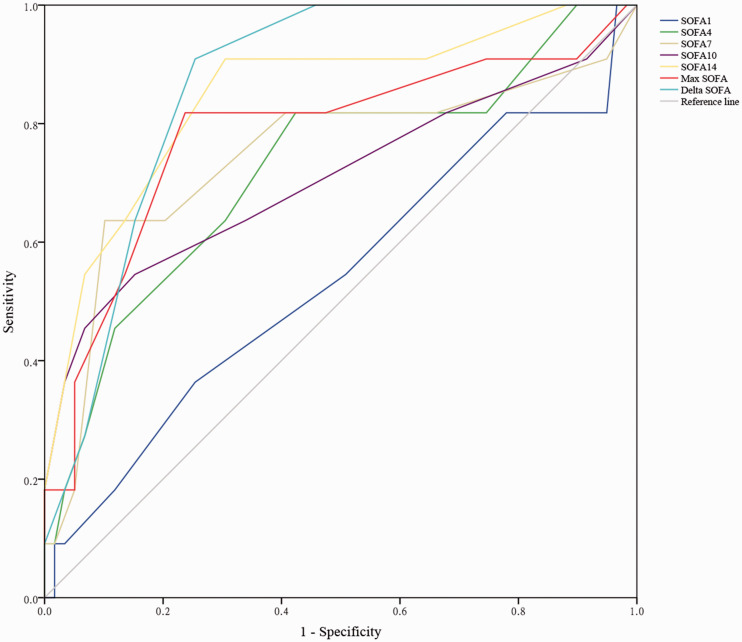
ROC curve of the SOFA score
To validate the predictive effect of the SOFA score on the prognosis of patients with severe AIS, we used the ROC curve to assess the total SOFA scores of patients on days 1, 4, 7, 10, and 14, the maximum SOFA score, and the ΔSOFA score. To create the ROC curve, the various SOFA scores and the survival status (death or survival) of the patients were used as test variables and a state variable, respectively. An AUC from 0.5 to 0.7 was considered as moderate discrimination, while 0.7 to 0.9 was considered as good discrimination. As shown in Figure 1, the AUCs for SOFA scores on days 1, 4, 7, 10, and 14 after hospitalization were 0.539 (95% CI: 0.337–0.740, P = 0.687), 0.722 (95% CI: 0.543–0.901, P = 0.020), 0.745 (95% CI: 0.550–0.940, P = 0.010), 0.708 (95% CI: 0.504–0.912, P = 0.029), and 0.849 (95% CI: 0.714–0.984, P < 0.001), respectively. The AUCs for the maximum SOFA and ΔSOFA scores were 0.866 (95% CI: 0.778–0.954, P < 0.001) and 0.782 (95% CI: 0.608–0.956, P = 0.003), respectively. Taken together, these data showed that for SOFA of patients on days 4, 7, 10, and 14, the maximum SOFA and ΔSOFA scores had good predictive value for the prognosis of patients with severe AIS.
Figure 1.
Receiver operator characteristic curve of patients with severe acute ischemic stroke
SOFA, sequential organ failure assessment; SOFA1, SOFA score on day 1; SOFA4, SOFA score on day 4; SOFA7, SOFA score on day 7; SOFA10, SOFA score on day 10; SOFA14, SOFA score on day 14; max SOFA, maximum SOFA score.
Discussion
In the present study, we found that urinary tract infection, mechanical ventilation, and osmotic therapy were risk factors for early death in patients with severe acute ischemic stroke. Moreover, the SOFA score had a good predictive value for the prognosis of these patients. Therefore, our findings should be useful in diagnosis and treatment of severe AIS.
We found that early death in patients with severe AIS was clearly associated with an increased risk of urinary tract infection. The mortality rate of urosepsis can be as high as 50%, mainly due to urinary tract infection.19 Patients with AIS are more likely to develop urinary tract infections. Indwelling catheterization is an effective method to solve urination disorders in patients with AIS. However, a biofilm is formed on the surface of the urinary catheter within 24 hours of indwelling catheterization.20,21 Formation of a biofilm subsequently facilitates bacterial adhesion and colonization, leading to the occurrence of urinary tract infections.22 More microbes are present in the urinary system of patients with AIS compared with patients without AIS.23 Furthermore, multiple studies have shown that patients with AIS have a low immunity.24,25 Reduced immunity may cause invasion of bacteria associated with the occurrence of urinary tract infection.
Our study showed that mechanical ventilation and osmotic therapy were risk factors associated with early death in patients with severe AIS. Mechanical ventilation is one of the key interventions for treatment of critically ill patients, but it has an adverse effect on the lungs and distant organs, including the kidneys, leading to further organ damage.26–28 Our findings of mechanical ventilation are consistent with previous reports in which mechanical ventilation was found to be an independent risk factor for acute kidney injury and for death in patients with cystic fibrosis.29,30 Similarly, previous studies have shown that osmotic therapy has certain side effects on the liver.31,32 These data may provide a basis for our observation that osmotic therapy was a risk factor for early death.
This study showed that the SOFA score had a predictive role in the prognosis of patients with severe AIS. The AUCs for SOFA scores on days 4, 7, 10, and 14 after hospitalization, and those for the maximum SOFA and ΔSOFA scores exceeded 0.7. The SOFA score was designed to develop an objective tool to quantify single and multiple organ failure of patients.17 The function of this model in critically ill patients has been validated by cohort studies.33,34 The SOFA score has a variety of characteristics that make it applicable in the emergency department and it can be easily performed and calculated at the patients’ bedside. The components of this scale involve clinical and laboratory parameters that are usually available and measured in the emergency department.35 To date, few studies have attempted to assess the accuracy of this model for predicting prognosis in patients with severe AIS.
In an effort to evaluate the accuracy of the SOFA score in patients with sepsis, Jones et al.36 found that the AUC of the SOFA scoring system in predicting the in-hospital mortality rate was 0.75 at admission and 0.84 on day 3. This finding indicated that this scoring system could provide the medical team with valuable prognostic information about in-hospital mortality of patients with sepsis. Tee et al.37 investigated the value of the SOFA score for predicting mortality in the intensive care unit and found that there was a significant correlation between the SOFA score at different time points and the mortality rate. Further analysis showed that SOFA was an appropriate tool for predicting mortality for patients with acute severe pancreatitis. Additionally, the SOFA score, along with a history of chemotherapy and change in mental status, were identified as predictors of 14-day mortality in patients with cancer.38 These results are consistent with our findings in determining the predictive value of the SOFA score for prognosis of patients with severe AIS. While the AUCs of the SOFA scoring system reported in various studies ranged from 0.70 to 0.87,39–41 we showed similar values between 0.722 to 0.866 in our study.
Several limitations of the present study should be considered when interpreting the results. First, this was a single-center study, and there was an inevitable problem of selective bias. Second, the sample size was small, and this limitation may have caused bias in statistics. Finally, all patients included in this study had severe AIS with disturbance of consciousness, thus limiting the applicability of the results.
In conclusion, urinary tract infection, mechanical ventilation, and osmotic therapy are risk factors for early death of patients with severe AIS. Furthermore, the SOFA score has a good predictive value for prognosis of those patients. Therefore, to improve the outcome of patients with severe AIS, physicians need to perform a urethral examination, as well as avoid mechanical ventilation and osmotic therapy applied to patients. Additionally, the SOFA score allows physicians to accurately determine the patient’s condition and make the best decisions on treatments.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iD
References
- 1.Demarin V. Stroke-a challenge in the diagnosis and therapy. Acta Med Croatica 2001; 55: 145–148. [PubMed] [Google Scholar]
- 2.Khoshnam SE, Winlow W, Farbood Y, et al. Emerging roles of microRNAs in ischemic stroke: as possible therapeutic agents. Stroke 2017; 19: 166–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee SH, Park MS, Choi SM, et al. Clinical features and subtypes of hemorrhagic stroke: analysis of the CNUH stroke registry. Chonnam Med J 2004; 40: 131–135. [Google Scholar]
- 4.Duan Y, Chen F, Lin L, et al. Leukoaraiosis rather than lacunes predict poor outcome and chest infection in acute ischemic stroke patients. Int J Clin Exp Med 2015; 8: 19304–19310. [PMC free article] [PubMed] [Google Scholar]
- 5.Mousavi-Mirzaei SM, Talebi A, Amirabadizadeh A, et al. Increasing the risk of stroke by opium addiction. J Stroke Cerebrovasc Dis 2019; 28: 1930–1935. [DOI] [PubMed] [Google Scholar]
- 6.Mousavi-Mirzaei SM, Khorasani EY, Amirabadizadeh A, et al. Comparison of blood lead concentrations in patients with acute ischemic stroke and healthy subjects. J Trace Elem Med Biol 2020; 61: 126532. [DOI] [PubMed] [Google Scholar]
- 7.Vincent JL, Moreno R, Takala J, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med 1996; 22: 707–710. [DOI] [PubMed] [Google Scholar]
- 8.Ferreira FL, Bota DP, Bross A, et al. Serial evaluation of the SOFA score to predict outcome in critically ill patients. JAMA 2001; 286: 1754–1758. [DOI] [PubMed] [Google Scholar]
- 9.Bota DP, Melot C, Ferreira FL, et al. The Multiple Organ Dysfunction Score (MODS) versus the Sequential Organ Failure Assessment (SOFA) score in outcome prediction. Intensive Care Med 2002; 28: 1619–1624. [DOI] [PubMed] [Google Scholar]
- 10.Huang W, Qin S, Sun Y, et al. Establishment of multiple organ dysfunction syndrome early warning score in patients with severe trauma and its clinical significance: A multicenter study. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 2018; 30: 41–46. [DOI] [PubMed] [Google Scholar]
- 11.Jawa R, Vosswinkel J, McCormack J, et al. 1509: ED QSOFA score: a simple predictor for adverse outcomes following admission for blunt trauma. Crit Care Med 2016; 44: 453. [Google Scholar]
- 12.Farzaneh E, Ghobadi H, Akbarifard M, et al. Prognostic factors in Acute aluminium phosphide poisoning: a risk-prediction nomogram approach. Basic Clin Pharmacol Toxicol 2018; 123: 347–355. [DOI] [PubMed] [Google Scholar]
- 13.Augustinho FC, Zocche TL, Borgonovo A, et al. Applicability of Sepsis-3 criteria and quick Sequential Organ Failure Assessment in patients with cirrhosis hospitalised for bacterial infections. Liver Int 2019; 39: 307–315. [DOI] [PubMed] [Google Scholar]
- 14.Kidwell CS, Warach S. Acute ischemic cerebrovascular syndrome: diagnostic criteria. Stroke 2003; 34: 2995–2998. [DOI] [PubMed] [Google Scholar]
- 15.Adams HP, Jr, Woolson RF, Clarke WR, et al. Design of the Trial of Org 10172 in Acute Stroke Treatment (TOAST). Control Clin Trials 1997; 18: 358–377. [DOI] [PubMed] [Google Scholar]
- 16.Burn J, Warlow C. Aspirin after stroke: a CT scan first?: the Oxfordshire Community Stroke Project (OCSP). Age Ageing 1990; 19: P25-c–P25. [Google Scholar]
- 17.Nair R, Bhandary NM, D'Souza AD, et al. Initial Sequential Organ Failure Assessment score versus Simplified Acute Physiology score to analyze multiple organ dysfunction in infectious diseases in intensive care unit. Indian J Crit Care Med 2016; 20: 210–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jain S, Guleria K, Suneja A, et al. Use of the Sequential Organ Failure Assessment score for evaluating outcome among obstetric patients admitted to the intensive care unit. Int J Gynaecol Obstet 2016; 132: 332–336. [DOI] [PubMed] [Google Scholar]
- 19.Scotland KB, Lo J, Grgic T, et al. Ureteral stent-associated infection and sepsis: pathogenesis and prevention: a review. Biofouling 2019; 35: 117–127. [DOI] [PubMed] [Google Scholar]
- 20.Gupta SS, Irukulla PK, Shenoy MA, et al. Successful strategy to decrease indwelling catheter utilization rates in an academic medical intensive care unit. Am J Infect Control 2017; 45: 1349–1355. [DOI] [PubMed] [Google Scholar]
- 21.Kostoula A, Dimitrios L, Phyllis C, et al. Comparison of Oligon catheters and chlorhexidine-impregnated sponges with standard multilumen central venous catheters for prevention of associated colonization and infections in intensive care unit patients: a multicenter, randomized, controlled study. Crit Care Med 2012; 40: 420–429. [DOI] [PubMed] [Google Scholar]
- 22.Sabir N, Ikram A, Zaman G, et al. Bacterial biofilm-based catheter-associated urinary tract infections: causative pathogens and antibiotic resistance. Am J Infect Control 2017; 45: 1101–1105. [DOI] [PubMed] [Google Scholar]
- 23.Ionita CC, Siddiqui AH, Levy EI, et al. Acute ischemic stroke and infections. J Stroke Cerebrovasc Dis 2011; 20: 1–9. [DOI] [PubMed] [Google Scholar]
- 24.Burtsev EM, Grinshteĭn VB, Nazarov SB. Changes of homeostasis and immunity in the acute period of ischemic stroke. Zh Nevrol Psikhiatr Im S S Korsakova 2001: 41–44. [PubMed] [Google Scholar]
- 25.Zhang F, Yan C, Wei C, et al. Vinpocetine inhibits NF-κB-dependent inflammation in acute ischemic stroke patients. Transl Stroke Res 2017; 9: 1–11. [DOI] [PubMed] [Google Scholar]
- 26.Sequeira TCA, BaHammam AS, Esquinas AM. Noninvasive ventilation in the critically ill patients with obesity hypoventilation syndrome: a review. J Intensive Care Med 2016; 32: 421–428. [DOI] [PubMed] [Google Scholar]
- 27.Duke GJ. Cardiovascular effects of mechanical ventilation. Crit Care Resusc 1999; 1: 388–399. [PubMed] [Google Scholar]
- 28.Chacon-Cabrera A, Rojas Y, Martínez-Caro L, et al. Influence of mechanical ventilation and sepsis on redox balance in diaphragm, myocardium, limb muscles, and lungs. Transl Res 2014; 164: 477–495. [DOI] [PubMed] [Google Scholar]
- 29.Van Den Akker JPC, Egal M, Groeneveld ABJ. Invasive mechanical ventilation as a risk factor for acute kidney injury in the critically ill: a systematic review and meta-analysis. Crit Care 2013; 17: R98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Efrati O, Bylin I, Segal E, et al. Invasive mechanical ventilation: risk factor for death in patients with cystic fibrosis admitted to intensive care unit – outcome and prolonged follow up. Pulmonology 2008; 7: S68. [DOI] [PubMed] [Google Scholar]
- 31.Diringer MN, Zazulia AR. Osmotic therapy: fact and fiction. Neurocrit Care 2004; 1: 219–233. [DOI] [PubMed] [Google Scholar]
- 32.Richling B. [Current status of treatment of the cerebral edema]. Anaesthesist 1987; 36: 191–196. [PubMed] [Google Scholar]
- 33.Qiao Q, Lu G, Li M, et al. Prediction of outcome in critically ill elderly patients using APACHE II and SOFA scores. J Int Med Res 2012; 40: 1114–1121. [DOI] [PubMed] [Google Scholar]
- 34.Bodin K. Serial evaluation of the MODS, SOFA and LOD scores to predict ICU mortality in mixed critically ill patients. J Med Assoc Thai 2008; 91: 1336–1342. [PubMed] [Google Scholar]
- 35.Macdonald SPJ, Arendts G, Fatovich DM, et al. Comparison of PIRO, SOFA, and MEDS Scores for predicting mortality in emergency department patients with severe sepsis and septic shock. Acad Emerg Med 2014; 21: 1257–1263. [DOI] [PubMed] [Google Scholar]
- 36.Jones AE, Trzeciak S, Kline JA. The Sequential Organ Failure Assessment score for predicting outcome in patients with severe sepsis and evidence of hypoperfusion at the time of emergency department presentation. Crit Care Med 2009; 37: 1649–1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tee Y, Fang H, Kuo I, et al. Serial evaluation of the SOFA score is reliable for predicting mortality in acute severe pancreatitis. Medicine (Baltimore) 2018; 97: e9654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee JS, Kwon OY, Choi HS, et al. Application of the Sequential Organ Failure Assessment (SOFA) score in patients with advanced cancer who present to the ED. Am J Emerg Med 2012; 30: 362–366. [DOI] [PubMed] [Google Scholar]
- 39.Li M, Xing X, Lu Z, et al. Comparison of scoring systems in predicting severity and prognosis of hypertriglyceridemia-induced acute pancreatitis. Dig Dis Sci 2020; 65: 1206–1211. [DOI] [PubMed] [Google Scholar]
- 40.Zhou H, Guo S, Lan T, et al. Risk stratification and prediction value of procalcitonin and clinical severity scores for community-acquired pneumonia in ED. Am J Emerg Med 2018; 36: 2155–2160. [DOI] [PubMed] [Google Scholar]
- 41.Rahmatinejad Z, Reihani H, Tohidinezhad F, et al. Predictive performance of the SOFA and mSOFA scoring systems for predicting in-hospital mortality in the emergency department. Am J Emerg Med 2019; 37: 1237–1241. [DOI] [PubMed] [Google Scholar]