Abstract
Background
The relationship between cholesterol levels and risk of venous thromboembolism (VTE) is uncertain. We set out to determine the effect of PCSK9 inhibition on the risk of VTE, explore potential mechanisms, and examine the efficacy in clinically and genetically defined risk subgroups.
Methods
We performed a post-hoc analysis of the FOURIER trial testing whether evolocumab reduces the risk of VTE events (deep venous thrombosis or pulmonary embolism). Data from FOURIER and ODYSSEY OUTCOMES were then combined in a meta-analysis to assess class effect of PCSK9 inhibition on the risk of VTE. We also analyzed baseline lipids in FOURIER to investigate potential mechanisms explaining the reduction in VTE with evolocumab. Finally, an exploratory genetic analysis was performed in FOURIER to determine whether a VTE polygenic risk score could identify high-risk patients who would derive the greatest VTE reduction from evolocumab.
Results
In FOURIER, the HR for VTE with evolocumab was 0.71 (95%CI 0.50–1.00, p=0.05), with no effect in the 1st year (HR 0.96, [0.57–1.62]) but a 46% reduction (HR 0.54 [0.33–0.88], p=0.014) beyond 1 year. A meta-analysis of FOURIER and ODYSSEY OUTCOMES demonstrated a 31% relative risk reduction in VTE with PCSK9 inhibition (HR 0.69 [0.53–0.90], p=0.007). There was no relation between baseline LDL-C levels and magnitude of VTE risk reduction. In contrast, in patients with higher baseline Lp(a) levels, evolocumab reduced Lp(a) by 33 nmol/L and risk of VTE by 48% (HR 0.52 [0.30–0.89], p=0.017), whereas in patients with lower baseline Lp(a) levels evolocumab reduced Lp(a) by only 7 nmol/L and had no effect on VTE risk (Pinteraction for HR 0.087, Pheterogeneity for ARR 0.037). Modeled as a continuous variable, there was a significant interaction between baseline Lp(a) concentration and magnitude of VTE risk reduction (P=0.04). A polygenic risk score identified patients who were at >2-fold increased risk for VTE and who derived greater relative (Pinteraction=0.04) and absolute VTE reduction (Pheterogeneity=0.009) compared to those without high genetic risk.
Conclusion
PCSK9 inhibition significantly reduces the risk of VTE. Lp(a) reduction may be an important mediator of this effect, a finding of particular interest given ongoing development of potent Lp(a) inhibitors.
Background
The relationship between cholesterol levels and the risk of venous thromboembolism (VTE) is uncertain. Observational studies have been mixed, with some finding an association between LDL-C levels and increased risk of VTE,1–6 while others failed to show a relationship.7–10 Recent genetic studies, however, have suggested a potential link. A Mendelian Randomization study, taking advantage of nature’s random allocation of genetic variants that predispose individuals to higher and lower cholesterol levels, found that individuals with a genetic predisposition to elevated LDL-C had a significantly increased risk of developing VTE.11 The JUPITER randomized clinical trial found a seemingly consistent result that high-intensity statin therapy in patients without hypercholesterolemia but with elevated hs-CRP levels reduced the incidence of VTE by 43%.12 However, the authors attributed these findings to statins’ pleiotropic effects, including potential antithrombotic or anti-inflammatory mechanisms, rather than LDL-C lowering. Other investigations have focused on the atherogenic and prothrombotic particle, lipoprotein(a) (Lp(a)), with some, but not all, studies suggesting it may be a potential mediator for VTE risk.13–19
The emergence of proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors allow an opportunity to directly test the benefit of a class of drugs that reduces both LDL-C and Lp(a) on the risk of VTE without other known non-lipid anti-inflammatory or antithrombotic effects.20, 21 In this study, we set out to determine if PCSK9 inhibitors reduce the risk of VTE, to explore the potential mechanism, and to examine the efficacy in clinically and genetically defined risk subgroups.
Methods
Study Design
We performed a post-hoc analysis of the FOURIER trial (Further Cardiovascular Outcomes Research With PCSK9 Inhibition in Subjects With Elevated Risk)20 testing whether evolocumab reduces VTE events compared to placebo. Summary level data from FOURIER and ODYSSEY OUTCOMES22 (which tested alirocumab) were then combined in a meta-analysis to assess the aggregate data for the effect of PCSK9 inhibition on the risk of VTE. This was followed by stratified baseline lipid analyses in the FOURIER trial to gain insights into whether the reduction in LDL-C or Lp(a) may be associated with the reduction in VTE observed with evolocumab. Finally, we performed an exploratory genetic analysis in FOURIER to determine whether a polygenic risk score for VTE could identify high-risk patients who would derive the greatest VTE risk reduction from evolocumab therapy.11
Study Populations
All patients randomized in the FOURIER trial, a trial of 27,564 patients with stable atherosclerosis and hyperlipidemia on statin therapy, were included in the primary and subgroup analyses of the effect of evolocumab on VTE risk. In the meta-analysis of FOURIER and ODYSSEY OUTCOMES (a trial of 18,924 patients 1–12 month post-acute coronary syndrome), the study population included all patients randomized in these two trials. In analyses examining the association between baseline lipid levels and the risk of VTE, analyses were restricted to the placebo arm of the FOURIER trial. In the genetic analysis, a subset of FOURIER patients who consented to genetic testing, passed genetic quality control, and were of European ancestry were included. Approval for this study was obtained by the local institutional review committee. Although data and study material will not be made universally available, we encourage parties interested in collaboration to contact the corresponding author directly.
Clinical Endpoints
The primary endpoint of interest was VTE, defined as either deep venous thrombosis and/or pulmonary embolism. VTE events during the trial were reported by the site study physician as per standard adverse event reporting for clinical trials. Physicians blinded to treatment assignment systematically reviewed the investigator reported adverse event database using preferred terms, lower level terms, and verbatim terms to identify and confirm VTE events.
Genotyping and Imputation
Our methods for genotyping and imputation in the FOURIER trial have previously been published.23 Briefly, genotyping was performed on the Infinium Global Array chip. Pre-imputation quality control was performed using PLINK v2.0.24 Imputation was accomplished using the Michigan Imputation server25 and TOPMed Freeze5 reference panel.26 Post-imputation quality control was performed, including assessment of cryptic relatedness. The 1000 Genomes phase 3 v5 reference panel27 and ADMIXTURE tool28 were used to identify individuals of European ancestry.
Genetic Risk Score
The polygenic risk score applied to this analysis was comprised of an initial list of 297 single nucleotide polymorphisms (SNPs) as reported by Klarin et al, which represent the most up to date results of single variants associated with venous thromboembolism.11 Of these, 273 SNPs were available for inclusion in the polygenic risk score following genotyping and imputation. The score was calculated using the genotype dosage for each allele, multiplied by its weight, and then summed across all variants. Those in the top one third of the genetic risk score were categorized as high genetic risk for VTE.
Statistical Analysis
To assess whether evolocumab significantly reduced VTE, cumulative event curves and hazard ratio between treatment and placebo arms were compared using the Kaplan-Meier method and Cox proportional hazards model, respectively. As was done in the parent trial, a landmark analysis at 1 year was performed as the full clinical benefit of lipid lowering therapy does not generally manifest until at least 1 year.20, 29 The meta-analysis was run using fixed effects given that both trials tested PCSK9 inhibition in similar populations, with similar reported effects of treatment on LDL-C.
A series of subgroup analyses based on baseline lipid analyses were performed. First, we tested whether tertiles of baseline LDL-C and Lp(a) were associated with VTE risk in the placebo arm. Then, we assessed treatment benefit of evolocumab stratified by baseline LDL-C and Lp(a) levels (dichotomized at the median) to create subgroups that differed in the absolute magnitude of LDL-C or Lp(a) lowering on the basis of baseline (pre-randomization) lipid values. Interaction testing for the magnitude of relative risk reductions and heterogeneity testing for the magnitude of absolute risk reductions in these subgroups was performed. To further explore the interaction between Lp(a) levels and efficacy of evolocumab two additional analyses were performed. First, patients were divided into quartiles of baseline Lp(a) levels and a meta-regression was performed to assess the reduction in VTE with evolocumab per unit absolute decrease in Lp(a). Second, a Cox proportional hazards model for VTE was created that contained terms for evolocumab, baseline Lp(a), and the interaction between the two. All p-values were two-sided with an alpha of 0.05.
For the genetic analysis, patients with high genetic risk for VTE (highest tertile) were compared to the remainder of the genetic cohort for both VTE risk prediction and treatment benefit. For risk prediction, Cox proportional hazards regression (assumptions tested and criteria were met) was used to calculate hazard ratios comparing high genetic risk vs those without high genetic risk in the placebo arm. Analyses were adjusted for age, sex, ancestry (principal components 1–4) and established clinical risk factors including obesity (BMI ≥30), smoking, heart failure, and diabetes. To assess treatment benefit, relative and absolute risk reductions were calculated across genetic risk groups, followed by gene x treatment interaction testing.
Results
Baseline Characteristics
Demographics of the FOURIER trial population are shown in Table 1. The average age was 63 years, 75% were male, 39% were obese, 28% were smokers, 23% had heart failure, and 37% had diabetes. The median LDL-C was 92 mg/dL and the median Lp(a) was 37 nmol/L. The median follow-up time was 2.2 years.
Table 1:
Baseline Characteristics in FOURIER Trial
| Evolocumab N=13,784 |
Placebo N=13,780 |
|
|---|---|---|
| Demographics | ||
| Age, yr (±SD) | 62.5 (9.1) | 62.5 (8.9) |
| Male | 10,397 (75) | 10,398 (76) |
| White | 11,748 (85) | 11,710 (85) |
| Obese (BMI >30) | 5,419 (39) | 5,523 (40) |
| Medical History | ||
| Myocardial infarction | 11,145 (81) | 11,206 (81) |
| Ischemic stroke | 2,686 (20) | 2,651 (19) |
| Peripheral artery disease | 1,858 (14) | 1,784 (13) |
| Heart Failure | 3224 (23) | 3170 (23) |
| Hypertension | 11,045 (80) | 11,039 (80) |
| Diabetes mellitus | 5,054 (37) | 5,027 (37) |
| Current cigarette use | 3,854 (28) | 3,923 (29) |
| Hormone Replacement Therapy | 518 (3.8) | 520 (3.8) |
| Median Lab Measures (IQR) | ||
| LDL cholesterol — mg/dl | 92 (80–109) | 92 (80–109) |
| Total cholesterol — mg/dl | 168 (151–188) | 168 (151–189) |
| HDL cholesterol — mg/dl | 44 (37–53) | 44 (37–53) |
| Triglycerides — mg/dl | 134 (101–183) | 133 (99–181) |
| Lipoprotein (a) — nmol/L | 37 (13–166) | 37 (13–164) |
Data shown are n(%) unless otherwise indicated.
BMI, body mass index; HDL, high density lipoprotein; LDL, low density lipoprotein; IQR, interquartile range; SD, standard deviation; Yr, years.
Effect of PCSK9 Inhibition on Risk of VTE
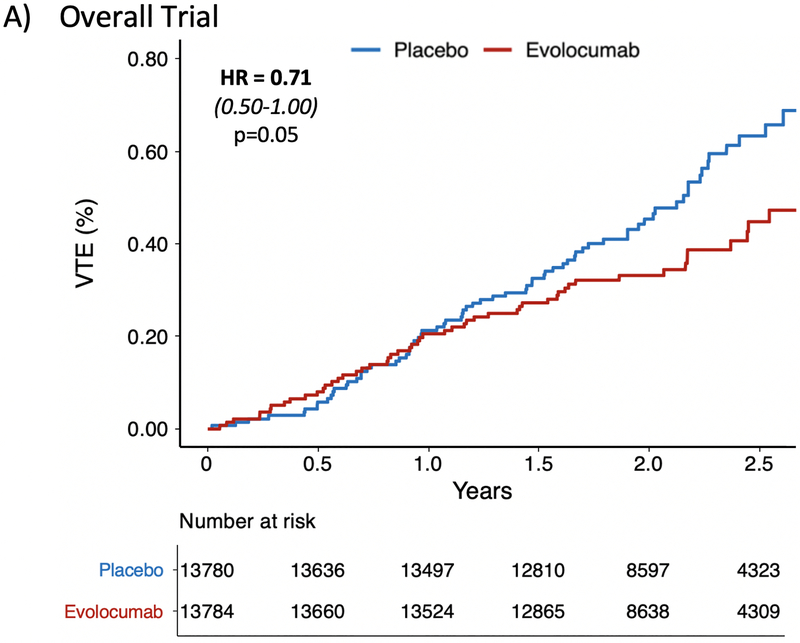
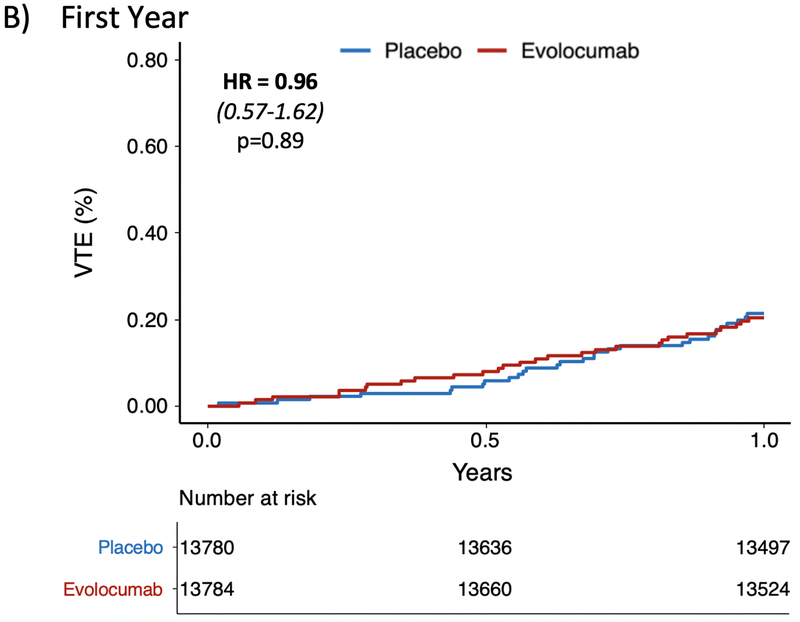
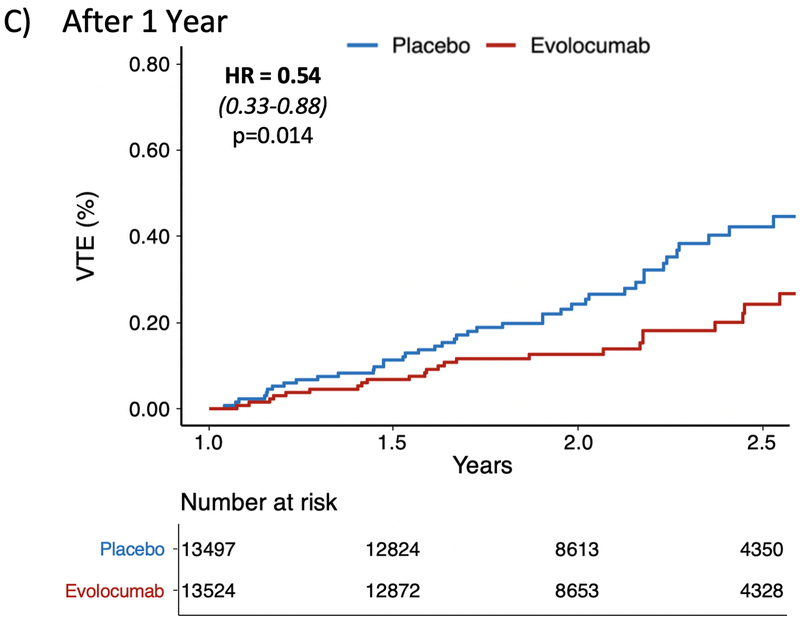
In the FOURIER trial, a total of 128 patients had a VTE event (72 DVT and 56 PE). The event rate in the placebo arm was 0.63% compared to 0.45% in the treatment arm. The HR for VTE with evolocumab was 0.71 (95% CI 0.50–1.00, p=0.05) (Figure 1A). Sensitivity analyses removing patients on baseline anticoagulation if anything strengthened this finding (HR 0.67 [95% CI 0.46,0.97], p=0.035), and total event analyses including the 12 recurrent VTE events were consistent (Supplemental Table 1). There was a late divergence of the curves after one year which was similar to the pattern observed with key primary and key secondary outcomes in the FOURIER trial. Specifically, there was no effect of evolocumab on VTE in the first year (HR 0.96 [95% CI 0.57,1.62], p=0.89) (Figure 1B) whereas there was a 46% reduction (HR 0.54 [95% CI 0.33,0.88], p=0.014) in a landmark analysis starting at 12 months (Figure 1C). Only 7 VTE events were preceded by an ASCVD event during the trial, and all were separated by at least 50 days. A sensitivity analysis removing these 7 cases remained consistent with the primary analysis (Supplemental Table 1).
Figure 1: Effect of Evolocumab on the Reduction in Venous Thromboembolism.
Panel (A) demonstrates a 29% reduction in the overall FOURIER trial (HR 0.71 [0.50–1.00], p=0.05). Panel (B) shows no difference in VTE during the first year (HR 0.96 [0.57–1.62], p=0.89). Panel (C) is a landmark analysis highlighting the 46% reduction in VTE beyond 1 year (HR 0.54 [0.33–0.88], p=0.014).
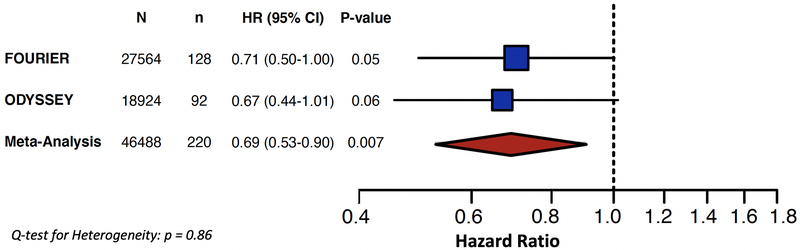
Next, we combined data from FOURIER and ODYSSEY OUTCOMES. In ODYSSEY OUTCOMES, the average age was 59 years, 75% were male, 24% were smokers, 15% had heart failure, and 29% had diabetes.21 There were 92 VTE events in ODYSSEY OUTCOMES, and there was a trend towards alirocumab reducing the risk of VTE (HR 0.67 [0.44–1.01], p=0.06).22 A meta-analysis of the two trials demonstrated a statistically significant 31% relative risk reduction in VTE with PCSK9 inhibition compared with placebo (HR 0.69 [0.53–0.90], p=0.007) (Figure 2).
Figure 2: Meta-analysis for the Effect of PSCK9 Inhibitors on Venous Thromboembolism.
A total of 46,488 patients with 220 VTE events were included from the FOURIER and ODYSSEY OUTCOMES trials. Both trials demonstrated a trend towards reduction in VTE and when combined in a meta-analysis there was a significant 31% relative risk reduction in VTE with PCSK9 inhibition (HR 0.69 [0.53–0.90], p=0.007).
Exploring Potential Lipid-related Mechanisms for VTE Reduction
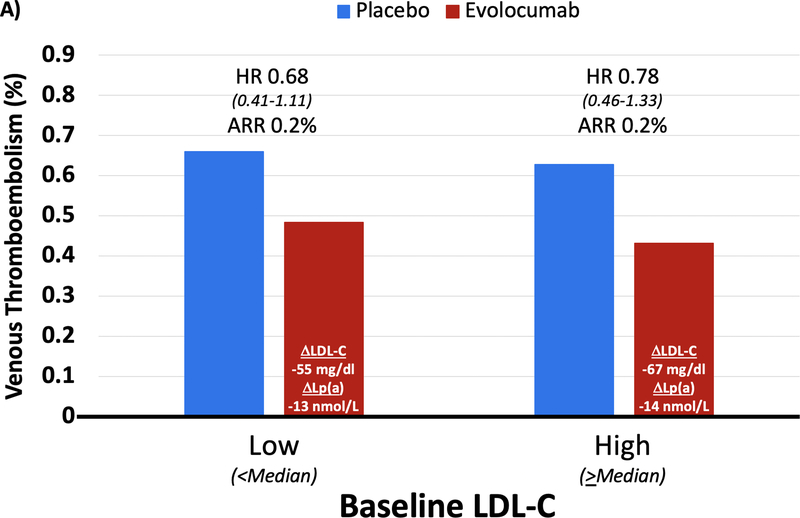
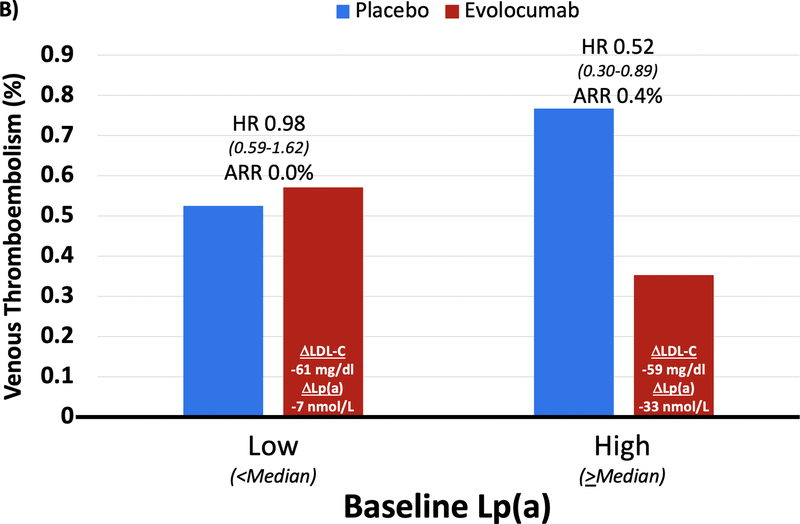
In the placebo arm of FOURIER, there was no trend for increased risk of VTE by baseline LDL-C; however, there appeared to be a pattern of increasing risk of VTE with higher levels of baseline Lp(a) (Supplemental Figure 1). When comparing efficacy of evolocumab in patients with baseline LDL-C above and below the median, there was a consistent reduction in VTE with evolocumab despite differences in the magnitude of LDL-C reduction (67 vs. 55 mg/dL; Figure 3). However, when comparing efficacy in patients stratified by baseline Lp(a) above and below the median, in patients with higher Lp(a) levels evolocumab reduced Lp(a) by 33 nmol/L and VTE risk by 48% (HR 0.52 [0.30–0.89], p=0.017), whereas in those below the median there was only a 7 nmol/L reduction in Lp(a) and no reduction in VTE risk (despite a similarly large reduction in LDL-C, 59 mg/dl vs 61 mg/dl, respectively, in the 2 groups) (Pinteraction for HR 0.087; Pheterogeneity for absolute risk reduction 0.037). When further dividing baseline Lp(a) into quartiles, LDL-C reduction remained stable across these groups (58–61 mg/dl), but as expected, Lp(a) reduction ranged from 2 to 34 nmol/L. A greater reduction in Lp(a) tended to be correlated with greater reduction in risk of VTE events (p=0.098) (Supplemental Figure 2). Moreover, when modeling a formal interaction term between the benefit of evolocumab and baseline Lp(a) level as a continuous variable, the term was significant (P=0.04).
Figure 3: Benefit of Evolocumab for Reducing VTE by Baseline LDL-C and Lp(a) Category.
(A) In High (≥ median) vs. Low (< median) baseline LDL-C groups, the reduction in LDL-C was 67 and 55 mg/dl, respectively, and the reduction in Lp(a) was 14 and 13 nmol/L. VTE reduction was similar in both groups. (B) In High (≥ median) vs. Low (< median) baseline Lp(a) groups, the reduction in Lp(a) was 33 and 7 nmol/L, respectively, and the reduction in LDL-C was 59 and 61 mg/dl. Patients with High baseline Lp(a) had greater VTE reduction (HR 0.52 [0.30–0.89], p=0.017) compared to those with Low baseline Lp(a) (Pinteraction for HR 0.087; Pheterogeneity for absolute risk reduction 0.037).
Polygenic Risk and the Benefit of Treatment with Evolocumab
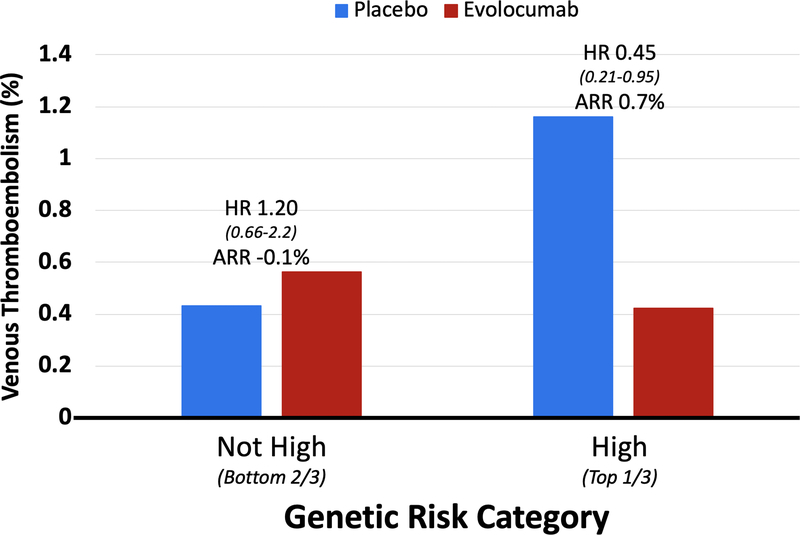
The genetic sub study included 14,298 patients from FOURIER (who were similar to the overall trial cohort; Supplemental Table 2) with 76 VTE events. Each increase by 1 standard deviation in genetic risk score carried a 57% greater risk of VTE (adjusted HR 1.57 [1.23–2.01], p=0.0003). More specifically, patients in the top one third of genetic risk carried a >2-fold increased risk of VTE compared to those in the lower two thirds (adjusted HR 2.31 [1.26–4.25], p=0.007). The predictive value of the genetic risk score remained when Lp(a) was included in the model (Supplemental Table 3). Patients in this high genetic risk group (top one third) also appeared to derive greater VTE reduction from evolocumab (Figure 4). They had a relative risk reduction of 55% (HR 0.45 [95% CI 0.21,0.95], p=0.035) compared with no apparent benefit in those without high genetic risk (HR 1.20 [95% CI 0.66,2.17], Pinteraction = 0.04); and an absolute risk reduction of 0.7% (95% CI 0.2,1.3) compared to −0.1% (95% CI −0.4,0.2) (Pheterogeneity= 0.009).
Figure 4: Benefit of Evolocumab for Reducing VTE by Genetic Risk Category.
Patients in the top one third of genetic risk had a 55% relative risk reduction (HR 0.45 [0.21–0.95], p=0.035) and 0.7% absolute risk reduction (95% CI 0.2,1.3). The VTE reduction was significantly greater in patients with high genetic risk compared to not-high genetic risk (Pinteraction= 0.04; Pheterogeneity= 0.009).
Discussion
This is the first study to demonstrate a significant reduction in VTE with PCSK9 inhibitors, evidenced by a consistent benefit across two PCSK9 inhibitor trials. These data also suggest that the reduction in VTE with PCSK9 inhibition may be mediated by a reduction in Lp(a) rather than LDL-C. In addition, the use of a polygenic risk score identified one-third of the population who was not only at >2-fold risk for VTE, but also appeared to derive the greatest relative and absolute reduction in VTE from evolocumab.
Our findings are novel in regard to PCSK9 inhibition in humans, but supported by a reduction in VTE in PSCK9 knockout animal models.30 As noted above, data from the JUPITER trial demonstrate that high-intensity statin therapy in patients without hypercholesterolemia but with inflammation reduced the risk of VTE.12 Moreover, a meta-analysis of multiple statin trials (which did not require inflammation at baseline) suggested a more modest effect of statins on VTE risk.31 These clinical trial data offer complementary insights into potential pathobiology and mechanism of benefit. High-intensity statin therapy reduces LDL-C and hs-CRP, but not Lp(a).32 PCSK9 inhibitors reduce LDL-C and Lp(a), but not hs-CRP.20, 21 Although both reduce LDL-C and there are some data for an association between LDL-C and VTE1–4, 11, the data are inconsistent7–10 and we did not see any relation in FOURIER between the magnitude of LDL-C lowering and the magnitude of VTE risk reduction. It may be that statins reduce the risk of VTE by reducing inflammation and PCSK9 inhibitors reduce the risk by reducing levels of Lp(a).
Along with its known atherogenicity, Lp(a) is thought to have prothrombotic properties, with multiple epidemiologic studies supporting its association with VTE13–17. These data have been somewhat tempered by genetic studies that have not supported a causal association.18, 19 However, our data suggests a trend of increased risk of VTE with higher levels of Lp(a), and we found that greater reductions in Lp(a) correlated with greater decreases in risk of VTE events. These observations are especially intriguing as both RNA interference (RNAi) and antisense oligonucleotide (ASO) therapies against Lp(a) progress to phase 3 clinical trials, and suggest that assessment of VTE in these trials may be warranted.
Further work is needed to confirm the mechanism for VTE reduction with PCSK9 inhibition. While these data suggest that the benefit may be associated with Lp(a), the potential pathway mediating this effect is uncertain. One possibility is the prothrombotic effect of Lp(a), although late divergence of the cumulative incidence curves resembles the pattern observed in atherosclerosis-mediated mechanisms. If an anti-thrombotic effect is operational, the emergence of this benefit may not follow the time course we expect from antiplatelet or anticoagulant medications with a direct effect on hemostatic/thrombotic factors and for which the effect is more immediate.
Although there was a substantial relative risk reduction in VTE with evolocumab, the absolute risk reduction was modest due to the low event rate. Therefore, given the current cost of the drug, prescribing PCSK9 inhibitors to this population for the prevention of VTE alone is likely not warranted. However, in patients with atherosclerotic cardiovascular disease being prescribed a PCSK9 inhibitor to reduce the risk of major adverse cardiovascular events, an additional benefit will be a reduction in the risk of VTE.
Limitations
VTE was not a prespecified endpoint in the FOURIER trial and the incidence was low; however, the total number of events were greater than any prior lipid-modifying trial reporting on VTE. The addition of ODYSSEY OUTCOMES data increased our power to allow us to definitively demonstrate a significant reduction in VTE with PCSK9 inhibition. Additionally, this study was performed in patients with established atherosclerotic disease participating in clinical trials, so may not be generalizable to all populations. While the primary analysis included patients from all ethnicities, the genetic analysis was limited to individuals of European ancestry in order to be consistent with the studies from which the genetic risk score was derived. The stratification of genetic risk by tertiles was exploratory and optimal thresholds for the VTE polygenic risk score need to be prospectively validated prior to clinical implementation. The observed association between genetic risk of VTE and treatment with PCSK9 will require future independent replication as additional clinical trial data becomes available.
Conclusion
PCSK9 inhibition significantly reduces VTE events. The association between degree of Lp(a) lowering and the magnitude of VTE reduction suggests Lp(a) may be a mediator of this effect, a finding of particular interest given ongoing development of potent Lp(a) inhibitors.
Supplementary Material
What is New?
This is the first study to demonstrate a significant reduction in venous thromboembolism (VTE) with PCSK9 (proprotein convertase subtilisin/kexin type 9) inhibition.
The reduction in VTE was associated with the degree of Lipoprotein(a) lowering, not LDL-C lowering, suggesting Lp(a) may be a mediator of VTE risk.
What are the Clinical Implications?
In patients with atherosclerotic cardiovascular disease being prescribed a PCSK9 inhibitor to reduce the risk of major adverse cardiovascular events, an additional benefit will be a reduction in the risk of VTE.
As both RNA interference (RNAi) and antisense oligonucleotide (ASO) therapies against Lp(a) progress to phase 3 clinical trials, assessment of VTE may be warranted.
Acknowledgements
NAM contributed to study design, literature search, statistical analysis, data interpretation, figures, and drafting of the manuscript. YG, GM MPB, BG and CR contributed to data preparation, study design, and statistical analysis. PSS, TRP, ACK, SAL, PTE, MOD, and RPG contributed to data interpretation and critical review of the manuscript. CTR and MSS contributed to study design, statistical analysis, data interpretation, figures, and critical review of the manuscript. CTR and MSS are the guarantors of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Sources of Funding
Amgen provided a research grant to the Brigham and Women’s Hospital for the conduct of the FOURIER trial. The ODYSSEY OUTCOMES trial was supported by Sanofi. No support was provided for this manuscript.
Disclosures
NAM: Dr. Marston reports no disclosures. YG: Dr. Gurmu is a member of the TIMI Study Group which has received institutional research grant support through Brigham and Women’s from: Abbott, Amgen, Aralez, AstraZeneca, Bayer HealthCare Pharmaceuticals, Inc., BRAHMS, Daiichi-Sankyo, Eisai, GlaxoSmithKline, Intarcia, Janssen, MedImmune, Merck, Novartis, Pfizer, Poxel, Quark Pharmaceuticals, Roche, Takeda, The Medicines Company, Zora Biosciences. GM: Dr. Melloni is a member of the TIMI Study Group which has received institutional research grant support through Brigham and Women’s from: Abbott, Amgen, Aralez, AstraZeneca, Bayer HealthCare Pharmaceuticals, Inc., BRAHMS, Daiichi-Sankyo, Eisai, GlaxoSmithKline, Intarcia, Janssen, MedImmune, Merck, Novartis, Pfizer, Poxel, Quark Pharmaceuticals, Roche, Takeda, The Medicines Company, Zora Biosciences. MPB: Dr. Bonaca discloses grant support from Amgen, AstraZeneca, Bayer, Sanofi and consulting fees from Amgen, AstraZeneca, Bayer, Sanofi. BG: Dr. Gencer reports research grant support from Geneva University Hospitals, Eugenio Litta and Arthemis Foundations. PS: Dr. Sever reports research grants and honoraria for speakers bureau- Amgen and Pfizer. TP: Dr. Pedersen reports speaker honoraria from Sanofi, Amgen, Consulting for Sanofi, Amgen, all modest. ACK: Dr. Keech reports grants and personal fees from Abbott, personal fees from Amgen, personal fees from AstraZeneca, grants and personal fees from Mylan, personal fees from Pfizer, grants from Sanofi, grants from Novartis, personal fees from Bayer, outside the submitted work. CR: Ms. Roselli is supported by a grant from Bayer AG to the Broad Institute focused on the development of therapeutics for cardiovascular disease. SAL: Dr. Lubitz reports grants from NIH, grants from AHA, grants from Boehringer Ingelheim, grants and personal fees from BMS/Pfizer, grants and personal fees from Bayer AG, personal fees from Quest diagnostics, outside the submitted work. PTE: Dr. Ellinor reports grants and personal fees from Bayer AG, personal fees from Novartis, personal fees from Quest Diagnostics, outside the submitted work. MLO: Dr. O’Donoghue reports institutional research grants from Amgen, Janssen, The Medicines Company, Eisai, GlaxoSmithKline, and Astra Zeneca. RPG: Dr. Giugliano reports grants from Amgen and Daiichi Sankyo, during the conduct of the study; personal fees from Akcea, grants and personal fees from Amarin, personal fees from American College of Cardiology, grants and personal fees from Amgen, personal fees personal fees from Bristol Myers Squibb, personal fees from CVS Caremark, grants and personal fees from Daiichi Sankyo, personal fees from GlaxoSmithKline, personal fees from Janssen, personal fees from Lexicon, grants and personal fees from Merck, personal fees from Pfizer, personal fees from Servier, outside the submitted work; and Institutional research grant to the TIMI Study Group at Brigham and Women’s Hospital for research he is not directly involved in from Abbott, Amgen, Aralez, AstraZeneca, Bayer HealthCare Pharmaceuticals, Inc., BRAHMS, Daiichi Sankyo, Eisai, GlaxoSmithKline, Intarcia, Janssen, MedImmune, Merck, Novartis, Pfizer, Poxel, Quark Pharmaceuticals, Roche, Takeda, The Medicines Company, Zora Biosciences. CRT: Dr. Ruff reports grants from Boehringer Ingelheim, grants from Daiichi Sankyo, grants from MedImmune, grants from National Institute of Health, personal fees from Bayer, personal fees from Bristol Myers Squibb, personal fees from Boehringer Ingelheim, personal fees from Daiichi Sankyo, personal fees from Janssen, personal fees from MedImmune, personal fees from Pfizer, personal fees from Portola, personal fees from Anthos, outside the submitted work; Dr. Ruff is a member of the TIMI Study Group, which has received institutional research grant support through Brigham and Women’s Hospital from: Abbott, Amgen, Aralez, AstraZeneca, Bayer HealthCare Pharmaceuticals, Inc., BRAHMS, Daiichi-Sankyo, Eisai, GlaxoSmithKline, Intarcia, Janssen, MedImmune, Merck, Novartis, Pfizer, Poxel, Quark Pharmaceuticals, Roche, Takeda, The Medicines Company, Zora Biosciences. MSS: Dr. Sabatine reports research grant support; Significant Abbott Laboratories, Amgen, AstraZeneca, Bayer, Critical Diagnostics, Daiichi-Sankyo, Eisai, Genzyme, Gilead, GlaxoSmithKline, Intarcia, Janssen Research and Development, Medicines Company, MedImmune, Merck, Novartis, Poxel, Pfizer, Quark pharmaceuticals, Roche Diagnostics, and Takeda and has received consulting fees; Modest from Alnylam, AstraZeneca, Bristol-Myers Squibb, CVS Caremark, Dyrnamix, Esperion, IFM Pharmaceuticals, Intarcia, Ionis, Janssen Research and Development, Medicines Company, MedImmune, Merck, MyoKardia, and Novartis. Consulting fees; Significant from Amgen.
Non-standard Abbreviations and Acronyms
- VTE
venous thromboembolism
- PCSK9
proprotein convertase subtilisin/kexin type 9
- LDL-C
low-density lipoprotein cholesterol
- hsCRP
high-sensitivity C-reactive protein
- Lp(a)
apolipoprotein(a)
- SNPs
single nucleotide polymorphisms
- BMI
body mass index
- DVT
deep vein thrombosis
- PE
pulmonary embolism
- HR
hazard ratio
- ASCVD
atherosclerotic cardiovascular disease
- RNAi
ribonucleic acid interference
- ASO
antisense oligonucleotide
References
- 1.Morelli VM, Lijfering WM, Bos MHA, Rosendaal FR and Cannegieter SC. Lipid levels and risk of venous thrombosis: results from the MEGA-study. Eur J Epidemiol. 2017;32:669–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Delluc A, Malecot JM, Kerspern H, Nowak E, Carre JL, Mottier D, Le Gal G and Lacut K. Lipid parameters, lipid lowering drugs and the risk of venous thromboembolism. Atherosclerosis. 2012;220:184–188. [DOI] [PubMed] [Google Scholar]
- 3.Mi Y, Yan S, Lu Y, Liang Y and Li C. Venous thromboembolism has the same risk factors as atherosclerosis: A PRISMA-compliant systemic review and meta-analysis. Medicine (Baltimore). 2016;95:e4495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zaccardi F, Kunutsor SK, Seidu S, Davies MJ and Khunti K. Is the lower risk of venous thromboembolism with statins related to low-density-lipoprotein reduction? A network meta-analysis and meta-regression of randomised controlled trials. Atherosclerosis. 2018;271:223–231. [DOI] [PubMed] [Google Scholar]
- 5.Kunutsor SK, Seidu S and Khunti K. Statins and primary prevention of venous thromboembolism: a systematic review and meta-analysis. Lancet Haematol. 2017;4:e83–e93. [DOI] [PubMed] [Google Scholar]
- 6.Kunutsor SK, Seidu S and Khunti K. Statins and secondary prevention of venous thromboembolism: pooled analysis of published observational cohort studies. Eur Heart J. 2017;38:1608–1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tsai AW, Cushman M, Rosamond WD, Heckbert SR, Polak JF and Folsom AR. Cardiovascular risk factors and venous thromboembolism incidence: the longitudinal investigation of thromboembolism etiology. Arch Intern Med. 2002;162:1182–1189. [DOI] [PubMed] [Google Scholar]
- 8.Everett BM, Glynn RJ, Buring JE and Ridker PM. Lipid biomarkers, hormone therapy and the risk of venous thromboembolism in women. J Thromb Haemost. 2009;7:588–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holst AG, Jensen G and Prescott E. Risk factors for venous thromboembolism: results from the Copenhagen City Heart Study. Circulation. 2010;121:1896–1903. [DOI] [PubMed] [Google Scholar]
- 10.van Schouwenburg IM, Mahmoodi BK, Gansevoort RT, Muntinghe FL, Dullaart RP, Kluin-Nelemans HC, Veeger NJ and Meijer K. Lipid levels do not influence the risk of venous thromboembolism. Results of a population-based cohort study. Thromb Haemost. 2012;108:923–929. [DOI] [PubMed] [Google Scholar]
- 11.Klarin D, Busenkell E, Judy R, Lynch J, Levin M, Haessler J, Aragam K, Chaffin M, Haas M, Lindstrom S, et al. Genome-wide association analysis of venous thromboembolism identifies new risk loci and genetic overlap with arterial vascular disease. Nat Genet. 2019;51:1574–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Glynn RJ, Danielson E, Fonseca FA, Genest J, Gotto AM Jr., Kastelein JJ, Koenig W, Libby P, Lorenzatti AJ, MacFadyen JG, et al. A randomized trial of rosuvastatin in the prevention of venous thromboembolism. N Engl J Med. 2009;360:1851–1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nowak-Gottl U, Junker R, Hartmeier M, Koch HG, Munchow N, Assmann G and von Eckardstein A. Increased lipoprotein(a) is an important risk factor for venous thromboembolism in childhood. Circulation. 1999;100:743–748. [DOI] [PubMed] [Google Scholar]
- 14.Marcucci R, Liotta AA, Cellai AP, Rogolino A, Gori AM, Giusti B, Poli D, Fedi S, Abbate R and Prisco D. Increased plasma levels of lipoprotein(a) and the risk of idiopathic and recurrent venous thromboembolism. Am J Med. 2003;115:601–605. [DOI] [PubMed] [Google Scholar]
- 15.Sofi F, Marcucci R, Abbate R, Gensini GF and Prisco D. Lipoprotein (a) and venous thromboembolism in adults: a meta-analysis. Am J Med. 2007;120:728–733. [DOI] [PubMed] [Google Scholar]
- 16.von Depka M, Nowak-Gottl U, Eisert R, Dieterich C, Barthels M, Scharrer I, Ganser A and Ehrenforth S. Increased lipoprotein (a) levels as an independent risk factor for venous thromboembolism. Blood. 2000;96:3364–3368. [PubMed] [Google Scholar]
- 17.Dentali F, Gessi V, Marcucci R, Gianni M, Grandi AM and Franchini M. Lipoprotein(a) as a Risk Factor for Venous Thromboembolism: A Systematic Review and Meta-analysis of the Literature. Semin Thromb Hemost. 2017;43:614–620. [DOI] [PubMed] [Google Scholar]
- 18.Kamstrup PR, Tybjaerg-Hansen A and Nordestgaard BG. Genetic evidence that lipoprotein(a) associates with atherosclerotic stenosis rather than venous thrombosis. Arterioscler Thromb Vasc Biol. 2012;32:1732–1741. [DOI] [PubMed] [Google Scholar]
- 19.Helgadottir A, Gretarsdottir S, Thorleifsson G, Holm H, Patel RS, Gudnason T, Jones GT, van Rij AM, Eapen DJ, Baas AF, et al. Apolipoprotein(a) genetic sequence variants associated with systemic atherosclerosis and coronary atherosclerotic burden but not with venous thromboembolism. J Am Coll Cardiol. 2012;60:722–729. [DOI] [PubMed] [Google Scholar]
- 20.Sabatine MS, Giugliano RP, Keech AC, Honarpour N, Wiviott SD, Murphy SA, Kuder JF, Wang H, Liu T, Wasserman SM, et al. Evolocumab and Clinical Outcomes in Patients with Cardiovascular Disease. N Engl J Med. 2017;376:1713–1722. [DOI] [PubMed] [Google Scholar]
- 21.Schwartz GG, Steg PG, Szarek M, Bhatt DL, Bittner VA, Diaz R, Edelberg JM, Goodman SG, Hanotin C, Harrington RA, et al. Alirocumab and Cardiovascular Outcomes after Acute Coronary Syndrome. N Engl J Med. 2018;379:2097–2107. [DOI] [PubMed] [Google Scholar]
- 22.Schwartz GG, Steg PG, Szarek M, Bittner VA, Diaz R, Goodman SG, Hanotin C, Harrington RA, Jukema JW, Letierce A, et al. Abstract 11173: Effect of Alirocumab on Peripheral Artery and Venous Thromboembolic Events, and Relation to Lipoprotein(a) Concentration. Circulation. 2019;140:A11173–A11173. [Google Scholar]
- 23.Marston NA, Kamanu FK, Nordio F, Gurmu Y, Roselli C, Sever PS, Pedersen TR, Keech AC, Wang H, Lira Pineda A, et al. Predicting Benefit From Evolocumab Therapy in Patients With Atherosclerotic Disease Using a Genetic Risk Score: Results From the FOURIER Trial. Circulation. 2020;141:616–623. DOI: 10.1161/CIRCULATIONAHA.119.043805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chang CC, Chow CC, Tellier LC, Vattikuti S, Purcell SM and Lee JJ. Second-generation PLINK: rising to the challenge of larger and richer datasets. Gigascience. 2015;4:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Das S, Forer L, Schonherr S, Sidore C, Locke AE, Kwong A, Vrieze SI, Chew EY, Levy S, McGue M, et al. Next-generation genotype imputation service and methods. Nat Genet. 2016;48:1284–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.NHLBI Trans-Omics for Precision Medicine. University of Washington, Seattle WA: 2019. https://www.nhlbiwgs.org. [Google Scholar]
- 27.Genomes Project C, Auton A, Brooks LD, Durbin RM, Garrison EP, Kang HM, Korbel JO, Marchini JL, McCarthy S, McVean GA, et al. A global reference for human genetic variation. Nature. 2015;526:68–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alexander DH, Novembre J and Lange K. Fast model-based estimation of ancestry in unrelated individuals. Genome Res. 2009;19:1655–1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cholesterol Treatment Trialists C, Mihaylova B, Emberson J, Blackwell L, Keech A, Simes J, Barnes EH, Voysey M, Gray A, Collins R, et al. The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: meta-analysis of individual data from 27 randomised trials. Lancet. 2012;380:581–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang H, Wang Q, Wang J, Guo C, Kleiman K, Meng H, Knight JS and Eitzman DT. Proprotein convertase subtilisin/kexin type 9 (PCSK9) Deficiency is Protective Against Venous Thrombosis in Mice. Sci Rep. 2017;7:14360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rahimi K, Bhala N, Kamphuisen P, Emberson J, Biere-Rafi S, Krane V, Robertson M, Wikstrand J and McMurray J. Effect of statins on venous thromboembolic events: a meta-analysis of published and unpublished evidence from randomised controlled trials. PLoS Med. 2012;9:e1001310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM Jr., Kastelein JJ, Koenig W, Libby P, Lorenzatti AJ, MacFadyen JG, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359:2195–2207. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.