Abstract
Neurofeedback training has been shown to influence behavior in healthy participants as well as to alleviate clinical symptoms in neurological, psychosomatic, and psychiatric patient populations. However, many real‐time fMRI neurofeedback studies report large inter‐individual differences in learning success. The factors that cause this vast variability between participants remain unknown and their identification could enhance treatment success. Thus, here we employed a meta‐analytic approach including data from 24 different neurofeedback studies with a total of 401 participants, including 140 patients, to determine whether levels of activity in target brain regions during pretraining functional localizer or no‐feedback runs (i.e., self‐regulation in the absence of neurofeedback) could predict neurofeedback learning success. We observed a slightly positive correlation between pretraining activity levels during a functional localizer run and neurofeedback learning success, but we were not able to identify common brain‐based success predictors across our diverse cohort of studies. Therefore, advances need to be made in finding robust models and measures of general neurofeedback learning, and in increasing the current study database to allow for investigating further factors that might influence neurofeedback learning.
Keywords: fMRI, functional neuroimaging, learning, meta‐analysis, neurofeedback, real‐time fMRI
Many real‐time fMRI neurofeedback studies report large inter‐individual differences in learning success, but the factors that cause this vast variability between participants remain unknown. Here, we used a meta‐analytic approach including data from 24 different neurofeedback studies with a total of 401 participants to determine whether levels of activity in target brain regions during pretraining functional localizer or no‐feedback runs could predict neurofeedback learning success. We were not able to identify common brain‐based success predictors across our diverse cohort of studies.
1. INTRODUCTION
During the last years, neurofeedback using real‐time functional magnetic resonance imaging (fMRI) has been gaining increasing attention in cognitive and clinical neuroscience. Real‐time fMRI‐based neurofeedback enables subjects to learn control over brain activity in localized regions of interest (ROIs). Brain areas that have been investigated in fMRI‐based neurofeedback studies include the anterior cingulate cortex (deCharms et al., 2005; Emmert et al., 2014; Gröne et al., 2015; Guan et al., 2014; Li et al., 2013), anterior insula (Yao et al., 2016), amygdala (Brühl et al., 2014; Gerin et al., 2016; Keynan et al., 2016; Nicholson et al., 2017; Paret et al., 2014; Young et al., 2014), auditory cortex (Emmert, Kopel, et al., 2017; Haller, Birbaumer, & Veit, 2010), defaultmodenetwork (DMN; McDonald et al., 2017), dorsolateral prefrontal cortex (Sherwood, Kane, Weisend, & Parker, 2016), hippocampus (Skouras et al., 2020), insula (Buyukturkoglu et al., 2015; Caria et al., 2007; Emmert et al., 2014; Frank et al., 2012; Zilverstand, Sorger, Sarkheil, & Goebel, 2015), motor cortex (Auer, Schweizer, Frahm, 2015; Blefari, Sulzer, Hepp‐Reymond, Kollias, & Gassert, 2015; Buyukturkoglu et al., 2013; Marins et al., 2015; Scharnowski et al., 2015; Yoo, Lee, O'Leary, Panych, & Jolesz, 2008), nucleusaccumbens (Greer, Trujillo, Glover, & Knutson, 2014), parahippocampalgyrus (Scharnowski et al., 2015), ventral tegmental area (MacInnes, Dickerson, Chen, & Adcock, 2016; Sulzer et al., 2013), and the visual cortex (Scharnowski, Hutton, Josephs, Weiskopf, & Rees, 2012; Shibata, Watanabe, Sasaki, & Kawato, 2011). More recently, functional brain networks have also been successfully trained employing connectivity‐informed neurofeedback in networks sub‐serving emotion regulation (Koush et al., 2015), attention (Koush et al., 2013), motor control (Liew et al., 2016; Megumi, Yamashita, Kawato, & Imamizu, 2015), craving (Kim, Yoo, Tegethoff, Meinlschmidt, & Lee, 2015), and executive control (Spetter et al., 2017).
Real‐time fMRI neurofeedback has been shown to improve behavioral and cognitive functions in healthy participants (e.g., Rota et al., 2009; Scharnowski et al., 2012; Scharnowski et al., 2015; Sherwood et al., 2016; Shibata et al., 2011), and to reduce clinical symptoms in neurological and psychiatric patient populations, such as patients suffering from adipositas (Frank et al., 2012), alcohol and nicotine addiction (Canterberry et al., 2013; Hanlon et al., 2013; Hartwell et al., 2016; Karch et al., 2015; Kim et al., 2015; Li et al., 2013), borderline personalitydisorder (Paret et al., 2016), chronic pain (deCharms et al., 2005; Guan et al., 2014), depression (Linden et al., 2012; Young et al., 2014; Young et al., 2017), Huntington's disease (Papoutsi et al., 2018; Papoutsi et al., 2020), obsessive compulsory disorder (Buyukturkoglu et al., 2015), Parkinson's disease (Buyukturkoglu et al., 2013; Subramanian et al., 2011), phobia (Zilverstand et al., 2015), post‐traumatic stress disorder (Gerin et al., 2016; Nicholson et al., 2017), and tinnitus (Emmert, Kopel, Koush, Maire, Senn, Van De Ville, et al., 2017; Haller et al., 2010). The increasing interest in real‐time fMRI NFB is also indicated by the rapidly rising number of publications in this field, which, according to a PubMed search (https://www.ncbi.nlm.nih.gov/pubmed/) using the search words “neurofeedback AND fMRI” rose from 11 studies published in 2009 to 70 studies being published in 2019 alone, with a total number of 430 publications to date.
However, not every individual can benefit from neurofeedback training and neurofeedback learning success differs substantially between individuals. In fact, many studies report participants who were unable to gain control over their own brain activity, even after multiple training sessions. In these studies, an average of about 38% of all participants failed to modulate their own brain activity and were not able to reach predefined goals after neurofeedback training (Bray, Shimojo, & O'Doherty, 2007; Chiew, LaConte, & Graham, 2012; deCharms et al., 2005; Johnson et al., 2012; Ramot, Grossman, Friedman, & Malach, 2016; Robineau et al., 2014; Scharnowski et al., 2012; Yoo et al., 2008). This failure to modulate brain activity, also referred to as the “neurofeedback inefficacy problem”(Alkoby, Abu‐Rmileh, Shriki, & Todder, 2017), leads to a reduction in overall efficiency of neurofeedback training and hampers translation to clinical interventions. To date, the factors that cause neurofeedback inefficacy as well as the large inter‐individual variability in neurofeedback learning success in the field of real‐time fMRI neurofeedback remain unknown.
Interestingly, neurofeedback studies based on electroencephalography(EEG) have reported very similar numbers of participants failing to gain control over their own brain activity (e.g., Enriquez‐Geppert et al., 2014; Zoefel, Huster, & Herrmann, 2011). However, despite intrinsic similarities shared by neurofeedback tasks across imaging modalities, EEG‐based and fMRI‐based neurofeedback differ substantially with regard to the underlying technology, methods and mechanisms. In this meta‐analysis, we focus selectively on fMRI‐based neurofeedback; for an overview of successful predictors in EEG‐based neurofeedback we refer interested readers to Alkoby et al. (2017).
Here, we investigate the influence of neural activity before neurofeedback training on neurofeedback learning success. In particular, we ask whether activity levels in the neurofeedback target region(s) during pretraining no‐feedbackruns—runs where participants modulate their brain activity in the targeted ROI without neurofeedback—or functional localizer runs can predict neurofeedback learning success in subsequent neurofeedback training runs. As pretraining brain activity already contains information on factors such as participant compliance and responsiveness to specific stimuli, we hypothesized that specific signal features (e.g., brain activity levels) extracted from the trained ROI during no‐feedback or localizer runs before neurofeedback training are correlated with the respective participant's success in modulating their own brain activity. To test this hypothesis, we performed a meta‐analysis on data from 24real‐time fMRI neurofeedback studies (see Table 1), including a range of different target brain areas (>20ROIs), participants (261healthy participants and 140patients), and neurofeedback training methods (activity‐based feedback as well as connectivity‐based feedback).
TABLE 1.
Overview of the studies that were included in the meta‐analysis
| Study ID/author | Type of NFB | Participants | ROIs | Pretraining run type | Pretraining task |
|---|---|---|---|---|---|
| 1/Auer et al. (2015) | Activity | Healthy (N = 16) | SMC | Functional localizer | Overt finger movements |
| 2/Blefari et al. (2015) | Activity | Healthy (N = 11) | M1 | Functional localizer | Active isometric pinching |
| 3/Emmert et al. (2017) | Activity | Tinnitus (N = 14) | Auditory cortex | Functional localizer | Pulsating 1 kHz tone |
| 4/Megumi et al. (2015) | Functional connectivity | Healthy (N = 12) | Left lateral parietal, left M1 | No‐feedback run | Finger tapping imagery |
| 5/Keynan et al. (in prep) | Activity | Healthy (N = 33) | Amygdala | Functional localizer | Hariri face recognition |
| 6/Kim et al. (2015) | Activity, functional connectivity | Tobacco use disorder (N = 14) | ACC, mPFC, OFC, PCC, precuneus | No‐feedback run | Resist urge to smoke while viewing smoking‐related video clips |
| 7/Kirschner et al. (2018) | Activity | Healthy (N = 27), cocaine use disorder (N = 24) | VTA | No‐feedback run | Reward imagery |
| 8/Kirschner et al. (in prep) | Activity | Schizophrenia (N = 14) | VTA | No‐feedback run | Reward imagery |
| 9/Koush et al. (2013) | Effective connectivity | Healthy (N = 7) | Visual cortex, SPL | Functional localizer | Flickering checkerboards |
| 10/Koush et al. (2015) | Effective connectivity | Healthy (N = 9) | Amygdala, dmPFC | No‐feedback run | Imagery of positive social situations |
| 11/Liew et al. (in prep.) | Functional connectivity | Healthy (N = 10) | Left PMC, left SMA | Functional localizer | Movement imagery |
| 12/MacInnes et al. (2016) | Activity | Healthy (N = 19) | VTA | No‐feedback run | Imagery of motivation |
| 13/Marins et al. (2015) | Activity | Healthy (N = 14) | Left PMC | ROI‐engaging run | Overt finger tapping |
| 14/McDonald et al. (2017) | Activity | Healthy (N = 16), psychiatric patients (N = 22) | Default mode network | ROI‐engaging run | Moral dilemma task |
| 15/Pamplona et al. (in prep) | Activity | Healthy (N = 15) | Default mode network, attention network | No‐feedback run | Attention‐related imagery |
| 16/Papoutsi et al. (2018) | Activity | Huntington's disease (N = 10) | SMA | Functional localizer | Fist clenching |
| 17/ Papoutsi et al. (2020) | Activity, functional connectivity | Huntington's disease (N = 16) | SMA, left striatum | No‐feedback run | Motor imagery |
| 18/Scharnowski et al. (2015) | Activity, differential | Healthy (N = 7) | SMA, PHC | Functional localizer | 1: Bimanual finger tapping, 2: Outdoor scenes versus faces |
| 19/Scharnowski et al. (2012) | Activity | Healthy (N = 10) | Visual cortex | Functional localizer | Flickering checkerboard |
| 20/Yao et al. (2016) | Activity | Healthy (N = 18) | Anterior insula | Functional localizer | Painful situations versus neutral pictures |
| 21/Sorger, Kamp, Weiskopf, Peters, and Goebel (2018) | Activity (levels) | Healthy (N = 10) | Individually different | Functional localizer | Individually different tasks |
| 22/Spetter et al. (2017) | Functional connectivity | Obesity (N = 8) | dlPFC, vmPFC | Functional localizer | Rating food images |
| 23/Young et al. (2017) | Activity | Depression (N = 18) | Amygdala | ROI‐engaging run | Happy imagery |
| 24/Zich et al. (2020) | Functional connectivity | Adolescents (N = 27) | Amygdala, dlPFC | Functional localizer | Social scenes task |
Note: We included data from 24 different studies, covering healthy subjects and patients, a large range of trained regions of interest, and activity‐based as well as connectivity‐based neurofeedback paradigms.
Abbreviations: ACC, anterior cingulate cortex, dlPFC; dorsolateral prefrontal cortex; mPFC, medial prefrontal cortex; M1, primary motor cortex; OFC, orbitofrontal cortex; PCC, posterior cingulate cortex; PMC, pre‐motor cortex; PHC, parahippocampal cortex; SMA, supplementary motor cortex; SMC, somatomotor cortex; SPL, superior parietal lobe, VTA, ventral tegmental area.
2. MATERIAL AND METHODS
2.1. Received data
This meta‐analysis required data that cannot be extracted from publications alone. Therefore, we reached out to authors of real‐time fMRI neurofeedback studies via the mailing list of the real‐time functional neuroimaging community, and by directly contacting authors of real‐time fMRI neurofeedback studies via e‐mail and at conferences. As we communicated all inclusion criteria in this search for data, only authors of suitable data sets reached out to us and no studies had to be excluded. Inclusion criteria were at least one task‐based functional run engaging the trained ROI/ROIs prior to neurofeedback training (e.g., a functional localizer run, a no‐feedback run, or a task engaging the target ROI that was not used for localization). For increased generalizability, we did not limit this study to a specific participant cohort, target ROI, or neurofeedback training method. A literature review revealed that, to date, 126 real‐time fMRI neurofeedback studies met these inclusion criteria and contained at least one task‐based functional run engaging the trained ROI(s).
Following our request, we received data from 24 independent neurofeedback studies with data from 261healthy participants [studies 1, 2, 4, 5, 7, 9–15, 18–21, 24] and 140 patients, including patients with alcohol abuse or dependence [14], anxiety disorder [14], cannabis abuse [14], cocaine use disorder [7], depression [14, 23], Huntington's disease [16, 17], obesity [22], obsessive–compulsive disorder [14], opioid abuse [14], schizophrenia [8], specific phobia [14], tinnitus [3], and tobacco use disorder [6]. 18studies conducted neurofeedback training on brain activity, while another eight studies provided connectivity‐based feedback (two studies investigated both activity—and connectivity‐based neurofeedback). We did not receive data from studies that performed neurofeedback based on other measures, such as multivariate pattern analysis. Brain areas that were targeted in these studies include the amygdala, anterior cingulate cortex (ACC), anterior insula, auditory cortex, dorsolateral prefrontal cortex (dlPFC), dorsomedialprefrontal cortex (dmPFC), medial prefrontal cortex (mPFC), orbitofrontal cortex (OFC), parahippocampal gyrus (PHG), posterior cingulate cortex (PCC), precuneus, premotor cortex (PMC), primary motor cortex (M1), somatomotor cortex (SMC), superior parietal lobule (SPL), supplementary motor area (SMA), ventral tegmental area (VTA), ventromedial prefrontal cortex (vmPFC), and the visual cortex (Figure 1). Table 1 provides an overview over all studies(Auer et al., 2015; Blefari et al, 2015; Emmert, Kopel, et al., 2017; Kim et al., 2015; Kirschner et al., 2018; Koush et al., 2015, Koush et al., 2013; MacInnes et al., 2016; Marins et al., 2015; McDonald et al., 2017; Megumi et al., 2015; Papoutsi et al., 2020, Papoutsi et al., 2018; Scharnowski et al., 2012, 2015; Skouras & Scharnowski, 2019; Sorger et al., 2018; Spetter et al., 2017; Yao et al., 2016; Young et al., 2017; Zich et al., 2020).
FIGURE 1.
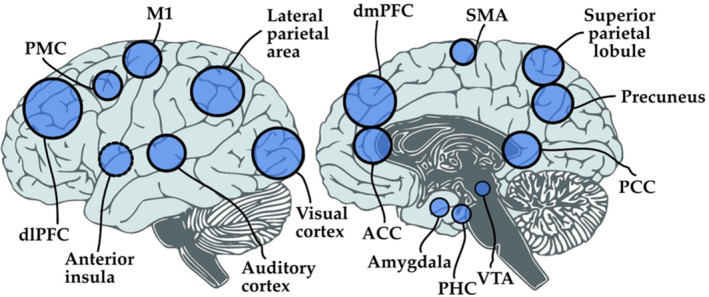
Schematic representation of areas targeted in the neurofeedback experiments. Studies included in this meta‐analysis trained activity within and connectivity between more than 20 different cortical and subcortical regions of interest that are associated with various behavioral functions. This figure is for overview purposes only and does not reflect the exact coordinates or shape of the chosen ROIs. Abbreviations: ACC, anterior cingulate cortex; dlPFC, dorsolateral prefrontal cortex; dmPFC, dorsomedial prefrontal cortex; M1, primary motor cortex; PCC, posterior cingulate cortex; PMC, pre‐motor cortex; PHC, parahippocampal cortex; SMA, supplementary motor cortex; VTA, ventral tegmental area
2.2. Received data on pretraining activity and neurofeedback learning success
We asked the authors to provide one value determining neurofeedback success for each neurofeedback training run, and one value determining pretraining brain activity levels within the ROI that was trained during neurofeedback. In particular, we asked for individual data for each participant of an experimental neurofeedback training group, excluding control groups such as receiving sham feedback or modulating brain regions of no interest. Most contributions consisted of data that were already fully analyzed and published.
For 23 studies [1–7, 9–24] we received fully processed neurofeedback success measures for each neurofeedback training run. For reasons of comparability to previously published results, neurofeedback success per run was defined as the measure of neurofeedback success that has been primarily assessed in the respective study and, for published data, has been previously reported in the corresponding publications. In one case (Kirschner et al. [in prep.]) [8], where raw data were provided, we calculated neurofeedback success based on standard general linear model (GLM) analyses, as described below. Overall neurofeedback learning success was then calculated based on these per‐run success measures (see below). In general, given the heterogeneity of the feedback measures (e.g., percent signal change, DCM Bayes Factor, etc.), aggregation of information is only possible at the level of learning curves based on study‐specific neurofeedback success measures.
For most studies, we also received fully processed beta values for average pretraining activity levels within the trained ROI. In some cases, we extracted these values using target ROI masks and contrast images of the corresponding pretraining run [3, 6, 9, 10, 18], or from raw data [7, 14].
2.3. Data analysis of raw data
For the study that shared the raw data, we analyzed the data using a standard preprocessing procedure in native space (slice time correction, motion correction, coregistration, spatial smoothingwith a Gaussian kernel of 6 mm full width at half maximum, no normalization) using SPM12 (http://www.fil.ion.ucl.ac.uk/spm/software/spm12/). We then performed first level GLM analyses on the neurofeedback as well as the pretraining runs to modelthe corresponding study's neurofeedback blocks or blocks engaging the ROI during pretraining runs, respectively.
To define pretraining activity, we extracted the average activity over all voxels within the trained ROI. When several ROIs were trained, the average over all ROIs was calculated. Activity was assessed by the beta weight representing the ROI‐engaging task during pretraining. For this study, neurofeedback learning success for each neurofeedback training run was assessed in the same way, using the beta value representing the corresponding study's neurofeedback blocks.
2.4. Meta‐analysis
To date, there is no consensus on how neurofeedback learning success should be defined and measured. Thus, in order to improve generalizability, we investigated the two most commonly used measures for assessing neurofeedback learning success(Thibault, MacPherson, Lifshitz, Roth, & Raz, 2018), namely (a) the slope of the learning curve (i.e., the regression line over the success measures for each training run), and (b) the difference between neurofeedback regulation success during the last and the first training run. Success measures of studies where participants had to perform down‐regulation were multiplied by −1. For each study, we then calculated the correlation between pretraining brain activity and these two success measures using Spearman correlations.
In addition, we investigated whether pretraining ROI activity levels might be more predictive of success during neurofeedback training runs that were performed in close temporal distance to the pretraining run. To this end, we performed correlation testing between pretraining activity levels and neurofeedback success in the very first training run.
First, we performed a meta‐analysis over all the 24 studies included here. Subsequently, we repeated the meta‐analysis for five different groupings of study data, to avoid confounds that may have been caused by differences between patients and healthy subjects, activity‐based and connectivity‐based neurofeedback paradigms, functional domains, or type of pretraining run:
Data from healthy subjects performing activity‐based neurofeedback.
Data from healthy subjects performing connectivity‐based neurofeedback.
Data from patients performing activity‐based neurofeedback.
Data split according to the functional domain of the trained ROI: (a) sensory areas, (b) motor areas, (c) reward areas, (d) emotion processing area/amygdala, (e) higher order cognitive processing areas/DMN and PFC.
Data split according to the type of pretraining run that was performed: (a) functional localizer run, (b) no‐feedback run, (c) ROI‐engaging run that was not used for localization.
Due to small sample sizes, further subdivisions of the data in (4) and (5) according to patients/healthy subjects and activity/connectivity measures were not performed. In general, meta‐analyses performed with small sample sizes, for instance for Group (2), should be read with caution. For this reason, we do not provide results for the grouping “patients performing connectivity‐based neurofeedback” which only consisted of three studies.
For each of these five groups as well as the entire sample (all data from all studies), we calculated overall meta‐correlations using a weighted (weights based on the number of participants included in the study) random‐effects model. All statistical meta‐analyses were performed using the meta package in R using the metacor function (www.cran.r-project.org/web/packages/metacor). Studies that included both patients and healthy subjects, and studies that investigated both connectivity‐ and activity‐based neurofeedback were split into several corresponding sub‐groups accordingly. One study that trained a different ROI for each participant [21] was not considered in the ROI‐based group split. Further, some of the studies included in the no‐feedback group or the ROI‐engaging paradigm group included a functional localizer scan in their experimental design but, due to data dropouts, the corresponding no‐feedback or ROI‐engaging paradigm runs were used to extract activity levels. In addition, we performed several analyses to quantify heterogeneity of effect sizes using the Meta‐Essentials tool (Suurmond & Hak, 2017).
3. RESULTS
3.1. Meta‐analysis over all studies
The meta‐analysis over the entire sample of all studies did not reveal a significant relationship between pretraining activity levels and neither of the two neurofeedback success measures (slope of the learning curve: r(27) = −0.02, p = .80; last versus first run: r(27) = −0.00, p = .94). Further, pretraining activity levels did not show a significant correlation to neurofeedback success during the very first neurofeedback run (r(27) = 0.08, p = .36). Heterogeneity analysesindicated low heterogeneity of effect sizes (Higgins, Thompson, Deeks, & Altman, 2003), both for the slope of the learning curve (Q = 30.13, Q‐df = 3.13, pQ = 0.31, I2 = 10.38%, T2 = 0.01, T = 0.10), and for the last versus first neurofeedback training run (Q = 27.61, Q‐df = 0.61, pQ = 0.43, I2 = 2.20%, T2 = 0.00, T = 0.04). Correlations between pretraining activity levels and success in the very first neurofeedback run were moderatelyheterogeneous across studies (Q = 49.35, Q‐df = 22.35, pQ = 0.005, I2 = 45.29%, T2 = 0.07, T = 0.27). Figures 2, 3, and 4 show forest plots for correlations between pretraining activity levels and the slope success measure, the difference between the last and the first runsuccess measures, and success during the very first neurofeedback run, respectively.
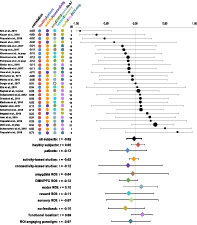
FIGURE 2.
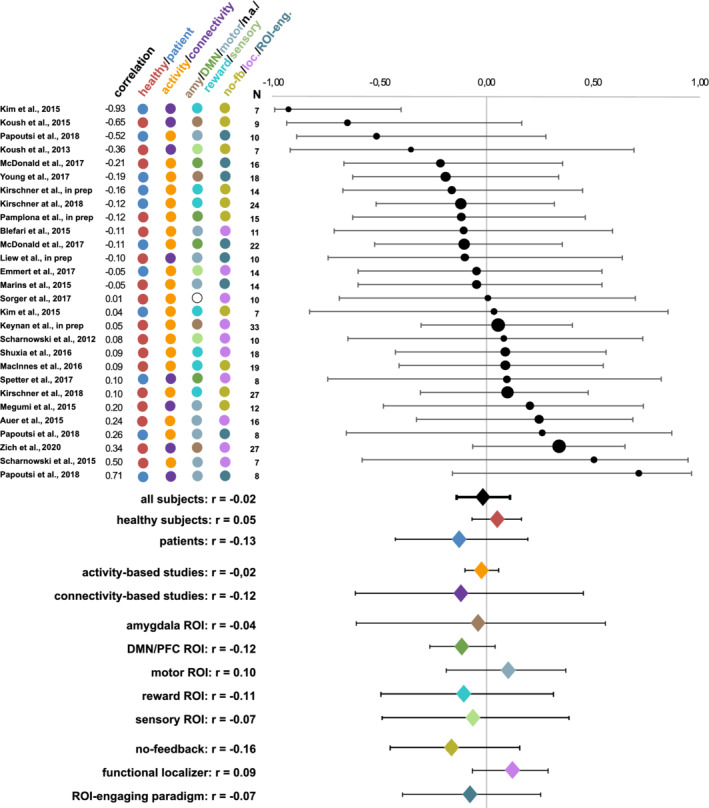
Averaged weighted Spearman correlations between pretraining activity levels and neurofeedback learning success as measured by the slope of the learning curve. Circle sizes represent the corresponding study's sample sizes. Further, the coloring scheme reflects the corresponding grouping of the subjects (healthy subjects/patients) and the studies (type of feedback, trained target region(s) and type of pretraining activity levels). Overall, no correspondence between pretraining activity levels and neurofeedback learning success was found. Abbreviations: amy, amygdala; DMN, default mode network; n.a., not applicable; no‐fb: no feedback; loc, localizer; ROI‐eng, ROI‐engaging
FIGURE 3.
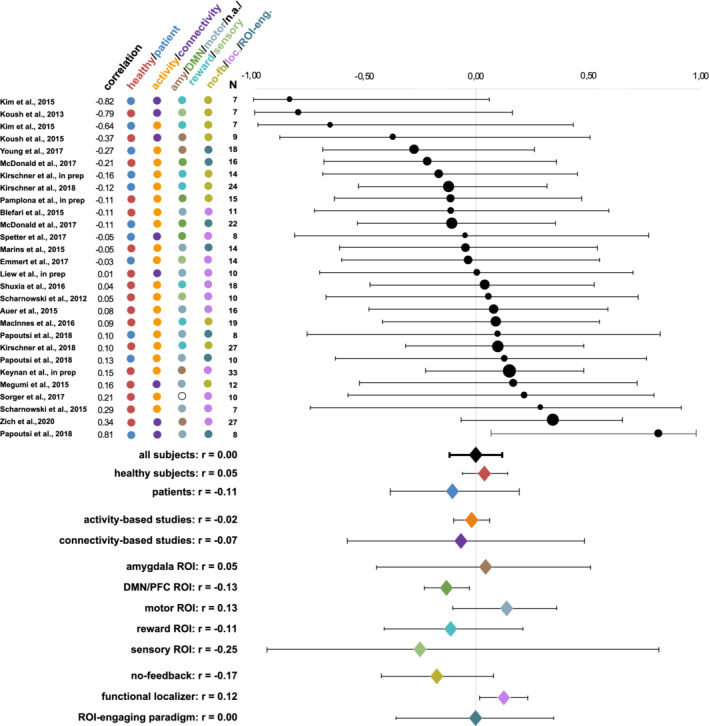
Averaged weighted Spearman correlations between pretraining activity levels and neurofeedback learning success as measured by the difference between neurofeedback success in the last and the first neurofeedback run. Circle sizes represent the corresponding study's sample sizes. Further, the coloring scheme reflects the corresponding grouping of the subjects (healthy subjects/patients) and the studies (type of feedback, trained target region(s) and type of pretraining activity levels). Overall, no correspondence between pretraining activity levels and neurofeedback learning success was found, except for when only investigating pretraining activity levels during a functional localizer run. Abbreviations: amy, amygdala; DMN, default mode network; n.a., not applicable; no‐fb, no feedback; loc, localizer; ROI‐eng, ROI‐engaging
FIGURE 4.
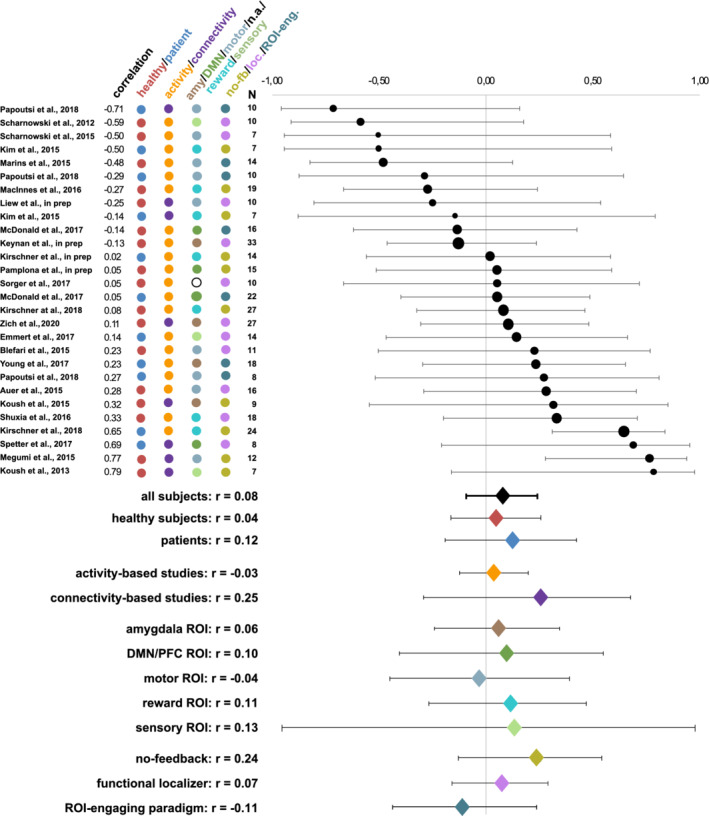
Averaged weighted Spearman correlations between pretraining activity levels and neurofeedback learning success during the first neurofeedback run. Circle sizes represent the corresponding study's sample sizes. Further, the coloring scheme reflects the corresponding grouping of the subjects (healthy subjects/patients) and the studies (type of feedback, trained target region(s) and type of pretraining activity levels). Overall, no correspondence between pretraining activity levels and neurofeedback success in the very first neurofeedback run was found. Abbreviations: amy, amygdala; DMN, default mode network; n.a., not applicable; no‐fb, no feedback; loc, localizer; ROI‐eng, ROI‐engaging
3.2. Activity‐based neurofeedback with healthy subjects
For activity‐based neurofeedback with healthy subjects, we found no significant relationship between pretraining activity levels and neurofeedback learning success for neither neurofeedback success measures; that is, neither based on the slope of the regression line over all neurofeedback runs (r = 0.04, p = .57), nor based on the difference between the last and the first run (r = 0.04, p = .56). Heterogeneity measures for activity‐based studies on healthy subjects were smaller than heterogeneity measures for all studies. They indicated very low heterogeneity of effect sizes, both for the slope‐based (Q = 3.24, Q‐df < 0, pQ = 0.99, I2 = 0.00%, T2 = 0.00, T = 0.00) and the difference‐based (Q = 2.36, Q‐df < 0, pQ = 1.00, I2 = 0.00%, T2 = 0.00, T = 0.00) neurofeedback learning success measure.
For activity‐based neurofeedback studies with healthy subjects, we also found no significant relationship between pretraining activity levels and success in the first neurofeedback run (r = −0.06, p = .49), with 6 of 12 studies even showing a negative correlation. Heterogeneity measures again showed low heterogeneity of effect sizes (Q = 12.45, Q‐df < 0, pQ = 0.33, I2 = 11.68%, T2 = 0.01, T = 0.10).
3.3. Connectivity‐based neurofeedback with healthy subjects
Similar to the results on activity‐based neurofeedback studies with healthy subjects, for connectivity‐based neurofeedback studies on healthy subjects, we again found no significant correlation between pretraining activity levels and neurofeedback learning success (slope of the regression line: r = −0.04, p = .85; last vs. first run difference: r = −0.06, p = .77). Heterogeneity measures for connectivity‐based studies on healthy subjects indicated a moderate heterogeneity of effect sizes, for the slope‐based (Q = 7.18, Q‐df < 0, pQ = 0.07, I2 = 58.24%, T2 = 0.17, T = 0.41) and for the difference‐based (Q = 8.41, Q‐df < 0, pQ = 0.08, I2 = 52.43%, T2 = 0.13, T = 0.36) neurofeedback learning success measure.
Pretraining activity levels were slightly predictive of neurofeedback success in the very first neurofeedback run (r = 0.38, p = .10). Heterogeneity analyses showed again moderate heterogeneity (Q = 9.99, Q‐df = 5.99, pQ = 0.04, I2 = 59.96%, T2 = 0.17, T = 0.41).
3.4. Activity‐based neurofeedback with patients
For activity‐based neurofeedback studies across different patient populations, we did not find a significant correlation between pretraining activity levels and neurofeedback learning success, for neither neurofeedback learning success measures (slope of the learning curve:r = −0.13, p = .20; last vs. first rundifference: r = −0.14, p = 0.19). Here, 6 of 8, and 7 out of 8 studies showed a slightly negative relationship, respectively. Heterogeneity of effects sizes was very low (slope: Q = 2.42, Q‐df < 0, pQ = 0.93, I2 = 0.00%, T2 = 0.00, T = 0.00; last vs. first difference: Q = 2.79, Q‐df = 0.79, pQ = 0.90, I2 = 0.00%, T2 = 0.00, T = 0.00). Pretraining activity levels in patients were also not predictive for neurofeedback success in the very first training run (r = 0.18, p = 0.18; heterogeneity measures: Q = 11.13, Q‐df = 4.13, pQ = 0.13, I2 = 37.08%, T2 = 0.05, T = 0.23).
3.5. Functional domain of the trained ROI
To investigate whether ROIs within specific functional domains would show stronger correlations than others, we clustered the studies based on the functional domain of the (main) neurofeedback target ROI(s). For neurofeedback success, as measured by the slope of the learning curve (see Table S1), we did not find significant effects for any of the assessed functional domains, that is, amygdala (emotion processing), DMN/PFC (mind wandering and higher cognitive functioning), motor functioning, reward processing, and other sensory domains. For neurofeedback success measured by the difference between success in the first and the last neurofeedback run (see Table S2), we found a negative correlation for studies that focused on DMN/PFC regulation (r = −0.13, p < .001). We did not find significant effects for any functional domain clusters when investigating the correlation between pretraining activity levels and neurofeedback success during the first neurofeedback run (see Table S3).
3.6. Type of pretraining run
Pretraining activity levels were either based on a no‐feedback run, a functional localizer run, or on another task engaging the ROI that was not used for localizing the ROI, for example, a finger tapping task when neurofeedback training was targeting the motor cortex [13]. Overall, studies with a functional localizer run showed a significant positive correlation between the localizer activity levels and neurofeedback learning success as measured by the difference between neurofeedback learning success in the last and the first neurofeedback run (r = 0.12, p = .003). However, this correlation was not significant when success was measured by the slope of the learning curve (r = 0.09, p = .20). For activity levels during other pretraining runs we did not observe a significant correlation with learning success. Further, none of the three types of pretraining run groups showed significant correlations between pretraining activity and the very first neurofeedback run (see Tables S4‐S6 for exact values).
4. DISCUSSION
Here, we performed a meta‐analysis with 24 different fMRI‐based neurofeedback studies to investigate whether pretraining activity levels can be used to predict neurofeedback learning success. In our data set of 401 subjects undergoing neurofeedback training, we did not find an overall significant relationship between these two measures, that is, ROI activity prior to neurofeedback training and neurofeedback learning success were not significantly correlated.
One of the reasons for not having found an overall relationship between pretraining activity and learning success might be that the studies included in this meta‐analysis are quite diverse in terms of, for example, the research question, the target ROI, the feedback signal and the population. On the other hand, heterogeneity analyses of effect sizes across all studies revealed that our sample of studies was sufficiently homogenous for a meta‐analysis and that the result was unlikely to be confounded by single studies. Nevertheless, we aimed at partly mitigating heterogeneity by repeating the analysis for different groups containing only healthy participants or patients, activity‐or connectivity‐based neurofeedback, only studies with the same type of pretraining run, and by grouping studies based on the functional domain of the trained ROI. Unfortunately, further subclassifications in, for instance, studies who performed up‐ vs. down‐regulation could not be performed due to too low sample sizes.
4.1. Differences between healthy subjects and patients
Neither healthy subjects nor patients showed a significant correlation between pretraining activity levels and neurofeedback learning success.
Interestingly, the majority of patient studies showed a negative correlation between neurofeedback learning success and pretraining activity levels, while we observed more positive correlations for studies with healthy subjects. This might be explained by symptom severity being associated with increased ROI activity, which again can influence a patient's neurofeedback learning performance. For example, patients suffering from substance use disorder who show highly increased craving‐induced brain activity levels might be less successful in down‐regulating craving‐related brain signals than addiction patients who only show mildly increased craving‐related brain activity. Increased brain activity levels in higher order brain areas might also be an indicator for decreased cognitive capacities – as the performed task constitutes a particular challenge to the patients, they might experience exhaustion during the following neurofeedback training runs. Further, aspects like differences in adaptation, motivation, deficits in sustained attention etc. that are often reported in specific patient populations, might also drive neurofeedback training success differences.
4.2. Activity‐ versus connectivity‐based neurofeedback
Neither activity‐ nor connectivity‐based neurofeedback studies showed a significant correlation between pretraining activity levels and neurofeedback learning success. Moreover, while heterogeneity measures for effect sizes of activity‐based neurofeedback studies showed very low heterogeneity, this was not the case for effect sizes of connectivity‐based neurofeedback studies. Here, heterogeneity measures of effect sizes revealed moderate heterogeneity, indicating that effect sizes in connectivity‐based studies might be too diverse to be grouped together in one meta‐analysis. This might be related to connectivity‐based neurofeedback studies still being sparse with overall limited samplesizes. Another confounding factor might be that for connectivity‐based studies pretraining activity levels are not as similar to neurofeedback success measures as for activity‐based studies. Consequently, future studies should investigate whether pretraining levels based on connectivity are more predictive for neurofeedback learning success in connectivity‐based neurofeedback studies and, in addition, whether effect sizes based on pretraining connectivity levels are less heterogeneous. In fact, a recent study found that DMN up‐regulation learning and downregulation learning scores are partly determined by pre‐neurofeedback resting‐state eigenvector centrality of the PCC/PCu (Skouras & Scharnowski, 2019). Further, another study observed resting state connectivity to be predictive for neurofeedback learning success in patients with obsessive–compulsive disorder (Dustin Scheinost et al., 2014). Functional and effective connectivity measures might even be a suitable predictor for activity‐based neurofeedback studies, as neurofeedback success is likely also influenced by the connectivity of the trained region(s) to other regions within the brain. For instance, Bassett and colleagues suggest that highly connected brain regions such as areas within the DMN, might be easier to train than less‐connected brain areas (Bassett & Khambhati, 2017). This is also in line with recent suggestions that connectivity‐based measures might be more promising for predicting complex higher order cognitive processes than measures based on single brain regions (see Horien, Greene, Constable, & Scheinost, 2020 for a review on this topic). Indeed, several activity‐based neurofeedback studies report concomitant changes in brain connectivity (Lee et al., 2011; Rota, Handjaras, Sitaram, Birbaumer, & Dogil, 2011; Scharnowski et al., 2014; Scheinost et al., 2013; Zotev et al., 2011; Zweerings et al., 2019).
Thus, future analyses should consider connectivity measures as predictors not just for connectivity‐based neurofeedback studies, but also for activity‐based studies.
4.3. Type of pretraining run
Interestingly, when grouping together studies based on the paradigm of the run during which pretraining activity levels were collected, we observed a significant positive correlation between pretraining activity levels and neurofeedback success (as measured by the difference in neurofeedback success between the last and the first neurofeedback run) for studies with a functional localizer run. This indicates that pretraining activity levels might indeed be linked to neurofeedback learning success, but only when the neurofeedback target ROI is completely activated during the pretraining run, as it is the case in functional localizer runs. In contrast, in no‐feedback and other ROI‐engaging paradigms (i.e., not functional localizers), the target ROI may be engaged by the pretraining paradigm, however some voxels within the specified ROI may not be specifically involved in the neural processes under investigation. Consequently, when extracting pretraining activity levels from no‐feedback and ROI‐engaging (but not functional localizer) pretraining runs, more voxels than those that reliably activate during the performed task contribute to the derived signal and, thus, limit its predictive power for neurofeedback success.
In contrast to functional localizer runs, no‐feedback runs (i.e., where the participants were performing the same task as during a neurofeedback run but without getting any feedback) did not predict neurofeedback learning success. Surprisingly, the no‐feedback runs performed just before the neurofeedback training commenced were not even predictive of performance during the very first neurofeedback training run. No‐feedback runs (also referred to as “transfer runs” when performed after neurofeedback training) are identical to the neurofeedback training runs (i.e., same ROI, same design, similar instructions, similar mental task, etc.) except that no feedback signal is presented. This indicates that providing feedback might only be a small experimental addition, but one that changes the paradigm significantly. Previous studies have already highlighted the discrepancy between pretraining no‐feedback runs and neurofeedback runs by showing that no‐feedback runs differ substantially from neurofeedback training runs in terms of functional connectivity changes (Haller et al., 2013), self‐regulation performance (Robineau et al., 2014), and signal‐to‐noise ratio (Papageorgiou, Lisinski, McHenry, White, & LaConte, 2013). This also indicates that the feedback has a stronger effect on neurofeedback training success than the actual task the participant is performing in the scanner. Indeed, recent implicit neurofeedback studies show that neurofeedback learning is possible even when participants are not informed what the neurofeedback signal represents and are not provided with mental strategy instructions that are related to the function of the target ROI (Cortese, Amano, Koizumi, Kawato, & Lau, 2016; Koizumi et al., 2017; Shibata et al., 2011; Taschereau‐Dumouchel et al., 2018). These findings show that neurofeedback runs are special in that they constitute their own specific experimental condition that is distinct from seemingly‐related conditions such as transfer runs without neurofeedback. Thus, they should be analyzed separately and, for example, performance during no‐feedback and training runs should not be combined in one continuous learning curve. This also indicates that, maybe, the very first neurofeedback run of a session might be a better predictor for neurofeedback learning than a no‐feedback run and should be investigated in future studies.
4.4. Neurofeedback learning measure
For the purpose of generalizability, we assessed neurofeedback learning success by (a) the slope of the regression line over the per‐run success measures, and (b) the difference between neurofeedback success during the last run compared to the first neurofeedback run. These two measures are frequently used in neurofeedback studies and they capture the efficiency of neurofeedback learning (slope) as well as the effect of neurofeedback learning (difference between the last and first run). These two measures are highly correlated with an average correlation of r = 0.78 across all studies. However, in the neurofeedback literature there is still no generally accepted best measure for assessing neurofeedback learning success. Other potential success measures are, for example, the difference between pre‐ and post‐training no‐feedback runs (e.g., Auer et al., 2015; Koush et al., 2015; MacInnes et al., 2016; Megumi et al., 2015), or the behavioral/clinical improvements (e.g., deCharms et al., 2005; Emmert, Kopel, et al., 2017; Linden et al., 2012; Scharnowski et al., 2015; Young et al., 2017). One might speculate that predictions might have been better if we had used an alternative neurofeedback learning measure. On the other hand, pretraining activity was not even predictive of the very first neurofeedback training run activity (Table S6) and for this comparison identical measures (i.e., ROI activity) were used.
The underlying problem with respect to defining a commonly accepted neurofeedback learning measure is that there is no established model of neurofeedback learning (Sitaram et al., 2016), thus making it difficult to define the key parameters involved in successful neurofeedback training. In addition, individual learning curves are quite diverse so that defining a one‐fits‐all learning measures that captures the multitude of manifestations of neurofeedback learning is very challenging. For that reason, running the analyses in parallel for two different neurofeedback performance measures is a pragmatic solution aiming to capture potential predictors of learning success.
4.5. Predicting neurofeedback learning success
Overall, we were not able to predict neurofeedback learning success from pretraining activity levels. However, when observing only studies that defined their neurofeedback target ROI(s) based on a functional localizer task, we identified a positive correlation between pretraining activity levels and neurofeedback learning success (i.e., slope‐based and difference‐based). These results indicate that neurofeedback performance is connected to pretraining activity levels, but only when all neurofeedback target voxels can be actively engaged by the functional pretraining task. Nevertheless, even for this group of neurofeedback training studies, we did not find any significant results for individual studies. Further, the weak correlation of r = 0.12 indicates that it is not possible to create considerably accurate predictions on which participants might be able to perform well during neurofeedback training and which participants will fail to do so.
Taken together, factors that can already be assessed in pretraining no‐feedback and localizer runs, such as noise levels, participant compliance, or the responsiveness of a particular ROI, are not the main causes for the large inter‐individual differences in neurofeedback learning success (Bray et al., 2007; Chiew et al., 2012; deCharms et al., 2005; Johnson et al., 2012; Robineau et al., 2014; Scharnowski et al., 2012; Yoo et al., 2008).
This poses the question of what other information might be useful as a predictor for neurofeedback learning success. Obvious candidates would be standardized questionnaires or behavioral tasks that could be used for participant selection without having to acquire imaging data. Unfortunately, evidence for the predictive power of such measures is sparse. While two studies found that the pain Coping Strategies Questionnaire (Rosenstiel & Keefe, 1983) and state anxiety scores (Spielberger, 2010) predict success in learning to regulate the ACC (Emmert et al., 2017) and emotion networks (Koush et al., 2015), respectively, another study did not find correlations between pretraining spatial orientation (Stumpf & Fay, 1983), creative imagination (Barber & Wilson, 1978), or mood scores (Zerssen, 1976) scores and success in learning to regulate pre‐motor and para hippocampal ROIs (Scharnowski et al., 2015). A recent systematic review on psychological factors that might influence neurofeedback learning success in EEG and fMRI studies argues that factors such as attention and motivation might play an important role in successful neurofeedback training runs (Cohen & Staunton, 2019). However, although these are likely candidates for affecting neurofeedback learning, a concrete empirical effect of these factors has so far only been reported in one fMRI‐based neurofeedback study(Chiew et al., 2012), showing a clear necessity for more empirical investigations on these factors.
In EEG neurofeedback, several factors have been observed to be correlated with neurofeedback learning success (Alkoby et al., 2017), but they were only reported in single EEG studies have not yet been tested in fMRI‐based neurofeedback studies. For instance, factors that seemed to have a positive influence on EEG‐based neurofeedback learning success were regular spiritual practice (Kober et al., 2017) or a relaxing attitude towards one's ability to control technological devices (Witte, Kober, Ninaus, Neuper, & Wood, 2013). Also, other brain‐based measures that are, for example, focused on areas more generally involved in self‐regulation (Emmert et al., 2016) or on connectivity rather than activity levels (Horien et al., 2020)might be suitable candidates that should be explored in future studies. The latter might be particularly relevant for connectivity‐based neurofeedback studies, but we were not able to test this due to lack of suitable data. Further possible candidates for predicting neurofeedback success might be factors that have already been identified to be predictive of cognitive and behavioral training success in non‐neurofeedback studies, for example, activity in areas related to stimulus encoding and motor control has been found to be predictive of motor learning (Herholz, Coffey, Pantev, & Zatorre, 2016), and activity in the motor network has been found to predict training‐related changes in working memory (Simmonite & Polk, 2019). Finally, very recent work by Skouras et al. indicates that neurofeedback learning performance can be influenced by biological factors such as genetic and anatomical predispositions (Skouras et al., 2019), thus demonstrating the complexity of the underlying processes and the need for using multimodal data sets.
Hence, currently, no robust predictors for neurofeedback learning success have been identified, and, even if predictions can be made, they are likely study‐specific (i.e., questionnaires that are specific to the trained ROI) and might not generalize across studies. Besides empirical studies, future studies using secondary mega‐analyses might be a promising tool to identify factors that influence neurofeedback learning.
5. CONCLUSION
Here, we aimed at finding general pretraining predictors for neurofeedback training success. We observed a slightly positive correlation between pretraining activity levels during a functional localizer run and neurofeedback learning success, but we were not able to identify common brain‐based success predictors across our diverse cohort of studies. In order to achieve the goal of finding predictors for neurofeedback learning success advances need to be made: in developing (a) models for neurofeedback learning, (b) establishing robust measures for neurofeedback learning, and (c) in increasing the database including acquired candidate measures across numerous studies. The reward of such a joint effort would be increased efficacy and cost‐effectiveness of this promising scientific and therapeutic method.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
Supporting information
Appendix S1: Supporting informaion
ACKNOWLEDGMENTS
This work was supported by the Forschungskredit of the University of Zurich (FK‐18‐030), the Foundation for Research in Science and the Humanities at the University of Zurich (STWF‐17‐012), the Baugarten Stiftung, and the Swiss National Science Foundation (BSSG10_155915, 32003B_166566, 100014_178841).
Haugg A, Sladky R, Skouras S, et al. Can we predict real‐time fMRI neurofeedback learning success from pretraining brain activity? Hum Brain Mapp. 2020;41:3839–3854. 10.1002/hbm.25089
Funding information Foundation for Research in Science and the Humanities at the University of Zurich, Grant/Award Number: STWF‐17‐012; Schweizerischer Nationalfonds zur Förderung der Wissenschaftlichen Forschung, Grant/Award Numbers: 32003B_166566, BSSG10_155915, 100014_178841; Forschungskredit of the University of Zurich, Grant/Award Number: FK‐18‐030
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- Alkoby, O. , Abu‐Rmileh, A. , Shriki, O. , & Todder, D. (2017). Can we predict who will respond to neurofeedback? A review of the inefficacy problem and existing predictors for successful EEG neurofeedback learning. Neuroscience, 378, 155–164. 10.1016/j.neuroscience.2016.12.050 [DOI] [PubMed] [Google Scholar]
- Auer, T. , Schweizer, R. , & Frahm, J. (2015). Training efficiency and transfer success in an extended real‐time functional MRI neurofeedback training of the Somatomotor cortex of healthy subjects. Frontiers in Human Neuroscience, 9(October), 547 10.3389/fnhum.2015.00547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber, T. X. , & Wilson, S. C. (1978). The Barber suggestibility scale and the creative imagination scale: Experimental and clinical applications. American Journal of Clinical Hypnosis, 21(2–3), 84–108. 10.1080/00029157.1978.10403966 [DOI] [PubMed] [Google Scholar]
- Bassett, D. S. , & Khambhati, A. N. (2017). A network engineering perspective on probing and perturbing cognition with neurofeedback. Annals of the New York Academy of Sciences, 1396, 126–143. 10.1111/nyas.13338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blefari, M. L. , Sulzer, J. , Hepp‐Reymond, M.‐C. , Kollias, S. , & Gassert, R. (2015). Improvement in precision grip force control with self‐modulation of primary motor cortex during motor imagery. Frontiers in Behavioral Neuroscience, 9(February), 1–11. 10.3389/fnbeh.2015.00018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray, S. , Shimojo, S. , & O'Doherty, J. P. (2007). Direct instrumental conditioning of neural activity using functional magnetic resonance imaging‐derived reward feedback. The Journal of Neuroscience, 27(28), 7498–7507. 10.1523/JNEUROSCI.2118-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brühl, A. B. , Scherpiet, S. , Sulzer, J. , Stämpfli, P. , Seifritz, E. , & Herwig, U. (2014). Real‐time neurofeedback using functional MRI could improve down‐regulation of amygdala activity during emotional stimulation: A proof‐of‐concept study. Brain Topography, 27(1), 138–148. 10.1007/s10548-013-0331-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buyukturkoglu, K , Rana, M. , Ruiz, S. , Hackley, S. A. , Soekadar, S. R. , Birbaumer, N. , & Sitaram, R. (2013). Volitional regulation of the supplementary motor area with fMRI‐BCI neurofeedback in Parkinson's disease: A pilot study. Paper presented at: 2013 6th International IEEE/EMBS Conference on Neural Engineering (NER) . 10.1109/NER.2013.6696025 [DOI]
- Buyukturkoglu, K. , Roettgers, H. , Sommer, J. , Rana, M. , Dietzsch, L. , Arikan, E. B. , … Ruiz, S. (2015). Self‐regulation of anterior insula with real‐time fMRI and its behavioral effects in obsessive‐compulsive disorder: A feasibility study. PLoS ONE, 10(8), 1–26. 10.1371/journal.pone.0135872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canterberry, M. , Hanlon, C. A. , Hartwell, K. J. , Li, X. , Owens, M. , LeMatty, T. , … George, M. S. (2013). Sustained reduction of nicotine craving with real‐time neurofeedback: Exploring the role of severity of dependence. Nicotine & Tobacco Research, 15(12), 2120–2124. 10.1093/ntr/ntt122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caria, A. , Veit, R. , Sitaram, R. , Lotze, M. , Weiskopf, N. , Grodd, W. , & Birbaumer, N. (2007). Regulation of anterior insular cortex activity using real‐time fMRI. NeuroImage, 35(3), 1238–1246. 10.1016/j.neuroimage.2007.01.018 [DOI] [PubMed] [Google Scholar]
- Chiew, M. , LaConte, S. M. , & Graham, S. J. (2012). Investigation of fMRI neurofeedback of differential primary motor cortex activity using kinesthetic motor imagery. NeuroImage, 61(1), 21–31. 10.1016/j.neuroimage.2012.02.053 [DOI] [PubMed] [Google Scholar]
- Cohen, K. , & Staunton, G. (2019). NeuroImage a systematic review of the psychological factors that in fl uence neurofeedback learning outcomes. NeuroImage, 185, 545–555. 10.1016/j.neuroimage.2018.10.021 [DOI] [PubMed] [Google Scholar]
- Cortese, A. , Amano, K. , Koizumi, A. , Kawato, M. , & Lau, H. (2016). Multivoxel neurofeedback selectively modulates confidence without changing perceptual performance. Nature Communications, 7, 13669 10.1038/ncomms13669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- deCharms, R. C. , Maeda, F. , Glover, G. H. , Ludlow, D. , Pauly, J. M. , Soneji, D. , … Mackey, S. C. (2005). Control over brain activation and pain learned by using real‐time functional MRI. Proceedings of the National Academy of Sciences, 102(51), 18626–18631. 10.1073/pnas.0505210102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmert, K. , Breimhorst, M. , Bauermann, T. , Birklein, F. , Rebhorn, C. , Van De Ville, D. , & Haller, S. (2017). Active pain coping is associated with the response in real‐time fMRI neurofeedback during pain. Brain Imaging and Behavior, 11(3), 712–721. 10.1007/s11682-016-9547-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmert, K. , Breimhorst, M. , Bauermann, T. , Birklein, F. , Van De Ville, D. , & Haller, S. (2014). Comparison of anterior cingulate vs. insular cortex as targets for real‐time fMRI regulation during pain stimulation. Frontiers in Behavioral Neuroscience, 8(October), 1–13. 10.3389/fnbeh.2014.00350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmert, K. , Kopel, R. , Koush, Y. , Maire, R. , Senn, P. , Van De Ville, D. , & Haller, S. (2017). Continuous vs. intermittent neurofeedback to regulate auditory cortex activity of tinnitus patients using real‐time fMRI ‐ a pilot study. NeuroImage: Clinical, 14, 97–104. 10.1016/j.nicl.2016.12.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmert, K. , Kopel, R. , Sulzer, J. , Brühl, A. B. , Berman, B. D. , Linden, D. E. J. , … Haller, S. (2016). Meta‐analysis of real‐time fMRI neurofeedback studies using individual participant data: How is brain regulation mediated? NeuroImage, 124, 806–812. 10.1016/j.neuroimage.2015.09.042 [DOI] [PubMed] [Google Scholar]
- Enriquez‐Geppert, S. , Huster, R. J. , Scharfenort, R. , Mokom, Z. N. , Zimmermann, J. , & Herrmann, C. S. (2014). Modulation of frontal‐midline theta by neurofeedback. Biological Psychology, 95(1), 59–69. 10.1016/j.biopsycho.2013.02.019 [DOI] [PubMed] [Google Scholar]
- Frank, S. , Lee, S. , Preissl, H. , Schultes, B. , Birbaumer, N. , & Veit, R. (2012). The obese brain athlete: Self‐regulation of the anterior insula in adiposity. PLoS ONE, 7(8), 3–8. 10.1371/journal.pone.0042570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerin, M. I. , Fichtenholtz, H. , Roy, A. , Walsh, C. J. , Krystal, J. H. , Southwick, S. , & Hampson, M. (2016). Real‐time fMRI neurofeedback with war veterans with chronic PTSD: A feasibility study. Frontiers in Psychiatry, 7(JUN), 1–11. 10.3389/fpsyt.2016.00111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer, S. , Trujillo, A. , Glover, G. H. , & Knutson, B. (2014). Control of nucleus accumbens activity with neurofeedback. NeuroImage, 96, 237–244. 10.1021/nl061786n.Core-Shell [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gröne, M. , Dyck, M. , Koush, Y. , Bergert, S. , Mathiak, K. A. , Alawi, E. M. , … Mathiak, K. (2015). Upregulation of the rostral anterior cingulate cortex can Alter the perception of emotions: fMRI‐based neurofeedback at 3 and 7 T. Brain Topography, 28(2), 197–207. 10.1007/s10548-014-0384-4 [DOI] [PubMed] [Google Scholar]
- Guan, M. , Ma, L. , Li, L. , Tong, L. , Zhang, Y. , Zheng, D. , … Shi, D. (2014). Self‐regulation of rACC activation in patients with postherpetic neuralgia: A preliminary study using real‐time fMRI neurofeedback. Ismrm, 22, 5889 10.7910/DVN/27368 [DOI] [Google Scholar]
- Haller, S. , Birbaumer, N. , & Veit, R. (2010). Real‐time fMRI feedback training may improve chronic tinnitus. European Radiology, 20(3), 696–703. 10.1007/s00330-009-1595-z [DOI] [PubMed] [Google Scholar]
- Haller, S. , Kopel, R. , Jhooti, P. , Haas, T. , Scharnowski, F. , Lovblad, K. O. , … Van De Ville, D. (2013). Dynamic reconfiguration of human brain functional networks through neurofeedback. NeuroImage, 81, 243–252. 10.1016/j.neuroimage.2013.05.019 [DOI] [PubMed] [Google Scholar]
- Hanlon, C. A. , Hartwell, K. J. , Canterberry, M. , Li, X. , Owens, M. , LeMatty, T. , … George, M. S. (2013). Reduction of cue‐induced craving through realtime neurofeedback in nicotine users: The role of region of interest selection and multiple visits. Psychiatry Research: Neuroimaging, 213(1), 79–81. 10.1016/j.pscychresns.2013.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwell, K. J. , Hanlon, C. A. , Li, X. , Borckardt, J. J. , Canterberry, M. , Prisciandaro, J. J. , … Brady, K. T. (2016). Individualized real‐time fMRI neurofeedback to attenuate craving in nicotine‐dependent smokers. Journal of Psychiatry and Neuroscience, 41(1), 48–55. 10.1503/jpn.140200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herholz, S. C. , Coffey, E. B. J. , Pantev, C. , & Zatorre, R. J. (2016). Dissociation of neural networks for predisposition and for training‐related plasticity in auditory‐motor learning. Cerebral Cortex, 26(7), 3125–3134. 10.1093/cercor/bhv138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins, J. P. , Thompson, S. G. , Deeks, J. J. , & Altman, D. G. (2003). Measuring inconsistency in meta‐analyses. Bmj, 327(7414), 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horien, C. , Greene, A. S. , Constable, R. T. , & Scheinost, D. (2020). Regions and connections: Complementary approaches to characterize brain organization and function. The Neuroscientist, 26(2), 117–133. 10.1177/1073858419860115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson, K. , Hartwell, K. J. , Lematty, T. , Borckardt, J. , Morgan, P. , Govindarajan, K. , … George, M. S. (2012). Intermittent “real‐time” fMRI feedback is superior to continuous presentation for a motor imagery task: A pilot study. Journal of Neuroimaging, 22(1), 58–66. 10.1111/j.1552-6569.2010.00529.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karch, S. , Keeser, D. , Hümmer, S. , Paolini, M. , Kirsch, V. , Karali, T. , … Pogarell, O. (2015). Modulation of craving related brain responses using real‐time fMRI in patients with alcohol use disorder. PLoS ONE, 10(7), e0133034 10.1371/journal.pone.0133034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keynan, J. N. , Meir‐Hasson, Y. , Gilam, G. , Cohen, A. , Jackont, G. , Kinreich, S. , … Hendler, T. (2016). Limbic activity modulation guided by functional magnetic resonance imaging–inspired electroencephalography improves implicit emotion regulation. Biological Psychiatry, 80(6), 490–496. 10.1016/j.biopsych.2015.12.024 [DOI] [PubMed] [Google Scholar]
- Kim, D.‐Y. , Yoo, S.‐S. , Tegethoff, M. , Meinlschmidt, G. , & Lee, J.‐H. (2015). The inclusion of functional connectivity information into fMRI‐based neurofeedback improves its efficacy in the reduction of cigarette cravings. Journal of Cognitive Neuroscience, 27(8), 1552–1572. 10.1162/jocn [DOI] [PubMed] [Google Scholar]
- Kirschner, M. , Sladky, R. , Haugg, A. , Stämp, P. , Jehli, E. , Hodel, M. , … Herdener, M. (2018). Self‐regulation of the dopaminergic reward circuit in cocaine users with mental imagery and neurofeedback. eBioMedicine, 37, 489–498. 10.1016/j.ebiom.2018.10.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kober, S. E. , Witte, M. , Ninaus, M. , Koschutnig, K. , Wiesen, D. , Zaiser, G. , … Wood, G. (2017). Ability to gain control over one' s own brain activity and its relation to spiritual practice: A multimodal imaging study. Frontiers in Human Neuroscience, 11(May), 1–12. 10.3389/fnhum.2017.00271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koizumi, A. , Amano, K. , Cortese, A. , Shibata, K. , Yoshida, W. , Seymour, B. , … Lau, H. (2017). Fear reduction without fear through reinforcement of neural activity that bypasses conscious exposure. Nature Human Behaviour, 1(1), 1–7. 10.1038/s41562-016-0006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koush, Y. , Meskaldji, D.‐E. , Pichon, S. , Rey, G. , Rieger, S. W. , Linden, D. E. J. , … Scharnowski, F. (2015). Learning control over emotion networks through connectivity‐based neurofeedback. Cerebral Cortex, 27(2), 1193–1202. 10.1093/cercor/bhv311 [DOI] [PubMed] [Google Scholar]
- Koush, Y. , Rosa, M. J. , Robineau, F. , Heinen, K. , Rieger, W. , Weiskopf, N. , … Scharnowski, F. (2013). Connectivity‐based neurofeedback: Dynamic causal modeling for real‐time fMRI. NeuroImage, 81, 422–430. 10.1016/j.neuroimage.2013.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, S. , Ruiz, S. , Caria, A. , Veit, R. , Birbaumer, N. , & Sitaram, R. (2011). Detection of cerebral reorganization induced by real‐time fMRI feedback training of insula activation: A multivariate investigation. Neurorehabilitation and Neural Repair, 25(3), 259–267. 10.1177/1545968310385128 [DOI] [PubMed] [Google Scholar]
- Li, X. , Hartwell, K. J. , Borckardt, J. , Prisciandaro, J. J. , Saladin, M. E. , Morgan, P. , … George, M. S. (2013). Volitional reduction of anterior cingulate cortex activity produces decreased Cue craving in smoking cessation: A preliminary real‐time fMRI study. Addiction Biology, 18(4), 739–748. 10.1111/j.1369-1600.2012.00449.x.Volitional [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liew, S. L. , Rana, M. , Cornelsen, S. , Fortunato De Barros Filho, M. , Birbaumer, N. , Sitaram, R. , … Soekadar, S. R. (2016). Improving motor Corticothalamic communication after stroke using real‐time fMRI connectivity‐based neurofeedback. Neurorehabilitation and Neural Repair, 30(7), 671–675. 10.1177/1545968315619699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden, D. E. J. , Habes, I. , Johnston, S. J. , Linden, S. , Tatineni, R. , Subramanian, L. , … Goebel, R. (2012). Real‐time self‐regulation of emotion networks in patients with depression. PLoS ONE, 7(6), e38115 10.1371/journal.pone.0038115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacInnes, J. J. , Dickerson, K. C. , Chen, N. k. , & Adcock, R. A. (2016). Cognitive Neurostimulation: Learning to volitionally sustain ventral tegmental area activation. Neuron, 89(6), 1331–1342. 10.1016/j.neuron.2016.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marins, T. F. , Rodrigues, E. C. , Engel, A. , Hoefle, S. , Basílio, R. , Lent, R. , … Tovar‐Moll, F. (2015). Enhancing motor network activity using real‐time functional MRI neurofeedback of left premotor cortex. Frontiers in Behavioral Neuroscience, 9(December), 1–12. 10.3389/fnbeh.2015.00341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald, A. R. , Muraskin, J. , Dam, N. T. V. , Froehlich, C. , Puccio, B. , Pellman, J. , … Craddock, R. C. (2017). The real‐time fMRI neurofeedback based stratification of default network regulation neuroimaging data repository. NeuroImage, 146(October 2016), 157–170. 10.1016/j.neuroimage.2016.10.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Megumi, F. , Yamashita, A. , Kawato, M. , & Imamizu, H. (2015). Functional MRI neurofeedback training on connectivity between two regions induces long‐lasting changes in intrinsic functional network. Frontiers in Human Neuroscience, 9, 160 10.3389/fnhum.2015.00160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson, A. A. , Rabellino, D. , Densmore, M. , Frewen, P. A. , Paret, C. , Kluetsch, R. , … Lanius, R. A. (2017). The neurobiology of emotion regulation in posttraumatic stress disorder: Amygdala downregulation via real‐time fMRI neurofeedback. Human Brain Mapping, 38(1), 541–560. 10.1002/hbm.23402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papageorgiou, T. D. , Lisinski, J. M. , McHenry, M. A. , White, J. P. , & LaConte, S. M. (2013). Brain‐computer interfaces increase whole‐brain signal to noise. Proceedings of the National Academy of Sciences, 110(33), 13630–13635. 10.1073/pnas.1210738110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papoutsi, M. , Magerkurth, J. , Josephs, O. , Pépés, S. E. , Ibitoye, T. , Reilmann, R. , … Tabrizi, S. J. (2020). Activity or connectivity? A randomized controlled feasibility study evaluating neurofeedback training in Huntington's disease. Brain Communications, (1). 10.1093/braincomms/fcaa049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papoutsi, M. , Weiskopf, N. , Langbehn, D. , Reilmann, R. , Rees, G. , & Tabrizi, S. J. (2018). Stimulating neural plasticity with real‐time fMRI neurofeedback in Huntington's disease: A proof of concept study. Human Brain Mapping, 39(3), 1339–1353. 10.1002/hbm.23921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paret, C. , Kluetsch, R. , Ruf, M. , Demirakca, T. , Hoesterey, S. , Ende, G. , & Schmahl, C. (2014). Down‐regulation of amygdala activation with real‐time fMRI neurofeedback in a healthy female sample. Frontiers in Behavioral Neuroscience, 8(September), 1–15. 10.3389/fnbeh.2014.00299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paret, C. , Kluetsch, R. , Zaehringer, J. , Ruf, M. , Demirakca, T. , Bohus, M. , … Schmahl, C. (2016). Alterations of amygdala‐prefrontal connectivity with real‐time fMRI neurofeedback in BPD patients. Social Cognitive and Affective Neuroscience, 11(6), 952–960. 10.1093/scan/nsw016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramot, M. , Grossman, S. , Friedman, D. , & Malach, R. (2016). Covert neurofeedback without awareness shapes cortical network spontaneous connectivity. Proceedings of the National Academy of Sciences, 113(17), E2413–E2420. 10.1073/pnas.1516857113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robineau, F. , Rieger, S. W. , Mermoud, C. , Pichon, S. , Koush, Y. , Van De Ville, D. , … Scharnowski, F. (2014). Self‐regulation of inter‐hemispheric visual cortex balance through real‐time fMRI neurofeedback training. NeuroImage, 100, 1–14. 10.1016/j.neuroimage.2014.05.072 [DOI] [PubMed] [Google Scholar]
- Rosenstiel, A. K. , & Keefe, F. J. (1983). The use of coping strategies in chronic low back pain patients: Relationship to patient characteristics and current adjustment. Pain, 17(1), 33–44. 10.1016/0304-3959(83)90125-2 [DOI] [PubMed] [Google Scholar]
- Rota, G. , Handjaras, G. , Sitaram, R. , Birbaumer, N. , & Dogil, G. (2011). Reorganization of functional and effective connectivity during real‐time fMRI‐BCI modulation of prosody processing. Brain and Language, 117(3), 123–132. 10.1016/j.bandl.2010.07.008 [DOI] [PubMed] [Google Scholar]
- Rota, G. , Sitaram, R. , Veit, R. , Erb, M. , Weiskopf, N. , Dogil, G. , & Birbaumer, N. (2009). Self‐regulation of regional cortical activity using real‐time fMRI: The right inferior frontal gyrus and linguistic processing. Human Brain Mapping, 30(5), 1605–1614. 10.1002/hbm.20621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharnowski, F. , Hutton, C. , Josephs, O. , Weiskopf, N. , & Rees, G. (2012). Improving visual perception through neurofeedback. Journal of Neuroscience, 32(49), 17830–17841. 10.1523/JNEUROSCI.6334-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharnowski, F. , Rosa, M. J. , Golestani, N. , Hutton, C. , Josephs, O. , Weiskopf, N. , & Rees, G. (2014). Connectivity changes underlying neurofeedback training of visual cortex activity. PLoS ONE, 9(3), e91090 10.1371/journal.pone.0091090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharnowski, F. , Veit, R. , Zopf, R. , Studer, P. , Bock, S. , Diedrichsen, J. , … Weiskopf, N. (2015). Manipulating motor performance and memory through real‐time fMRI neurofeedback. Biological Psychology, 108, 85–97. 10.1016/j.biopsycho.2015.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheinost, D. , Stoica, T. , Saksa, J. , Papademetris, X. , Constable, R. T. , Pittenger, C. , & Hampson, M. (2013). Orbitofrontal cortex neurofeedback produces lasting changes in contamination anxiety and resting‐state connectivity. Translational Psychiatry, 3(November 2012), e250 10.1038/tp.2013.24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheinost, D. , Stoica, T. , Wasylink, S. , Gruner, P. , Saksa, J. , Pittenger, C. , & Hampson, M. (2014). Resting state functional connectivity predicts neurofeedback response. Frontiers in Behavioral Neuroscience, 8(September), 338 10.3389/fnbeh.2014.00338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwood, M. S. , Kane, J. H. , Weisend, M. P. , & Parker, J. G. (2016). Enhanced control of dorsolateral prefrontal cortex neurophysiology with real‐time functional magnetic resonance imaging (rt‐fMRI) neurofeedback training and working memory practice. NeuroImage, 124, 214–223. 10.1016/j.neuroimage.2015.08.074 [DOI] [PubMed] [Google Scholar]
- Shibata, K. , Watanabe, T. , Sasaki, Y. , & Kawato, M. (2011). Perceptual learning incepted by decoded fMRI neurofeedback without stimulus presentation. Science, 334(6061), 1413–1415. 10.1126/science.1212003.Perceptual [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmonite, M. , & Polk, T. A. (2019). Independent components of neural activation associated with 100 days of cognitive training. Journal of Cognitive Neuroscience, 31(2), 808–820. 10.1162/jocn [DOI] [PubMed] [Google Scholar]
- Sitaram, R. , Ros, T. , Stoeckel, L. E. , Haller, S. , Scharnowski, F. , Lewis‐Peacock, J. , … Sulzer, J. (2016). Closed‐loop brain training: The science of neurofeedback. Nature Neuroscience, 18, 86–100. 10.1038/nrn.2016.164 [DOI] [PubMed] [Google Scholar]
- Skouras, S. , & Scharnowski, F. (2019). NeuroImage the effects of psychiatric history and age on self‐regulation of the default mode network. NeuroImage, 198(June 2018), 150–159. 10.1016/j.neuroimage.2019.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skouras, S. , Torner, J. , Anderson, P. , Koush, Y. , Falcon, C. , Minguillon, C. , & Molinuevo, J. (2019). The effect of APOE genotype and streamline density volume, on hippocampal CA1 down‐regulation: a real‐time fMRI virtual reality neurofeedback study. bioRxiv, https://www.biorxiv.org/content/10.1101/643577v1. [Google Scholar]
- Skouras, S. , Torner, J. , Andersson, P. , Koush, Y. , Falcon, C. , Minguillon, C. , … Molinuevo, J. L. (2020). Earliest amyloid and tau deposition modulate the influence of limbic networks during closed‐loop hippocampal downregulation. Brain: A Journal of Neurology, 143(3), 976–992. 10.1093/brain/awaa011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorger, B. , Kamp, T. , Weiskopf, N. , Peters, J. C. , & Goebel, R. (2018). N EUROSCIENCE when the brain takes ‘BOLD’ steps: Real‐time fMRI neurofeedback can further enhance the ability to gradually self‐regulate regional brain activation. Neuroscience, 378, 71–88. 10.1016/j.neuroscience.2016.09.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spetter, M. S. , Malekshahi, R. , Birbaumer, N. , Lührs, M. , van der Veer, A. H. , Scheffler, K. , … Hallschmid, M. (2017). Volitional regulation of brain responses to food stimuli in overweight and obese subjects: A real‐time fMRI feedback study. Appetite, 112, 188–195. 10.1016/j.appet.2017.01.032 [DOI] [PubMed] [Google Scholar]
- Spielberger, C. D. (2010). State‐trait anxiety inventory, Hoboken, New Jersey: John Wiley & Sons, Inc. [Google Scholar]
- Stumpf, H. , & Fay, E. (1983). Schlauchfiguren: ein Test zur Beurteilung des räumlichen Vorstellungsvermögens. Hogrefe: Verlag Für Psychologie. [Google Scholar]
- Subramanian, L. , Hindle, J. V. , Johnston, S. , Roberts, M. V. , Husain, M. , Goebel, R. , & Linden, D. (2011). Real‐time functional magnetic resonance imaging neurofeedback for treatment of Parkinson's disease. Journal of Neuroscience, 31(45), 16309–16317. 10.1523/JNEUROSCI.3498-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulzer, J. , Sitaram, R. , Blefari, M. L. , Kollias, S. , Birbaumer, N. , Stephan, K. E. , & Gassert, R. (2013). Neurofeedback‐mediated self‐regulation of the dopaminergic midbrain. NeuroImage, 75, 176–184. 10.1016/B978-0-12-384719-5.00424-X [DOI] [PubMed] [Google Scholar]
- Suurmond, R. , & Hak, T. (2017). Introduction, comparison, and validation of meta ‐ essentials: A free and simple tool for meta ‐ analysis. Res Synth Methods, 8(4), 537–553. 10.1002/jrsm.1260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taschereau‐Dumouchel, V. , Cortese, A. , Chiba, T. , Knotts, J. D. , Kawato, M. , & Lau, H. (2018). Towards an unconscious neural reinforcement intervention for common fears. Proceedings of the National Academy of Sciences, 115(13), 201721572–201723475. 10.1073/pnas.1721572115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibault, R. T. , MacPherson, A. , Lifshitz, M. , Roth, R. R. , & Raz, A. (2018). Neurofeedback with fMRI: A critical systematic review. NeuroImage, 172(September 2017), 786–807. 10.1016/j.neuroimage.2017.12.071 [DOI] [PubMed] [Google Scholar]
- Witte, M. , Kober, S. E. , Ninaus, M. , Neuper, C. , & Wood, G. (2013). Control beliefs can predict the ability to up‐regulate sensorimotor rhythm during neurofeedback training. Frontiers in Human Neuroscience, 7(August), 1–8. 10.3389/fnhum.2013.00478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao, S. , Becker, B. , Geng, Y. , Zhao, Z. , Xu, X. , Zhao, W. , … Kendrick, K. M. (2016). Voluntary control of anterior insula and its functional connections is feedback‐independent and increases pain empathy. NeuroImage, 130, 230–240. 10.1016/j.neuroimage.2016.02.035 [DOI] [PubMed] [Google Scholar]
- Yoo, S.‐S. , Lee, J.‐H. , O'Leary, H. , Panych, L. , & Jolesz, F. A. (2008). Neurofeedback fMRI‐mediated learning and consolidation of regional brain activation during motor imagery. International Journal of Imaging Systems and Technology, 18(1), 69–78. 10.1002/ima.20139.Neurofeedback [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young, K. D. , Siegle, G. J. , Zotev, V. , Phillips, R. , Misaki, M. , Yuan, H. , … Bodurka, J. (2017). Randomized clinical trial of real‐time fMRI amygdala neurofeedback for major depressive disorder: Effects on symptoms and autobiographical memory recall. American Journal of Psychiatry, 20, 748–755. 10.1176/appi.ajp.2017.16060637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young, K. D. , Zotev, V. , Phillips, R. , Misaki, M. , Yuan, H. , Drevets, W. C. , & Bodurka, J. (2014). Real‐time fMRI neurofeedback training of amygdala activity in patients with major depressive disorder. PLoS ONE, 9(2), e88785 10.1371/journal.pone.0088785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerssen, D. (1976). Die Befindlichkeitsskala (BS)—ein einfaches Instrument zur Objektivierung von Befindlichkeitsstörungen, insbesondere im Rahmen von Längsschnittuntersuchungen. Arzneimittelforschung, 20, 915–918. [PubMed] [Google Scholar]
- Zilverstand, A. , Sorger, B. , Sarkheil, P. , & Goebel, R. (2015). fMRI neurofeedback facilitates anxiety regulation in females with spider phobia. Frontiers in Behavioral Neuroscience, 9(June), 1–12. 10.3389/fnbeh.2015.00148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoefel, B. , Huster, R. J. , & Herrmann, C. S. (2011). Neurofeedback training of the upper alpha frequency band in EEG improves cognitive performance. NeuroImage, 54(2), 1427–1431. 10.1016/j.neuroimage.2010.08.078 [DOI] [PubMed] [Google Scholar]
- Zotev, V. , Krueger, F. , Phillips, R. , Alvarez, R. P. , Simmons, W. K. , Bellgowan, P. , … Bodurka, J. (2011). Self‐regulation of amygdala activation using real‐time FMRI neurofeedback. PLoS ONE, 6(9), e24522 10.1371/journal.pone.0024522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweerings, J. , Hummel, B. , Keller, M. , Zvyagintsev, M. , Schneider, F. , Klasen, M. , & Mathiak, K. (2019). Neurofeedback of core language network nodes modulates connectivity with the default‐mode network: A double‐blind fMRI neurofeedback study on auditory verbal hallucinations. NeuroImage, 189, 533–542. 10.1016/j.neuroimage.2019.01.058 [DOI] [PubMed] [Google Scholar]
- Zich, C. , Johnstone, N. , Lührs, M. , Lisk, S. , Haller, S. P. W. , Lipp, A. , & Kadosh, K. C. (2020). Modulatory effects of dynamic fMRI‐based neurofeedback on emotion regulation networks in adolescent females. NeuroImage, 117053 10.1016/j.neuroimage.2020.117053. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Supporting informaion
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


