Abstract
Alveolar soft part sarcoma (ASPS), epithelioid sarcoma (ES), and clear cell sarcoma (CCS) are known to be chemoresistant tumors. The aim of this study was to investigate the effect of pazopanib on these chemoresistant tumors. This study is designed as a single‐arm, multicenter, investigator‐initiated phase II trial. Patient enrollment was undertaken between July 2016 and August 2018 at 10 hospitals participating in the Japanese Musculoskeletal Oncology Group. The primary end‐point is the CBR (CBR, including complete or partial response and stable disease) at 12 weeks after treatment with pazopanib according to RECIST. Eight patients were enrolled within the period. The histological subtypes were 5 ASPS, 2 ES, and 1 CCS. The median follow‐up period was 22.2 (range, 4.9‐24.9) months. All patients initially received pazopanib 800 mg once daily. The CBRs were 87.5% (7 of 8) and 75.0% (6 of 8) according to RECIST and Choi criteria at 12 weeks after pazopanib treatment, respectively. The CBRs at 12 weeks according to RECIST were 80.0%, 100.0%, and 100.0% in ASPS, ES, and CCS, respectively. Partial response was observed in 1 ASPS according to RECIST and 3 ASPS and 1 ES according to Choi criteria at 12 weeks after pazopanib treatment. This study documented antitumor activity of pazopanib, especially in ASPS. These results support the frontline use of pazopanib for ASPS. Prospective data collection is desired using both RECIST and Choi criteria for these rare chemoresistant tumors.
Keywords: alveolar soft part sarcoma, chemoresistant tumor, clear cell sarcoma, epithelioid sarcoma, pazopanib
This prospective study, carried out among the Japanese reference centers for treatment of sarcomas, confirmed the value of pazopanib in patients with metastatic or unresectable chemoresistant sarcomas (alveolar soft part sarcoma, epithelioid sarcoma, and clear cell sarcoma). Pazopanib showed antitumor activity in most patients with alveolar soft part sarcoma according to RECIST and Choi criteria. These results support the frontline use of pazopanib for alveolar soft part sarcoma.

Abbreviations
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- ASPS
alveolar soft part sarcoma
- CCS
clear cell sarcoma
- Choi
Choi criteria
- CR
complete response
- CT
computed tomography
- ES
epithelioid sarcoma
- JMOG
Japanese Musculoskeletal Oncology Group
- OS
overall survival
- PD
progressive disease
- PFS
progression‐free survival
- PR
partial response
- SD
stable disease
1. INTRODUCTION
Alveolar soft part sarcoma, ES, and CCS have different genetic backgrounds of ASPL‐TFE3 translocation, 1 inactivation of INI1, 2 and EWSR1‐ATF1 translocation, 3 respectively, and primarily affect young adults. 4 , 5 , 6 The mainstay of management for localized disease is wide surgical resection, but there are few effective chemotherapeutic treatments for patients with locally advanced or metastatic disease. Prognosis is poor in advanced or metastatic disease despite systemic treatments, and the median PFS for first‐line systemic treatments was reported as 7 months in ASPS, 7 4 months in ES, 8 and 11 weeks in CCS. 9 The median OS was reported as 40 months, 4 10.8 months, 8 and 8.9 months 10 in patients with locally advanced or metastatic ASPS, ES, and CCS, respectively.
Alveolar soft part sarcoma, ES, and CCS are known to be chemoresistant tumors that are resistant to cytotoxic agents. The response rates after use of various chemotherapies have been reported as 7%‐17%, 11 , 12 10%‐12%, 5 , 8 and 4%‐23% 6 , 9 in ASPS, ES, and CCS, respectively. Effective systemic treatment is particularly desired for these 3 soft tissue sarcoma subtypes.
Molecular targeted therapy has recently been tried for these conventionally chemotherapy‐resistant sarcomas of ASPS, 13 , 14 ES, 15 and CCS. 16 Pazopanib is a small molecule multitargeting tyrosine kinase inhibitor that inhibits vascular endothelial growth factor receptor‐1, ‐2, and ‐3, platelet‐derived growth factor receptor‐a and ‐b, and c‐Kit. 17 The effectiveness of pazopanib for nonadipocytic soft tissue sarcoma was determined in the phase III, randomized, double‐blinded PALETTE trial. 18 Pazopanib was approved by regulatory agencies and widely used for soft tissue sarcomas in clinical practice, but there is little information about the effectiveness of pazopanib for these histological subtypes, especially for ES and CSC. 19 The aim of this study was to investigate the effect of pazopanib on chemoresistant sarcomas including ASPS, ES, and CCS.
2. MATERIALS AND METHODS
2.1. Study design
This study was undertaken to investigate the effect of pazopanib on chemoresistant sarcomas (ASPS, ES, and CCS; cohort 2). We aimed to enroll 23 cases considering feasibility rather than statistical considerations because of the extreme rarity of each of these chemoresistant sarcomas. This trial is a single‐arm, multicenter, investigator‐initiated phase II trial. This study was approved by the ethics committee of Nagoya University Graduate School and School of Medicine (Nagoya, Japan) in January 2016, and the registration number was 2015‐0371, and also approved by each study site’s institutional review board. This trial was registered in the UMIN Clinical Trials Registry as UMIN000019303 (http://www.umin.ac.jp/ctr/index.htm).
2.2. Patients
The patient registration was discontinued in August 2018. Eight patients with chemoresistant sarcoma were eligible and included in our study at hospitals participating in the JMOG between July 2016 and August 2018. All participating hospitals are referral sarcoma centers in Japan. The key inclusion criteria were patients with unresectable or metastatic chemoresistant tumors including ASPS, ES, and CCS, disease progression within 6 months, and measurable tumors according to RECIST version 1.1. 20 The key exclusion criteria were age under 18 years, cerebrovascular disease, gastrointestinal disorder, bleeding tendency, hypersensitivity, and pregnancy. Entry criteria included ECOG performance status of 0 or 1 (http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcv20_4‐30‐992.pdf), no/asymptomatic central nervous system metastases or leptomeningeal metastases, and adequate bone marrow function (absolute neutrophil count 1500 cells/μL or more, platelets 10×104 per μL or more, and hemoglobin 9 g/dL or more), renal function (serum creatinine 1.5 mg/dL or less, or, if more than 1.5 mg/dL, calculated creatinine clearance greater than 50 mL/min), hepatic function (bilirubin 1.5× upper limit of normal or less, aspartate aminotransferase and alanine aminotransferase 2.5× upper limit of normal or less), and cardiac function (based on the institution’s lower limit of normal [left ventricular ejection fraction assessed by multigated acquisition scan or echocardiogram], normal 12‐lead electrocardiogram [no prolongation of corrected QT interval greater than 480 ms] and no history of any of the following in the past 6 months: cardiac angioplasty or stenting, myocardial infarction, unstable angina, coronary artery bypass graft surgery, symptomatic peripheral vascular disease, or class III or IV congestive heart failure, as defined by the New York Heart Association). Blood pressure had to be below 140/90 mm Hg, spontaneously or controlled with antihypertensive medication. Anticoagulant therapy was permitted with stable coagulation tests. Patients with recent (within 6 months) thromboembolic events who were stable, taking anticoagulation drugs for at least 6 weeks, were eligible. Written and informed consent was obtained from all patients. All 8 patients were treated with pazopanib and analyzed in our study.
2.3. Treatments and assessment procedures
All 8 patients were treated with pazopanib (Votrient; Novartis Pharmaceuticals) orally at a dose of 800 mg once daily. Dose modifications for adverse events were made according to the protocol. Treatment was continued until disease progression (according to both RECIST 20 and modified Choi criteria [Choi] 21 , 22 ), unacceptable toxic effects, withdrawal of consent, or death. Enhanced CT was carried out at base line, and at 3, 7, and 12 weeks after the initiation of treatment and at 8‐week intervals thereafter. Tumor responses were evaluated using investigator‐assessed RECIST 20 and Choi. 21 , 22 Clinical assessments, including medical history and physical examination, and laboratory assessment, of safety, were carried out at baseline, at 1, 3, 5, 7, 9, and 12 weeks after the initiation of treatment, and at 4‐week intervals thereafter. Adverse events were graded according to the NCI’s Common Terminology Criteria for Adverse Events version 4.03 (https://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/CTCAE_4.03.xlsx). A serious adverse event was defined as any untoward medical occurrence at any dose that results in death, is life‐threatening, requires inpatient hospitalization or causes prolongation of existing hospitalization, results in persistent or significant disability/incapacity, might have caused a congenital anomaly/birth defect, or requires intervention to prevent permanent impairment or damage.
2.4. Statistical analysis
Data collection using electronic data capture and analysis were carried out in the Center for Advanced Medicine and Clinical Research at Nagoya University Hospital. The primary end‐point of this study was the clinical benefit rate (including complete or partial response and stable disease) at 12 weeks after treatment with pazopanib according to RECIST. The secondary end‐points were the clinical benefit rate at 12 weeks after pazopanib treatment according to Choi, response rate at 12 weeks after treatment according to RECIST and Choi, PFS according to RECIST and Choi, OS, and safety. Progression‐free survival was defined as the time from study entry until disease progression or death due to any cause, and OS as the time from study entry until death due to any cause. Estimated PFS and OS rates were calculated using Kaplan‐Meier product limit methods. Follow‐up period was defined as the time from study entry until last visit at the time of data cut‐off. Data cut‐off was undertaken on 1 September 2019, and the median follow‐up period was 22.2 (range, 4.9‐24.9) months. The relative dose intensity is the ratio of “delivered” to “planned” dose intensity and is expressed as a percentage.
3. RESULTS
3.1. Demographic characteristics
The histological subtypes were 5 ASPS, 2 ES, and 1 CCS. There were 4 male patients and 4 female patients with a median age of 32.5 (range, 18‐76) years. Patient demographics are summarized in Table 1. The sites of primary tumor were extremity in 7 and viscera in 1. The median treatment period of pazopanib was 11.0 (range, 1.0‐24.9) months. In 1 patient (case 5), pazopanib treatment was stopped after progression of disease according to RECIST and Choi criteria; in another patient (case 3) it was stopped after progression of disease only according to RECIST. Treatment was discontinued due to side‐effects (pneumothorax, 1 [case 1]; fatigue, 1 [case 2]; and infection, 1 [case 6]) in 3 patients (37.5%) receiving pazopanib, and dose reductions were undertaken in 4 (50.0%). The relative dose intensity was 87.9%. The median follow‐up period after using pazopanib was 22.2 (range, 4.9‐24.9) months. At the last follow‐up, 3 patients had continuously received pazopanib. Three patients experienced disease progression according to both the RECIST and Choi criteria during the follow‐up period. After the discontinuation of pazopanib treatment, 3 patients were treated with systemic therapies, which included 2 pazopanib rechallenges outside of this trial (case 1 and case 6) and 1 nivolumab in a clinical trial (case 2).
TABLE 1.
Demographics of 8 patients with metastatic or unresectable chemoresistant sarcomas
| Characteristics | Value (range) or No. of patients (%) |
|---|---|
| Sex | |
| Male | 4 (50.0) |
| Female | 4 (50.0) |
| ECOG performance status | |
| 0 | 7 (87.5) |
| 1 | 1 (12.5) |
| Age, years | |
| Median (range) | 32.5 (18‐76) |
| Tumor status | |
| Unresectable | 2 (25.0) |
| Metastasis | 6 (75.0) |
| Size of primary tumor, cm | |
| ≤5 | 3 (37.5) |
| >5 | 5 (62.5) |
| Anatomic sites of primary tumors | |
| Extremity | 7 (87.5) |
| Viscera | 1 (12.5) |
| Depth of primary tumors | |
| Superficial | 0 (0.0) |
| Deep | 8(100.0) |
| Histological subtype | |
| Alveolar soft part sarcoma | 5 (62.5) |
| Epithelioid sarcoma | 2 (25.0) |
| Clear cell sarcoma | 1 (12.5) |
| Follow‐up, mo | |
| Median (range) | 22.2(4.9‐24.9) |
3.2. Clinical outcomes
The treatment outcomes for individual patients are shown in Table 2 and Figure 1. Treatment outcomes after starting pazopanib are summarized in Table 3. Responses according to RECIST were PR in 1 (12.5%), SD in 6 (75.0%), and PD in 1 (12.5%). Responses according to Choi were PR in 4 (50.0%), SD in 2 (25.0%), and PD in 2 (25.0%) at 12 weeks after starting pazopanib. The clinical benefit rates were 87.5% (7 of 8 patients) and 75.0% (6 of 8 patients) according to the RECIST and Choi criteria, respectively, at 12 weeks after pazopanib treatment. In terms of histological subtype, the clinical benefit rates at 12 weeks according to RECIST were 80.0% (4 of 5 patients), 100.0% (2 of 2 patients), and 100.0% (1 of 1 patient) in ASPS, ES, and CCS, respectively. The clinical benefit rates at 12 weeks according to Choi were 80.0% (4 of 5 patients), 100.0% (2 of 2 patients), and 0.0% (0 of 1 patient) in ASPS, ES, and CCS, respectively. The response rates were 12.5% (1 of 8 patients) and 50.0% (4 of 8 patients) according to RECIST and Choi criteria, respectively, at 12 weeks after treatment with pazopanib. In terms of histological subtype, the response rates at 12 weeks according to RECIST were 20.0% (1 of 5 patients), 0.0% (0 of 2 patients), and 0.0% (0 of 1 patient). The response rates at 12 weeks according to Choi were 60.0% (3 of 5 patients), 50.0% (1 of 2 patients), and 0.0% (0 of 1 patient) in ASPS, ES, and CCS, respectively. The median decreases in tumor diameter and tumor density from baseline were 11.0% and 30.5% at 12 weeks (Figure 2). Median PFS in RECIST and Choi, and OS were 15.7 months, 15.7 months, and not reached, respectively (Table 3 and Figure 3). In terms of histological subtype, the median PFS according to RECIST was 15.6, 4.9, and 10.3 months, and the median PFS according to Choi criteria was 15.6 months, not reached, and 3.0 months in ASPS, ES, and CCS, respectively. The median OS was not reached in ASPS or ES, and 23.2 months in CCS.
TABLE 2.
Patient characteristics and treatment outcomes for individuals with metastatic or unresectable chemoresistant sarcomas treated with pazopanib
| Case | Age (y)/sex | ECOG PS | Histological subtype | Site of primary tumor | Metastatic sites | No. of prior chemotherapy regimens | Response to pazopanib at 12 wk (RECIST) | PFS, mo (RECIST)/PD or non‐PD | Response to pazopanib at 12 wk (Choi) | PFS, mo (Choi)/PD or non‐PD | OS, mo | Final status |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 22/Male | 1 | ES | Foot | None | 1 | SD | 23.6/non‐PD | SD | 23.6/non‐PD | 23.6 | AWD |
| 2 | 49/Female | 0 | CCS | Small intestine | None | 1 | SD | 10.3/PD | PD | 3.0/PD | 23.2 | DOD |
| 3 | 76/Female | 0 | ES | Shoulder | Lung | 0 | SD | 4.9/PD | PR | 4.9/non‐PD | 4.9 | AWD |
| 4 | 32/Male | 0 | ASPS | Thigh | Lung, pancreas, kidney, pelvis | 2 | SD | 24.9/non‐PD | SD | 24.9/non‐PD | 24.9 | AWD |
| 5 | 18/Male | 0 | ASPS | Buttock | Lung | 0 | SD | 15.6/PD | PR | 15.6/PD | 24.2 | AWD |
| 6 | 33/Female | 0 | ASPS | Thigh | Lung | 0 | PD | 3.1/PD | PD | 1.7/PD | 21.2 | AWD |
| 7 | 27/Male | 0 | ASPS | Elbow | Lung, bone, lymph nodes | 1 | PR | 14.7/non‐PD | PR | 14.7/non‐PD | 14.7 | AWD |
| 8 | 36/Female | 0 | ASPS | Thigh | Lung | 1 | SD | 15.3/non‐PD | PR | 15.3/non‐PD | 15.3 | AWD |
ASPS, alveolar soft part sarcoma; AWD, alive with disease; CCS, clear cell sarcoma; Choi, Choi criteria; DOD, dead of disease; ES, epithelioid sarcoma; OS, overall survival; PD, progressive disease; PFS, progression‐free survival; PR, partial response; PS, performance status; SD, stable disease.
FIGURE 1.
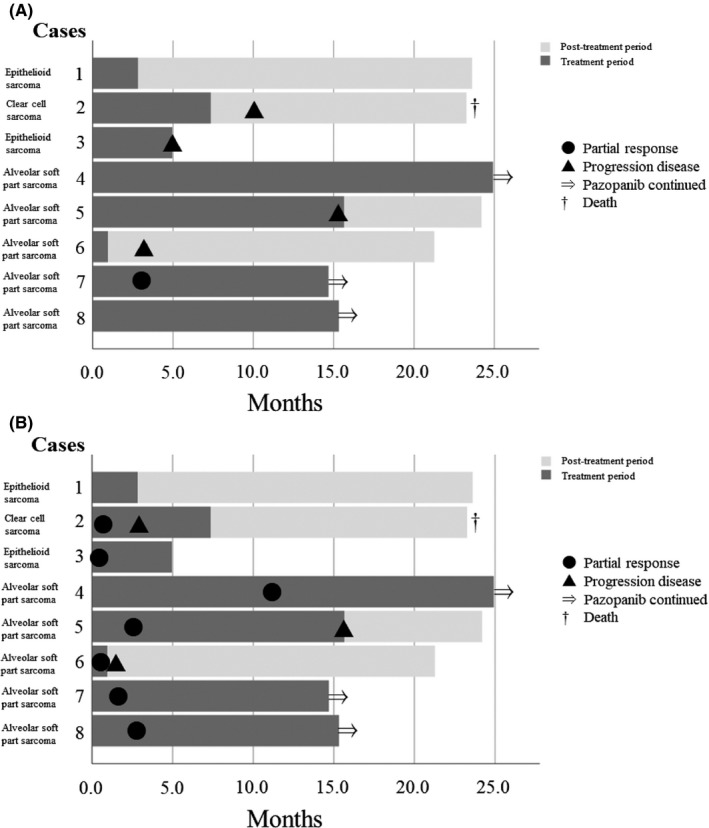
Swimmer’s plot of the clinical course of 8 patients with metastatic or unresectable chemoresistant sarcomas treated with pazopanib. A, Swimmer’s plot using RECIST. B, Swimmer’s plot using Choi criteria
TABLE 3.
Summary of treatment outcomes for 8 patients with metastatic or unresectable chemoresistant sarcomas treated with pazopanib
| Category | No. of patients (%) or value (for each histological subtype) |
|---|---|
| Response to pazopanib at 12 wk (RECIST), n = 8 | |
| PR | 1 (12.5%) |
| SD | 6 (75.0%) |
| PD | 1 (12.5%) |
| Response rate at 12 wk (RECIST) | 12.5% (ASPS 20.0%, ES 0.0%, CCS 0.0%) |
| Clinical benefit rate at 12 wk (RECIST) | 87.5% (ASPS 80.0%, ES 100.0%, CCS 100.0%) |
| Median PFS (RECIST), mo | 15.7 (ASPS 15.6, ES 4.9, CCS 10.3) |
| Response to pazopanib at 12 wk (Choi), n = 8 | |
| PR | 4 (50.0%) |
| SD | 2 (25.0%) |
| PD | 2 (25.0%) |
| Response rate at 12 wk (Choi) | 50.0% (ASPS 60.0%, ES 50.0%, CCS 0.0%) |
| Clinical benefit rate at 12 wk (Choi) | 75.0% (ASPS 80.0%, ES 100.0%, CCS 0.0%) |
| Median PFS (Choi), mo | 15.7 (ASPS 15.6, ES not reached, CCS 3.0) |
| Median OS, mo | Not reached (ASPS not reached, ES not reached, CCS 23.2) |
ASPS, alveolar soft part sarcoma; CCS, clear cell sarcoma; Choi, Choi criteria; ES, epithelioid sarcoma; OS, overall survival; PD, progressive disease; PFS, progression‐free survival; PR, partial response; SD, stable disease.
FIGURE 2.
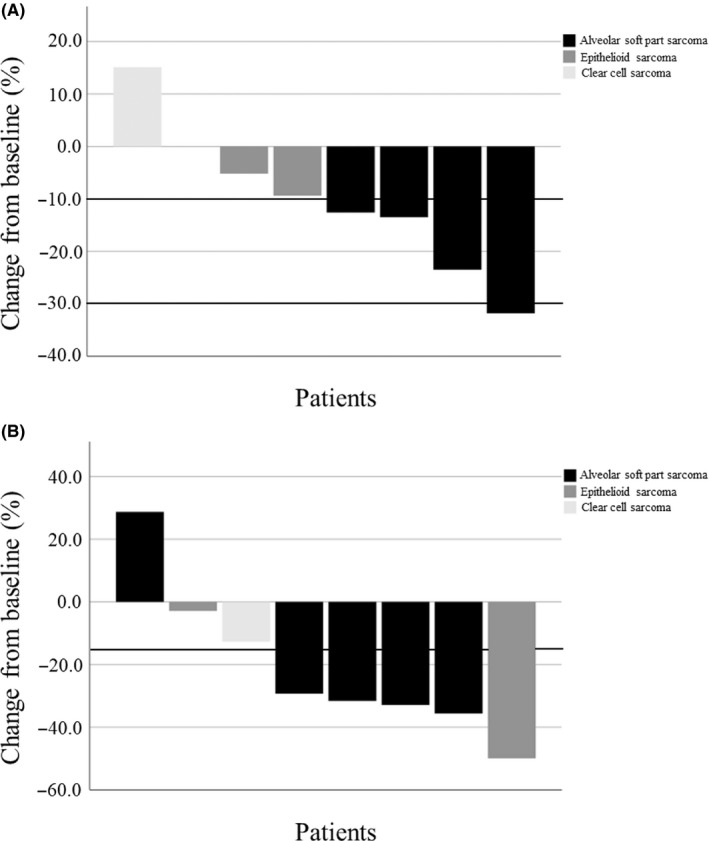
Response to treatment with pazopanib at 12 weeks in 8 patients with metastatic or unresectable chemoresistant sarcomas. A, Change in tumor diameter from baseline, B, Change in tumor density from baseline
FIGURE 3.
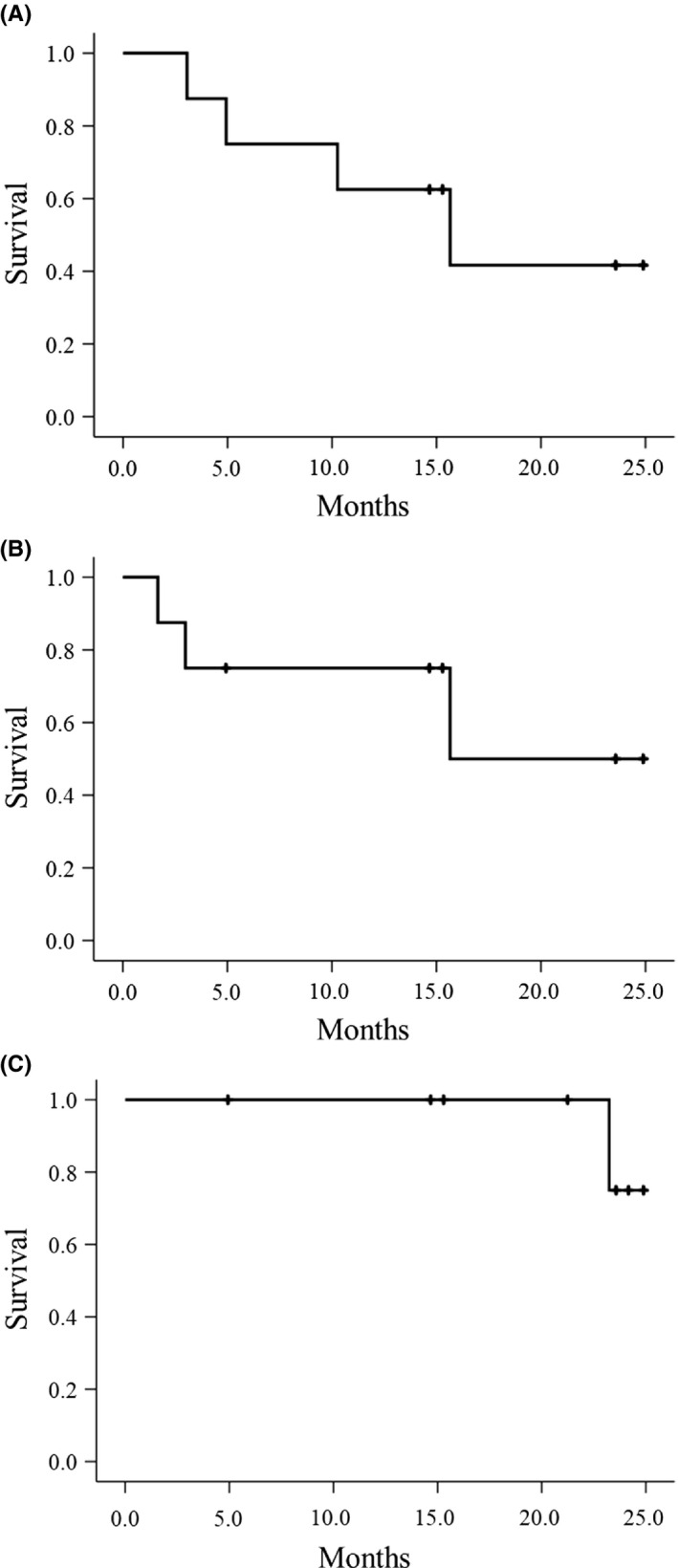
Kaplan‐Meier curves for survival among 8 patients with metastatic or unresectable chemoresistant sarcomas treated with pazopanib. A, Progression‐free survival according to RECIST. B, Progression‐free survival according to Choi criteria. C, Overall survival
3.3. Safety
Table 4 shows the main adverse events. The most common adverse events were increased ALT (75.0%, 6 of 8), increased AST (62.5%, 5 of 8), hypertension, fatigue, nausea, and anorexia (25.0%, 2 of 8, for each). Grade 3 adverse events such as increased ALT in 2, increased AST in 2, hypertension in 1, fatigue in 1, decreased platelet count in 1, increased lipase in 1, pneumothorax in 1, pancreatitis in 1, diarrhea in 1, and back pain in 1, were observed. Serious adverse events were observed in 3 (pneumothorax in 1, pancreatitis in 1, and fatigue and back pain in 1), but there were no fatal adverse events.
TABLE 4.
Adverse events in 8 patients with metastatic or unresectable chemoresistant sarcomas treated with pazopanib a
| All grades | Grade 3 | Grade 4 | |
|---|---|---|---|
| ALT increased | 6 (75.0) | 2 (25.0) | 0 (0.0) |
| AST increased | 5 (62.5) | 2 (25.0) | 0 (0.0) |
| Hypertension | 2 (25.0) | 1 (12.5) | 0 (0.0) |
| Fatigue | 2 (25.0) | 1 (12.5) | 0 (0.0) |
| Nausea | 2 (25.0) | 0 (0.0) | 0 (0.0) |
| Anorexia | 2 (25.0) | 0 (0.0) | 0 (0.0) |
| Platelet count decreased | 1 (12.5) | 1 (12.5) | 0 (0.0) |
| Lipase increased | 1 (12.5) | 1 (12.5) | 0 (0.0) |
| Pneumothorax | 1 (12.5) | 1 (12.5) | 0 (0.0) |
| Pancreatitis | 1 (12.5) | 1 (12.5) | 0 (0.0) |
| Diarrhea | 1 (12.5) | 1 (12.5) | 0 (0.0) |
| Back pain | 1 (12.5) | 1 (12.5) | 0 (0.0) |
Data are shown as n (%).
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase.
Adverse events with a frequency of 20% or higher or grade 3 or higher.
4. DISCUSSION
This study targeted chemoresistant tumors of ASPS, ES, and CCS. The median PFS for first‐line systemic treatments was reported as 7 months, 7 4 months, 8 and 11 weeks 9 in ASPS, ES, and CCS, respectively. For these tumors, conventional chemotherapy cannot be expected to be effective, and so there is a great need for devising effective new systemic treatments. In our study, the primary end‐point of clinical benefit rate in RECIST was 87.5% (7 of 8 patients). In histological subtype, the clinical benefit rates at 12 weeks in RECIST were 80.0%, 100.0%, and 100.0% in ASPS, ES, and CCS, respectively, indicating relatively good short‐term disease control in these chemoresistant tumors. In soft tissue sarcoma, the association between prognosis and amount of tumor shrinkage has not been found conclusive based on previous reports, 23 , 24 and we used the clinical benefit rate (CR + PR + SD) at 12 weeks according to RECIST as the primary end‐point to evaluate the short‐term efficacy of pazopanib.
Advantages of this study include the prospective observation using both RECIST and Choi criteria. Choi criteria were initially proposed as new CT response criteria 21 based on the correlation between CT density and PET in patients with metastatic gastrointestinal stromal tumor treated with imatinib mesylate. Subsequently, they were applied to high‐grade soft tissue sarcomas, with a relationship between the Choi criteria and pathologic response observed. 22 Evaluation based on the Choi criteria seems to be of some benefit in patients with soft tissue sarcomas who will be treated with pazopanib. Tumor shrinkage after pazopanib treatment is usually inferior to that achieved with cytotoxic chemotherapy in soft tissue sarcomas, 25 whereas a significant decrease in tumor density was noted in responders after pazopanib treatment in soft tissue sarcomas. 26 In our study, the clinical benefit rate at 12 weeks was 87.5% according to RECIST, compared to 75.0% according to Choi criteria, whereas the response rate at 12 weeks was 12.5% according to RECIST, compared to 50.0% according to Choi criteria. As shown in Figure 1, 3 cases of ASPS (cases 5, 6, and 8) and 1 each of ES (case 3) and CCS (case 2) showed PR in Choi criteria, but not in RECIST. Although long‐term follow‐up will be needed to assess the relationship between Choi’s evaluation and OS, investigations of the relationship between Choi’s evaluation and OS and/or histological response will be required.
After the initiation of this study, some prospective and retrospective reports on the effectiveness of pazopanib in ASPS were published. Kim et al reported a phase II trial of pazopanib in patients with metastatic ASPS. 27 Six patients were enrolled in the study, with 1 patient achieving PR, and 5 patients showing SD according to RECIST (response rate 16.7%). With a median follow‐up of 33 months, median PFS was 5.5 months and median OS was not reached. Stacchiotti et al undertook a retrospective study to evaluate the activity of pazopanib on metastatic ASPS. 14 In 29 evaluable patients, the best responses were 1 CR, 7 PR, 17 SD, and 4 PD according to RECIST (response rate 27.6%). At the median follow‐up of 19 months, median PFS was 13.6 months, and median OS was not reached. In the retrospective JMOG study to evaluate clinical outcomes in Japanese patients with relapsed soft tissue sarcoma, 4 PR, 4 SD, and 1 PD (response rate 44.4%) were observed according to RECIST in 9 evaluable ASPS patients after pazopanib treatment. 19 In our study, the response rate of ASPS was 20.0% in RECIST, which is comparable to the results of previous reports. The best response rate of ASPS using RECIST was 35.7% (5 of 14) after pazopanib treatment in combined results of previous 19 and this JCOG studies. Median PFS was 15.6 months according to both RECIST and Choi criteria in patients with ASPS, which is also comparable to the results obtained in a previous study. Alveolar soft part sarcoma has highly vascular properties and abnormal expression of genes related to angiogenesis, 28 leading to hope that antiangiogenic drugs including pazopanib will be effective against it. Indeed, other antiangiogenic drugs, such as cediranib and sunitinib, have similarly shown activity against ASPS. 13 , 29 The response rates after treatment with cediranib or sunitinib were reported as 19.4% and 55.6%, respectively, according to RECIST. A recent study proposed that antiangiogenics such as pazopanib be made available for clinical use as first‐line medical options for an extremely rare disease such as ASPS in which cytotoxics are clearly ineffective. 14 Metastatic ASPS could be especially resistant to chemotherapy, 30 and so first‐line use of pazopanib should be considered.
There are relatively few reports in patients with ES. In a multiinstitutional case series of ES, 18 patients were treated with pazopanib. 31 There were no objective responses (response rate 0.0%) according to RECIST, and the median PFS was 3 months. In another multiinstitutional case series of ES, 1 PR, 4 SD, and 4 PD (response rate 11.1%) were observed according to RECIST in 9 ES patients after pazopanib treatment. 8 In contrast, 1 PR was observed according to Choi criteria in 2 patients with advanced ES in our study. This suggested that pazopanib might have some activity in patients with ES. Recently, a phase II multicenter study of the EZH2 inhibitor tazemetostat was undertaken in adults with INI1‐negative ES (NCT02601950). This trial included 62 ES patients and the response rate was 15% after using tazemetostat (https://www.ascopost.com/issues/march‐10‐2020/tazemetostat‐for‐advanced‐epithelioid‐sarcoma/), indicating the feasibility of clinical trials in this rare tumor.
There are very few reports of pazopanib in CCS. Only a single preclinical study showed activity of pazopanib on a CCS cell line in vitro and in vivo. 32 In our study, there were no objective responses according to either RECIST or Choi criteria in a patient with CCS. In 26 MET‐positive CCS cases, 1 patient (3.8%) achieved a confirmed PR and 17 (65.4%) had SD after treatment with crizotinib. 16 In our study, the number of CCS cases was small, precluding the drawing of any definitive conclusions. Further prospective evaluation will be needed in patients with CCS who will be treated with pazopanib.
As shown in Figure 1, 3 of 5 patients with ASPS were still receiving pazopanib at the final follow‐up. This could partially be attributed to the slow progressive nature of this tumor, but the PFS in ASPS was reported as 7 months for first‐line systemic treatments. 7 All 3 patients have been on treatment for more than 14 months, indicating the relatively greater efficacy of pazopanib for ASPS. In this study, there were no patients whose tumors became resectable after treatment with pazopanib. This could be because many patients had metastatic disease. The poor tumor shrinkage after treatment with pazopanib was also shown in the PALETTE trial, and the response rate in RECIST was 6% after treatment of pazopanib in nonadipocytic soft tissue sarcoma. 18 As shown in Figure 2, pazopanib showed relatively good tumor shrinkage and good decrease of tumor density in most of the 5 patients with ASPS. In contrast, little tumor shrinkage and 1 decrease of tumor density were observed in 2 patients with ES after treatment with pazopanib. The effect of pazopanib on ASPS is clear, but it might be worth trying if no other treatment is available for ES.
In the PFS analysis, median PFS according to both RECIST and Choi was 15.7 months after pazopanib treatment. In our study, only 3 of 8 patients received pazopanib as first‐line treatment, but better PFS was observed. The median PFS according to RECIST was 15.6, 4.9, and 10.3 months in ASPS, ES, and CCS, respectively, and better PFS was observed especially in ASPS. In the survival analysis, 1 patient died during the follow‐up with the median follow‐up period being 22.2 (range, 4.9‐24.9) months. In our study, 1 case each of ES and CCS (case 1 and case 2) stopped pazopanib treatment relatively early, but survived for more than 20 months. These 2 cases were treated with pazopanib rechallenge and nivolumab respectively, but it is not clear to what extent these treatments contributed to the prolongation of survival.
Pneumothorax is known as an adverse effect in the treatment with pazopanib. 19 In this study, 1 patient developed pneumothorax after pazopanib treatment and required insertion of a chest catheter. This patient had no further problems, but caution is needed because pazopanib causes bleeding and inhibits wound healing due to its inhibition of vascular endothelial growth factor.
There were some limitations in our study. The first is the small number of patients enrolled. The study initially planned for 23 patient enrollments, but only 8 were enrolled. The rarity of these chemoresistant sarcomas likely accounts for the difficulty in achieving adequate patient enrollment. At the same time, ASPS and CCS were studied in another clinical trial using nivolumab (clinical trial ID: UMIN000023665), which might also have affected the registration in our trial. Indeed, 1 patient with CCS was treated with nivolumab in this trial after disease progression. Second, we were not able to undertake statistical hypothesis testing in this study. It was difficult to set the statistical hypothesis testing due to the rarity of these chemoresistant sarcomas and the lack of historical studies, especially in ES and CCS. Third, this study included mixed subtypes of sarcoma and there was a clear bias in the histological subtypes registered in our study. There were some reports of the effectiveness of pazopanib in ASPS before and during the registration period, 14 , 19 and it seems that the registration of ASPS had increased. Alveolar soft part sarcoma is a relatively slowly progressing sarcoma with a better prognosis reported than the other 2 sarcomas, 4 and there is a possibility that it could have affected the better clinical outcomes in our study. Fourth, treatment after progression was determined by the attending physicians, and the use of pazopanib was not banned. Two patients received pazopanib rechallenge after discontinuation of pazopanib. Finally, postoperative treatment can affect survival, but the information related to this was limited.
This study showed antitumor activity of pazopanib in ASPS. These results support the front‐line use of pazopanib for ASPS. Prospective data collection is desired using both RECIST and Choi criteria for these rare chemoresistant tumors.
CONFLICT OF INTEREST
All authors declare no conflict of interest.
ETHICAL APPROVAL
This study was approved by the ethics committee of Nagoya University Graduate School and School of Medicine (Nagoya, Japan) in January 2016, and the registration number was 2015‐0371, and also approved by each study site's Institutional Review Board. This trial was registered in the UMIN Clinical Trials Registration as UMIN000019303 (http://www.umin.ac.jp/ctr/index.htm). All patients provided written informed consent.
ACKNOWLEDGMENTS
This work was supported by Novartis Pharmaceuticals Corporation. This study was designed as an investigator‐initiated trial and the study sponsor was not involved in study design, provision of drugs, data collection/analysis, writing of the report, or in the decision to submit the article for publication. We thank the patients who participated in our study, members of JMOG participating institutions, Data and Safety Monitoring Committee (Dr H. Sugiura, Dr A. Nagano, and Dr Y. Shido), the Center for Advanced Medicine and Clinical Research in Nagoya University Hospital (Dr Y. Kuwatsuka, Ms F. Sugiura, and Ms M. Ito), and employees of Novartis Pharmaceuticals Corporation (Mr T. Yonezu, Ms M. Endo, Mr R. Kano, and Ms M. Machida) for their contributions to this study.
Urakawa H, Kawai A, Goto T, et al. Phase II trial of pazopanib in patients with metastatic or unresectable chemoresistant sarcomas: A Japanese Musculoskeletal Oncology Group study. Cancer Sci. 2020;111:3303–3312. 10.1111/cas.14542
Contributor Information
Hiroshi Urakawa, Email: urakawa@med.nagoya-u.ac.jp.
Yoshihiro Nishida, Email: ynishida@med.nagoya-u.ac.jp.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Williams A, Bartle G, Sumathi VP, et al. Detection of ASPL/TFE3 fusion transcripts and the TFE3 antigen in formalin‐fixed, paraffin‐embedded tissue in a series of 18 cases of alveolar soft part sarcoma: useful diagnostic tools in cases with unusual histological features. Virchows Arch. 2011;458:291‐300. [DOI] [PubMed] [Google Scholar]
- 2. Modena P, Lualdi E, Facchinetti F, et al. SMARCB1/INI1 tumor suppressor gene is frequently inactivated in epithelioid sarcomas. Cancer Res. 2005;65:4012‐4019. [DOI] [PubMed] [Google Scholar]
- 3. Zucman J, Delattre O, Desmaze C, et al. EWS and ATF‐1 gene fusion induced by t(12;22) translocation in malignant melanoma of soft parts. Nat Genet. 1993;4:341‐345. [DOI] [PubMed] [Google Scholar]
- 4. Portera CA Jr, Ho V, Patel SR, et al. Alveolar soft part sarcoma: clinical course and patterns of metastasis in 70 patients treated at a single institution. Cancer. 2001;91:585‐591. [DOI] [PubMed] [Google Scholar]
- 5. Levy A, Le Pechoux C, Terrier P, et al. Epithelioid sarcoma: need for a multimodal approach to maximize the chances of curative conservative treatment. Ann Surg Oncol. 2014;21:269‐276. [DOI] [PubMed] [Google Scholar]
- 6. Kawai A, Hosono A, Nakayama R, et al. Clear cell sarcoma of tendons and aponeuroses: a study of 75 patients. Cancer. 2007;109:109‐116. [DOI] [PubMed] [Google Scholar]
- 7. Flores RJ, Harrison DJ, Federman NC, et al. Alveolar soft part sarcoma in children and young adults: A report of 69 cases. Pediatr Blood Cancer. 2018;65:e26953. [DOI] [PubMed] [Google Scholar]
- 8. Touati N, Schoffski P, Litiere S, et al. European organisation for research and treatment of cancer soft tissue and bone sarcoma group experience with advanced/metastatic epithelioid sarcoma patients treated in prospective trials: clinical profile and response to systemic therapy. Clin Oncol. 2018;30:448‐454. [DOI] [PubMed] [Google Scholar]
- 9. Jones RL, Constantinidou A, Thway K, et al. Chemotherapy in clear cell sarcoma. Med Oncol. 2011;28:859‐863. [DOI] [PubMed] [Google Scholar]
- 10. Gonzaga MI, Grant L, Curtin C, Gootee J, Silberstein P, Voth E. The epidemiology and survivorship of clear cell sarcoma: a National Cancer Database (NCDB) review. J Cancer Res Clin Oncol. 2018;144:1711‐1716. [DOI] [PubMed] [Google Scholar]
- 11. Reichardt P, Lindner T, Pink D, Thuss‐Patience PC, Kretzschmar A. Dorken B. Chemotherapy in alveolar soft part sarcomas. What do we know? Eur J Cancer. 2003;39:1511‐1516. [DOI] [PubMed] [Google Scholar]
- 12. Orbach D, Brennan B, Casanova M, et al. Paediatric and adolescent alveolar soft part sarcoma: A joint series from European cooperative groups. Pediatr Blood Cancer. 2013;60:1826‐1832. [DOI] [PubMed] [Google Scholar]
- 13. Judson I, Morden JP, Kilburn L, et al. Cediranib in patients with alveolar soft‐part sarcoma (CASPS): a double‐blind, placebo‐controlled, randomised, phase 2 trial. Lancet Oncol. 2019;20:1023‐1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Stacchiotti S, Mir O, Le Cesne A, et al. Activity of pazopanib and trabectedin in advanced alveolar soft part sarcoma. Oncologist. 2018;23:62‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schuetze SM, Bolejack V, Choy E, et al. Phase 2 study of dasatinib in patients with alveolar soft part sarcoma, chondrosarcoma, chordoma, epithelioid sarcoma, or solitary fibrous tumor. Cancer. 2017;123:90‐97. [DOI] [PubMed] [Google Scholar]
- 16. Schoffski P, Wozniak A, Stacchiotti S, et al. Activity and safety of crizotinib in patients with advanced clear‐cell sarcoma with MET alterations: European Organization for Research and Treatment of Cancer phase II trial 90101 'CREATE'. Ann Oncol. 2017;28:3000‐3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bukowski RM. Pazopanib: a multikinase inhibitor with activity in advanced renal cell carcinoma. Expert Rev Anticancer Ther. 2010;10:635‐645. [DOI] [PubMed] [Google Scholar]
- 18. van der Graaf WT, Blay JY, Chawla SP, et al. Pazopanib for metastatic soft‐tissue sarcoma (PALETTE): a randomised, double‐blind, placebo‐controlled phase 3 trial. Lancet. 2012;379:1879‐1886. [DOI] [PubMed] [Google Scholar]
- 19. Nakamura T, Matsumine A, Kawai A, et al. The clinical outcome of pazopanib treatment in Japanese patients with relapsed soft tissue sarcoma: A Japanese Musculoskeletal Oncology Group (JMOG) study. Cancer. 2016;122:1408‐1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228‐247. [DOI] [PubMed] [Google Scholar]
- 21. Choi H, Charnsangavej C, Faria SC, et al. Correlation of computed tomography and positron emission tomography in patients with metastatic gastrointestinal stromal tumor treated at a single institution with imatinib mesylate: proposal of new computed tomography response criteria. J Clin Oncol. 2007;25:1753‐1759. [DOI] [PubMed] [Google Scholar]
- 22. Stacchiotti S, Collini P, Messina A, et al. High‐grade soft‐tissue sarcomas: tumor response assessment–pilot study to assess the correlation between radiologic and pathologic response by using RECIST and Choi criteria. Radiology. 2009;251:447‐456. [DOI] [PubMed] [Google Scholar]
- 23. Grunwald V, Litiere S, Young R, et al. Absence of progression, not extent of tumour shrinkage, defines prognosis in soft‐tissue sarcoma ‐ An analysis of the EORTC 62012 study of the EORTC STBSG. Eur J Cancer. 2016;64:44‐51. [DOI] [PubMed] [Google Scholar]
- 24. Zer A, Prince RM, Amir E, Abdul RA. Evolution of randomized trials in advanced/metastatic soft tissue sarcoma: end point selection, surrogacy, and quality of reporting. J Clin Oncol. 2016;34:1469‐1475. [DOI] [PubMed] [Google Scholar]
- 25. Kim JH, Park HS, Heo SJ, et al. Differences in the efficacies of pazopanib and gemcitabine/docetaxel as second‐line treatments for metastatic soft tissue sarcoma. Oncology. 2019;96:59‐69. [DOI] [PubMed] [Google Scholar]
- 26. Esser M, Kloth C, Thaiss WM, et al. CT‐morphologic and CT‐textural patterns of response in inoperable soft tissue sarcomas treated with pazopanib‐a preliminary retrospective cohort study. Br J Radiol. 2019;92:20190158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kim M, Kim TM, Keam B, et al. A phase II trial of pazopanib in patients with metastatic alveolar soft part sarcoma. Oncologist. 2019;24:e20‐e29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Stockwin LH, Vistica DT, Kenney S, et al. Gene expression profiling of alveolar soft‐part sarcoma (ASPS). BMC Cancer. 2009;9:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Stacchiotti S, Negri T, Zaffaroni N, et al. Sunitinib in advanced alveolar soft part sarcoma: evidence of a direct antitumor effect. Ann Oncol. 2011;22:1682‐1690. [DOI] [PubMed] [Google Scholar]
- 30. Ogose A, Yazawa Y, Ueda T, et al. Alveolar soft part sarcoma in Japan: multi‐institutional study of 57 patients from the Japanese Musculoskeletal Oncology Group. Oncology. 2003;65:7‐13. [DOI] [PubMed] [Google Scholar]
- 31. Frezza AM, Jones RL, Lo Vullo S, et al. Anthracycline, gemcitabine, and pazopanib in epithelioid sarcoma: a multi‐institutional case series. JAMA Oncol. 2018;4:e180219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Outani H, Tanaka T, Wakamatsu T, et al. Establishment of a novel clear cell sarcoma cell line (Hewga‐CCS), and investigation of the antitumor effects of pazopanib on Hewga‐CCS. BMC Cancer. 2014;14:455. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


