Abstract
Cell line‐derived xenograft (CDX) models created by implanting cancer cell lines into immunodeficient mice have contributed largely to the development of cancer drug therapies. However, cell lines often lose their original biological characteristics through many passages and cancer tissues in CDX models have many cancer cells and few cancer stromal cells, therefore CDX models are currently considered not suitable for predicting the results of clinical studies. Conversely, patient‐derived xenograft (PDX) models are gaining importance, as human cancer biological characteristics and microenvironments are recreated by implanting tumor tissue into immunodeficient mice. These highly expected, evidently beneficial PDX models have been used in some basic research and are becoming more generalized. However, quality control and quality assurance criteria have not been established for them, and challenges and problems in the utilization of valuable PDX models in drug development have yet to be clarified. In this report, we conducted a questionnaire survey among researchers in Japanese academic institutions and pharmaceutical companies to understand the current status of PDX models in Japan. Based on the questionnaire results, we summarized the situations surrounding respondent's utilization and quality control in the development of anticancer drugs and proposed several measures to facilitate the utilization of PDX models in the development of anticancer drugs.
Keywords: animal model, drug development, patient‐derived xenograft model, questionnaire, regulatory science
We conducted a questionnaire survey among researchers in Japanese academic institutions and pharmaceutical companies to understand the current status of PDX models in Japan. Based on the results of the survey, we proposed several measures to facilitate the utilization of PDX models in the development of anticancer drugs. It is hoped that this report provides information to undertake research using PDX models for the development of anticancer drugs.
1. BACKGROUND/OBJECTIVE
Advances in molecular and cellular biology in recent years have helped elucidate many intracellular signals involved in cancer development, progression, invasion, and metastasis, and cancer drug therapies are now making remarkable progress. This is largely attributable to cancer cells (cell lines) derived from patients with cancer and cell line‐derived xenograft (CDX) models created by implanting these cancer cell lines into immunodeficient mice, such as nude mice. Among many candidate drugs, those showing cytocidal effects are identified by in vitro assays using cell lines, and only the ones demonstrating excellent intratumoral drug distribution, antitumor effects, and acceptable toxicity in preclinical studies with CDX models lead to clinical studies. Reducing research resources and development costs are important for a drug development process. Cell lines ensured via methods such as DNA analysis are commonly used in a preclinical research. However, the use of cell lines that acquired genetic variation through many passages and lost their original biological characteristics often yields inconsistent results between preclinical studies and clinical studies. Of the anticancer drugs demonstrating efficacy in preclinical studies, reportedly only c. 5% are approved by the US Food and Drug Administration (FDA). 1
A solid tumor is comprised not only of cancer cells and understanding the cancer microenvironment is important. 2 Among the components of the cancer microenvironment, stromal cells surrounding cancer cells, such as tumor endothelial cells (TECs), cancer‐associated fibroblasts (CAFs), and tumor‐associated macrophages (TAMs) promote cancer progression and metastasis, 3 , 4 , 5 and understanding the interaction between cancer cells and stromal cells is crucial. In general, CDX models created by implanting cancer cell lines into immunodeficient mice have many cancer cells and few cancer stromal cells and are thereby considered not suitable for predicting the results of clinical studies. Therefore, patient‐derived xenograft (PDX) models, in which human cancer microenvironments are recreated by implanting tumor tissue into immunodeficient mice, are gaining importance. 6
Through the successive development of severe immunodeficient mice, from nude mice in the 1980s, to SCID mice in the 1990s, to NOD/SCID mice in the 2000s, to NOG/NSG mice in the 2010s, success rates of PDX models have increased, prompting the global launch of EurOPDX in 2013, which is a large‐scale consortium consisting of 18 centers located across Europe and the United States. The National Cancer Institute (NCI) also announced a shift in its drug screening methodology from the traditional NCI‐60 panel using 60 different human cancer cell lines to PDX models. 7 Thus, the PDX has become one of the most‐spotlighted themes in in vivo research. However, unlike cell lines and CDX models, PDX models are currently used by a limited number of researchers in anticancer drug research. To allow any researcher to use PDX models in drug development, further studies are needed to overcome their limitations.
In drug development in academia and pharmaceutical companies, there has been a gradual increase in the use of PDX models created by the implantation of human tissue into immunodeficient mice. These highly expected, evidently beneficial PDX models have been used in some basic research and are becoming generalized. However, in Japan, quality control and quality assurance criteria have not been established for them, and challenges and problems in the utilization of valuable PDX models in drug development have yet to be clarified.
Therefore, we conducted a "questionnaire survey on PDX models" as part of a research project on the Research on Regulatory Science of Pharmaceuticals and Medical Devices funded by the Japan Agency for Medical Research and Development (AMED) titled “Survey and research of usefulness and issue on the PDX model for the drug development (AMED‐PDX research)”. The AMED‐PDX research intends to investigate and organize the current status and issues of PDX models in the development of drugs, such as anticancer drugs, and make proposals to promote the utilization of PDX models in drug development. As part of the AMED‐PDX research, this questionnaire survey was conducted among researchers in Japanese academic institutions and pharmaceutical companies with the aim of better understanding the current status of PDX models in Japan by investigating the situations surrounding their utilization and quality control in the development of anticancer drugs.
2. ANIMAL FACILITIES AND RESEARCHERS INCLUDED IN THIS SURVEY
Subjects of this questionnaire survey were animal facility administrators and researchers at 284 academic institutions including the Japanese Association of Laboratory Animal Facilities of National University Corporations, the Japanese Association of Laboratory Animal facilities of Public and private universities, and the Animal Experiment Facility Council under the jurisdiction of the Ministry of Health, Labor and Welfare, as well as researchers at research and development divisions of 19 pharmaceutical companies in Japan. We prepared 4 types of questionnaire forms, 1 each for animal facility administrators (4 items), academic researchers (22 items), researchers at the research division of a pharmaceutical company (22 items), and researchers at the development division of a pharmaceutical company (22 items).
Responses were obtained from 129 academic institutions (response rate: 45.4%) and 8 pharmaceutical companies (response rate: 42.1%). The questionnaire items related to animal facilities were completed by all the 129 animal facility administrators. Regarding the questionnaire items related to the use of PDX models, 79 researchers from 26 institutions responded. Of these, 19 researchers from 14 institutions answered "PDX models used" and "anticancer drugs developed," while 21 researchers from 7 institutions answered "PDX models not used" and "anticancer drugs developed" (6 out of 21 researchers did not answer most of the questions). There were 9 respondents from pharmaceutical companies, 5 from research divisions (basic research) and 4 from development divisions. PDX models were used by 13.2% (17) of all academic institutions, and 75.0% (6) of all pharmaceutical companies.
3. MOUSE STRAINS USED IN PDX MODELS AND HOUSING ENVIRONMENTS FOR THE MICE
Various immunodeficient mice are used in PDX models to implant human cancer tissue. Results of this survey revealed that various mouse strains are used in PDX models, including nude mice, SCID mice, NOD/SCID mice to SCID/beige mice, NOG or NSG mice, Rag2 knockout (KO) mice, and Rag2 KO/Jak3 KO mice. The most frequently used models are nude mice and NOG or NSG mice; the former is easy to handle as they do not have fur, while the latter are genetically modified, severely immunocompromised mice with a high success rates of tumor transplantation. Both nude mice and NOG or NSG mice were used in 12 facilities (70.6%); all other facilities except for 1, used either nude mice or NOG or NSG mice. As for other strains, SCID mice (6 facilities, 35.3%) and NOD/SCID mice (5 facilities, 29.4%) were used by a relatively high number of facilities. In contrast, SCID/beige mice, Rag2 KO mice, and Rag2 KO/Jak3 KO mice were used only by 2 facilities (11.8%), one facility (5.9%), and one facility (5.9%), respectively. These were similar to the trend of the mouse strains used in recent PDX models found in a literature search. 8 As shown in Table S1, the majority of the respondents used either nude mice because of the cost and ease of handling or NOG or NSG mice because they are the most immune deficient, allowing for more efficient engraftment of human‐derived cancer tissue. Positioned between these 2 mouse strains, SCID mice and NOD/SCID mice are readily available, and widely used strains. However, with increasing usage of NOG or NSG mice, the use of SCID mice and NOD/SCID mice in PDX models is reducing. SCID/beige mice and Rag2 KO mice are sold by a limited number of breeders, and Rag2 KO mice and Rag2 KO/Jak3 KO mice require individual researchers to maintain the strains and are therefore used only by a very small number of facilities. Particularly, when mouse strains are maintained by researchers, they require the quality control and quality assurance of the mouse strains, including their microbiological and genetic quality, which explains why these types of strains are used sparingly. If it is absolutely necessary to use such strains, it is important to obtain a steady supply under a controlled system by the breeder.
As for immunodeficient mice, they require a controlled, specific pathogen free (SPF) housing environment. Depending on whether there is the possibility of harboring undiagnosed and unknown pathogenic microorganisms arising from the use of human‐derived tissue, the mice may need to be handled in a conventional or infectious environment (including P2A laboratories and laboratories for the biological or chemical hazards). PDX model mice were housed under SPF conditions in 14 facilities, conventional conditions in 3 facilities, and infectious conditions in one facility (Figure 1A). Differential pressure in the mouse room was controlled with positive pressure in 13 facilities (76.5%) and with negative pressure in 3 facilities (17.6%) according to the state of immunodeficiency in mice (Figure 1B). Regarding the housing rack, many used isolation racks or individual ventilation system racks capable of controlling differential pressure, with a HEPA filter (defined as an air filter that can collect at least 99.97% of particles 0.3 μm in size) installed on the exhaust side. The rack was kept at positive pressure in 10 facilities (62.5%) and at negative pressure in 6 facilities (37.5%) (Figure 1C). For PDX models in which immunodeficient mice were implanted with patient‐derived tissue that may have unknown microbial infection, it is basically recommended that they be housed in a negative pressure rack with a HEPA filter installed on the exhaust side under microbiologically controlled SPF conditions 9 , 10 ; conversely, PDX mice were also housed in individual ventilation system racks at positive pressure in many animal facilities of contract research organization (CRO) or academic institutions. Thus, the rack with positive pressure can be used complying with facilities' standard operating procedures (SOPs), according to BSL2 guideline. In many facilities, cages, food, and drinking water were sterilized before use (Figure 1D), and waste was handled according to BSL2 guidelines (Figure 1E).
FIGURE 1.
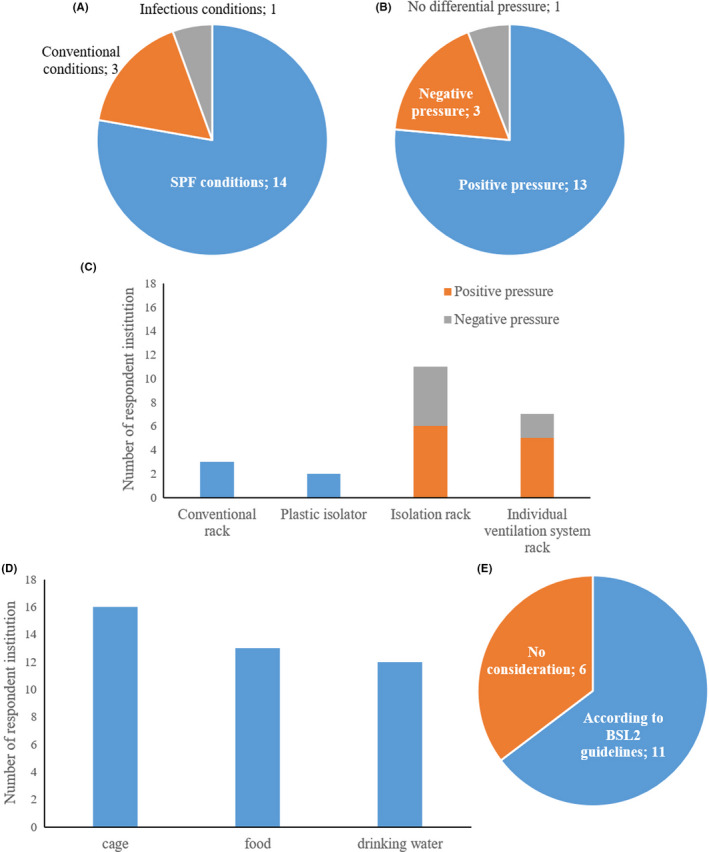
Housing environments of PDX models in Japanese facilities. A, Feeding conditions. B, Pressure regulations. C, Housing rack. D, Sterilization of materials before use. E, Handling of waste
4. STANDARD OPERATING PROCEDURE PREPARATION STATUS AND QUALITY CONTROL ITEMS IN THE CREATION OF PDX MODELS
Of the 19 academic researchers who answered "PDX models used" and "anticancer drugs developed," 18 created PDX models from human tumor tissue by themselves. Among them, 5 (27.8%) answered that they had their own SOPs to follow for the management of PDX models, and 12 researchers (66.7%) said that they had set procedures for the creation of PDX models but had no SOPs.
To facilitate more widespread use of PDX models that are readily available, it is important to develop an integrated quality control/quality assurance system among researchers. Additionally, information accompanying PDX models is also important to enhance the quality of the PDX models. The more detailed the information about a PDX model is, the greater its value becomes, by which the quality is also assured. However, recording all items is effort intensive. In Japan, there is no unified view on what information should accompany a PDX model. Disclosing and disseminating the minimal information to be recorded will help improve the quality of PDX models in Japan beyond a certain level. Based on the survey results from the 19 researchers of 14 academic institutions who answered "PDX models used" and "anticancer drugs developed," the information was classified into essential/recommended/optional/basically unnecessary/unnecessary (Table 1). "Essential" was defined as items recorded by 15‐19 researchers (78.9%‐100%), "recommended" as items recorded by 10‐14 (52.6%‐73.7%), “optional” as items recorded by 5‐9 (26.3%‐47.4%), "basically unnecessary" as items recorded by 1‐4 (5.3%‐21.1%), and "unnecessary" as items recorded by none of the 19 researchers.
TABLE 1.
Contents of quality assurance of PDX model
| Module | Field | Recommendation |
|---|---|---|
| Patient information | Age | Essential |
| Gender | Essential | |
| Disease name | Essential | |
| HLA type | Basically unnecessary | |
| History of treatment | Recommend | |
| Medical history | Recommend | |
| Presence/absence of infection | Recommend | |
| State of consent | Essential | |
| IRB number for the research | Recommend | |
| Ethnicity/race | Basically unnecessary | |
| Tumor information | Primary tumor tissue of origin | Essential |
| Primary/metastatic lesion type | Recommend | |
| Specimen tumor tissue | Recommend | |
| Pathological diagnosis | Essential | |
| Clinical stage(eg TNM classification) | Recommend | |
| Status of gene expression(eg EGFR, HER2) | Optional | |
| Type of specimens (eg surgical sample, biopsy, ascites fluid) | Recommend | |
| Quality assurance information | Specimens for which quality assurance is performed | |
| Original cancer tissue | Recommend | |
| PDX tissue (at establishment) | Recommend | |
| PDX tissue (during passages) | Optional | |
| Contents of quality assurance | ||
| Genomic information | Optional | |
| Gene expression information | Optional | |
| Histopathological features | Recommend | |
| ’Omics information | Basically unnecessary | |
| Model creation information | Name of the mouse strain | |
| At establishment | Recommend | |
| During passages | Optional | |
| Sex of the mouse | Essential | |
| Passage conditions | ||
| Differences among mouse strains | Optional | |
| Injection site (subcutaneous or orthotopic) | Optional | |
| Passage number of the PDX tissue | Essential | |
| Tissue storage conditions | ||
| Storage temperature | Basically unnecessary | |
| Storage number | Optional | |
| Tumor engraftment rate | Recommend | |
| Doubling time of tumor | Optional | |
| Strain immune system humanized? | Basically unnecessary | |
| Others | Published information on the PDX model in academic conferences or papers | Recommend |
| Confirmation of contamination | Basically unnecessary | |
| Sensitivity to anticancer drugs | Optional | |
Regarding the information associated with the creation of a PDX model, the items for which more than half of the researchers said they would record as patient information were: age, gender, disease name, history of treatment, medical history, presence/absence of infection, status of consent, and research number approved by the Institutional Review Board (IRB) for which specimens were collected; as tumor information were: primary organ name, primary/metastatic lesion type, name of the organ from which the specimens were acquired, pathological diagnosis, clinical stage such as TNM classification, and type of specimens such as surgically collected or biopsy specimens; as quality assurance information were: results of comparing histopathological characteristics between original cancer tissue and the PDX model at establishment; as model creation information were: the name of the animal strain at establishment, sex of the animal, passage number of the PDX tissue, and tumor engraftment rate; and as other information were: published information on the PDX model in academic conferences or papers. The above‐mentioned items are considered the minimal information to be recorded.
PDX Minimal Information (PDX‐MI) has been proposed in other countries, and it summarizes the essential/recommended items to be recorded, including clinical information, information on the PDX model creation, quality assurance of the PDX model, and information on drug sensitivity testing that used the PDX model. 11
5. PASSAGE NUMBERS OF PDX MODELS USED IN STUDIES AND HUMANIZED PDX MODELS
Regarding the passage numbers of the PDX models used by the 19 academic researchers who answered "PDX models used" and "anticancer drugs developed," where the passage number at the time of establishment is set as P0, the 1st passage (P1) was used by 4 (9.3%), the 2nd passage (P2) was used by 7 (16.3%), the 3rd passage (P3) was used by 9 (20.9%), the 4th passage (P4) was used by 7 (16.3%), and the 5th to 9th passage (P5‐P9) was used by 10 (23.3%). The results revealed that the use of P3 or P5‐P9 was more frequent than P2 or less due to the number of PDXs stored and for more stable model creation. There were 6 researchers (14.0%) who said they used PDXs that had been passaged 10 times or more (Figure 2A). Conversely, many of the basic researchers at pharmaceutical companies who have not established their own PDXs used P3 or later, particularly P5‐P9 (Figure 2B). Some studies showed that the additional passages caused a change in the original genetic features, tumor growth rates, and populations of tumor cells in tumor tissues. 12 , 13 While, there are several reports that the histological characteristics, gene expression pattern, and differentiation grade were maintained even when the passage number of PDX models was 10 times or more. 14 , 15 , 16 , 17 Therefore, the adequate quality evaluations of PDX models according to a purpose of each research (eg hematoxylin eosin staining, protein expression, gene expression, and genetic mutation) should be performed when PDX models passaged 10 times or more were used.
FIGURE 2.
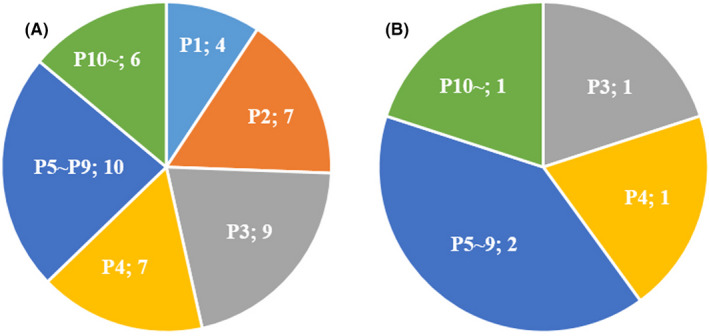
Passage numbers. A, Passage numbers of PDX models used by the academic researchers who answered “PDX models used” and “anticancer drugs developed.” B, Passage numbers of PDX models used by the basic researchers at pharmaceutical companies who have not established own PDXs
In general, humanized mice used in PDX models often indicate mice that have been transplanted with human immune cells. They were created by transplanting peripheral blood mononuclear cells (PBMCs) or hematopoietic stem cells (HSCs) into irradiated mice. 18 , 19 , 20 , 21 The number of academic researchers using these humanized mice was 4 (21.1%). Conversely, none of the researchers said that they used human‐derived cancer stroma or human‐derived intestinal flora, even though they were included in the options.
6. HOW ARE PDX MODELS CURRENTLY USED IN THE DEVELOPMENT OF ANTICANCER DRUGS AND HOW WILL THEY BE USED IN THE FUTURE?
Currently, academia researchers use PDX models in pharmacology studies (17, 89.5%) and elucidation of the true nature of tumors (11, 57.9%) (Figure 3A). However, PDX models used by researchers at pharmaceutical companies (3 from each of research and development divisions) are for pharmacology studies (6, 100%) (Figure 3B).
FIGURE 3.
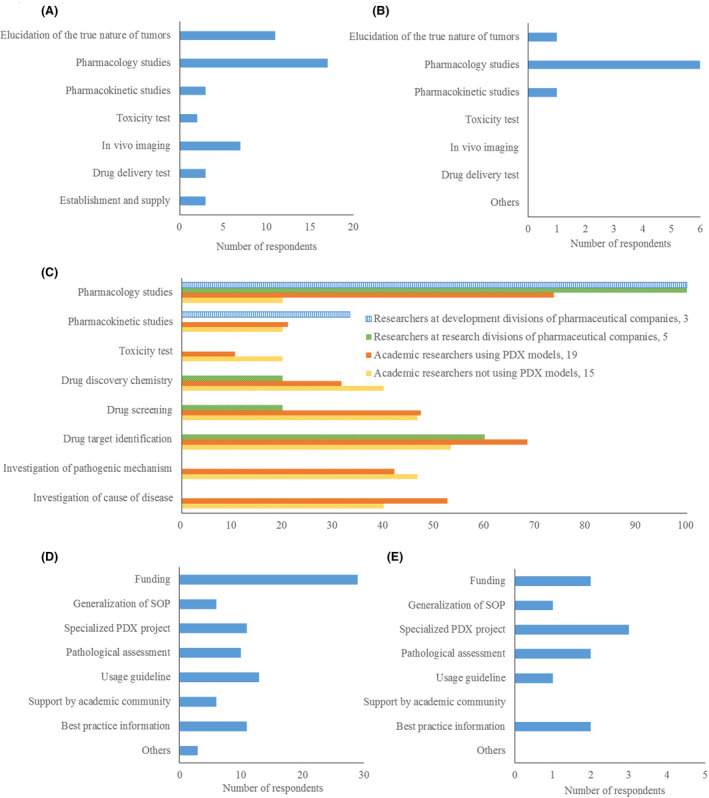
Purpose of using PDX models in the development of anticancer drugs and required supports for the utilization of PDX models. A, Current purpose of academic researchers (total respondents: 19). B, Current purpose of researchers in pharmaceutical companies (total respondents: 6). C, Future purpose of researchers in each group. Total number of respondents was 3 (researchers at development divisions of pharmaceutical companies), 5 (researchers at research divisions of pharmaceutical companies), 19 (academic researchers using PDX models), and 15 (academic researchers not using PDX models). D, Results from the questionnaire on required support of academic researchers (total respondents: 34). E, Results from the questionnaire on required support of researchers from research divisions of pharmaceutical companies (total respondents: 5)
Regarding the studies for which the use of PDX models was considered appropriate, including ones currently not undertaken, at least half of researchers in each group answered as follows: researchers at development divisions of pharmaceutical companies chose pharmacology studies (3, 100%); researchers at research divisions of pharmaceutical companies chose pharmacology studies (5, 100%) and drug target identification (3, 60%); academic researchers using PDX models chose pharmacology studies (14, 73.7%), drug target identification (13, 68.4%) and investigation of the cause of disease (10, 52.6%); and academic researchers not using PDX models chose drug target identification (8, 53.3%) (Figure 3C).
7. SUPPORT SYSTEM FOR THE UTILIZATION OF PDX MODELS
Academia researchers said they needed "funding" support (29, 85.3%). All studies require research funding, and PDX models are not particularly expensive compared with others. Outsourcing a pharmacology study with an established PDX model to a CRO costs several million yen, but the US NCI provides PDX tissues for US academic researchers for US$250, not significantly different from cell lines purchased from the American Type Culture Collection (ATCC) or the Japanese Collection of Research Bioresources Cell Bank (JCRB). The use of PDX models requires covering the costs of housing and using mice, but the same is true for CDX models. Conversely, if establishing a PDX model from patient‐derived tissue, the actual cost can be several hundred thousand yen per strain; therefore, funding support associated with the establishment is necessary (Figure 3D).
Researchers from research divisions of pharmaceutical companies said that support is needed for "PDX projects specialized in rare cancers or common cancers in Asia" (3, 60%) (Figure 3E). The PDX Finder (http://www.pdxfinder.org/) is a search site for PDX models mainly provided by CROs and EurOPDX. As PDX models registered in the site (2888 models as of March 2020) are mostly of Western origin, pharmaceutical companies developing anticancer drugs in Japan are seeking PDX libraries of Japanese origin. Japanese‐derived PDX libraries that are currently available include J‐PDX led by National Cancer Center and F‐PDX led by Fukushima Medical University, and it is important to establish consistently in the future a system allowing for stable supply of Japanese‐derived PDXs (Table S2).
8. PROPOSALS FOR THE UTILIZATION OF PATIENT‐DERIVED XENOGRAFT MODELS IN THE DEVELOPMENT OF ANTICANCER DRUGS
Based on these questionnaire results, we propose the following measures to facilitate the utilization of PDX models in the development of anticancer drugs.
It is recommended that PDX models are housed in a negative pressure rack with a HEPA filter installed on the exhaust side under microbiologically controlled SPF conditions. However, a positive pressure rack can be used according to facilities' SOP as long as housing conditions comply with BSL2.
In the creation of PDX models, it is necessary to develop SOPs and establish a system allowing for the appropriate management of the minimal information to be recorded.
PDX models up to 9 passages are recommended to be used for an experiment. PDX models with 10 or more passages should not be used without an appropriate quality assessment.
Humanized PDX models engrafted with PBMCs or HSCs are promising for the development of some anticancer drugs, such as immune‐related anticancer drugs. However, improvements in humanized mice models are still required to observe an adequate immune response as the models do not fully reflect human immune systems.
Although PDX models for the development of anticancer drugs are mainly intended for pharmacology studies, they can also be applied to basic research, such as the identification of potential drug targets and elucidation of disease pathogenesis.
It is important to establish a system that allows Japanese‐derived PDX libraries to deliver a stable supply of PDX to researchers consistently in the future, and funding support for the costs associated with the establishment and distribution of PDXs is essential.
DISCLOSURE
The authors have no conflicts of interest.
ETHICAL APPROVAL
We asked the Ethical Committee for clinical studies and animal experiments before this questionnaire. They said that this study can be conducted without approvals by the Ethical Committee for the Institution (IRB) and the Committee for Ethics of Animal Experiment, because no human subjects or animals are used in this study.
Supporting information
Table S1
Table S2
ACKNOWLEDGMENTS
This work was funded by the Japan Agency for Medical Research and Development (AMED; Research on Regulatory Science of Pharmaceuticals and Medical Devices grant number 19mk0101121h0002 to Y. Koga) and by the National Cancer Center Research and Development Fund (grant number 31‐A‐8 to Y. Koga).
Tsumura R, Koga Y, Hamada A, et al. Report of the use of patient-derived xenograft models in the development of anticancer drugs in Japan. Cancer Sci. 2020;111:3386–3394. 10.1111/cas.14564
REFERENCES
- 1. Hutchinson L, Kirk R. High drug attrition rates–where are we going wrong? Nat Rev Clin Oncol. 2011;8:189‐190. [DOI] [PubMed] [Google Scholar]
- 2. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646‐674. [DOI] [PubMed] [Google Scholar]
- 3. Maishi N, Hida K. Tumor endothelial cells accelerate tumor metastasis. Cancer Sci. 2017;108:1921‐1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ishii G, Ochiai A, Neri S. Phenotypic and functional heterogeneity of cancer‐associated fibroblast within the tumor microenvironment. Adv Drug Delivery Rev. 2016;99:186‐196. [DOI] [PubMed] [Google Scholar]
- 5. Komohara Y, Takeya M. CAFs and TAMs: maestros of the tumour microenvironment. J Pathol. 2017;241:313‐315. [DOI] [PubMed] [Google Scholar]
- 6. Hidalgo M, Amant F, Biankin AV, et al Patient‐derived xenograft models: an emerging platform for translational cancer research. Cancer Discovery. 2014;4:998‐1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ledford H. US cancer institute to overhaul tumour cell lines. Nature. 2016;530:391. [DOI] [PubMed] [Google Scholar]
- 8. Koga Y, Ochiai A. Systematic review of patient‐derived xenograft models for preclinical studies of anti‐cancer drugs in solid tumors. Cells. 2019;8:418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. World Health Organization . Laboratory Biosafety Manual. 3rd ed Geneva, Switzerland: World Health Organization; 2004. [Google Scholar]
- 10. Uthamanthil R, Tinkey P, de Stanchina D. Patient Derived Tumor Xenograft Models: Promise, Potential and Practice. 1st ed Cambridge, MA: Academic Press; 2016. [Google Scholar]
- 11. Meehan TF, Conte N, Goldstein T, et al. PDX‐MI: minimal information for patient‐derived tumor xenograft models. Cancer Res. 2017;77:e62‐e66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pearson AT, Finkel KA, Warner KA, et al. Patient‐derived xenograft (PDX) tumors increase growth rate with time. Oncotarget. 2016;7:7993‐8005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ben‐David U, Ha G, Tseng YY, et al. Patient‐derived xenografts undergo mouse‐specific tumor evolution. Nat Genet. 2017;49:1567‐1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nishime C, Ohnishi Y, Suemizu H, et al. Gallbladder small cell carcinoma Xenograft established by serial transplantation in nude mice. Anticancer Res. 2006;26:79‐83. [PubMed] [Google Scholar]
- 15. Mischek D, Steinborn R, Petznek H, et al. Molecularly characterised xenograft tumour mouse models: valuable tools for evaluation of new therapeutic strategies for secondary liver cancers. J Biomed Biotechnol. 2009;2009:437284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lin D, Wyatt AW, Xue H, et al. High fidelity patient‐derived xenografts for accelerating prostate cancer discovery and drug development. Cancer Res. 2014;74:1272‐1283. [DOI] [PubMed] [Google Scholar]
- 17. Kuwata T, Yanagihara K, Iino Y, et al. Establishment of novel gastric cancer patient‐derived xenografts and cell lines: pathological comparison between primary tumor, patient‐derived, and cell‐line derived xenografts. Cells. 2019;8:585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rosato RR, Davila‐Gonzalez D, Choi DS, et al. Evaluation of anti‐PD‐1‐based therapy against triple‐negative breast cancer patient‐derived xenograft tumors engrafted in humanized mouse models. Breast Cancer Research: BCR. 2018;20:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Williams JA. Using PDX for preclinical cancer drug discovery. The evolving field. J Clin Med. 2018;7:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yao LC, Aryee KE, Cheng M, Kaur P, Keck JG, Brehm MA. Creation of PDX‐bearing humanized mice to study immuno‐oncology. Methods Mol Biol (Clifton, NJ). 2019;1953:241‐252. [DOI] [PubMed] [Google Scholar]
- 21. Meraz IM, Majidi M, Meng F, et al. An improved patient‐derived xenograft humanized mouse model for evaluation of lung cancer immune responses. Cancer Immunol Res. 2019;7:1267‐1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Table S2


