Abstract
Myeloid-derived suppressor cells (MDSC) are a heterogeneous population of immature myeloid cells with suppressive function on immune response. In this review, we discuss recent studies about mechanisms of expansion and suppressive function of MDSCs during inflammation, infection and autoimmune diseases, as well as pro-angiogenic and pro-metastatic functions of these cells in tumor development. Further, we focus on novel studies of MDSCs and therapeutic approaches to eliminate these cells in cancer.
Keywords: MDSC, Cancer, Inflammation
INTRODUCTION
Myeloid-derived suppressor cells (MDSC) are identified as a heterogeneous cell population in mice and humans under pathological conditions, including cancer, autoimmune diseases, transplantation, and infection diseases. These cells are of myeloid origin, and are comprised of myeloid progenitors and immature myeloid cells (IMCs). Under physiological conditions, these cells are generated in the bone marrow (BM) and differentiated into mature macrophages, dendritic cells (DCs), and granulocytes, and are present to a lesser extent in the spleen. The different progenitor cells that form this population demonstrate a broad range of morphology and functional capacity. In contrast, in pathological conditions, there is a dramatic expansion of cells with the same phenotype and immunesuppressive activity in various tissues (Youn et al., 2008) (Gabrilovich and Nagaraj, 2009). These immature cell populations with potent immunesuppressive activity are important as negative regulators of immune responses.
During chronic inflammation and cancer, immature cell populations significantly expand owing to a partial block in their differentiation into mature myeloid cells. The patho-physiological relevance of MDSCs is attributed to their ability to suppress T-cell responses (Nagaraj et al., 2010; Qu et al., 2009a; Qu et al., 2011b). MDSCs regulate innate immune responses by modulating cytokine production of macrophages and up-regulating production of immunesuppressive factors, such as nitric oxide (NO) and reactive oxygen species (ROS) (Gabrilovich and Nagaraj, 2009; Sinha et al., 2007b) (Ostrand-Rosenberg and Sinha, 2009; Peranzoni et al., 2010). MDSCs suppress proliferation and cytokine secretion in both T lymphocytes and Natural Killer (NK) cells, as well as induce apoptosis in T cell subsets (Bronte et al., 1998). Recent reports demonstrated that MDSCs were expanded in mouse bone marrow during chronic inflammation where they over-express the anti-inflammatory cytokine IL-10 and suppress T-cell responses (Bunt et al., 2009; Delano et al., 2007). The MDSC accumulation in inflammatory settings suggests that increased MDSC numbers may be a common anti-inflammatory control mechanism for the maintenance of homeostasis. Further, MDSCs can regulate inflammatory responses through multiple mechanisms resulting in modulation of both innate and adaptive immunity.
MDSC Definition
In mice, MDSCs are generally characterized as Gr-1+CD11b+ cells. Gr-1 antigen is considered to be a marker of granulocytic differentiation, and the antibodies bind to two epitopes, Ly6G and Ly6C (Bronte, 2009). The use of these epitope-specific antibodies has led to the identification of two MDSC subsets, monocytic, and granulocytic subsets, which have different morphological and functional features. As both Ly6C and Ly6G epitopes are bound by anti-Gr-1 antibodies, the relative intensity of Gr-1 staining is indicative of monocytic (low or intermediate) and granulocytic (high) subsets (Youn et al., 2012). In both humans and mice, MDSCs are marked by high expression of CD11b (a common myeloid cell surface marker).
Following studies of MDSCs, new functional markers such as CD16, CD31, CD40, CD49b, CD115, CD80, and F4/80 were found in mouse model (Gabrilovich and Nagaraj, 2009; Nagaraj et al., 2010). Some of these markers have been demonstrated to play an important role in MDSC suppression of the T cell response. In particular, studies have suggested that interactions between CD40 and CD80 on MDSCs and cognate receptors on T cells are required for suppression of T cell activity (Pan et al., 2010; Yang et al., 2006).
In tumor-bearing mice, CD11b+Ly6GlowLy6Chigh monocytic MDSCs and CD11b+Ly6Ghigh Ly6Clow granulocytic MDSCs utilize different suppressive mechanisms (Youn et al., 2008) (Table 1). CD11b+Ly6GlowLy6Chigh monocytic MDSCs produce very little reactive oxygen species (ROS) but produce a high level of nitiric oxide (NO), and consist of IMCs with the ability to differentiate into macrophages and DCs (Gabrilovich and Nagaraj, 2009). This subset of MDSCs mediates immune suppression through the production of NO and arginase (Rodriguez et al., 2005; Youn et al., 2012). In contrast, CD11b+Ly6GhighLy6Clow granulocytic MDSCs express a high level of ROS and very little NO, and are the largest population of MDSCs in tumor-bearing mice, representing 75% of all MDSCs (Youn et al., 2012).
Table 1:
The phenotypes of MDSCs in mice and humans.
| Monocytic MDSCs | Granulocytic MDSCs | |
|---|---|---|
| Mice | CD11b+Ly6GlowLy6Chigh | CD11b+Ly6GhighLy6Clow |
| Humans | CD11b+CD33+CD14+ | CD11b+CD33+CD14− |
Suppression by granulocytic MDSCs is mediated via ROS and H2O2 (Youn et al., 2008). Granulocytic MDSCs do not survive in culture and do not differentiate into macrophages and DCs (Youn et al., 2008). Recent data show that the functions of tumor-related granulocytic MDSCs are different from those of normal mature neutrophils, even though they share certain cell surface markers (Fridlender et al., 2012; Youn et al., 2012).
It has been suggested to define human MDSCs in cancer patients by the combinations of functional markers, such as CD14, CD33, CD11b, CD66b, and low expression of CD15 (Lechner et al., 2011; Nagaraj and Gabrilovich, 2010; Rodriguez et al., 2009) (Table 1). In different cancer patients, there are different types of MDSCs. In hepatocellular carcinoma (HCC) patients, the monocytic subset of MDSCs was observed to induce expansion of a population of IL-10 dependent regulatory T cells (Tregs) (Hoechst et al., 2008). In lung cancer patients, granulocytic MDSCs were revealed to be CD15+CD14 CD33b+, and were found to reduce the expression of T cell receptor (TCR) in CD8+ T lymphocytes in vitro (Nagaraj and Gabrilovich, 2010). These data suggest that the phenotypes and functions of MDSCs in humans need further study to fully understand the differences in populations between cancer patients.
Development and Expansion of MDSCs
Cytokines, growth factors, microbial products and other factors released in pathological microenvironments (such as cancer tissues), have been shown to be involved in the induction and expansion of MDSCs with suppressive activity (Gabrilovich and Nagaraj, 2009). Among these molecules, GM-CSF, PGE2 and VEGF have been shown to mediate MDSC accumulation (Bunt et al., 2006; Bunt et al., 2007; Gabrilovich et al., 1998; Huang et al., 2007; Kamiyama et al., 2006; Melani et al., 2003; Serafini et al., 2004a; Sinha et al., 2007b; Solheim et al., 2007; Varga et al., 2008). Most of these mediators activate signaling pathways in MDSCs that involve NF-κB and STATs, especially STAT3 (Gabrilovich and Nagaraj, 2009; Qu et al., 2011a).
Furthermore, MDSCs accumulate in response to some tumor vaccine models in mice, promoting tumor progression and negating the effects of the vaccines, and can also play a role in refractoriness to anti-VEGF tumor therapy (Prins et al., 2002; Serafini et al., 2004a; Shojaei et al., 2007). In contrast, factors that promote myeloid-lineage cell differentiation reduce MDSC population expansion (Almand et al., 2001) (Kusmartsev et al., 2003).
STAT3 plays important roles in the expansion, differentiation and function of MDSCs. Inhibition of STAT3 in vitro abolishes the suppressive activity of MDSCs (Qu et al., 2011a). Several pathways downstream of STAT3 may be involved in the regulation of MDSC expansion and function. Two downstream molecules, S100A8 and S100A9, are involved in the accumulation and function of MDSCs, particularly granulocytic MDSCs. This protein heterodimer participates in the formation of the NADPH oxidase (NOX2) complex that is responsible for production of ROS in MDSCs. In the absence of S100A9, MDSC accumulation is inhibited in the spleen of tumor-bearing mice. MDSC surface glycoprotein receptors signal for the effects of S100A9 and S100A8 through activation of the NF-κB pathway (Sinha et al., 2008).
Recently, novel regulatory mechanisms for the expansion and activation of MDSCs were described. There is emerging evidence that individual microRNAs (miRNAs) become part of a complex regulatory network involving other miRNAs and transcriptional factors that cooperate to mediate development of MDSCs. miRNAs are abundant small molecules of ~22 nucleotides that reprogram gene expression by targeting mRNA degradation and translation disruption (O’Connell et al., 2012). Thus, miRNAs could couple with transcription factors in development and differentiation of MDSCs, contributing to cancer-associated inflammation (Chaudhuri et al., 2011; O’Connell et al., 2007).
The inflammatory response involves profound myeloid proliferation and, through miRNAs such as miR-155, miR-223 and miR-a146, may prove to create a microenvironment suitable for cancer formation and development if not resolved in a timely manner (Fujita et al., 2011; Taganov et al., 2006). miR-146a plays a negative regulatory role in the development of myeloid cells because its deletion in mice results in a myeloproliferative disorder with a massive accumulation of MDSCs in the secondary lymphoid organs. This defect seems to result from an excessive proliferative capacity of MDSCs in the absence of miR-146a caused by increased CSF-1R expression, producing a more robust proliferation of MDSCs in response to M-CSF without a change in the number of myeloid progenitors (Zhao et al., 2011).
In addition, two other miRNAs, miR-155 and miR-223, have also been implicated in the development of MDSCs in mouse models (Johnnidis et al., 2008; O’Connell et al., 2008). Interestingly, miR-155, which, like miR-146a, can also be induced by ligands of toll-like receptors, such as lipopolysaccharide (LPS), was shown to cause myeloproliferation. Chronic up-regulation of miR-155 resulted in the hyperplasia of myeloid cells and suggests a link between inflammation and cancer (O’Connell et al., 2007). Given the possible roles of miRNAs in MDSC differentiation and expansion, future research is needed to investigate whether miRNAs have other roles in regulating immune responses by influencing MDSC expansion and activation.
MDSC Mediated Antigen-specific Immune Suppression
MDSCs mediate antigen-specific and/or antigen non-specific suppression of T cell responses. Some in vitro studies have demonstrated an antigen non-specific nature of MDSC-mediated inhibition of T cells (Gabrilovich and Nagaraj, 2009; Kusmartsev et al., 2003). However, many data demonstrated that MDSCs are capable of taking up antigens, processing them, and presenting them to T cells (Kusmartsev et al., 2004; Movahedi et al., 2008; Nagaraj et al., 2007). MHC class I-restricted MDSC-mediated CD8+ T cell suppression has been shown in tumor models (Bronte et al., 1999; Kusmartsev et al., 2005). Further, treatment with MHC class I-specific antibody can block MDSC inhibition of CD8+ T cell response in some cancer models (Gabrilovich et al., 2001).
Mechanisms of MDSC Suppressive Function
MDSCs inhibit immune responses by mediating other immune cells, such as T cells, macrophages, NK cells, dendritic cells (DCs), and Tregs (Almand et al., 2001; Bronte et al., 2000; Bronte et al., 2003a; Bronte et al., 2003b; Bronte et al., 1998; Ezernitchi et al., 2006; Gabrilovich and Nagaraj, 2009; Kusmartsev and Gabrilovich, 2005; Kusmartsev et al., 2005; Kusmartsev et al., 2004; Liu et al., 2007; Mazzoni et al., 2002; Melani et al., 2003; Serafini et al., 2006a; Serafini et al., 2004a; Serafini et al., 2004b; Serafini et al., 2006b; Serafini et al., 2008). Multiple mechanisms have been implicated in MDSC mediated immune suppression for T lymphocytes. Suppressive functions are mediated through combinations of several major molecular players, including inducible nitric oxide synthetase (iNOS), arginase-1 (Arg1), cyclo-oxygenase 2 (COX-2), prostaglandin E2 (PGE2) and TGF-β (Li et al., 2009) (Bogdan, 2011) (Yang et al., 2008). Tumor-related MDSCs induce apoptosis of CD8+ T cells, promoted by IL-4 (Bronte et al., 2000; Bronte et al., 2003a; Bronte et al., 1998). One study has shown that MDSCs mediate T cell anergy through antigen processing and presentation within the tumor environment (Kusmartsev et al., 2005).
Evidence indicates that MDSCs inhibit NK cell cytotoxicity in a cell-cell contact dependent manner (Liu et al., 2007). The cell-cell contact is also necessary for T cell activity inhibition (De Wilde et al., 2009; Ezernitchi et al., 2006; Mazzoni et al., 2002). Sinha et al. showed that a reduction in the numbers of MDSCs and an increase in levels of M1 macrophages could promote the rejection of metastases (Sinha et al., 2005). MDSCs produce IL-10, which in conjunction with a cell-cell contact dependent mechanism, leads to decreased production of IL-12 by macrophages, resulting in the skewing of immune response from an anti-tumor type 1 response, to a pro-tumor type 2 response (Sinha et al., 2007a).
Some data indicate that MDSCs can differentiate into tumor associated macrophages (TAMs), which can mediate the death of T cells and immune suppression. CD11b+Gr-1 + MDSCs from the spleens of tumor-bearing mice can migrate to the tumor site and become F4/80+ TAMs (Kusmartsev et al., 2004). Both MDSCs and TAMs suppress CD8+ T cell function in a non-specific manner at the tumor site. MDSCs produce high levels of Arg1 and NO, whereas TAMs up-regulate the expression of either Arg1 or iNOS, but not of both proteins, dependent on the tumor microenvironment (Mantovani et al., 2002). TAMs also inhibit T cell function by secreting cytokines such as IL-1β and TGF-β (Kusmartsev and Gabrilovich, 2005; Kusmartsev et al., 2005; Sica and Bronte, 2007).
The suppressive activity of MDSCs is associated with the metabolism of L-arginine, particularly for monocytic MDSCs. Both Arg1 and iNOS use L-arginine as a common substrate to produce urea or NO, respectively. NO produced by iNOS has been shown to block T cell differentiation, cytokine production, and proliferation (Bogdan, 2011). In addition to direct signaling effects through NO, iNOS can also decrease available levels of its substrate L-arginine, a process that can be further accelerated by the metabolic activities of Arg-1. Up-regulation of Arg1 activity leads to depletion of L-arginine from the pathological microenvironment, which leads to low expression of the TCR and inhibits the cell-cycle in T cells to suppress T cell proliferation (Rodriguez et al., 2003). This metabolic targeting is not unique to L-arginine, as a recent study demonstrated additional metabolic targets utilized in MDSC-mediated suppression, such as cystine and cysteine (Srivastava et al., 2010).
ROS produced by the NADPH oxidase complex is the main regulator responsible for granulocytic MDSCs suppression. Inhibition of ROS production using an upstream inhibitor in vitro abolished MDSC mediated suppression (Corzo et al., 2009). The combination of ROS and NO suppresses CD8+ T cell responses by producing peroxynitrite, which leads to nitration and nitrosylation of several amino acids, affecting protein function in target cells (Corzo et al., 2009; Nagaraj et al., 2007). Some reports have shown that high levels of peroxynitrite resulted in the nitration of the TCR and inhibition of subsequent signaling pathways (Nagaraj et al., 2007). MDSCs can also mediate the down-regulation of the ζ chain of the TCR to suppress T cell function (Bronstein-Sitton et al., 2003; Ezernitchi et al., 2006). Furthermore, one study found that MDSCs upregulate heme-oxygenase-1 and IL-10 in response to LPS stimulation, mediating the immune suppressive activity of MDSCs (De Wilde et al., 2009).
Tumor cells produce COX-2, an enzyme in the pathway that produces inflammatory molecules such as PGE2, whose receptor was found on MDSCs. PGE2 has been implicated in MDSC function, leading to upregulation of arginase 1 and regulation of tumor angiogenesis (Fujita et al., 2011; Kamiyama et al., 2006; Rodriguez et al., 2005). Accordingly, COX-2 inhibitors can abrogate production of Arg1 and enhance immune response to suppress tumor growth (Talmadge et al., 2007; Zea et al., 2005).
TGF-β is a secreted ligand that has been intimately linked to the regulation of tumor initiation, progression and metastasis. Normally, TGF-β contributes to homeostasis and tumor suppression. However, recently, it was found that the TGF-β signaling pathway is associated with tumor cell motility, invasion and metastasis (Fu et al., 2009; Lu et al., 2011b). Diminished TGF-β signaling in breast tumor cells resulted in the recruitment of MDSCs to the invasive front of the tumor tissue. This is regulated through increased CXCL5/CXCR2 and SDF-1/CXCR4 chemokine axes signals. In turn, these myeloid cells produce large quantities of MMPs and TGF-β1, thus promoting tumor invasion and metastasis (Yang et al., 2008). In addition, MSDCs produce TGF-β to suppress NK responses (Li et al., 2009). MDSC can also directly impact the prevalence of Tregs through IFNγ dependent production of IL-10 or iNOS (Huang et al., 2006). Thus, MDSCs can participate in both acute control of T cell responses and the induction of Tregs capable of potentiating long-term suppression.
MDSCs and Autoimmunity
MDSCs have been studied for more than 20 years in tumor-bearing mice and patients with cancer. Recently, accumulating evidence has shown that MDSCs also regulate immune responses during bacterial and parasitic infections, acute and chronic inflammation and autoimmune diseases. An increased number of MDSCs was found in a mouse model of experimental autoimmune encephalomyelitis (EAE); inflammation induced the development of MDSCs that produced ROS and NO to suppress T cell function (Zhu et al., 2007). Similar findings were observed in experimental autoimmune uveoretinitis (EAU), and in inflammatory bowel disease models (Greten et al., 2011; Haile et al., 2010; Kerr et al., 2008). Interestingly, increased numbers of MDSCs were also observed in normal mice following immunization with protein or peptide antigens given together with CFA or staphylococcal enterotoxins (Gabrilovich and Nagaraj, 2009). These observations suggest that the development of MDSCs is contributed to many inflammatory diseases.
MDSCs and Transplantation
Recently, a critical role for CD11b+CD115+Gr-1+ monocytic MDSCs was identified in a cardiac transplant model, using CD40 ligand blockade and donor specific splenocyte transfusion to induce tolerance in the recipient (Garcia et al., 2010). The types of cells were identified after some inflammatory cells (such as monocytes, macrophages, and neutrophils) were removed. Mechanisms of action by which these cells exert their immune regulatory function included antigen-nonspecific T cell suppression and development of Tregs (Adeegbe et al., 2011). On the other hand, an increased frequency of MDSCs may favor the development of tumors in transplant patients (Adeegbe et al., 2011; Lees et al., 2011). MDSCs may contribute to patients’ immune suppression, suggesting that their characterization and enumeration post-transplantation may be important in informing the dosing and eventually the minimization or withdrawal of immunosuppressive drugs.
MDSCs in Cancer
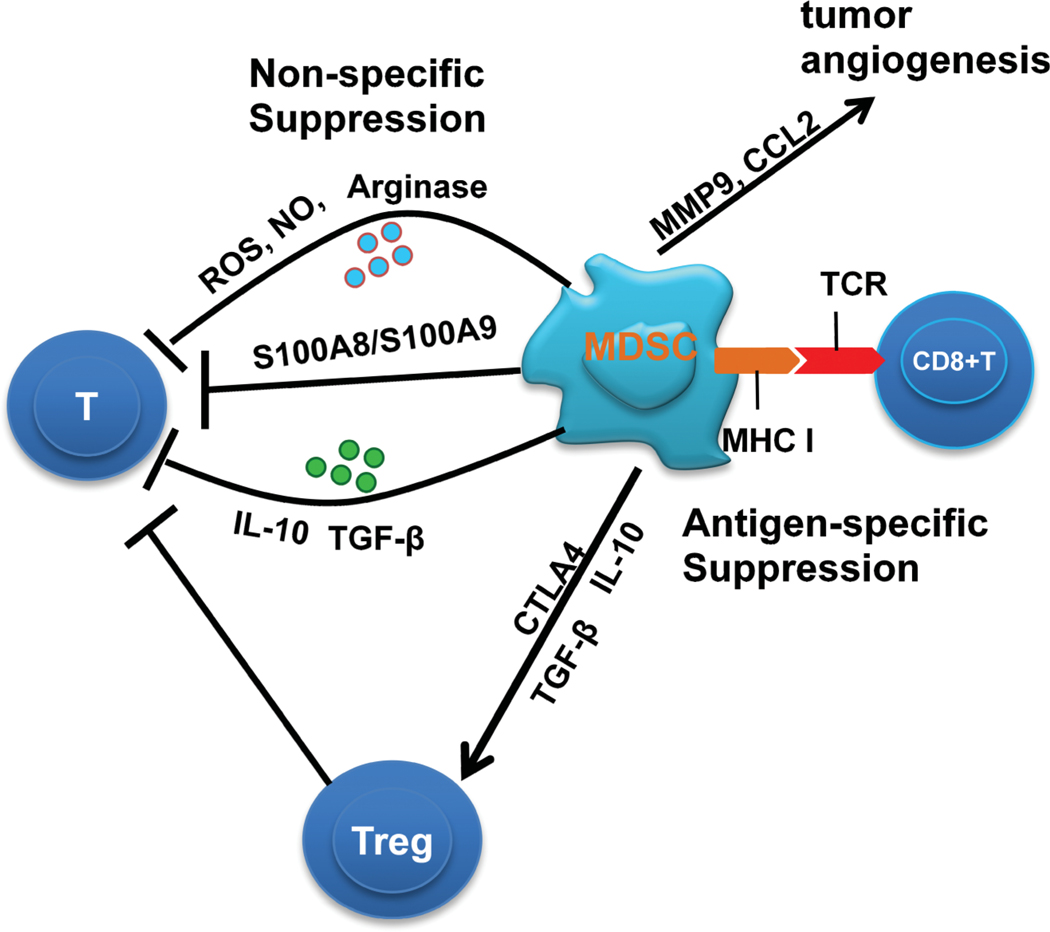
MDSC promote tumor escape by inhibiting T cell responses in vivo (Lesokhin et al., 2012; Lu et al., 2011a) (Figure 1). MDSC accumulation has been demonstrated to participate in the suppression of immune responses to tumor antigens (Almand et al., 2001) (Boutte et al., 2011; Bronte et al., 2000). In tumor-bearing mice, MDSCs accumulate in the bone marrow, peripheral blood (PB) and secondary immune tissues (such as spleen and lymph nodes), and increased numbers of MDSCs were detected in the blood of most cancer patients (Gabrilovich and Nagaraj, 2009; Youn et al., 2008).
Figure 1:
The suppression mechanisms of MDSC in the tumor environment.
MDSCs produce reactive oxygen species (ROS) and nitric oxide (NO) which inhibit T and NK cells. The constitutive overactivation of Stat3 is associated with an increased proliferation of MDSCs, and promotes the suppressive function for T cells, through the up-regulation of its many target genes, such as S100A8 and S100A9. MDSCs produce IL-10 and TGF-β to inhibit immune function in a non-specific manner. MDSCs induce Treg expansion via secretion of IL-10 and TGF-β. In some cancer models, the induction of Treg by MDSCs was associated with the expression of cytotoxic lymphocyte 4 antigen (CTLA4). Tregs inhibit T and NK cells. MDSCs can take up, process and present antigens to antigen-specific CD8+ T cells. Through cell-cell contact, MDSCs cause nitration of an amino acid on the T-cell receptor (TCR) on the surface of T cells. T cells then become unresponsive to antigen-specific stimulation. MDSCs contribute to tumor angiogenesis by production of factors such as MMP9 and CCL2.
The STAT family of transcription factors plays major roles in MDSC function and altered immune response to cancer. In particular, the relationship between tumor cells and MDSCs appears to be mediated via STAT3 signal transduction, whose target genes include matrix metalloprotease-9 (MMP9) and VEGF. The constitutive overactivation of STAT3 is also associated with an increased proliferation and survival of myeloid progenitors, and promotes the suppressive function against T cells, possibly through the upregulation of its many target genes, such as S100A8, S100A9, cyclin D1 and c-myc (Sinha et al., 2008). Recently, some novel signaling molecules were found to induce MDSC expansion via the STAT3 pathway. Myeloid cell specific overexpression of apoptosis inhibitor 6 (Api6) induced constitutive activity of STAT3 in myeloid cells and systemic expansion of MDSCs. In the lung, this led to chronic inflammation and lung adenocarcinoma (Qu et al., 2009a).
In addition, matrix metalloproteinase-12 (MMP12) overexpression in epithelial cells has been reported in inflammation-triggered lung cancer. During the tumorigenesis process, MMP12 upregulation activated the expression of STAT3 and its downstream genes on epithelial cells to stimulate chronic inflammation and tumor formation (Qu et al., 2009b). The ablation of STAT3 using conditional knockout mice or selective inhibitors dramatically reduced the expansion of MDSCs and improved T cell responses in tumor-bearing mice (Kujawski et al., 2010). The selective STAT3 inhibitor JSI-124 also downregulated STAT3 activity in MDSCs and dramatically reduced their presence in tumor-bearing mice (Nefedova and Gabrilovich, 2007).
The treatment of tumor-bearing mice with JSI-124 substantially enhanced the effect of cancer immunotherapy and anti-tumor immune responses. Some reports demonstrated that specific miRNAs can inhibit the expression of STAT3 in cancer, such as miR-17-5p and miR-20a, which target the STAT3 3’UTR (Zhang et al., 2011). Although MDSCs accumulate in a tumor microenvironment, tumor-associated factors downregulate the expression of miR-17-5p and miR-20a, and promote the STAT3-associated suppressive function of MDSCs, which contain higher levels of STAT3 and components of the NADPH oxidase complex. Thus, miR-17-5p and miR-20a might potentially be used as targets in immunotherapy strategies to inhibit function of MDSCs via reducing STAT3 expression.
Tumor derived MDSCs promote tumor growth, whereas an infusion of MDSCs from the spleen of tumor-bearing mice does not distinctly affect tumor growth as compared with non-infused mice. These data indicate that tumor and spleen-derived MDSCs have different functions, even though they have similar morphology and phenotypes. Tumor MDSCs have no alteration of ROS level, compared with those from naive CD11b+Gr-1+ IMC, but have a high level of NO and Arg1. Tumor MDSCs suppressed both antigen-specific and nonspecific T cells. In contrast, splenic MDSCs contain high levels of ROS and modest levels of NO and Arg1 activity, and only suppress nonspecific T cells (Corzo et al., 2010; Qu et al., 2011b).
These functions are thought to be related to the fact that MDSCs express MHC class I, but not MHC class II, and are mediated by cell-cell contact (Corzo et al., 2009). Tumor MDSCs promote more metastasis and invasion of tumor cells, compared with splenic MDSCs (Yang et al., 2008). In the tumor-bearing host, MDSCs are distinctly increased in not only spleen and other lymph organs, but also in other organs, including lungs and livers, where MDSCs could facilitate tumor metastasis to these organ sites (Qu et al., 2009b; Younos et al., 2011).
In different tumor models, MDSCs induce increased numbers of Tregs through different mechanisms. In colon tumor models, CD11b+Gr-1+CD115+ MDSCs induce Treg expansion via secretion of IL-10 and TGF-β. Interestingly, the production of NO was not required for MDSC induction of Tregs, whereas NO produced by iNOS has been shown to be involved in the T cell dysfunction induced by MDSCs (Huang et al., 2006). In contrast, in an ovarian cancer model, the induction of Tregs by MDSCs was associated with the expression of cytotoxic lymphocyte 4 antigen (CTLA4) (Yang et al., 2006).
These reports demonstrate that MDSCs can directly induce Treg expansion and cooperate to promote tumor progression. However, a high percentage of Tregs was invariable throughout tumor growth and was not related to the kinetics of accumulation of the MDSCs (Movahedi et al., 2008), suggesting that MDSCs were not required for some cancer models. MDSCs and Tregs might be linked in the common regulatory network that is involved in both myeloid cell and lymphocyte through the mechanism, which needs be confirmed in the future studies.
In addition to affecting immune function, MDSCs play an important role in tumor angiogenesis. MDSCs promote increased vascularization in tumors, leading to decreased apoptosis of tumor cells, and decreased hypoxic and necrotic regions within tumors, correlating with increased tumor burden (Yang et al., 2004). The pro-angiogenic function of MDSCs has been linked in part to MMP9 expression, which led to increased VEGF/VEGFR2 association, suggesting that MDSC-produced MMP9 enhanced the bioavailablilty of VEGF (Yang et al., 2004). Furthermore, culturing endothelial cells on a collagen matrix with MDSCs led to increased tube formation compared to endothelial cells alone (Kujawski et al., 2008).
Kujawski et al. demonstrated that this pro-angiogenic function of MDSCs was due to STAT3-dependent production of VEGF and basic FGF, both of which are potent angiogenic factors (Kujawski et al., 2008). A recent study found that MDSCs in the tumor microenvironment produced CCL2, which was known to function as an angiogenic factor (Boelte et al., 2011; Salcedo et al., 2000). Blocking CCL2 produced by MDSCs led to decreased endothelial cell migration (Boelte et al., 2011).
These studies suggest that MDSCs contribute to angiogenesis by production of factors which add to or alter the tumor microenvironment in a pro-angiogenic manner. Interestingly, a subset of MDSCs has been shown to express CD31, a marker for endothelial cells (Bronte et al., 2000), and when under angiogenic conditions, some MDSCs can be trans-differentiated into endothelial cells, indicated by cobblestone shape, VEGFR2 and VE-cadherin expression, and low-density lipoprotein uptake, indicating that there are other mechanisms through which MDSCs can modulate vascular formation (Min et al., 2011; Yang et al., 2004).
As previously mentioned, MDSCs are an immature population of cells. When MDSCs were induced to differentiate into DCs in mice in vivo, immune responses were improved (Li et al., 2004; Park et al., 2011). In addition, in both mice and humans, treatment with all-trans retinoic acid (ATRA), a factor that induces differentiation of myeloid cells, mediated the decrease in IMCs while enhancing DCs and T cell responses (Mirza et al., 2006; Yu et al., 2003). Thus, determining the mechanisms of differentiation and function of these cells, and applying this knowledge to treatments, may have an impact on patient survival.
In addition to differentiating the MDSCs, several treatments appear promising in limiting the effect of MDSCs. Gemcitabine, a nucleoside analog used for chemotherapy, reduces the number of MDSCs in tumor-bearing mice, leading to increased immune response to the tumor cells (Ko et al., 2007; Suzuki et al., 2005). Sunitinib, a tyrosine kinase inhibitor, reduced MDSC levels and enhanced immune response to tumors in human patients, suggesting that targeting the factors that lead to accumulation of MDSCs would be therapeutic (Hanson et al., 2009). In addition, phosphodiesterase-5 inhibitors, such as sildenafil, are able to decrease the production of both Arg1 and iNOS, blocking the tumor-promoting functions of MDSCs and enhancing immune response in the mice (Serafini et al., 2006b). Another drug, nitroaspirin, shown to inhibit production of ROS, also inhibits Arg1 and iNOS, and therefore prevents MDSC suppressive functions (De Santo et al., 2005). Several of the drugs that have been found to target MDSCs are already in use for cancer treatment. When used in combination with other tumor therapies, these drugs should not only affect the tumor cells themselves, but also prevent the tumor enhancing capabilities of MDSCs, leading to a better prognosis for the patient.
CONCLUSIONS
We have reviewed the development, differentiation and function of MDSCs, and discussed the regulatory mechanisms and functions of MDSCs in different pathological conditions. Although MDSCs play important roles in clinical human diseases, many questions remain to be elucidated, and are listed here:
How does the nature of MDSCs compare with monocytes and neutrophils?
In humans, the phenotypic characterization of these cells needs to be further developed.
How do we enhance MDSC-based therapies in cancer patients?
REFERENCES
- Adeegbe D, Serafini P, Bronte V, Zoso A, Ricordi C, Inverardi L (2011). In vivo induction of myeloid suppressor cells and CD4(+)Foxp3(+) T regulatory cells prolongs skin allograft survival in mice. Cell Transplant. 20:941–954. [DOI] [PubMed] [Google Scholar]
- Almand B, Clark JI, Nikitina E, van Beynen J, English NR, Knight SC, Carbone DP, Gabrilovich DI (2001). Increased production of immature myeloid cells in cancer patients: a mechanism of immunosuppression in cancer. J. Immunol. 166:678–689. [DOI] [PubMed] [Google Scholar]
- Boelte KC, Gordy LE, Joyce S, Thompson MA, Yang L, Lin PC (2011). Rgs2 mediates pro-angiogenic function of myeloid derived suppressor cells in the tumor microenvironment via upregulation of MCP-1. PloS One 6:e18534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdan C (2011). Regulation of lymphocytes by nitric oxide. Meth. Mol. Biol 677:375–393. [DOI] [PubMed] [Google Scholar]
- Boutte AM, McDonald WH, Shyr Y, Yang L, Lin PC (2011). Characterization of the MDSC proteome associated with metastatic murine mammary tumors using label-free mass spectrometry and shotgun proteomics. PloS One 6: e22446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronstein-Sitton N, Cohen-Daniel L, Vaknin I, Ezernitchi AV, Leshem B, Halabi A, Houri-Hadad Y, Greenbaum E, Zakay-Rones Z, Shapira L, Baniyash M. (2003). Sustained exposure to bacterial antigen induces interferon-gamma-dependent T cell receptor zeta down-regulation and impaired T cell function. Nat. Immunol 4:957–964. [DOI] [PubMed] [Google Scholar]
- Bronte V. (2009). Myeloid-derived suppressor cells in inflammation: Uncovering cell subsets with enhanced immunosuppressive functions. Eur. J. Immunol 39:2670–2672. [DOI] [PubMed] [Google Scholar]
- Bronte V, Apolloni E, Cabrelle A, Ronca R, Serafini P, Zamboni P, Restifo NP, Zanovello P. (2000). Identification of a CD11b(+)/Gr-1( +)/CD31(+) myeloid progenitor capable of activating or suppressing CD8(+) T cells. Blood 96: 3838–3846. [PMC free article] [PubMed] [Google Scholar]
- Bronte V, Chappell DB, Apolloni E, Cabrelle A, Wang M, Hwu P, Restifo NP (1999). Unopposed production of granulocyte-macrophage colony-stimulating factor by tumors inhibits CD8+ T cell responses by dysregulating antigen-presenting cell maturation. J. Immunol 162:5728–5737. [PMC free article] [PubMed] [Google Scholar]
- Bronte V, Serafini P, De Santo C, Marigo I, Tosello V, Mazzoni A, Segal DM, Staib C, Lowel M, Sutter G, et al. (2003a). IL-4-induced arginase 1 suppresses alloreactive T cells in tumor-bearing mice. J. Immunol 170:270–278. [DOI] [PubMed] [Google Scholar]
- Bronte V, Serafini P, Mazzoni A, Segal DM, Zanovello P. (2003b). L-arginine metabolism in myeloid cells controls T-lymphocyte functions. Trends Immunol. 24:302–306. [DOI] [PubMed] [Google Scholar]
- Bronte V, Wang M, Overwijk WW, Surman DR, Pericle F, Rosenberg SA, Restifo NP (1998). Apoptotic death of CD8+ T lymphocytes after immunization: induction of a suppressive population of Mac-1+/Gr-1+ cells. J. Immunol 161:5313–5320. [PMC free article] [PubMed] [Google Scholar]
- Bunt SK, Clements VK, Hanson EM, Sinha P, and Ostrand-Rosenberg S. (2009). Inflammation enhances myeloid-derived suppressor cell cross-talk by signaling through Toll-like receptor 4. J. Leukocyte Biol. 85:996–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunt SK, Sinha P, Clements VK, Leips J, Ostrand-Rosenberg S. (2006). Inflammation induces myeloid-derived suppressor cells that facilitate tumor progression. J. Immunol 176:284–290. [DOI] [PubMed] [Google Scholar]
- Bunt SK, Yang L, Sinha P, Clements VK, Leips J, Ostrand-Rosenberg S. (2007). Reduced inflammation in the tumor microenvironment delays the accumulation of myeloid-derived suppressor cells and limits tumor progression. Cancer Res. 67: 10019–10026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhuri AA, So AY, Sinha N, Gibson WS, Taganov KD, O’Connell RM, Baltimore D. (2011). MicroRNA-125b potentiates macrophage activation. J. Immunol 187:5062–5068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corzo CA, Condamine T, Lu L, Cotter MJ, Youn JI, Cheng P, Cho HI, Celis E, Quiceno DG, Padhya T, et al. (2010). HIF-1alpha regulates function and differentiation of myeloid-derived suppressor cells in the tumor microenvironment. J. Exper. Med 207:2439–2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corzo CA, Cotter MJ, Cheng P, Cheng F, Kusmartsev S, Sotomayor E, Padhya T, McCaffrey TV, McCaffrey JC, Gabrilovich DI (2009). Mechanism regulating reactive oxygen species in tumor-induced myeloid-derived suppressor cells. J. Immunol 182, 5693–5701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Santo C, Serafini P, Marigo I, Dolcetti L, Bolla M, Del Soldato P, Melani C, Guiducci C, Colombo MP, Iezzi M, et al. (2005). Nitroaspirin corrects immune dysfunction in tumor-bearing hosts and promotes tumor eradication by cancer vaccination. Proc. Nat’l. Acad. Sci. USA 104:4185–4190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Wilde V, Van Rompaey N, Hill M, Lebrun JF, Lemaitre P, Lhomme F, Kubjak C, Vokaer B, Oldenhove G, Charbonnier LM, et al. (2009). Endotoxin-induced myeloid-derived suppressor cells inhibit alloimmune responses via heme oxygenase-1. Am. J. Transplant 9:2034–2047. [DOI] [PubMed] [Google Scholar]
- Delano MJ, Scumpia PO, Weinstein JS, Coco D, Nagaraj S, Kelly-Scumpia KM, O’Malley KA, Wynn JL, Antonenko S, Al-Quran SZ, et al. (2007). MyD88-dependent expansion of an immature GR-1(+)CD11b(+) population induces T cell suppression and Th2 polarization in sepsis. J. Exper. Med 204:1463–1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezernitchi AV, Vaknin I, Cohen-Daniel L, Levy O, Manaster E, Halabi A, Pikarsky E, Shapira L, Baniyash M. (2006). TCR zeta down-regulation under chronic inflammation is mediated by myeloid suppressor cells differentially distributed between various lymphatic organs. J. Immunol 177:4763–4772. [DOI] [PubMed] [Google Scholar]
- Fridlender ZG, Sun J, Mishalian I, Singhal S, Cheng G, Kapoor V, Horng W, Fridlender G, Bayuh R, Worthen GS, Albelda SM (2012). Transcriptomic analysis comparing tumor-associated neutrophils with granulocytic myeloid-derived suppressor cells and normal neutrophils. PloS One 7: e31524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu H, Hu Z, Wen J, Wang K, Liu Y. (2009). TGF-beta promotes invasion and metastasis of gastric cancer cells by increasing fascin1 expression via ERK and JNK signal pathways. Acta Biochim. Biophys. Sinica 41:648–656. [DOI] [PubMed] [Google Scholar]
- Fujita M, Kohanbash G, Fellows-Mayle W, Hamilton RL, Komohara Y, Decker SA, Ohlfest JR, Okada H. (2011). COX-2 blockade suppresses gliomagenesis by inhibiting myeloid-derived suppressor cells. Cancer Res. 71:2664–2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabrilovich D, Ishida T, Oyama T, Ran S, Kravtsov V, Nadaf S, Carbone DP (1998). Vascular endothelial growth factor inhibits the development of dendritic cells and dramatically affects the differentiation of multiple hematopoietic lineages in vivo. Blood 92:4150–4166. [PubMed] [Google Scholar]
- Gabrilovich DI, Nagaraj S. (2009). Myeloid-derived suppressor cells as regulators of the immune system. Nature Rev. Immunol. 9:162–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabrilovich DI, Velders MP, Sotomayor EM, Kast WM (2001). Mechanism of immune dysfunction in cancer mediated by immature Gr-1+ myeloid cells. J. Immunol 166:5398–5406. [DOI] [PubMed] [Google Scholar]
- Garcia MR, Ledgerwood L, Yang Y, Xu J, Lal G, Burrell B, Ma G, Hashimoto D, Li Y, Boros P, et al. (2010). Monocytic suppressive cells mediate cardiovascular transplantation tolerance in mice. J. Clin. Investig 120:2486–2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greten TF, Manns MP, Korangy F. (2011). Myeloid derived suppressor cells in human diseases. Inter. Immunopharmacol 11:802–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haile LA, Gamrekelashvili J, Manns MP, Korangy F, Greten TF (2010). CD49d is a new marker for distinct myeloid-derived suppressor cell subpopulations in mice. J. Immunol 185:203–210. [DOI] [PubMed] [Google Scholar]
- Hanson EM, Clements VK, Sinha P, Ilkovitch D, Ostrand-Rosenberg S. (2009). Myeloid-derived suppressor cells down-regulate L-selectin expression on CD4+ and CD8+ T cells. J. Immunol 183:937–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoechst B, Ormandy LA, Ballmaier M, Lehner F, Kruger C, Manns MP, Greten TF, Korangy F. (2008). A new population of myeloid-derived suppressor cells in hepatocellular carcinoma patients induces CD4(+)CD25(+)Foxp3(+) T cells. Gastroenterology 135:234–243. [DOI] [PubMed] [Google Scholar]
- Huang B, Pan PY, Li Q, Sato AI, Levy DE, Bromberg J, Divino CM, Chen SH (2006). Gr-1+CD115+ immature myeloid suppressor cells mediate the development of tumor-induced T regulatory cells and T-cell anergy in tumor-bearing host. Cancer Res. 66:1123–1131. [DOI] [PubMed] [Google Scholar]
- Huang Y, Chen X, Dikov MM, Novitskiy SV, Mosse CA, Yang L, Carbone DP (2007). Distinct roles of VEGFR-1 and VEGFR-2 in the aberrant hematopoiesis associated with elevated levels of VEGF. Blood 110:624–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnnidis JB, Harris MH, Wheeler RT, Stehling-Sun S, Lam MH, Kirak O, Brummelkamp TR, Fleming MD, Camargo FD (2008). Regulation of progenitor cell proliferation and granulocyte function by microRNA-223. Nature 451: 1125–1129. [DOI] [PubMed] [Google Scholar]
- Kamiyama M, Pozzi A, Yang L, DeBusk LM, Breyer RM, and Lin PC (2006). EP2, a receptor for PGE2, regulates tumor angiogenesis through direct effects on endothelial cell motility and survival. Oncogene 25:7019–7028. [DOI] [PubMed] [Google Scholar]
- Kerr EC, Raveney BJ, Copland DA, Dick AD, Nicholson LB (2008). Analysis of retinal cellular infiltrate in experimental autoimmune uveoretinitis reveals multiple regulatory cell populations. J. Autoimmun. 31:354–361. [DOI] [PubMed] [Google Scholar]
- Ko HJ, Kim YJ, Kim YS, Chang WS, Ko SY, Chang SY, Sakaguchi S, Kang CY (2007). A combination of chemoimmunotherapies can efficiently break self-tolerance and induce antitumor immunity in a tolerogenic murine tumor model. Cancer Res. 67:7477–7486. [DOI] [PubMed] [Google Scholar]
- Kujawski M, Kortylewski M, Lee H, Herrmann A, Kay H, and Yu H. (2008). Stat3 mediates myeloid cell-dependent tumor angiogenesis in mice. J. Clin. Investig 118:3367–3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawski M, Zhang C, Herrmann A, Reckamp K, Scuto A, Jensen M, Deng J, Forman S, Figlin R, Yu H. (2010). Targeting STAT3 in adoptively trans-ferred T cells promotes their in vivo expansion and antitumor effects. Cancer Res. 70:9599–9610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusmartsev S, Cheng F, Yu B, Nefedova Y, Sotomayor E, Lush R, Gabrilovich D. (2003). All-trans-retinoic acid eliminates immature myeloid cells from tumor-bearing mice and improves the effect of vaccination. Cancer Res. 63:4441–4449. [PubMed] [Google Scholar]
- Kusmartsev S, Gabrilovich DI (2005). STAT1 signaling regulates tumor-associated macrophage-mediated T cell deletion. J. Immunol 174:4880–4891. [DOI] [PubMed] [Google Scholar]
- Kusmartsev S, Nagaraj S, Gabrilovich DI (2005). Tumor-associated CD8+ T cell tolerance induced by bone marrow-derived immature myeloid cells. J. Immunol 175:4583–4592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusmartsev S, Nefedova Y, Yoder D, Gabrilovich DI (2004). Antigen-specific inhibition of CD8+ T cell response by immature myeloid cells in cancer is mediated by reactive oxygen species. J. Immunol. 172:989–999. [DOI] [PubMed] [Google Scholar]
- Lechner MG, Megiel C, Russell SM, Bingham B, Arger N, Woo T, Epstein AL (2011). Functional characterization of human Cd33+ and Cd11b+ myeloid-derived suppressor cell subsets induced from peripheral blood mononuclear cells co-cultured with a diverse set of human tumor cell lines. J. Trans. Med. 9:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lees JR, Azimzadeh AM, Bromberg JS (2011). Myeloid derived suppressor cells in transplantation. Curr. Opin. Immunol. 23:692–697. [DOI] [PubMed] [Google Scholar]
- Lesokhin AM, Hohl TM, Kitano S, Cortez C, Hirschhorn-Cymerman D, Avogadri F, Rizzuto GA, Lazarus JJ, Pamer EG, Houghton AN, et al. (2012). Monocytic CCR2(+) myeloid-derived suppressor cells promote immune escape by limiting activated CD8 T-cell infiltration into the tumor microenvironment. Cancer Res. 72:876–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Han Y, Guo Q, Zhang M, Cao X. (2009). Cancer-expanded myeloid-derived suppressor cells induce anergy of NK cells through membrane-bound TGF-beta 1. J. Immunol. 182:240–249. [DOI] [PubMed] [Google Scholar]
- Li Q, Pan PY, Gu P, Xu D, Chen SH (2004). Role of immature myeloid Gr-1+ cells in the development of antitumor immunity. Cancer Res. 64:1130–1139. [DOI] [PubMed] [Google Scholar]
- Liu C, Yu S, Kappes J, Wang J, Grizzle WE, Zinn KR, Zhang HG (2007). Expansion of spleen myeloid suppressor cells represses NK cell cytotoxicity in tumor-bearing host. Blood 109:4336–4342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu T, Ramakrishnan R, Altiok S, Youn JI, Cheng P, Celis E, Pisarev V, Sherman S, Sporn MB, Gabrilovich D. (2011a). Tumor-infiltrating myeloid cells induce tumor cell resistance to cytotoxic T cells in mice. J. Clin. Investig. 121:4015–4029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Jiang F, Zheng X, Katakowski M, Buller B, To SS, Chopp M. (2011b). TGF-beta1 promotes motility and invasiveness of glioma cells through activation of ADAM17. Oncol. Repts. 25:1329–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. (2002). Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 23:549–555. [DOI] [PubMed] [Google Scholar]
- Mazzoni A, Bronte V, Visintin A, Spitzer JH, Apolloni E, Serafini P, Zanovello P, and Segal DM (2002). Myeloid suppressor lines inhibit T cell responses by an NO-dependent mechanism. J. Immunol. 168:689–695. [DOI] [PubMed] [Google Scholar]
- Melani C, Chiodoni C, Forni G, Colombo MP (2003). Myeloid cell expansion elicited by the progression of spontaneous mammary carcinomas in c-erbB-2 transgenic BALB/c mice suppresses immune reactivity. Blood 102:2138–2145. [DOI] [PubMed] [Google Scholar]
- Min Y, Ghose S, Boelte K, Li J, Yang L, Lin PC (2011). C/EBP-delta regulates VEGF-C autocrine signaling in lymphangiogenesis and metastasis of lung cancer through HIF-1alpha. Oncogene 30:4901–4909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirza N, Fishman M, Fricke I, Dunn M, Neuger AM, Frost TJ, Lush RM, Antonia S, Gabrilovich DI (2006). All-trans-retinoic acid improves differentiation of myeloid cells and immune response in cancer patients. Cancer Res. 66:9299–9307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Movahedi K, Guilliams M, Van den Bossche J, Van den Bergh R, Gysemans C, Beschin A, De Baetselier P, Van Ginderachter JA (2008). Identification of discrete tumor-induced myeloid-derived suppressor cell subpopulations with distinct T cell-suppressive activity. Blood 111:4233–4244. [DOI] [PubMed] [Google Scholar]
- Nagaraj S, Gabrilovich DI (2010). Myeloid-derived suppressor cells in human cancer. Cancer J. 16:348–353. [DOI] [PubMed] [Google Scholar]
- Nagaraj S, Gupta K, Pisarev V, Kinarsky L, Sherman S, Kang L, Herber DL, Schneck J, Gabrilovich DI (2007). Altered recognition of antigen is a mechanism of CD8+ T cell tolerance in cancer. Nature Med. 13:828–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaraj S, Schrum AG, Cho HI, Celis E, Gabrilovich DI (2010). Mechanism of T cell tolerance induced by myeloid-derived suppressor cells. J. Immunol. 184:3106–3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nefedova Y, Gabrilovich DI (2007). Targeting of Jak/STAT pathway in antigen presenting cells in cancer. Curr. Cancer Drug Targets 7:71–77. [DOI] [PubMed] [Google Scholar]
- O’Connell RM, Rao DS, Baltimore D. (2012). MicroRNA regulation of inflammatory responses. Ann. Rev. Immunol 30:295–312. [DOI] [PubMed] [Google Scholar]
- O’Connell RM, Rao DS, Chaudhuri AA, Boldin MP, Taganov KD, Nicoll J, Paquette RL, Baltimore D. (2008). Sustained expression of microRNA-155 in hematopoietic stem cells causes a myeloproliferative disorder. J. Exper. Med 205, 585–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connell RM, Taganov KD, Boldin MP, Cheng G, Baltimore D. (2007). MicroRNA-155 is induced during the macrophage inflammatory response. Proc. Natl. Acad. Sci. USA 104:1604–1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrand-Rosenberg S, Sinha P. (2009). Myeloid-derived suppressor cells: linking inflammation and cancer. J. Immunol 182:4499–4506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan PY, Ma G, Weber KJ, Ozao-Choy J, Wang G, Yin B, Divino CM, Chen SH (2010). Immune stimulatory receptor CD40 is required for T-cell suppression and T regulatory cell activation mediated by myeloid-derived suppressor cells in cancer. Cancer Res. 70:99–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park YS, Bae JH, Son CH, Lee KS, Kim W, Jung MH, Yang K, Kim SH, Kang CD (2011). Cyclophosphamide potentiates the antitumor effect of immunization with injection of immature dendritic cells into irradiated tumor. Immunol. Invest 40:383–399. [DOI] [PubMed] [Google Scholar]
- Peranzoni E, Zilio S, Marigo I, Dolcetti L, Zanovello P, Mandruzzato S, Bronte V. (2010). Myeloid-derived suppressor cell heterogeneity and subset definition. Curr. Opin. Immunol 22:238–244. [DOI] [PubMed] [Google Scholar]
- Prins RM, Scott GP, Merchant RE, Graf MR (2002). Irradiated tumor cell vaccine for treatment of an established glioma. II. Expansion of myeloid suppressor cells that promote tumor progression. Cancer Immunol. Immunother. 51:190–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu P, Du H, Li Y, Yan C. (2009a). Myeloid-specific expression of Api6/AIM/Sp alpha induces systemic inflammation and adenocarcinoma in the lung. J. Immunol 182:1648–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu P, Du H, Wang X, Yan C. (2009b). Matrix metalloproteinase 12 overexpression in lung epithelial cells plays a key role in emphysema to lung bronchioalveolar adenocarcinoma transition. Cancer Res. 69:7252–7261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu P, Yan C, Blum JS, Kapur R, and Du H. (2011a). Myeloid-specific expression of human lysosomal acid lipase corrects malformation and malfunction of myeloid-derived suppressor cells in lal−/− mice. J. Immunol 187:3854–3866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu P, Yan C, Du H. (2011b). Matrix metalloproteinase 12 overexpression in myeloid lineage cells plays a key role in modulating myelopoiesis, immune suppression, and lung tumorigenesis. Blood 117:4476–4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez PC, Ernstoff MS, Hernandez C, Atkins M, Zabaleta J, Sierra R, Ochoa AC (2009). Arginase I-producing myeloid-derived suppressor cells in renal cell carcinoma are a subpopulation of activated granulocytes. Cancer Res. 69:1553–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez PC, Hernandez CP, Quiceno D, Dubinett SM, Zabaleta J, Ochoa JB, Gilbert J, Ochoa AC (2005). Arginase I in myeloid suppressor cells is induced by COX-2 in lung carcinoma. J. Exper. Med 202:931–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez PC, Zea AH, Ochoa AC (2003). Mechanisms of tumor evasion from the immune response. Cancer Chemother. Biolog. Respon. Modif. 21:351–364. [DOI] [PubMed] [Google Scholar]
- Salcedo R, Ponce ML, Young HA, Wasserman K, Ward JM, Kleinman HK, Oppenheim JJ, Murphy WJ (2000). Human endothelial cells express CCR2 and respond to MCP-1: Direct role of MCP-1 in angiogenesis and tumor progression. Blood 96:34–40. [PubMed] [Google Scholar]
- Serafini P, Borrello I, Bronte V. (2006a). Myeloid suppressor cells in cancer: recruitment, phenotype, properties, and mechanisms of immune suppression. Semin. Cancer Biol. 16:53–65. [DOI] [PubMed] [Google Scholar]
- Serafini P, Carbley R, Noonan KA, Tan G, Bronte V, Borrello I. (2004a). High-dose granulocyte-macrophage colony-stimulating factor-producing vaccines impair the immune response through the recruitment of myeloid suppressor cells. Cancer Res. 64:6337–6343. [DOI] [PubMed] [Google Scholar]
- Serafini P, De Santo C, Marigo I, Cingarlini S, Dolcetti L, Gallina G, Zanovello P, Bronte V. (2004b). Derangement of immune responses by myeloid suppressor cells. Cancer Immunol. Immunother. CII 53:64–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serafini P, Meckel K, Kelso M, Noonan K, Califano J, Koch W, Dolcetti L, Bronte V, Borrello I. (2006b). Phosphodiesterase-5 inhibition augments endogenous anti-tumor immunity by reducing myeloid-derived suppressor cell function. J. Exp. Med 203:2691–2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serafini P, Mgebroff S, Noonan K, and Borrello I. (2008). Myeloid-derived suppressor cells promote cross-tolerance in B-cell lymphoma by expanding regulatory T cells. Cancer Res. 68:5439–5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shojaei F, Wu X, Malik AK, Zhong C, Baldwin ME, Schanz S, Fuh G, Gerber HP, and Ferrara N. (2007). Tumor refractoriness to anti-VEGF treatment is mediated by CD11b+Gr1+ myeloid cells. Nat. Biotechnol 25:911–920. [DOI] [PubMed] [Google Scholar]
- Sica A, and Bronte V. (2007). Altered macrophage differentiation and immune dysfunction in tumor development. J. Clin. Investig 117:1155–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha P, Clements VK, Bunt SK, Albelda SM, Ostrand-Rosenberg S. (2007a). Cross-talk between myeloid-derived suppressor cells and macrophages subverts tumor immunity toward a type 2 response. J. Immunol 179:977–983. [DOI] [PubMed] [Google Scholar]
- Sinha P, Clements VK, Fulton AM, Ostrand-Rosenberg S. (2007b). Prostaglandin E2 promotes tumor progression by inducing myeloid-derived suppressor cells. Cancer Res. 67:4507–4513. [DOI] [PubMed] [Google Scholar]
- Sinha P, Clements VK, Ostrand-Rosenberg S. (2005). Reduction of myeloid-derived suppressor cells and induction of M1 macrophages facilitate the rejection of established metastatic disease. J. Immunol 174:636–645. [DOI] [PubMed] [Google Scholar]
- Sinha P, Okoro C, Foell D, Freeze HH, Ostrand-Rosenberg S, and Srikrishna G. (2008). Proinflammatory S100 proteins regulate the accumulation of myeloid-derived suppressor cells. J. Immunol 181:4666–4675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solheim JC, Reber AJ, Ashour AE, Robinson S, Futakuchi M, Kurz SG, Hood K, Fields RR, Shafer LR, Cornell D, et al. (2007). Spleen but not tumor infiltration by dendritic and T cells is increased by intravenous adenovirus-Flt3 ligand injection. Cancer Gene Ther. 14:364–371. [DOI] [PubMed] [Google Scholar]
- Srivastava MK, Sinha P, Clements VK, Rodriguez P, Ostrand-Rosenberg S. (2010). Myeloid-derived suppressor cells inhibit T-cell activation by depleting cystine and cysteine. Cancer Res. 70:68–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki E, Kapoor V, Jassar AS, Kaiser LR, Albelda SM (2005). Gemcitabine selectively eliminates splenic Gr-1+/CD11b+ myeloid suppressor cells in tumor-bearing animals and enhances antitumor immune activity. Clin. Cancer Res. 11: 6713–6721. [DOI] [PubMed] [Google Scholar]
- Taganov KD, Boldin MP, Chang KJ, Baltimore D. (2006). NF-kappaB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc. Natl. Acad. Sci. USA 103:12481–12486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talmadge JE, Hood KC, Zobel LC, Shafer LR, Coles M, Toth B. (2007). Chemoprevention by cyclooxygenase-2 inhibition reduces immature myeloid suppressor cell expansion. Int. Immunopharmacol. 7:140–151. [DOI] [PubMed] [Google Scholar]
- Varga G, Ehrchen J, Tsianakas A, Tenbrock K, Rattenholl A, Seeliger S, Mack M, Roth J, Sunderkoetter C. (2008). Glucocorticoids induce an activated, anti-inflammatory monocyte subset in mice that resembles myeloid-derived suppressor cells. J. Leukoc. Biol 84:644–650. [DOI] [PubMed] [Google Scholar]
- Yang L, DeBusk LM, Fukuda K, Fingleton B, Green-Jarvis B, Shyr Y, Matrisian LM, Carbone DP, Lin PC (2004). Expansion of myeloid immune suppressor Gr+CD11b+ cells in tumor-bearing host directly promotes tumor angiogenesis. Cancer Cell 6:409–421. [DOI] [PubMed] [Google Scholar]
- Yang L, Huang J, Ren X, Gorska AE, Chytil A, Aakre M, Carbone DP, Matrisian LM, Richmond A, Lin PC, Moses HL (2008). Abrogation of TGF beta signaling in mammary carcinomas recruits Gr-1+CD11b+ myeloid cells that promote metastasis. Cancer Cell 13:23–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang R, Cai Z, Zhang Y, Yutzy W. H. t., Roby KF, Roden RB (2006). CD80 in immune suppression by mouse ovarian carcinoma-associated Gr-1+CD11b+ myeloid cells. Cancer Res. 66:6807–6815. [DOI] [PubMed] [Google Scholar]
- Youn JI, Collazo M, Shalova IN, Biswas SK, Gabrilovich DI (2012). Characterization of the nature of granulocytic myeloid-derived suppressor cells in tumor-bearing mice. J. Leukocyte Biol. 91:167–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youn JI, Nagaraj S, Collazo M, and Gabrilovich DI (2008). Subsets of myeloid-derived suppressor cells in tumor-bearing mice. J. Immunol 181:5791–5802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younos I, Donkor M, Hoke T, Dafferner A, Samson H, Westphal S, Talmadge J. (2011). Tumor- and organ-dependent infiltration by myeloid-derived suppressor cells. Inter. Immunopharmacol 11:816–826. [DOI] [PubMed] [Google Scholar]
- Yu B, Kusmartsev S, Cheng F, Paolini M, Nefedova Y, Sotomayor E, Gabrilovich D. (2003). Effective combination of chemotherapy and dendritic cell administration for the treatment of advanced-stage experimental breast cancer. Clin. Cancer Res. 9:285–294. [PubMed] [Google Scholar]
- Zea AH, Rodriguez PC, Atkins MB, Hernandez C, Signoretti S, Zabaleta J, McDermott D, Quiceno D, Youmans A, O’Neill A, et al. (2005). Arginase-producing myeloid suppressor cells in renal cell carcinoma patients: a mechanism of tumor evasion. Cancer Res. 65:3044–3048. [DOI] [PubMed] [Google Scholar]
- Zhang M, Liu Q, Mi S, Liang X, Zhang Z, Su X, Liu J, Chen Y, Wang M, Zhang Y, et al. (2011). Both miR-17–5p and miR-20a alleviate suppressive potential of myeloid-derived suppressor cells by modulating STAT3 expression. J. Immunol 186:4716–4724. [DOI] [PubMed] [Google Scholar]
- Zhao JL, Rao DS, Boldin MP, Taganov KD, O’Connell RM, Baltimore D. (2011). NF-kappaB dysregulation in microRNA-146a-deficient mice drives the development of myeloid malignancies. Proc. Natl. Acad. Sci. USA 108:9184–9189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu B, Bando Y, Xiao S, Yang K, Anderson AC, Kuchroo VK, Khoury SJ (2007). CD11b+Ly-6C(hi) suppressive monocytes in experimental autoimmune encephalomyelitis. J. Immunol 179:5228–5237. [DOI] [PubMed] [Google Scholar]