Abstract
The ILAE Task Force on Women and Pregnancy conducted a survey among ILAE Chapters of their use of guidelines or recommendations for the management of women with epilepsy during pregnancy. A web‐based questionnaire including 10 questions was sent to the 118 ILAE Chapters in December 2017 with repeated reminders until the end of February 2018. In total, 77 chapters (65%) responded, although not to all questions. Out of those responding, 68% reported having guidelines or recommendations, 34% of which were from 2014 or earlier. At least 20% of the guidelines did not include information on possible risk to cognitive development, information regarding specific risks with specific antiepileptic drugs, nor recommendations regarding selection of antiepileptic drugs. Among those responding to the question, 91% reported that recommendations were made regarding folate supplementation, but the recommended dose ranged from 0.4 mg/d to 4 mg/d or more; 34% did not include recommendations regarding drug level monitoring during pregnancy, and 19% did not include guidelines on breastfeeding. Our survey demonstrates that there is a need for the development of up‐to‐date, globally applicable recommendations for the management of epilepsy during pregnancy.
Keywords: epilepsy, guidelines, pregnancy, survey
Key Points.
Many of the guidelines and recommendations in use are outdated.
Many guidelines lack recommendations on important aspects such as drug selection.
Recommendations vary between countries.
There is an urgent need for the development of up‐to‐date, globally applicable recommendations.
1. INTRODUCTION
The mission of the ILAE Task Force (TF) on Women and Pregnancy is to facilitate optimal management for women with epilepsy who are pregnant or of childbearing potential. One way to achieve this goal is by the use of evidence‐based guidelines. There are however no official, globally approved or ILAE endorsed guidelines, and it is not known on what physicians in different countries base their management of pregnant women with epilepsy.
An important action of the ILAE TF on Women and Pregnancy was therefore to document among ILAE Chapters the use of guidelines or recommendations for the management of women with epilepsy during pregnancy. The aim was to get an overview of the resources that already exist and are in use in different parts of the world, and to review to what extent the messages in the different guidelines are consistent and up to date.
2. METHODS
This study employed a short questionnaire which was designed by members of the TF with the aim of obtaining key information about guideline existence and use while not being too long or complex to complete. An invitation to participate in this web‐based survey was mailed to the indicated contact persons of all ILAE Chapters in December 2017 with two reminders until the end of February 2018 to those not responding. The survey included 10 questions on different aspects of recommendations for the management of women with epilepsy during pregnancy. The questions were mainly meant to investigate which issues that were covered by the guidelines. A few questions were designed to enquire about some selected specific management recommendations, if such were included in the guidelines, and if so what the recommendations were. The 10 questions are displayed in Table 1.
Table 1.
Answers by ILAE Chapters to seven questions in the guideline survey
| Responders (n) | Yes, N (%) | |
|---|---|---|
| Do you have guidelines or recommendations in your country for the management of women with epilepsy during pregnancy? | 73 | 50 (68%) |
| Which organization has issued the guidelines or recommendations that are in use in your country? | 58 | NA |
| Which year were the recommendations last updated? | 56 | NA |
| Does the document make specific recommendations on AED selections? | 58 | 45 (78%) |
| Does it include recommendations on folate supplementation? | 57 | 52 (91%) |
| If the recommended folate dose varies, please list the factors affecting the dose recommendations. | 24 | NA |
| Do your guidelines make recommendations on drug level monitoring during pregnancy? | 58 | 38 (66%) |
| Do your guidelines provide information on specific fetal risks associated with specific AED treatments? | 58 | 46 (79%) |
| Does the information on risks with specific AEDs include the possible risks to cognitive development? | 58 | 43 (74%) |
| Do your guidelines include information about breastfeeding? | 59 | 48 (81%) |
Abbreviation: NA, not applicable.
3. RESULTS
Out of 118 invited chapters, 77 (65%) responded to the invitation to participate in the survey. Only 73 answered the question whether they had guidelines and the chapters responded to a variable degree to the other 9 questions. Chapters participating in the survey are depicted in Figure 1, demonstrating that the poorest representation was from the African continent. The answers to the questions are summarized in Table 1. Of 73 chapters responding to the first question, 50 (68%) reported that in their country they have guidelines or recommendations for the management of women with epilepsy during pregnancy. Among those with recommendations, approximately 35% reported that these were issued by the national ILAE Chapter, 10% by the national neurological society, and 5% by national drug agencies. A substantial proportion (35%) referred to guidelines issued by organizations from other than their own country, in particular the American Academy of Neurology/American Epilepsy Society 1 , 2 , 3 or the UK's National Institute for Health and Care Excellence (NICE) guidelines. 4
Figure 1.
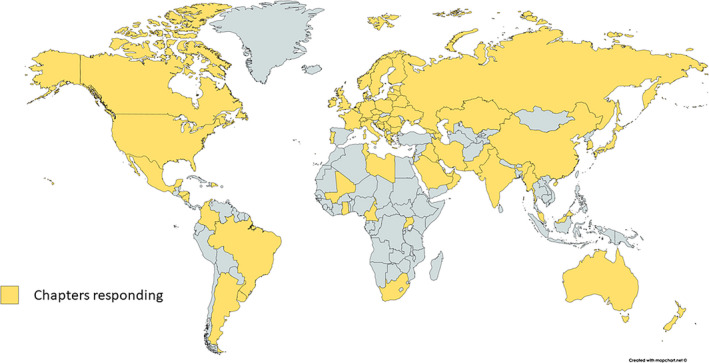
Countries of ILAE Chapters that responded to the guidelines survey
Figure 2 shows the year when the guidelines used by different chapters were issued. Some were as old as 15 years and a substantial proportion were issued before the year when the European Medicines Agency (EMA) first published their tightened restrictions in 2014 on the use of valproate in female patients 5 and before FDA's warnings in 2013. 6
Figure 2.
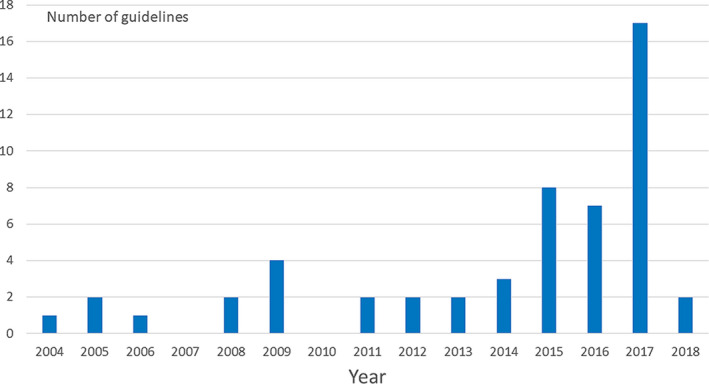
The year of publication of guidelines used in countries responding to the survey
Interestingly, 22% of the documents were reported not to include any specific recommendations on AED selections, and 26% did not include information on the possible risks to cognitive development posed by in utero AED exposure (Table 1). Most of the responding chapters, 52 out of 57 (91%) reported that their guidelines included recommendations regarding folate supplementation (Table 1). The majority favoured 4 mg/d or more, but recommended doses ranged from 0.4 mg/d to 4 or higher (Figure 3). Of the 52 that made recommendations on folate supplementation, 24 indicated that various factors had an impact on the dose that was recommended. Four responders reported that a higher folate dose (4‐5 mg/d) was recommended if there was a family history or personal history in prior pregnancy of malformations (in particular neural tube defects). A few responders each mentioned that this high dose was preferred for women on treatment with valproate or carbamazepine, with “old generation” AEDs, or with enzyme inducing AEDs. Four chapters used low dose (0.4‐1 mg/d) before pregnancy and high dose (3‐5 mg/d) during pregnancy.
Figure 3.
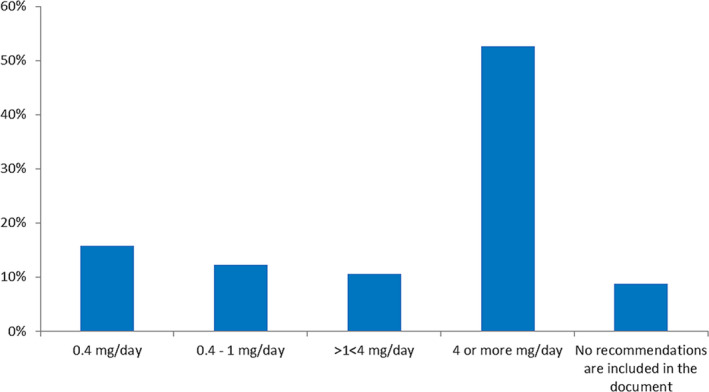
Recommendations on folate supplementation based on 57 responding chapters
4. DISCUSSION
Among the 77 chapters responding to this survey, the majority reported having guidelines or recommendations for the management of women with epilepsy during pregnancy. The guidelines came from different sources, although most had been developed or endorsed by the local chapter. It is concerning that a significant proportion of the guidelines were old, and clearly published before availability of more recent relevant data including the latest regulatory restrictions on valproate use. In more than 20% of the guidelines, no information was provided on possible risk to cognitive development, which may reflect that they are of old date. Similar proportions of guidelines failed to indicate specific risks with specific AEDs, and made no recommendations regarding AED selection. Over the last decade, substantial differential risks across AED treatments have been documented, and the teratogenic risk of some AEDs extend beyond congenital malformations alone. Using outdated or overly general guidelines may put patients and/or their offspring at risk when detailed, AED‐specific information is now available.
More than 30% made no recommendations regarding drug level monitoring, and 19% did not include guidance regarding breastfeeding. While the vast majority addressed folate supplementation, the specific recommendations varied considerably as to dose and timing. The latter is probably a reflection of the lack of conclusive data when it comes to optimal dose for folate supplementation for women treated with AEDs. 7
While acknowledging that we do not know what the situation is in countries of chapters that did not respond to the survey, we see no reason to assume that availability of guidelines is more prevalent among non‐responders compared to responding chapters. Our survey therefore clearly demonstrates that there is a need for the development of up‐to‐date, globally applicable recommendations for the management of epilepsy during pregnancy. While there are limitations in the present evidence base, which leave clinicians and their patients without adequate information, the ILAE Task Force on Women and Pregnancy has recently published expert opinion recommendations in anticipation of more evidence‐based guidelines in the future. 7 Further, this TF is working with the ILAE Wikipedia team to create a series of pages containing guidance on pregnancy in women with epilepsy and their care which will be monitored and updated as new data and developments become available.
CONFLICT OF INTERESTS
ILAE statement: This report was written by experts selected by the International League Against Epilepsy (ILAE) and was approved for publication by the ILAE. Opinions expressed by the authors, however, do not necessarily represent official policy or position of the ILAE. TT has received speaker's honoraria to his institution from Eisai, Sanofi, Sun Pharma, UCB, and Sandoz, and research support from Stockholm County Council, EU, CURE, GSK, UCB, Eisai, and Bial. DB and SK report no disclosures. The authors confirm that this work is consistent with the Journal's guidelines for ethical publication.
ACKNOWLEDGMENTS
KM is funded by NIH NINDS, NICHD #2U01‐NS038455 and has also received research support from Sunovion Pharmaceuticals, and travel support from Eisai. The Epilepsy Study Consortium pays Dr Meador's university for his research consultant time related to Eisai, GW Pharmaceuticals, NeuroPace, Novartis, Supernus, Upsher‐Smith Laboratories, and UCB Pharma. PBP is funded by NIH NINDS, NICHD #2U01‐NS038455 and has also received research support from Epilepsy Foundation, and honoraria and travel support from American Epilepsy Society, American Academy of Neurology, Epilepsy Foundation, National Institutes of Health, and academic institutions for CME lectures. RB is funded by a National Institute for Health Research grant (PDF‐2013‐06‐041). The views expressed in this publication are those of the author(s) and not necessarily those of the NHS, the National Institute for Health Research or the Department of Health. RB has received a consultancy fee from UCB Pharma on one occasion, for a matter unrelated to this subject area.
Tomson T, Battino D, Bromley R, et al. Global Survey of Guidelines for the Management of Epilepsy in Pregnancy: A report from the International League Against Epilepsy Task Force on Women and Pregnancy. Epilepsia Open. 2020;5:366–370. 10.1002/epi4.12420
REFERENCES
- 1. Harden CL, Meador KJ, Pennell PB, Allen Hauser W, Gronseth GS, French JA, et al. Management issues for women with epilepsy‐Focus on pregnancy (an evidence‐based review): II. Teratogenesis and perinatal outcomes: Report of the Quality Standards Subcommittee and Therapeutics and Technology Subcommittee of the American Academy of Neurology and the American Epilepsy Society. Epilepsia. 2009;50(5):1237–46. [DOI] [PubMed] [Google Scholar]
- 2. Harden CL, Pennell PB, Koppel BS, Hovinga CA, Gidal B, Meador KJ, et al. Practice Parameter update: management issues for women with epilepsy–focus on pregnancy (an evidence‐based review): vitamin K, folic acid, blood levels, and breastfeeding. Report of the Quality Standards Subcommittee and Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology and American Epilepsy Society. Epilepsia. 2009;50(5):1247–55. [DOI] [PubMed] [Google Scholar]
- 3. Harden CL, Hopp J, Ting TY, Pennell PB, French JA, Allen Hauser W, et al. Management issues for women with epilepsy‐Focus on pregnancy (an evidence‐based review): I. Obstetrical complications and change in seizure frequency: Report of the Quality Standards Subcommittee and Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology and the American Epilepsy Society. Epilepsia. 2009;50(5):1229–36. [DOI] [PubMed] [Google Scholar]
- 4. NICE . Epilepsies: diagnosis and management. Available at: http://www.nice.org.uk/guidence/cg137. Accessed February 1, 2020.
- 5. European Medicines Agency . Assessment report. Procedure under Article 31 of Directive 2001/83/EC resulting from pharmacovigilance data. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/Referrals_document/Valproate_and_related_substances_31/Recommendation_provided_by_Pharmacovigilance_Risk_Assessment_Committee/WC500177352.pdf. Accessed February 2020.
- 6. FDA Drug Safety Communication . Valproate Anti‐seizure Products Contraindicated for Migraine Prevention in Pregnant Women due to Decreased IQ Scores in Exposed Children. (Issued June 5, 2013). Available at: https://www.fda.gov/Drugs/DrugSafety/ucm350684.htm. Accessed February 2020.
- 7. Tomson T, Battino D, Bromley R, Kochen S, Meador K, Pennell P, et al. Management of epilepsy in pregnancy: a report from the International League Against Epilepsy Task Force on Women and Pregnancy. Epileptic Disord. 2019;21(6):497–517. [DOI] [PubMed] [Google Scholar]


