Abstract
Structural DNA nanotechnology enables DNA to be used as nanomaterials for novel nanostructure construction with unprecedented functionalities. Artificial DNA nanostructures can be designed and generated with precisely controlled features, resulting in its utility in bionanotechnological and biomedical applications. A tetrahedral DNA nanostructure (TDN), the most popular DNA nanostructure, with high stability and simple synthesis procedure, is a promising candidate as nanocarriers in drug delivery and bioimaging platforms, particularly in precision medicine as well as diagnosis for cancer therapy. Recent evidence collectively indicated that TDN successfully enhanced cancer therapeutic efficiency both in vitro and in vivo. Here, we summarize the development of TDN and highlight various aspects of TDN applications in cancer therapy based on previous reports, including anticancer drug loading, photodynamic therapy, therapeutic oligonucleotides, bioimaging platforms, and other molecules and discuss a perspective in opportunities and challenges for future TDN‐based nanomedicine.
Keywords: cancer, DNA nanostructure, nanotechnology, targeted therapy, tetrahedron
This review summarizes the development of tetrahedral DNA nanostructure (TDN) and highlights various aspects of TDN applications in cancer therapy based on previous reports, including anticancer drug loading, photodynamic therapy, therapeutic oligonucleotides, and bioimaging platforms, and discuss a perspective in opportunities and challenges for future TDN‐based nanomedicine.
1. INTRODUCTION
Nanotechnology has played a crucial role in scientific research and development of novel materials and devices with unprecedented properties. Precise manipulation and accurate control on a nanometer scale over sizes and shapes of nanomaterials are still very challenging. DNA is one of these macromolecules that have been used as building blocks for nanoscale construction in many biological and nanotechnological applications. The foundation of the structural DNA nanotechnology field was laid out after Nadrian C Seeman had proposed the immobile DNA junction in the early 1980s. 1 DNA nanostructures become promising nanomaterials for various applications due to their significant advantages, such as specific self‐assembly ability through Watson‐Crick base pairing, precise control over size and shape, biocompatibility and biodegradability, and functional modifications with other molecules. So far, a wide variety of DNA nanostructures have been produced in 2‐D or 3‐D conformations, such as cube, 2 truncated octahedron, 3 tube, 4 , 5 tetrahedron, 6 icosahedron, 7 2D‐DNA arrays, 8 , 9 and DNA origami nanostructures. 10 , 11 , 12 Among these reported DNA nanostructures, tetrahedral DNA nanostructures (TDN) have been widely utilized for biomedical purposes.
As a promising candidate in various bionanotechnological applications, especially in the nanomedical field, not only can TDN serve as a carrier to deliver different cargoes, such as drugs, nucleic acids, and enzymes, but it can also serve as a bioimaging agent for specific molecule detection. In this review, we will summarize different aspects of current TDN applications in cancer therapy and provide a perspective into future direction of TDN‐based nanomedicine.
2. DESIGN AND CHARACTERIZATION OF TETRAHEDRAL DNA NANOSTRUCTURES
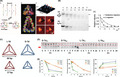
Since Turberfield and coworkers first introduced the single‐step synthesis of TDN, 6 it has become one of the most popular DNA nanostructures with a wide range of applications. Normally, TDN is composed of only 4 oligonucleotides, which self‐assemble into a well‐defined tetrahedral shape, as shown in Figure 1A, through base pairing. 13 Similar to other DNA nanostructures, the formation of TDN is simply carried out by a thermal annealing process with all 4 DNA oligonucleotides mixed together in an appropriate buffer. Multiple analytical techniques can be applied for verification and characterization of successful TDN construction. So far, gel electrophoresis in a nondenaturing condition is the simplest procedure used to confirm designed cDNA binding by visualizing band shift. 14 , 15 , 16 , 17 , 18 , 19 , 20 The dynamic light scattering technique has been widely used to determine the size and zeta‐potential of TDN 15 , 21 , 22 , 23 and the homogeneity of the nanostructures in solution as it directly measures the hydrodynamic size in a precise nanometer range. 24 Atomic force microscopy and transmission electron microscopy can be used to directly visualize TDN structure and conformation. 14 In all, these techniques provide useful information and confirmation of the TDN structure and allow a better understanding of their overall properties.
FIGURE 1.
Tetrahedral DNA nanostructure (TDN) structure and stability. A, Left panel, formation of the TDN nanostructure. Middle panel, space filling model of TDN. Right panel, atomic force microscopy images of TDN. Reproduced with permission. 13 B, In vitro stability of TDN against DNase I compared with the DNA duplex. Reproduced with permission. 28 C, L‐TDN (red) and D‐TDN (blue) in large and small size (30 bp and 17 bp, respectively). Reproduced with permission. 22 D, In vitro stability of D‐TDN and L‐TDN against serum nuclease. Reproduced with permission. 22 E, Left panel, in vivo stability of D‐TDN and L‐TDN estimated using fluorescence resonance energy transfer (FRET) signals. Middle panel, fluorescent intensity of TDN in blood samples. Right panel, fluorescent intensity of TDN in urine samples. Reproduced with permission 22
3. STABILITY – IN VITRO AND IN VIVO
For therapeutic purposes, the stability of TDN is among the most essential requirements for the development of drug delivery carriers to avoid rapid degradation by nucleases or early elimination from the circulation system. Previous studies showed that the presence of divalent Mg2+ is important for structural integrity of DNA‐based nanostructures. 25 The divalent ions could help in balancing an interhelical electrostatic repulsion and also stabilizing the stacked form of the 4‐way Holliday junction. 26 However, Mg2+ is known as the cofactor of various nucleases that are involved in DNA degradation, 27 so its presence might lead to the instability of the DNA nanostructures in the presence of such enzymes. A variety of conditions that mimic the physiological environment have been used to test TDN stability and TDN‐environment interactions. Several studies have examined the in vitro stability of TDN when incubated with various enzymes by gel electrophoresis. 21 , 28 , 29 , 30 Figure 1B shows that TDN exhibited higher stability than the normal duplex in the presence of DNase I. In 2011, by labeling TDN with Cy3 and Cy5, Walsh et al 31 used fluorescence resonance energy transfer (FRET) to investigate the structural integrity of TDN in living HEK cells and found that TDN remained intact for at least 48 hours. In addition, Kim et al 22 claimed that different forms of nucleotides can affect TDN stability. They constructed TDN from L‐DNA (L‐TDN), instead of natural D‐DNA (D‐TDN) (Figure 1C), and claimed that L‐TDN is more stable than D‐TDN (Figure 1D). They also examined the in vivo stability of L‐TDN using FRET and the results showed that L‐TDN was more stable and remained in the bloodstream longer than D‐TDN due to a decrease in renal clearance, as shown in Figure 1E. This might be because the unnatural backbone of L‐DNA is insensitive to nucleases in serum.
4. CELLULAR UPTAKE AND INTRACELLULAR FATES OF TDN
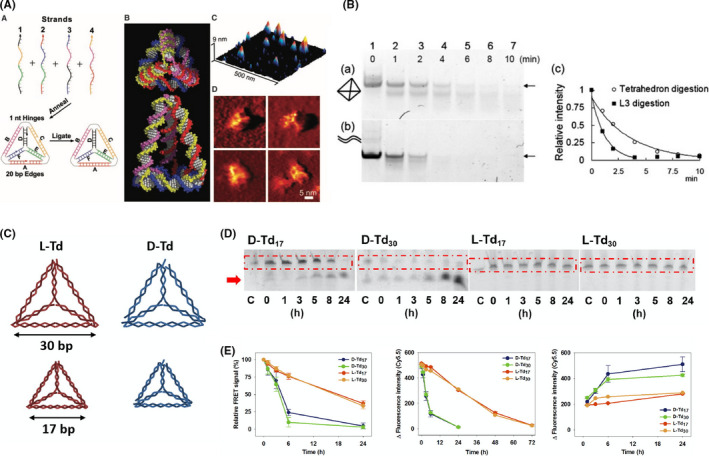
In order for effective use as nanocarriers in drug delivery applications, TDN need to be taken up by target cells. Therefore, understanding not only the routes of TDN internalization but also the fate of TDN inside the cell is necessary. Most cell types use the endocytic process to communicate between intracellular and extracellular compartments. Endocytosis leads to the engulfment of cargoes through membrane invagination by endocytic machineries to form a vesicle inside the cell. 32 , 33 Previous reports showed that nanoparticle uptake relies on many factors, including size, shape, charge, and cell type. 34 In general, DNA is usually impenetrable across the negatively‐charged plasma membrane without the aid of transfection reagents. Although the mechanisms of the cellular uptake of TDN are not well understood, 35 many studies reported that various DNA nanostructures can be taken up by cells without transfection reagents. It has been shown that smaller TDN was more rapidly internalized into cells than larger ones. 36 Walsh et al 31 studied the transfection efficiency of naked TDN and Lipofectin‐encapsulated TDN in mammalian cells. They found that TDN effectively entered mammalian cells both with and without transfection reagents and remained intact in the cytoplasm up to at least 48 hours (Figure 2A,B). Previous reports indicated that TDN entered the cell by endocytosis by way of the caveolin‐dependent pathway. 14 , 37 Using the single‐particle tracking technique to study the transportation pathways of TDN in HeLa cells, it has been shown that TDN entered the cell by fusing with the plasma membrane over approximately 87 seconds, as shown in Figure 2C and subsequently reached the lysosomes. 37 Also, the intracellular motility of TDN was microtubule‐dependent (Figure 2D), implicating that TDN was transported in a highly regulated manner. Furthermore, by adding nuclear localization signals, the subcellular fate of TDN can be targeted to the nucleus instead of lysosomes, like unmodified TDN. Later, another study indicated that the kinetics of the TDN internalization process is cell type‐dependent and that the localization of TDN in lysosomes does not alter lysosome functions. 38 Moreover, TDN does not affect cell cycle progression, resulting in evenly distributed TDN in 2 daughter cells.
FIGURE 2.
Cellular uptake and internalization of tetrahedral DNA nanostructures (TDN). A, Transfection efficiency of TDN with and without Lipofectin estimated by flow cytometry. Reproduced with permission. 31 B, Intracellular localization of TDN with and without Lipofectin. Green, centrin‐GFP; red, Cy5‐labeled TDN. Reproduced with permission. 31 C, Internalization of TDN at different time points. Green, DiO‐labeled lipid; red, Cy3‐labeled TDN. Reproduced with permission. 38 D, Colocalization of TDN (red) and microtubulin (green) in HeLa cells. Reproduced with permission 38
5. APPLICATIONS AS DELIVERY VEHICLES IN CANCER THERAPY
5.1. Targeted delivery
Different kinds of targeting molecules have been modified onto TDN to construct a targeted drug delivery system that enhances tissue specificity and prevents adverse side‐effects from nonspecific interactions. Aptamers have been used extensively for TDN modification due to their high affinity and high specificity to their targets. In general, aptamers, short synthetic ssDNA or RNA oligonucleotides, are developed using the systematic evolution of ligands by exponential enrichment (SELEX). 39 Aptamers can form a 3‐D structure 40 that specifically binds to targets ranging from small molecules to whole cells. 41 , 42 , 43 , 44 Other targeting moieties, such as folic acid, 45 cell‐penetrating peptides 15 , 46 and Affibody molecules, 47 have also been used to modify the TDN for specificity improvement.
5.2. Anticancer drugs
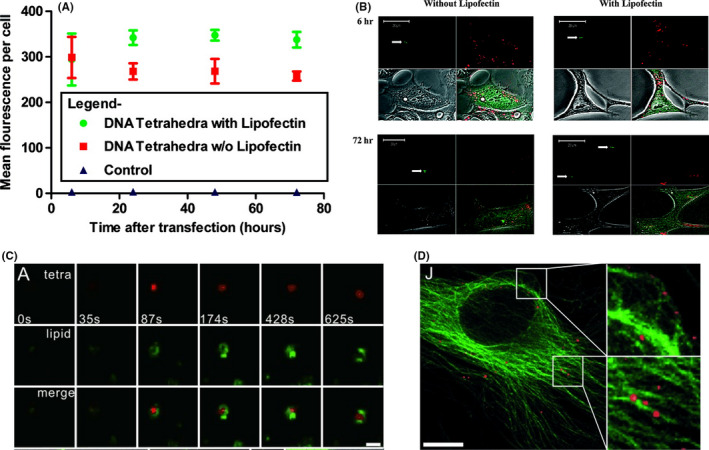
Tetrahedral DNA nanostructures have been utilized as nanocarriers for different types of cargoes. Chemotherapeutic drugs with planar conformations can be easily loaded by intercalating into the DNA duplex. Not only can they allow specific delivery of chemotherapeutic drugs to target cells, but these DNA nanocarriers can also overcome drug resistance in various cancer cell types. Kim and colleagues demonstrated that doxorubicin (dox)‐loaded TDN can circumvent drug resistance in MDR breast cancer cells. 14 It has also been shown that dox‐loaded TDN accumulated inside MDR cells more than free dox, as TDN might avoid the recognition of drug efflux pumps. A MUC1 aptamer is widely used as it specifically binds to Mucin 1 proteins, which are highly expressed in many cancerous cells. Using coculture experiments, Dai et al 48 have shown that MUC1‐aptamer‐modified TDN with dox exhibited high specificity to MUC1‐overexpressing MCF‐7 cells with little binding to MUC1‐negative MDA‐MB‐231 cells (Figure 3A). Recently, 2 different aptamers, MUC1 – specific to Mucin 1 and AS1411 – specific to nucleolin, were functionalized onto dox‐loaded TDN to enhance specificity and chemotherapeutic efficacy. 49 In addition, Liu et al 50 utilized an sgc8c aptamer to specifically deliver dox to protein tyrosine kinase 7 (PTK7)‐overexpressing cells. They showed that aptamer modification can increase cytotoxic effects in PTK7‐positive cells.
FIGURE 3.
Tetrahedral DNA nanostructure (TDN) applications as delivery vehicles in cancer therapy. A, MUC1 aptamer‐modified TDN for selective doxorubicin (Dox) delivery to breast cancer cells. Reproduced with permission. 49 B, AS1411 aptamer‐modified TDN for selective 5‐fluorouracil (5‐FU) delivery to breast cancer cells. Reproduced with permission. 52 C, Methylene blue (MB)‐loaded TDN for in vitro and in vivo photodynamic therapy. Reproduced with permission. 59 D, Delivery of unmethylated CpG motifs by TND for the immunostimulatory effect. TLR9, Toll‐like receptor 9. Reproduced with permission. 62 E, Antisense oligonucleotide (ASO) delivery by nuclear localization signal peptide (NLS)‐modified TDN for protooncogene c‐raf silencing. Reproduced with permission 23
Xie and coworkers investigated the effects of paclitaxel (PTX)‐loaded TDN on PTX‐resistant non‐small‐cell lung cancer cells and found that PTX‐loaded TDN can overcome drug resistance by downregulating the expression of mdr1 gene and P‐glycoprotein, resulting in reduced drug efflux from cancer cells. 16 The same group also reported the use of AS1411‐aptamer‐modified TDN to deliver the antimetabolite 5‐fluorouracil to specific breast cancer cells (Figure 3B). 51 The modified TDN showed the effect on cell cycle and apoptotic genes and proteins. Moreover, Chen and coworkers developed the anticancer metal complex, [Ir(ppy)2phen]+PF6 (Ir)‐loaded TDN, to inhibit vascular mimicry (VM) formation, which enhances tumor migration and metastasis in glioma. 52 They found that metal complex‐loaded TDN showed antimetastasis ability by reducing the expression of VM‐associated proteins.
5.3. Photodynamic therapy
Photodynamic therapy (PDT) is considered as a recently developed cancer treatment. Upon light exposure, the photosensitizer (PS) can undergo 1 of 2 types of reaction, type I or type II reaction, 53 resulting in the generation of free radicals and reactive oxygen species, which cause oxidative stress and cell damage. There are many PS, such as phthalocyanine, 54 hypericin, 55 methylene blue (MB), 56 and pheophorbide‐a, 57 that can be used in PDT. However, most PS have low solubility, poor cell/tissue penetration, and lack of selectivity, leading to low PDT efficacy. To overcome these drawbacks, several nanocarriers have been used to solve these problems. In 2016, Kim and coworkers utilized TDN as a carrier for MB delivery (Figure 3C). 58 They reported that TDN can enhance cellular uptake of MB and increase the stability of MB under reducing intracellular conditions. The MB‐loaded TDN can cause photo‐induced cytotoxicity both in vitro and in vivo. In addition, PDT can be applied to enhance synergistic effects with other treatments. As a proof of concept, Chen et al 59 developed DNA nanodevice‐based, sense‐and‐treat strategy by synergistic chemotherapy and PDT to detect and kill circulating tumor cells.
5.4. Therapeutic oligonucleotides
Due to the specificity and therapeutic property of AS1411 aptamer, it has been shown that AS1411 aptamer‐modified TDN is more specific to cancer cells than TDN and can inhibit tumor cell growth. 29 , 60 Moreover, aptamer‐modified TDN have different effects on cell growth and cell cycle in different cells as they inhibit cell cycle progression and cell growth in tumor cells while promoting cell cycle progression of normal cells in hypoxic condition. Alternatively, Fan and coworkers designed a multivalent DNA nanostructure for delivery of an unmethylated CpG motif (Figure 3D). 61 These motifs are found in bacterial and viral DNA and recognized by Toll‐like receptor 9, which results in immunostimulatory effects. They found that when compared with CpG alone, CpG‐TDN has more potential to induce the secretion of inflammatory cytokines like tumor necrosis factor‐α and interleukins (IL‐6 and IL‐12). These data also indicated that TDN can shield CpG from degradation by nucleases and serves as a nanocarrier for functional nucleic acid delivery.
Suppression of gene expression has been widely used in cancer therapy because this method provides a precise target gene suppression while avoiding undesired effects. As a proof of concept, Lee et al 62 designed TDN with folate and siRNA for silencing the target luciferase gene in tumors. In vivo experiments showed that, after tail vein injection into nude mice, folate‐modified TDN with antiluciferase siRNA highly accumulated in tumor and kidney, whereas less accumulated in heart, lung, spleen, and liver. By observing luciferase activity in folate receptor‐overexpressing KB cells in tumor xenograft mice, they revealed that at 48 hours post‐injection these modified TDN can reduce bioluminescent intensity up to 60%, whereas the decrease of a bioluminescent intensity of folic acid‐conjugated antiluciferase siRNAs was not observed. To suppress specific gene expression, Ding and his group use double‐bundle TDN to deliver antisense oligonucleotide (ASO) against protooncogene c‐raf for inhibiting cancer cell proliferation (Figure 3E). 23 In the reducing environment, disulfide bond in ASO will be cleaved and released from TDN to target c‐raf mRNA, leading to the inhibition of cell proliferation. Moreover, deoxyribozymes or DNAzymes, which are nucleic acids with catalytic activities, can recognize and cleave their substrates and are considered as therapeutic agents for a variety of diseases. 63 , 64 Meng and coworkers developed TDN to deliver a DNAzyme Dz13 for gene regulation. 17 This DNAzyme can cleave c‐Jun mRNA, which is involved in cell proliferation. They found that TDN‐Dz13 displayed high cellular penetration and subsequently decreased cell proliferation by c‐Jun mRNA‐silencing activity. These studies revealed the advantage of TDN as a carrier for gene therapy.
5.5. Other molecules
Peptide nucleic acid (PNA) can specifically hybridize with complementary DNA or RNA and resulting in the inhibition of gene expression. In 2018, Zhang and coworkers reported PNA delivery using TDN as a carrier in methicillin‐resistant Staphylococcus aureus. 65 The ftsZ‐asPNA delivered by TDN effectively targeted the ftsZ gene, which is involved in bacterial cell division, resulting in bacterial growth reduction in a concentration‐dependent manner. This offers a platform for delivery of DNA antibacterial drugs to combat bacterial resistance. In addition, many researchers consider enzymes to be therapeutic agents because of their high substrate specificity. However, the lack of cell‐penetrating properties has made it very challenging for therapeutic use of enzymes. To improve cellular uptake, Kim and coworkers have modified TDN with streptavidin (STV) to use as a multiple enzyme delivery platform for tumor targeted therapy. 66 It was found that STV‐modified TDN showed high cell‐penetrating properties and effectively delivered complex enzymes to the target. Moreover, they reported that the hybrid can also be applied for tumor targeted delivery in vivo. In addition, Liu et al proposed a platform for synthetic vaccine development using TDN assembling with STV and CpG adjuvant to stimulate the immune response in vivo. They showed that TDN delivered the model antigen to the lysosome after 2 hours and promoted model antigen internalization by antigen presenting cells in a time‐dependent manner and significantly increased the immunogenicity of the model antigen. 67
6. APPLICATIONS AS CELLULAR IMAGING PLATFORMS IN CANCER THERAPY
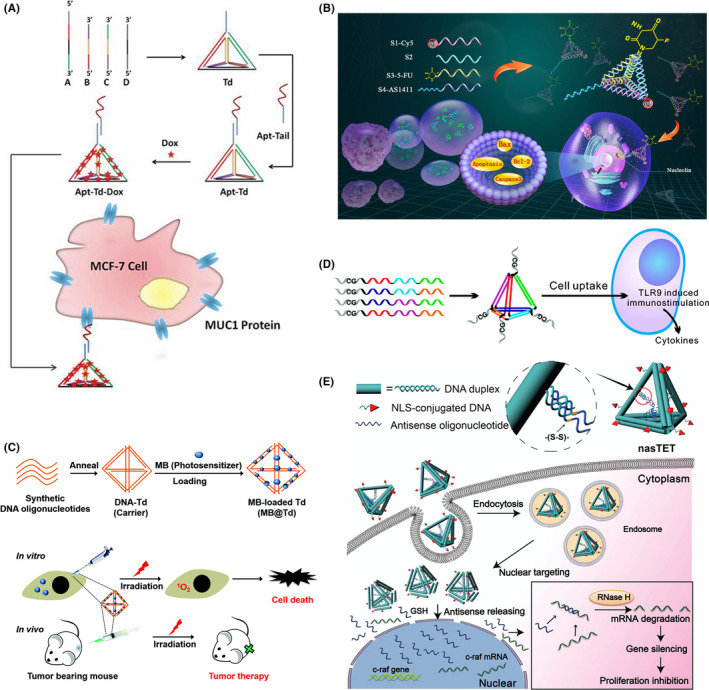
Tetrahedral DNA nanostructures can be functionalized with a wide variety of fluorophores to be used as biosensing and bioimaging platforms. In 2016, Fan and coworkers constructed multiple‐armed TDN for dual‐modality in vivo tumor imaging (Figure 4A). 68 Tetrahedral DNA nanostructures were decorated with different probes, near‐infrared (NIR) dye Dylight‐755 (Dy) and isotope 99mTc for NIR fluorescence and single‐photon emission computed tomography (SPECT) (Figure 4B). Along with folic acid (FA) as a targeting ligand, they showed that FA‐Dy‐99mTc‐TDN can simultaneously use both NIR and SPECT for in vivo imaging. Also, to show that the targeted drug delivery system can be coupled with cancer cell imaging, Liu and coworkers modified dox‐loaded TDN with 2 different aptamers, MUC1 and AS1411. 49 The MUC1 aptamer was modified with a quencher molecule. As the modified TDN were formed, the quencher was adjacent to Cy5, resulting in no fluorescence signal. In the presence of MUC1‐positive cells, the MUC1 aptamer binds to MUC1 protein, leading to enhanced fluorescence signals, which can be used for cancer cell imaging.
FIGURE 4.
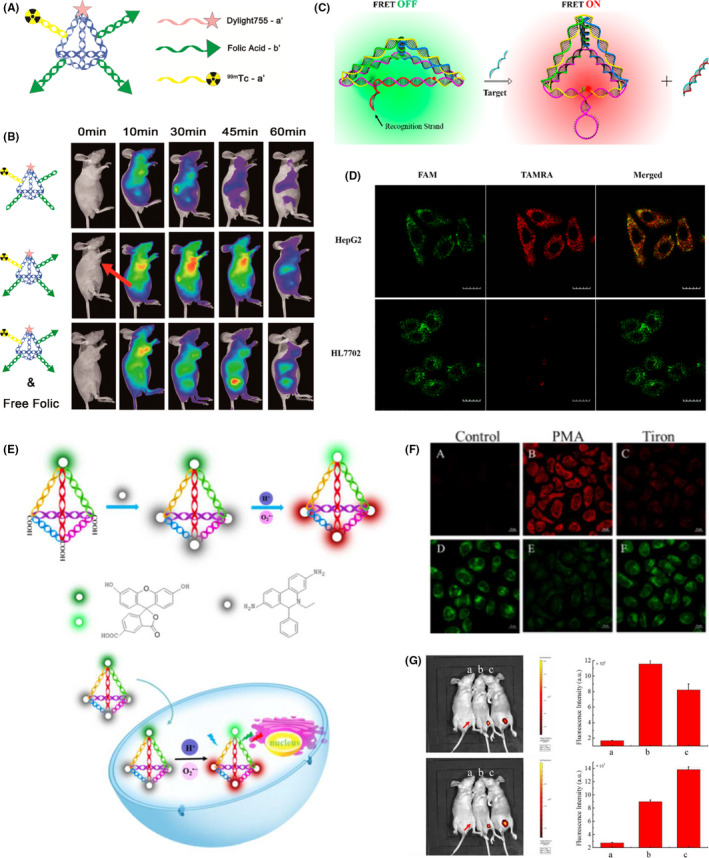
Tetrahedral DNA nanostructures (TDN) as bioimaging platforms. A, Multiple‐armed TDN modified with Dylight 755 fluorescent dye (Dy), folic acid (FA), and radioactive isotope 99mTc for tumor‐targeting imaging. Reproduced with permission. 69 B, Fluorescent imaging of in vivo biodistribution of Dy‐99mTc‐TDN, FA‐Dy‐99mTc‐TDN, and FA‐Dy‐99mTc‐TDN and free folic acid (FA) within 1 h in KB tumor‐bearing nude mice. Coadministration of FA‐Dy‐99mTc‐TDN and free FA served as control. Reproduced with permission. 69 C, TDN‐based molecular beacon for intracellular TK1 mRNA detection. FRET, fluorescence resonance energy transfer. Reproduced with permission. 20 D, Intracellular TK1 mRNA detection in TK1‐positive HepG2 cells and TK1‐negative HL7702 cells by TDN‐based molecular beacon. Green, fluorescein (FAM, fluorescent donor); red, carboxytetramethylrhodamine (TAMRA, fluorescent acceptor). Reproduced with permission. 20 E, TDN‐based nanoprobe for simultaneous detection of pH and superoxide anion () in living cells and in vivo. Green, FAM (pH‐sensitive fluorophore); red, hydroethidine (HE, ‐sensitive fluorophore). Reproduced with permission. 18 F, Simultaneous intracellular imaging of and pH in HeLa cells by TDN‐based nanoprobe. a, d, Control. b, e, Phorbol myristate acetate (PMA) induced production treated cells before incubated with TDN nanoprobe. c, f, Tiron, scavenger, treated cells after PMA treatment. a‐f, All conditions were excited at 488 nm. a‐c, Emission wavelength was collected at 560‐630 nm (HE channel, red). d‐f, Emission wavelength was collected at 500‐540 nm (FAM channel, green). Reproduced with permission. 18 G, In vivo fluorescence imaging of and pH in lipopolysaccharide (LPS)‐induced inflammatory mice model. a, Control (LPS alone). b, TDN nanoprobe injection. c, TDN nanoprobe injection after LPS treatment. a, Emission wavelength collected at 500‐540 nm (FAM channel) after excitation at 480 nm. c, Emission wavelength collected at 580‐620 nm (HE channel) after excitation at 480 nm. b, d, Quantification of fluorescence intensity from (a) and (c). Reproduced with permission 18
Furthermore, TDN could be modified as a biosensing platform to study the intracellular activity of telomerase, which are upregulated in various cancer types. 69 Chen and coworkers developed a TDN probe for in situ fluorescence imaging using FRET. 70 Their results indicated that the TDN probe had good sensitivity with the detection limit of approximately 90 cells/mL and it also exhibited higher selectivity to cancer cells than normal cells. In 2019, Miao and coworkers proposed another approach to detect the intracellular telomerase activity using TDN functionalized with FRET. 19 Data showed that the DNA nanoprobe had the limit of detection at a single cell level. These data confirmed that the DNA nanoprobe can also be applied for intracellular telomerase imaging.
Tumor‐related mRNA has received much attention and been used as a representative tumor biomarker for cancer diagnosis. Recently, mRNA of the TK1 gene, which is involved in cell division and tumor cell growth, 71 was chosen as a target for tumor biomarker detection. In 2016, Wang and coworkers designed a TDN‐based molecular beacon for detection of tumor‐related mRNA and ATP in living cells (Figure 4C). 20 As shown in Figure 4D, the TDN‐based biosensor can be used to detect TK1 mRNA in HepG2 and HL7702 cells. Also, Li et al 18 developed a TDN‐based nanoprobe for detection of pH and superoxide anion () that are involved in various diseases, including cancers (Figure 4E). The TDN was modified with 2 fluorophores, fluorescein (for pH sensing) and hydroethidine (for sensing). Data showed that the DNA nanoprobe can simultaneously allow the visualization of pH and without toxicity in living cells and mouse models with high sensitivity and image resolution (Figure 4F,G). Recently, Xie and coworkers developed a TDN‐based biosensor for imaging of Ago2, the essential protein for RNAi mechanism, in a single cell and RNase H detection. 72 They showed that the biosensor could be used with high selectivity for Ago2/microRNA‐21 imaging and to assess the concentration of Ago2 in a single cell. This suggested that the TDN‐based biosensor can be used for further diagnosis and treatment of diseases.
7. CONCLUSION AND PERSPECTIVE
Overall, the field of DNA nanotechnology has emerged as a crucial tool in a variety of biological and biomedical applications. Among different types of DNA nanostructures, TDN have been a major research focus due to their structural and biological features. This review summarized recent data that collectively indicate a great potential for TDN as drug delivery systems, biosensing and bioimaging platforms, and even biological regulators. Nevertheless, chemically modified TDN still need to meet necessary requirements before they can be utilized in clinical trials, including in vivo stability, suitable pharmacodynamic and pharmacokinetic properties, and long‐term cytotoxicity. This challenge awaits future researchers to tackle and continue the development of TDN and other DNA nanomaterials with real diagnostic and therapeutic value.
CONFLICT OF INTEREST
The authors have no conflict of interest.
ACKNOWLEDGMENTS
This work was supported by Burapha University (AU) and Mahidol University under the New Discovery and Frontier Research Grant (TK).
Duangrat R, Udomprasert A, Kangsamaksin T. Tetrahedral DNA nanostructures as drug delivery and bioimaging platforms in cancer therapy. Cancer Sci. 2020;111:3164–3173. 10.1111/cas.14548
Contributor Information
Anuttara Udomprasert, Email: anuttara@go.buu.ac.th.
Thaned Kangsamaksin, Email: thaned.kan@mahidol.edu.
REFERENCES
- 1. Seeman NC. Nucleic acid junctions and lattices. J Theor Biol. 1982;99:237‐247. [DOI] [PubMed] [Google Scholar]
- 2. Chen J, Seeman NC. Synthesis from DNA of a molecule with the connectivity of a cube. Nature. 1991;350:631‐633. [DOI] [PubMed] [Google Scholar]
- 3. Zhang Y, Seeman NC. Construction of a DNA‐truncated octahedron. J Am Chem Soc. 1994;116:1661‐1669. [Google Scholar]
- 4. Rothemund PWK, Ekani‐Nkodo A, Papadakis N, Kumar A, Fygenson DK, Winfree E. Design and characterization of programmable DNA nanotubes. J Am Chem Soc. 2004;126:16344‐16352. [DOI] [PubMed] [Google Scholar]
- 5. Mathieu F, Liao S, Kopatsch J, Wang T, Mao C, Seeman NC. Six‐helix bundles designed from DNA. Nano Lett. 2005;5:661‐665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Goodman RP, Berry RM, Turberfield AJ. The single‐step synthesis of a DNA tetrahedron. Chem Commun. 2004;12:1372‐1373. [DOI] [PubMed] [Google Scholar]
- 7. Zhang C, Su M, He Y, et al. Conformational flexibility facilitates self‐assembly of complex DNA nanostructures. Proc Natl Acad Sci USA. 2008;105:10665‐10669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Winfree E, Liu F, Wenzler LA, Seeman NC. Design and self‐assembly of two‐dimensional DNA crystals. Nature. 1998;394:539‐544. [DOI] [PubMed] [Google Scholar]
- 9. Majumder U, Rangnekar A, Gothelf KV, Reif JH, LaBean TH. Design and construction of double‐decker tile as a route to three‐dimensional periodic assembly of DNA. J Am Chem Soc. 2011;133:3843‐3845. [DOI] [PubMed] [Google Scholar]
- 10. Rothemund PWK. Folding DNA to create nanoscale shapes and patterns. Nature. 2006;440:297‐302. [DOI] [PubMed] [Google Scholar]
- 11. Dietz H, Douglas SM, Shih WM. Folding DNA into twisted and curved nanoscale shapes. Science. 2009;325:725‐730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Han D, Pal S, Nangreave J, Deng Z, Liu Y, Yan H. DNA origami with complex curvatures in three‐dimensional space. Science. 2011;332:342‐346. [DOI] [PubMed] [Google Scholar]
- 13. Goodman RP, Schaap IAT, Tardin CF, et al. Rapid chiral assembly of rigid DNA building blocks for molecular nanofabrication. Science. 2005;310:1661‐1665. [DOI] [PubMed] [Google Scholar]
- 14. Kim K‐R, Kim D‐R, Lee T, et al. Drug delivery by a self‐assembled DNA tetrahedron for overcoming drug resistance in breast cancer cells. Chem Commun. 2013;49:2010‐2012. [DOI] [PubMed] [Google Scholar]
- 15. Tian T, Li J, Xie C, et al. Targeted imaging of brain tumors with a framework nucleic acid probe. ACS Appl Mater Interfaces. 2018;10:3414‐3420. [DOI] [PubMed] [Google Scholar]
- 16. Xie X, Shao X, Ma W, et al. Overcoming drug‐resistant lung cancer by paclitaxel loaded tetrahedral DNA nanostructures. Nanoscale. 2018;10:5457‐5465. [DOI] [PubMed] [Google Scholar]
- 17. Meng L, Ma W, Lin S, Shi S, Li Y, Lin Y. Tetrahedral DNA nanostructure‐delivered DNAzyme for gene silencing to suppress cell growth. ACS Appl Mater Interfaces. 2019;11:6850‐6857. [DOI] [PubMed] [Google Scholar]
- 18. Li NA, Wang M, Gao X, et al. A DNA tetrahedron nanoprobe with controlled distance of dyes for multiple detection in living cells and in vivo . Anal Chem. 2017;89:6670‐6677. [DOI] [PubMed] [Google Scholar]
- 19. Meng F, Chai H, Ma X, Tang Y, Miao P. FRET investigation toward DNA tetrahedron‐based ratiometric analysis of intracellular telomerase activity. J Mater Chem B. 2019;7:1926‐1932. [DOI] [PubMed] [Google Scholar]
- 20. Xie N, Huang J, Yang X, et al. Competition‐mediated FRET‐switching DNA tetrahedron molecular beacon for intracellular molecular detection. ACS Sens. 2016;1:1445‐1452. [Google Scholar]
- 21. Tay CY, Yuan L, Leong DT. Nature‐inspired DNA nanosensor for real‐time in situ detection of mRNA in living cells. ACS Nano. 2015;9:5609‐5617. [DOI] [PubMed] [Google Scholar]
- 22. Kim K‐R, Kim HY, Lee Y‐D, et al. Self‐assembled mirror DNA nanostructures for tumor‐specific delivery of anticancer drugs. J Control Release. 2016;243:121‐131. [DOI] [PubMed] [Google Scholar]
- 23. Yang J, Jiang Q, He L, et al. Self‐assembled double‐bundle DNA tetrahedron for efficient antisense delivery. ACS Appl Mater Interfaces. 2018;10:23693‐23699. [DOI] [PubMed] [Google Scholar]
- 24. Oh N, Park JH. Endocytosis and exocytosis of nanoparticles in mammalian cells. Int J Nanomedicine. 2014;9:51‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Martin TG, Dietz H. Magnesium‐free self‐assembly of multi‐layer DNA objects. Nat Commun. 2012;3:1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Duckett DR, Murchie AIH, Lilley DMJ. The role of metal ions in the conformation of the four‐way DNA junction. EMBO J. 1990;9:583‐590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yang W, Lee JY, Nowotny M. Making and breaking nucleic acids: Two‐Mg2+‐ion catalysis and substrate specificity. Mol Cell. 2006;22:5‐13. [DOI] [PubMed] [Google Scholar]
- 28. Keum JW, Bermudez H. Enhanced resistance of DNA nanostructures to enzymatic digestion. Chem Commun. 2009;45:7036‐7038. [DOI] [PubMed] [Google Scholar]
- 29. Charoenphol P, Bermudez H. Aptamer‐targeted DNA nanostructures for therapeutic delivery. Mol Pharmaceutics. 2014;11:1721‐1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Xie N, Huang J, Yang X, et al. A DNA tetrahedron‐based molecular beacon for tumor related mRNA detection in living cells. Chem Commun. 2016;52:2346‐2349. [DOI] [PubMed] [Google Scholar]
- 31. Walsh AS, Yin H, Erben CM, Wood MJA, Turberfield AJ. DNA cage delivery to mammalian cells. ACS Nano. 2011;5:5427‐5432. [DOI] [PubMed] [Google Scholar]
- 32. Lee DS, Qian H, Tay CY, Leong DT. Cellular processing and destinies of artificial DNA nanostructures. Chem Soc Rev. 2016;45:4199‐4225. [DOI] [PubMed] [Google Scholar]
- 33. Behzadi S, Serpooshan V, Tao W, et al. Cellular uptake of nanoparticles: Journey inside the cell. Chem Soc Rev. 2017;46:4218‐4244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sahay G, Alakhova DY, Kabanov AV. Endocytosis of nanomedicines. J Control Release. 2010;145:182‐195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Angell C, Xie S, Zhang L, Chen Y. DNA nanotechnology for precise control over drug delivery and gene therapy. Small. 2016;12:1117‐1132. [DOI] [PubMed] [Google Scholar]
- 36. Kang JH, Kim KR, Lee H, Ahn DR, Ko YT In vitro and in vivo behavior of DNA tetrahedrons as tumor‐targeting nanocarriers for doxorubicin delivery. Colloids Surf B Biointerfaces. 2017;157:424‐431. [DOI] [PubMed] [Google Scholar]
- 37. Liang LE, Li J, Li Q, et al. Single‐particle tracking and modulation of cell entry pathways of a tetrahedral DNA nanostructure in live cells. Angew Chem Int Ed. 2014;53:7745‐7750. [DOI] [PubMed] [Google Scholar]
- 38. Xia K, Kong H, Cui Y, et al. Systemic study in mammalian cells showing no adverse response to tetrahedral DNA nanostructure. ACS Appl Mater Interfaces. 2018;10:15442‐15448. [DOI] [PubMed] [Google Scholar]
- 39. Tuerk C, Gold L. Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science. 1990;249:505‐510. [DOI] [PubMed] [Google Scholar]
- 40. Stoltenburg R, Reinemann C, Strehlitz B. SELEX‐A (r)evolutionary method to generate high‐affinity nucleic acid ligands. Biomol Eng. 2007;24:381‐403. [DOI] [PubMed] [Google Scholar]
- 41. Willner I, Zayats M. Electronic aptamer‐based sensors. Angew Chem Int Ed. 2007;46:6408‐6418. [DOI] [PubMed] [Google Scholar]
- 42. Kaur H, Bruno JG, Kumar A, Sharma TK. Aptamers in the therapeutics and diagnostics pipelines. Theranostics. 2018;8:4016‐4032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Shangguan D, Cao Z, Meng L, et al. Cell‐specific aptamer probes for membrane protein elucidation in cancer cells. J Proteome Res. 2008;7:2133‐2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zhu G, Zhang H, Jacobson O, et al. Combinatorial screening of DNA aptamers for molecular imaging of HER2 in cancer. Bioconjug Chem. 2017;28:1068‐1075. [DOI] [PubMed] [Google Scholar]
- 45. Zhang G, Zhang Z, Yang J. DNA tetrahedron delivery enhances doxorubicin‐induced apoptosis of HT‐29 colon cancer cells. Nanoscale Res Lett. 2017;12:495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Xia Z, Wang P, Liu X, et al. Tumor‐penetrating peptide‐modified DNA tetrahedron for targeting drug delivery. Biochemistry. 2016;55:1326‐1331. [DOI] [PubMed] [Google Scholar]
- 47. Zhang Y, Jiang S, Zhang D, Bai X, Hecht SM, Chen S. DNA‐affibody nanoparticles for inhibiting breast cancer cells overexpressing HER2. Chem Commun. 2017;53:573‐576. [DOI] [PubMed] [Google Scholar]
- 48. Dai B, Hu Y, Duan J, Yang XD. Aptamer‐guided DNA tetrahedron as a novel targeted drug delivery system for MUC1‐expressing breast cancer cells in vitro . Oncotarget. 2016;7:38257‐38269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Liu X, Wu L, Wang L, Jiang W. A dual‐targeting DNA tetrahedron nanocarrier for breast cancer cell imaging and drug delivery. Talanta. 2018;179:356‐363. [DOI] [PubMed] [Google Scholar]
- 50. Liu M, Ma W, Li Q, et al. Aptamer‐targeted DNA nanostructures with doxorubicin to treat protein tyrosine kinase 7‐positive tumours. Cell Prolif. 2019;52:e12511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zhan Y, Ma W, Zhang Y, et al. DNA‐based nanomedicine with targeting and enhancement of therapeutic efficacy of breast cancer cells. ACS Appl Mater Interfaces. 2019;11:15354‐15365. [DOI] [PubMed] [Google Scholar]
- 52. Tian Y, Huang Y, Gao P, Chen T. Nucleus‐targeted DNA tetrahedron as a nanocarrier of metal complexes for enhanced glioma therapy. Chem Commun. 2018;54:9394‐9397. [DOI] [PubMed] [Google Scholar]
- 53. Debele TA, Peng S, Tsai HC. Drug carrier for photodynamic cancer therapy. Int J Mol Sci. 2015;16:22094‐22136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Miller J, Baron E, Scull H, et al. Photodynamic therapy with the phthalocyanine photosensitizer Pc 4: the case experience with preclinical mechanistic and early clinical‐translational studies. Toxicol Appl Pharmacol. 2007;224:290‐299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Barathan M, Mariappan V, Shankar EM, Abdullah BJ, Goh KL, Vadivelu J. Hypericin‐photodynamic therapy leads to interleukin‐6 secretion by HepG2 cells and their apoptosis via recruitment of BH3 interacting‐domain death agonist and caspases. Cell Death Dis. 2013;4:e697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Obstoy B, Salaun M, Bohn P, Veresezan L, Sesboue R, Thiberville L. Photodynamic therapy using methylene blue in lung adenocarcinoma xenograft and hamster cheek pouch induced squamous cell carcinoma. Photodiagnosis Photodyn Ther. 2016;15:109‐114. [DOI] [PubMed] [Google Scholar]
- 57. Tang PM, Liu XZ, Zhang DM, Fong WP, Fung KP. Pheophorbide a based photodynamic therapy induces apoptosis via mitochondrial‐mediated pathway in human uterine carcinosarcoma. Cancer Biol Ther. 2009;8:533‐539. [DOI] [PubMed] [Google Scholar]
- 58. Kim KR, Bang D, Ahn DR. Nano‐formulation of a photosensitizer using a DNA tetrahedron and its potential for in vivo photodynamic therapy. Biomater Sci. 2016;4:605‐609. [DOI] [PubMed] [Google Scholar]
- 59. Chen N, Qin S, Yang X, Wang Q, Huang J, Wang K. “Sense‐and‐treat” DNA nanodevice for synergistic destruction of circulating tumor cells. ACS Appl Mater Interfaces. 2016;8:26552‐26558. [DOI] [PubMed] [Google Scholar]
- 60. Li Q, Zhao D, Shao X, et al. Aptamer‐modified tetrahedral DNA nanostructure for tumor‐targeted drug delivery. ACS Appl Mater Interfaces. 2017;9:36695‐36701. [DOI] [PubMed] [Google Scholar]
- 61. Li J, Pei H, Zhu B, et al. Self‐assembled multivalent DNA nanostructures for noninvasive intracellular delivery of immunostimulatory CpG oligonucleotides. ACS Nano. 2011;5:8783‐8789. [DOI] [PubMed] [Google Scholar]
- 62. Lee H, Lytton‐Jean AKR, Chen YI, et al. Molecularly self‐assembled nucleic acid nanoparticles for targeted in vivo siRNA delivery. Nat Nanotechnol. 2012;7:389‐393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Gong L, Zhao Z, Lv Y‐F, et al. DNAzyme‐based biosensors and nanodevices. Chem Commun. 2015;51:979‐995. [DOI] [PubMed] [Google Scholar]
- 64. Chan CWS, Khachigian LM. DNAzymes and their therapeutic possibilities. Intern Med J. 2009;39:249‐251. [DOI] [PubMed] [Google Scholar]
- 65. Zhang Y, Ma W, Zhu Y, et al. Inhibiting methicillin‐resistant Staphylococcus aureus by tetrahedral DNA nanostructure‐enabled antisense peptide nucleic acid delivery. Nano Lett. 2018;18:5652‐5659. [DOI] [PubMed] [Google Scholar]
- 66. Kim K‐R, Hwang D, Kim J, et al. Streptavidin‐mirror DNA tetrahedron hybrid as a platform for intracellular and tumor delivery of enzymes. J Control Release. 2018;280:1‐10. [DOI] [PubMed] [Google Scholar]
- 67. Liu X, Xu Y, Yu T, et al. A DNA nanostructure platform for directed assembly of synthetic vaccines. Nano Lett. 2012;12:4254‐4259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Jiang D, Sun Y, Li J, et al. Multiple‐armed tetrahedral DNA nanostructures for tumor‐targeting, dual‐modality in vivo imaging. ACS Appl Mater Interfaces. 2016;8:4378‐4384. [DOI] [PubMed] [Google Scholar]
- 69. Shay JW. Role of telomeres and telomerase in aging and cancer. Cancer Discov. 2016;6:584‐593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Feng QM, Zhu MJ, Zhang TT, Xu JJ, Chen HY. A novel DNA tetrahedron‐hairpin probe for in situ “off‐on” fluorescence imaging of intracellular telomerase activity. Analyst. 2016;141:2474‐2480. [DOI] [PubMed] [Google Scholar]
- 71. Brabender J, Danenberg KD, Metzger R, et al. Epidermal growth factor receptor and HER2‐neu mRNA expression in non‐small cell lung cancer is correlated with survival. Clin Cancer Res. 2001;7:1850‐1855. [PubMed] [Google Scholar]
- 72. Zhang K, Huang W, Huang Y, et al. DNA tetrahedron based biosensor for Argonaute2 assay in single cells and human immunodeficiency virus type‐1 related ribonuclease H detection in vitro . Anal Chem. 2019;91:7086‐7096. [DOI] [PubMed] [Google Scholar]


