Supplemental digital content is available in the text.
Key Words: cerebral physiology, focal seizure, generalized seizure, hemispheric cerebral oximetry, rcSO2 (regional cerebral oxygen saturation), neuroresuscitation, seizure, seizure activity
Background
Sustained neuronal activity during seizures causes cellular perturbations, alterations in cerebral physiology, and potentially neurological injury, a neurological emergency. With variable clinical manifestations of seizures, frequent failure of seizure recognition by providers in pediatric and developmentally challenged patients can increase seizure complications. Neuroresuscitation should include rapid cerebral physiology assessment for increased seizure recognition and optimal neurological outcomes. In neurological emergencies, cerebral oximetry has demonstrated its utility in altered cerebral physiology and a standard combat neurological assessment tool. During adult seizures, cerebral oximetry (regional cerebral oxygen saturation [rcSO2]) has been shown as a useful neurological assessment tool, but research is lacking in pediatric emergency department (PED) seizure patients.
Objective
The aim of this study was to identify trends in rcSO2 readings for patients presenting to the PED with seizure activity and in the postseizure state in order to evaluate usefulness of rcSO2 as a neurological assessment tool in pediatric seizure patients.
Methods
This was a PED observational case series comparing hemispheric rcSO2 readings in first-time clinically evident generalized and focal seizure patients to first-time postseizure patients with no PED seizures.
Results
Generalized or focal seizure (n = 185) hemispheric rcSO2 revealed significant differences compared with nonseizure and controls' rcSO2 readings (n = 115) (P < 0.0001). Generalized and focal seizure rcSO2's were either less than 60% or greater than 80% compared with nonseizure rcSO2 (P < 0.0001). Ipsilateral focal seizure rcSO2 correlated to seizure side (P < 0.0001) and was less than the contralateral rcSO2 (P < 0.0001), with interhemispheric rcSO2 discordance greater than 16 (P < 0.0001). Seizure to preseizure rcSO2 discordance was as follows: generalized 15.2, focal: left 19.8, right 20.3 (P < 0.0001).
Conclusions
Hemispheric during-seizure rcSO2 readings significantly correlated with generalized and focal seizures and reflected altered cerebral physiology. Ipsilateral focal seizure rcSO2 readings correlated to the focal side with wide interhemispheric rcSO2 discordance. All postseizure rcSO2 readings returned to preseizure readings, showing altered cerebral physiology resolution. Overall, in generalized or focal seizure, rcSO2 readings were less than 60% or greater than 80%, and in focal seizure, interhemispheric rcSO2 discordance was greater than 10. During seizures, hemispheric rcSO2 readings demonstrated its potential pediatric seizure utility. Utilizing rcSO2 readings related to seizure activity could expedite pediatric and developmentally challenged patients' seizure recognition, cerebral assessment, and interventions especially in pharmacoresistant seizures.
WHAT IS ALREADY KNOWN ON THIS SUBJECT
Pediatric seizure is common in the pediatric emergency department (PED) and clinically presents in multiple forms. Sustained neuronal activity causes cellular perturbations and alterations in cerebral physiology, potentially causing neurological injury. In limited adult and pediatric cerebral oximetry seizure studies, change in multichannel regional cerebral oxygen saturation (rcSO2) reading correlated with seizure activity in non-ED settings.
WHAT THIS STUDY ADDS
This study showed significantly decreased or increased hemispheric rcSO2 readings compared with preseizure and postseizure events during generalized and focal seizure. During seizure activity, there was an alteration in cerebral hemispheric cerebral tissue oxygenation and physiology. Also in the postseizure period, these altered hemispheric during-seizure rcSO2 readings return to preseizure readings. In focal seizure patients, the ipsilateral rcSO2 readings correlated to the hemispheric focal seizure side and were significantly different from the contralateral rcSO2 readings.
INTRODUCTION
Pediatric seizures presenting to the pediatric emergency department (PED) or emergency department (ED) can be generalized (which can be easily identifiable), focal, convulsive, or nonconvulsive, or status epilepticus.1–4 Subtle clinical or nonconvulsive seizure activity can be unrecognized by emergency medical services (EMS) and ED providers, given the lack of overt clinical signs and symptoms. Seizures can cause neuronal compromise and altered cerebral physiology, be pharmacoresistant, and cause neurological complications, a neurological emergency.1–8 Seizure manifestations are variable, and failure to recognize and treat prolongs the seizure activity, thus increasing the neurological complications.3–8 These “hard to clinically recognize” seizures can be exacerbated in the neonate, nonverbal pediatric patients and especially nonverbal/developmentally challenged patients.
A false seizure assumption is that the brain's physiology is not altered if there is global glucose and oxygen normalcy.6–12 Persistent cerebral physiology abnormalities can continue during the seizure and can be undetected by current ED monitoring systems.2–12 Neuroresuscitation should strive to quickly assess for altered cerebral physiology coupled with immediate therapeutic response assessment.2–12 These objectives are paramount to neuronal preservation and improved neurological outcomes.3–14 With a rapid objective cerebral physiology assessment tool's correlation with the patient's corresponding clinical neurological activity, this seizure misperceptions could be significantly decreased and augment the provider's critical thinking and decision making, which ED or EMS monitoring systems currently lack.7–15
During seizures, especially those with underlying neuronal mitochondria dysfunction, an increase in neuronal metabolism occurs causing regional oxygen and glucose depletion, resulting in regional tissue hypoxia and altered cerebral physiology.2–9,16–22 Cerebral oximetry (rcSO2) detects and reflects regional dynamic cerebral physiologic changes (tissue perfusion, oxygenation metabolism, and oxygen extraction).12–15,23–25 Normal pediatric hemispheric (left and right) rcSO2 is 60% to 80%. A low rcSO2 reading less than 60% or a high rcSO2 reading greater than 80% or an interhemispheric discordance rcSO2 reading greater than 10 represents abnormal hemispheric cerebral physiology and pathology and an increased hypoxic brain injury risk.12–15,23–26 In pediatric and adult neurological emergencies, cerebral oximetry has been validated for identifying altered hemispheric cerebral physiology and changes, neurological insults, and cerebral pathology detection.12–15,23–26 Adult cerebral oximetry seizure studies suggest that cerebral rcSO2 differences exist between generalized seizure and nonseizure rcSO2 readings but have never been investigated in pediatric patients.15,16,23–27
This preliminary hemispheric cerebral rcSO2 study's primary aim was to investigate the relationship of hemispheric cerebral rcSO2 readings to clinically evident generalized and/or focal seizure activity in PED seizure patients with no prior seizure history. These seizure rcSO2 readings were also compared with readings of PED postseizure patients with clinically evident seizures prior to PED arrival and no seizures during their PED stay.4–12 The secondary aim was to investigate generalized and focal seizures' hemispheric rcSO2 reading time pattern behavior. We hypothesized that there would be significantly different hemispheric rcSO2 readings during these clinically evident seizure events compared with the nonseizure events. Analysis for cerebral oximetry age matching, sensitivity, specificity, and positive and negative predictive values for seizures was not the scope of this preliminary study.
METHODS
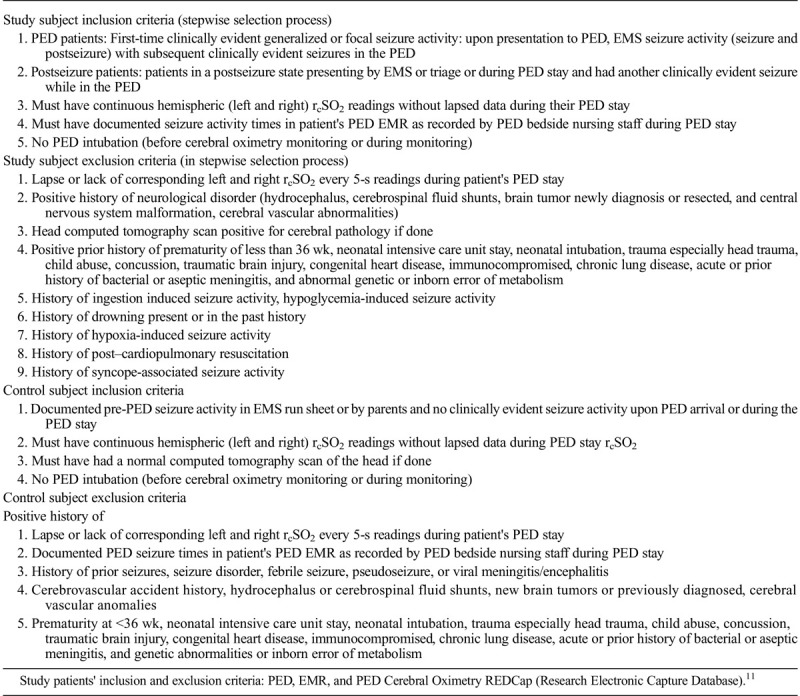
This is an observational retrospective case series from 2009 to 2016 in 2 academic PEDs (Arkansas Children's Hospital and Monroe Carrol Vanderbilt Children’s Hospital) (annual census: 56,000 and 55,000 patient visits) examining hemispheric rcSO2 readings in 2 PED clinically evident seizure groups: focal and generalized patients and nonseizure PED patients. The seizure groups were (1) PED patients presenting with seizure activity and (2) PED patients who developed clinically evident seizure activity during their PED stay with continuous hemispheric rcSO2 readings. The control patients had clinically evident seizure activity prior to their PED arrival and no subsequent seizure activity during their PED stay and had continuous hemispheric rcSO2 readings.12,14 To avoid potential underlying abnormal cerebral physiology causing underlying cerebral oximetry sampling bias, the seizure and control patients' inclusion and exclusion criteria were selected with the objective of underlying normalcy of the patients' nervous system (vascular and architecture) and cerebral physiology. More detailed study and control patient inclusion and exclusion criteria are presented in Table 1A.10 The study and control patients were selected by one of the primary investigators from the PED (Arkansas Children’s Hospital and Monroe Carrol Vanderbilt Children's Hospital) Cerebral Oximetry REDCap (Research Electronic Data Capture) database and screened with the inclusion and exclusion criteria of neurological normalcy, seizure activity, and continuous hemispheric rcSO2 readings (Table 1A).10
TABLE 1A.
Study and Control Patients' Inclusion and Exclusion Criteria
Patients meeting inclusion criteria had their PED electronic medical record (EMR) abstracted by one of the primary investigators for demographic data, EMS seizure activity, seizure activity upon PED presentation, generalized or focal seizure, seizure frequency, and various other seizure parameters. Patients' EMRs were reviewed for the neurologist's final diagnosis and final hospital or PED discharge diagnosis. The acknowledgement of patients' clinically evident seizure activity (whether tonic clonic or fine motor seizure activity) was by the PED attending physician or pediatric emergency medicine fellow and documented as seizure activity only in the patient's PED nursing EMR. The patient's clinically evident seizure recorded times from the PED nursing EMR were matched to the patient's cerebral oximetry hemispheric rcSO2 times by one of the one of the investigators. Correlating subclinical seizure activity to the patient's hemispheric cerebral oximetry readings was not a study objective. The patient's rcSO2 readings were not blinded to the PED providers and often not recorded in the PED EMR. The 2 PEDs had identical seizure protocols: normalizing blood sugar, supplemental oxygen, and anticonvulsants, and protocols were driven by the patient's clinically evident seizure activity, not by rcSO2 recording protocol.
Health Insurance Portability and Accountability Act compliance for all aspects of patient data was maintained. The institutional review board at each facility granted study approval including a waiver of informed consent, and this study was registered at ClinicalTrials.gov (NCT 02950181).
Cerebral Oximetry Sampling
At both facilities, hemispheric cerebral oximetry monitoring was standard during pediatric neurological emergencies 24 hours, 7 days a week. Patients had left and right forehead cerebral oximetry probes (INVOS 5100C Medtronic, Minneapolis, MN) with simultaneous rcSO2 recordings at 5-second intervals during their PED stay and did not delay patient's resuscitation endeavors. The patient's seizure times were recorded in the cerebral oximetry device as event marks.
Statistical Analysis
Single patient data consist of hemispheric rcSO2 readings at 5-second intervals over the patient's PED stay. Patient groups were formed according to the median rcSO2 in the first 5 minutes after PED arrival as less than 60%, between 60% and 80%, and greater than 80%, as adult cerebral oximetry seizure studies show an increase or decrease in regional cerebral blood flow (CBF) occurs just before and during a seizure.16–18,24–27 Patient rcSO2 data were split in periods around clinically evident seizure events as (1) 10 to 5 minutes before seizure start, (2) 5 to 0 minutes before seizure start, (3) during seizure, (4) 0 to 5 minutes after seizure ends, and (5) 0 to 10 minutes after seizure end. For each patient, 5-second rcSO2 reading data for periods defined were collapsed as median rcSO2, yielding a single number per period per patient. Analyses of rcSO2 behavior over periods defined around seizure events and corresponding comparisons of groups of interest (eg, control vs seizure, rcSO2 levels upon arrival in PED <60%, 60%–80%, >80%) were carried out by a mixed model (nonparametric repeated measures) followed by Tukey procedure. P < 0.05 was considered statistically significant; corresponding confidence intervals are provided. Medians and first (Q1) and third (Q3) quartiles were obtained. Setup of analytical data sets and analyses were carried out in SAS 9.4 (SAS Institute Inc, Cary, NC).
RESULTS
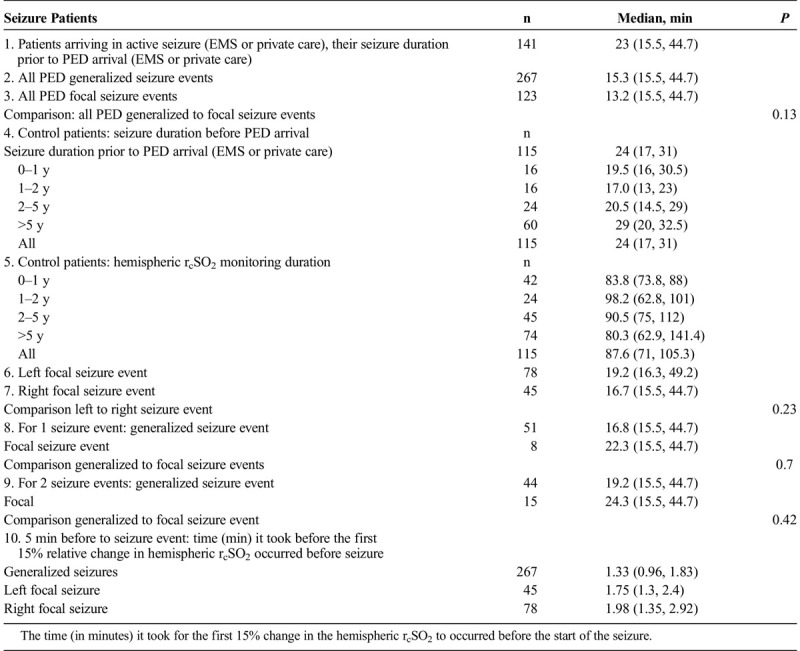
From 2012 to 2016, there were 185 PED patients (59.4% were male) with seizure activity (548 seizure events), simultaneous and continuous hemispheric rcSO2 monitoring duration of 167.6 minutes (101.4, 210.3 minutes) (Table 1B). There were 115 control patients with 52.6% males, and their rcSO2 monitoring duration was 87.6 minutes (71, 105.3 minutes). The seizure patients' median age was 2.13 years (0.84, 4.1 years), and the control subjects' median age was 5.17 years (1.9, 9.4 years) (P < 0.0001). With no age-specific hemispheric rcSO2 readings, these statistical age differences did not affect the hemispheric seizure rcSO2 analysis. In the seizure group, 167 patients had 334 generalized seizure events, 106 patients had 214 focal seizure events (48 patients had 68 left-sided focal seizure activity, and 75 patients had 146 right-sided focal seizure activity).
TABLE 1B.
Study and Control Patients' Clinical Evident Seizure Duration (inMinutes) and Comparison Between the Study and Control Groups (P) Duration of Seizure Prior to PED Arrival, by Age, Generalized Seizures, Focal Seizures, and Frequency of Seizure Events: Generalized, Left Focal, and Right Focal
Seizure patients' PED EMR diagnoses were new-onset seizure activities, generalized seizure, left focal seizure, right focal seizure, complex febrile seizure, simple febrile seizure, partial complex seizure, and convulsive status epilepticus. No seizure or control patients had a diagnosis of stroke or cerebral pathology on head computed tomography or magnetic resonance imaging scan. The control patients' final diagnoses were seizure, new-onset seizure, and simple and complex febrile seizures. No patient had positive bacterial or viral meningitis. For study and control patients, the PED EMR recorded no hypoxemic periods, intubations, bradycardia events, abnormal electrolytes, hypoglycemia, or positive toxicology screen in either group.
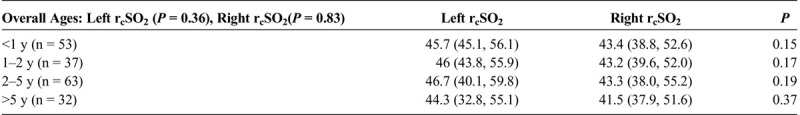
The patients 'seizure duration demonstrated no statistical significance between the groups (P = 0.13) (Table 1B). The relative left and right rcSO2 first 15% relative changes (showing earliest significant rcSO2 time change before the start of seizure) from 5 minutes before seizure to the seizure start for generalized, left focal and right focal, are shown in Table 1B. Comparing hemispheric rcSO2 reading for all seizures (generalized and focal), a strong significance for lower hemispheric rcSO2 readings during seizure events compared with the preseizure and postseizure readings was noted (P < 0.001) (Table 1C). For patients with multiple seizure events (generalized and focal), comparing their first to last seizure events' first 10-minute seizure rcSO2 readings showed no statistical difference (left, P = 0.37; right, P = 0.18) (Table 1C). The various age parameters were not significant (Table 1D).
TABLE 1C.
Comparison Analysis of All Left and Right Seizure's rcSO2 Readings (as Independent Events) in All Generalized and Focal Seizure Patient Events
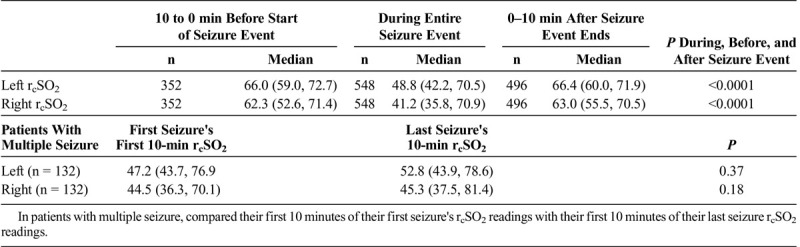
TABLE 1D.
Comparison of the Left and Right Seizure Event rcSO2 Readings by Overall Age and by Age Grouping (<1, 1–2, 2–5, >5 Years)
Figures 1A to C represent the time plots for generalized and left and right focal seizures hemispheric rcSO2 seizure compared with controls. Figures 2A and B represent individual generalized and left and right focal seizure patients' graphic representations of their hemispheric rcSO2 readings.
FIGURE 1.
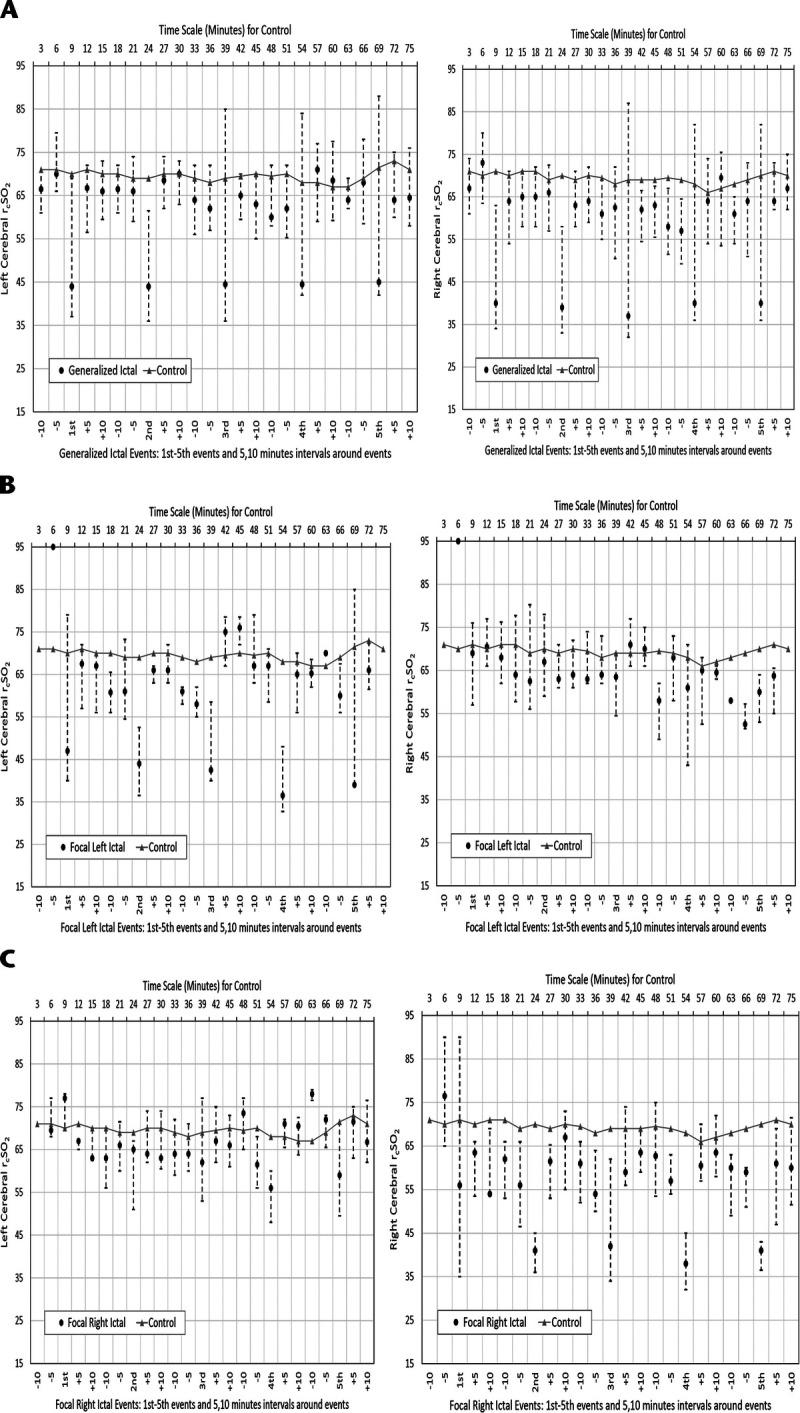
A–C, Left and right cerebral rcSO2 for generalized clinically evident seizure events versus control. Median (●) and interquartile range; 5-minute surrounding intervals are as follows: −10 (10 to 5 minutes prior to start of seizure event), −5 (5 to 0 minutes prior to start of seizure event), +5 (0–5 minutes after ending of seizure event), +10 (5–10 minutes after ending of seizure event). A–C, Left and right cerebral all rcSO2: A, clinically evident general seizure; B, clinically evident left focal seizure; C, clinically evident right focal seizure.
FIGURE 2.
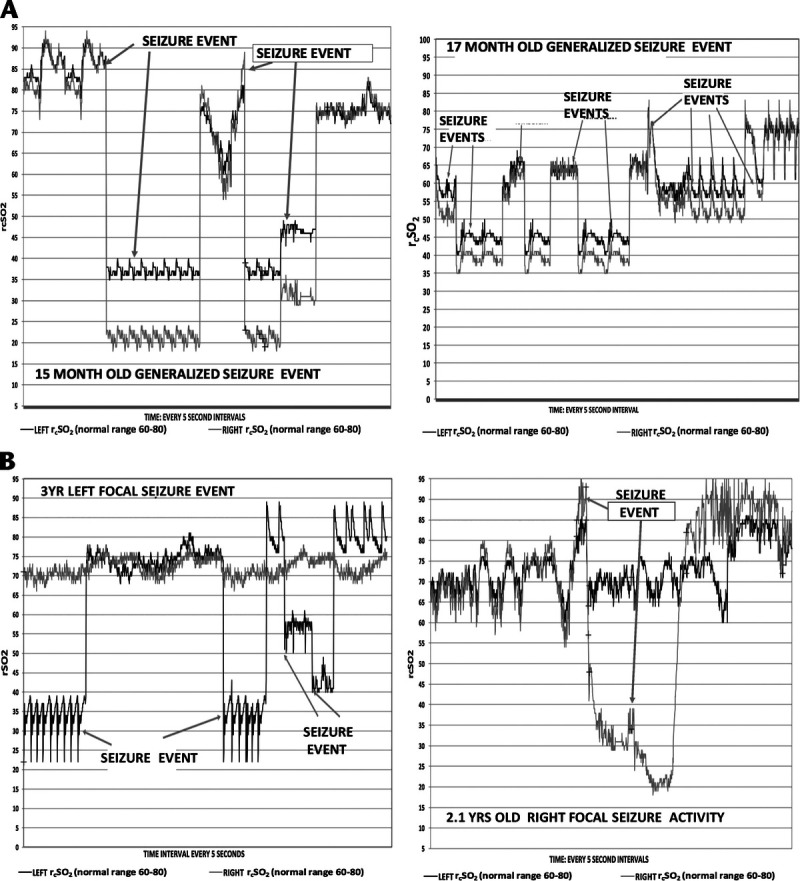
A, Fifteen-month and 17-month-old patients with clinically evident generalized seizures (seizure event) with left and right rcSO2 graph with clinically evident seizure (events and nonseizure events time graph). B, Left, A 3-year-old with clinically evident left focal seizure with left and right rcSO2 graph with clinically evident seizure events and nonseizure events time graph. B, Right, A 2.1-year-old patient with clinically evident right focal seizure with left and right rcSO2 graph with clinically evident seizure events and nonseizure events time graph.
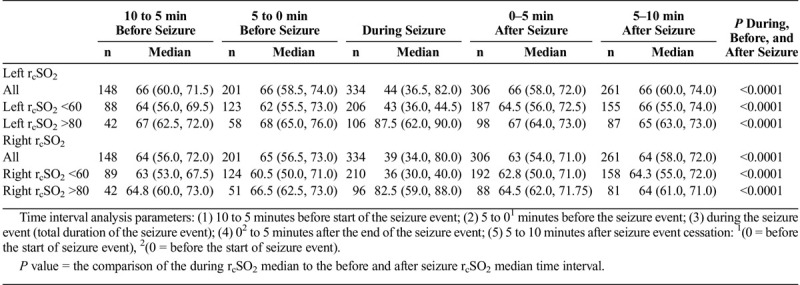
In the seizure event time analysis, for the seizure and control patients, the preseizure hemispheric and interhemispheric rcSO2 readings demonstrated no statistical significance (Table 2). For the during-seizure event, in the seizure and control patients’ hemispheric rcSO2 readings, the generalized and focal during-seizure rcSO2 readings were significantly lower or higher (P < 0.0001) than controls and focal during-seizure rcSO2 readings. Comparing the postseizure hemispheric and interhemispheric rcSO2 readings between seizure and control patients, there was statistical significance, but clinically both groups' rcSO2 readings were within the normal rcSO2 reading range (Table 2). Comparing the multiple seizure events (as independent events) during the seizure hemispheric rcSO2 readings with that of control patients, there was a strong discordance between the groups with lower median hemispheric seizure rcSO2 reading occurrence (P < 0.0001) (Table 2).
TABLE 2.
Seizure Versus Control Patients' Hemispheric (Left and Right) rcSO2 Readings With Corresponding Comparison Time Analysis: Control Patients' rcSO2 Versus PED Seizure rcSO2 Readings at Various Time Intervals Analysis
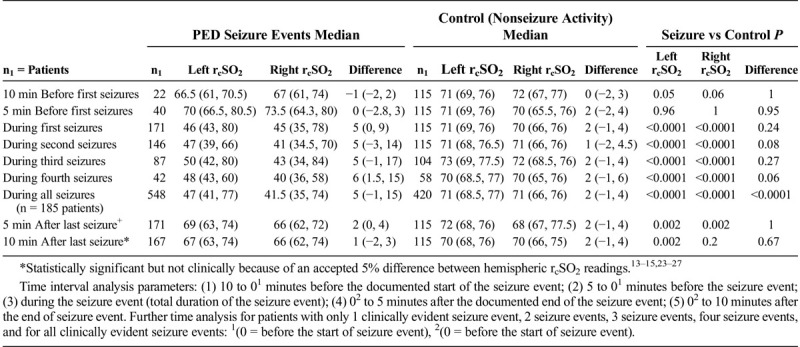
Patients with multiple seizures all had significant postictal times between repetitive seizure intervals, generalized, 20 minutes (11, 27 minutes); left focal seizures, 22 minutes (13, 28 minutes); and right focal seizure, 22 minutes (16, 25 minutes). These multiple seizure types were either repetitive focal seizures, generalized progressing to focal seizures, or repetitive generalized seizures. All multiple-seizures patients had appropriate PED seizure protocol adherence.
For the various seizure time parameter analysis in the generalized and focal seizures by their hemispheric rcSO2 readings and initial abnormal rcSO2 readings (<60% and >80%), the preseizure, seizure, and postseizure rcSO2 readings for the all generalized and focal seizures showed that during seizure rcSO2 readings had a stronger significant difference compared with the preseizure and postseizure rcSO2 reading times (P < 0.0001) and not the preseizure to postseizure times (P = 0.99) (Tables 3A, 3B, and 3C).
TABLE 3A.
Comparing All Generalized Hemispheric (Left, Right [Using All Generalized Seizure rcSO2 Readings as Independent Events]) During the Seizure rcSO2 Readings to Various Nonseizure rcSO2 Readings Time Intervals by All Their Seizure rcSO2 Readings (as Independent Events) and by Their Initial Hemispheric Seizure rcSO2 Readings Less Than 60% and Greater Than 80% With Same Time Comparisons
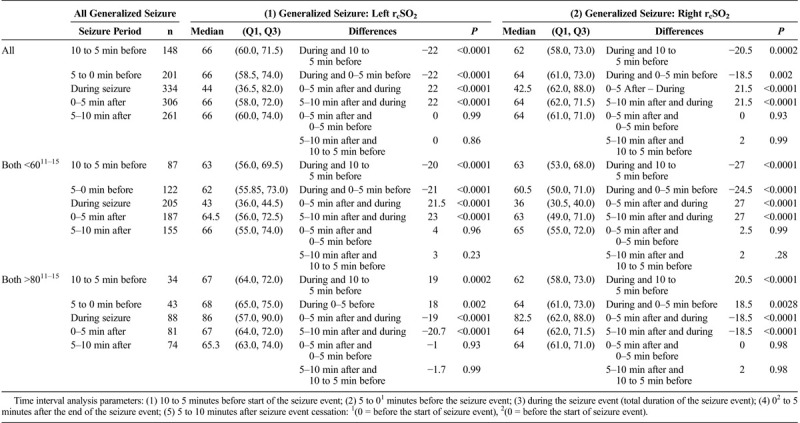
TABLE 3B.
All Left Focal Hemispheric (Left, Right [Using All Left and Right Focal Seizure rcSO2 Readings as Independent Events]) During the Seizure rcSO2 Readings to Various Nonseizure rcSO2 Readings Time Intervals by All Their Seizure rcSO2 Readings (as Independent Events) and by Their Initial Hemispheric Seizure rcSO2 Readings of Less Than 60% and Greater Than 80%
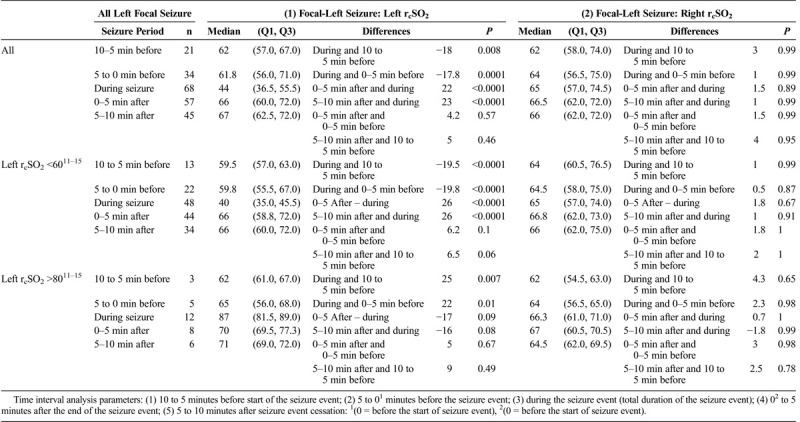
TABLE 3C.
All Right Focal Hemispheric (Left, Right [Using All Left and Right Focal Seizure rcSO2 Readings by All Their Seizure rcSO2 Readings (as Independent Events), and by Their Initial Hemispheric Seizure rcSO2 Readings of Less Than 60% and Greater Than 80% With Same Time Comparisons
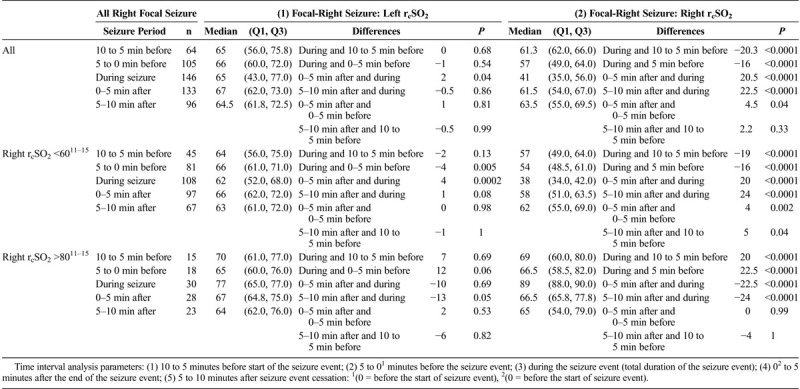
Focal ipsilateral seizure rcSO2 readings compared with generalized ipsilateral rcSO2 readings showed no statistical difference (Table 4A). Simultaneously, the focal contralateral rcSO2 readings were statistically higher than the corresponding generalized seizure rcSO2 (P < 0.0001) (Table 4A). The focal ipsilateral rcSO2 readings had either a lower rcSO2 reading compared with the contralateral rcSO2 with a wide interhemispheric difference and were statistically significant (P < 0.0001) (Table 4B). For left to right focal seizure rcSO2 reading comparison, right focal seizure rcSO2 readings were significantly less than the left focal seizure rcSO2 readings (P < 0.0001) (Table 4A and 4B). With increasing focal seizure frequency, these focal seizure rcSO2 reading findings were consistent with persistent correlation to the focal seizure side (P < 0.0001) (Table 4B). The focal interhemispheric rcSO2 reading discordances, whether single or multiple seizures, were consistently wide (P < 0.0001) (Table 4B). Comparing the overall left focal contralateral (right) rcSO2 readings median rcSO2 64 (57.7, 77.7) to the corresponding control’s (right) rcSO2 readings 70 (64.0, 76.0) there was no median difference detected (P = 0.15). Whereas the right focal contralateral rcSO2 side (left) rcSO2 64 (56, 70) median compared to the control’s (left) side rcSO2 70 (65, 75) had a significant difference (P < 0.001).
TABLE 4A.
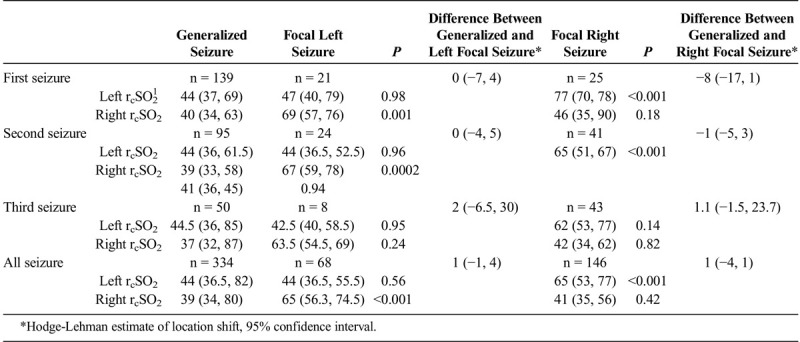
Comparison Analysis of Hemispheric (Left and Right) Seizure rcSO2 Readings Between Generalized Seizure Compared With Left Focal and Right Focal Seizure rcSO2 Readings (n1 = patients, I = Total Number of Seizure Events) by Their First, Second, Third, and All Seizure Events (as independent events) and the Difference Between General and Focal (left and right) Seizure rcSO2 Readings
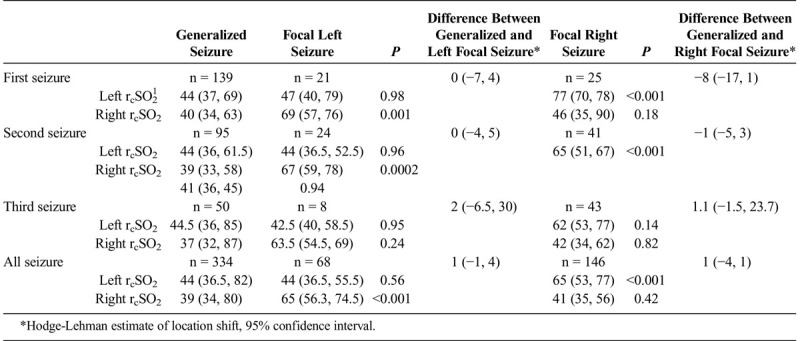
TABLE 4B.
Comparison Analysis of Left and Right Seizure rcSO2 Readings Between Left and Right Focal Seizure rcSO2 Readings and Between Hemispheric rcSO2 Reading Side Differences (During Focal Events) (n1 = Patients, I = Total Number of Seizure Events) by Their First, Second, and Third Seizures and in All focal Seizures' rcSO2 Readings (as Independent Events) and the Differences Between the Focal Seizure rcSO2 Readings
For all generalized seizure rcSO2 readings during seizure event times compared between non-seizure event times, a wide median with significant difference was noted (P < 0.0001) (Table 5A). Refining this comparison time by initial abnormal hemispheric rcSO2 readings (<60% or >80%) during seizure times to other times, a wide median with a significant difference occurred (P < 0.0001) (Table 5A). For generalized and focal seizures between preseizures and during seizures, their hemispheric delta rcSO2 reading change was for general seizures: left 10 and right 18.5, and left focal 19.8 and right focal 20.3, all demonstrating significant differences (P < 0.0001) (Table 5B). However, the preseizure to postseizure hemispheric delta rcSO2 change demonstrated no significance (P = 0.7) (Table 5B). The delta rcSO2 changes for generalized and focal seizures by initial hemispheric abnormal rcSO2 readings (<60% or >80) demonstrated a wider significant difference (P < 0.0001) (Table 5B). In multiple seizures, similar delta rcSO2 change parameter results demonstrated similar differences (P < 0.0001). (For readers interested in the generalized seizure rcSO2 frequency, analysis for the first, second, and third seizure events is presented in Supplementary Tables 5C, D, E [C, 1 seizure event; D, patient with 2 seizures: second seizure event; and E, patients with 3 seizures, third seizure event, and their statistical analysis], http://links.lww.com/PEC/A217).
TABLE 5A.
Patients With Multiple Generalized Seizures: Comparative Time Analysis of Their Hemispheric (Left and Right) Seizure rcSO2 Readings by Time Intervals: All Their Seizure rcSO2 Readings (as Independent Events) and by Their Initial Hemispheric Seizure rcSO2 Readings of Less Than 60% and Greater Than 80% With Same Time Comparisons
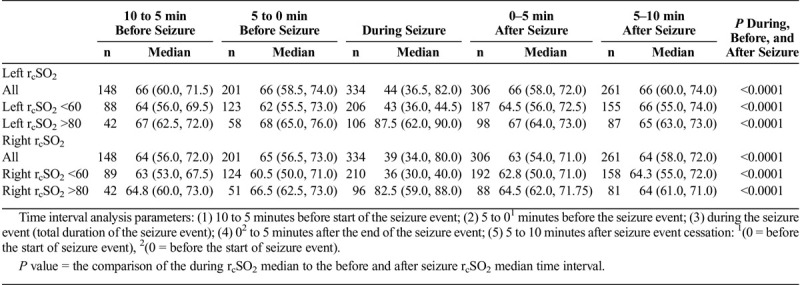
TABLE 5B.
Comparative Time Analysis of Delta Difference of Seizure rCSO2 Readings in Generalized, Left and Right Focal Hemispheric and Abnormal Seizure (Both rCSO2 <60,11–15 Both rCSO2 <8011–15) rcSO2 Readings (Differences Between Seizure Times) and by Their Initial Hemispheric Seizure rcSO2 Readings of Less Than 60% and Greater Than 80% With Same Time Comparisons. Overall: A positive value = increase, negative value = decrease. 1. Generalized seizure event had a rcSO2 change of left −9.8, right −10.7 2. Left focal event had a left rcSO2 change of −17.8. 3. Right focal event had a right rcSO2 change of −20.3
DISCUSSION
This preliminary PED hemispheric cerebral oximetry study during clinically evident generalized and focal seizure activity demonstrates the following findings. Overall, hemispheric during-seizure rcSO2 readings significantly correlated with generalized and focal seizures. In generalized and focal seizure activity, during the seizure, hemispheric rcSO2 readings revealed significant cerebral tissue oxygenation alteration during the seizure (increase or decrease) compared with preseizure, and in the postseizure, it returned to baseline. These findings correlated with prior cerebral oximetry seizure studies.16–20,24–27 In the generalized and focal seizure patients, during the seizure phase, there were 2 trends noted during the seizure phase: rcSO2 readings of less than 60 or greater than 80%, and with seizures with greater than 80% readings decreasing over time.16–20,24–27 In either left or right focal seizure, hemispheric rcSO2 readings had a strong correlation to the seizure side with a wide interhemispheric discordance rcSO2. As in similar cerebral oximetry studies, these focal and general seizures' rcSO2 reading changes demonstrate that nonfrontal hemispheric abnormal regional cerebral activity has a dynamic interplay with and can affect the frontal hemispheric cerebral physiology. In all groups, generalized seizures, focal seizures, and controls, upon seizure resolution or postictal phase, the postseizure rcSO2 readings return to preseizure rcSO2 readings or normal range, 60% to 80%, demonstrating resolution of the cerebral seizure's abnormal physiology. With increased generalized or focal seizure frequency, there was a persistent strong correlation for the during-seizure rcSO2 reading change compared with preseizure and postseizure rcSO2 readings, postseizure rcSO2 readings, and returning to preseizure readings across all seizure frequencies. For all the seizure rcSO2 readings, there was a significant rcSO2 reading change by 2 to 3 minutes before the documented clinically evident seizure start time, demonstrating a hemispheric cerebral physiological change occurrence before clinical evidence. Overall, hemispheric seizure rcSO2 readings highly correlated with clinically evident (generalized or focal) seizures and demonstrate hemispheric cerebral oxygenation and physiology alteration during seizures. Also, by utilizing left and right cerebral oximetry sensors, detection of these unilateral and bilateral hemispheric cerebral physiological alterations was possible.
Seizure activity is related to neuronal mitochondrial dysfunction, producing abnormal neuronal cellular metabolism, further mitochondrial damage, tissue hypoxia, persistent neuronal dysfunction, and increased regional cerebral oxygen and glucose consumption all during the seizure event.1–4,16–20,25–27 A compensatory action is an increase in regional CBF and oxygenation.16–20,25–27 However, when this increased regional CBF becomes inadequate to meet the seizure's metabolic demand, decreased regional tissue oxygenated hemoglobin occurs.16–20,25–27 This study's generalized and focal seizure hemispheric rcSO2 readings during single or multiple seizure events followed similar patterns.24–26 From this study, some seizure patients' hemispheric rcSO2 readings initially rose to greater than 80% and then declined during their seizure. These seizure rcSO2 readings pattern probably reflected that the increased CBF became inadequate for the seizure's metabolic demand, causing a tissue oxygenation deficit as indicated by the lower rcSO2 readings.16–20,25–27
In left or right focal seizure, compared with generalized seizure events, there were no significant differences between the focal seizure rcSO2 readings and the generalized ipsilateral seizure rcSO2 readings. In either left or right focal seizure, there were significant interhemispheric rcSO2 discordance readings, which was consistent over multiple focal seizure events. This significant focal seizure interhemispheric discordance was significant and like other pediatric neurological cerebral oximetry findings in strokes, hydrocephalus, epidural, and subdurals.12–14
The ED patient's documented seizure times compared with the rcSO2 readings did have a high correlation, which could be due to 77% of the patients having clinically evident seizures upon PED presentation. Knowledge of a patient's prior seizure could heighten the PED staff's seizure awareness, leading to quicker seizure interpretation, quicker subsequent seizures detection, and improved seizure documentation.
In summary, in the acute setting, once the seizure patient's airway, breathing, circulation, and glycemic state are stabilized, focus should shift to rapid seizure termination and restoring normal cerebral physiology to minimize the abnormal hemispheric cerebral physiology, energy depletion, and neurological injury.1–4,16–20,23–27 In either generalized or focal seizure, rcSO2 readings correlated with seizure event and demonstrated a consistent decrease or increase, probably reflecting a decrease or increase in regional cerebral oxygen and physiology during the seizure compared with either their preseizure or postseizure time.16–20,24–27 Postseizure rcSO2 readings approximated their preseizure rcSO2 readings, demonstrating a resolution of the abnormal hemispheric cerebral oxygen and physiology. In the focal seizure events, ipsilateral rcSO2 strongly correlated with the focal seizure side and in the postseizure phase returned to the preseizure readings. This study demonstrates that there is a dynamic hemispheric cerebral physiology change occurring during clinically evident seizure events, and this change may indicate physical neuronal compromise and potential for neuronal injury. These hemispheric frontal lobe rcSO2 readings are dynamic, have an interplay with other cerebral regions, and can be affected by other abnormal regional cerebral activity as shown by focal and generalized seizure rcSO2 readings. From this vast pool of left and right cerebral oximetry readings in the generalized and focal seizures patients, if the hemispheric rcSO2 readings are less than 60% or greater than 80%, or the interhemispheric discordance rcSO2 readings are greater than 10 with no obvious signs of clinically evident seizure for the nonneurologist, further investigation for possible clinical and/or subclinical seizures is warranted. Because seizure recognition is greatly impaired in neonates, pediatric patients, and nonverbal or developmentally challenged patients, cerebral oximetry has shown its potential as an objective neurological assessment tool and possibly decreases misperception of clinically evident seizure recognition. This study should not be interpreted as a replacement for the diagnostic electroencephalogram (EEG) system, but rather as an adjunct acute-setting neurological assessment tool for the health care provider. Cerebral oximetry has shown its potential utilization in neonate and pediatric seizure research as an adjunct assessment tool. Further research is needed to investigate the potential use of hemispheric and interhemispheric discordant rcSO2 readings as a predictive tool for anticonvulsants therapeutic effectiveness especially in pharmacoresistant seizures.
LIMITATIONS
Documentation and detection of pediatric seizures can have sampling error due to the PED staff's clinically evident seizure recognition experience. Current hemispheric cerebral oximetry probes physically prevent ambulatory EEG forehead probe placement; therefore, correlating clinical seizures to EEG seizures with simultaneous hemispheric rcSO2 readings cannot be accomplished.
CONCLUSIONS
This PED preliminary hemispheric cerebral oximetry seizure study during clinically evident general and focal seizures showed altered cerebral oximetry rcSO2 readings and significantly correlated with seizures and reflected abnormal seizure cerebral physiology. In generalized seizures, hemispheric rcSO2 readings were either significantly decreased (<60%) or increased (>80%) compared with their preseizure and postseizure readings and nonseizure patients. During focal seizures, ipsilateral rcSO2 readings correlated to the seizure side, and interhemispheric discordance rcSO2 readings differed significantly (>10). Postseizure rcSO2 readings returned to preseizure readings, showing altered cerebral physiology resolution. Overall, cerebral oximetry during generalized and focal seizure rcSO2 readings were less than 60% or greater than 80%, and in focal seizures their interhemispheric rcSO2 discordance was greater than 10, demonstrating its potential pediatric seizure utility. This PED seizure cerebral oximetry study substantiates further investigation in subclinical seizure detection and anticonvulsant therapeutic effectiveness especially in pharmacoresistant seizures. This study advances cerebral oximetry functionality as an adjunct neurological assessment tool in pediatric neuroresuscitation.
Supplementary Material
Footnotes
The abstract of this study was presented at the 2015 American Academy of Pediatrics National NEC meeting, Pediatric Emergency Medicine section on October 2016.
Disclosure: The authors declare no conflict of interest.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.pec-online.com).
REFERENCES
- 1.Othal J Folbergrov J Kovacs R, et al. . Epileptic focus and alteration of metabolism. Int Rev Neurobiol. 2014;114:209–243. [DOI] [PubMed] [Google Scholar]
- 2.Zsurka G, Kunz WS. Mitochondrial dysfunction and seizures: the neuronal energy crisis. Lancet Neurol. 2015;14:956–966. [DOI] [PubMed] [Google Scholar]
- 3.Morimoto K, Fahnestock M, Racine RJ. Kindling and status epilepticus models of epilepsy: rewiring the brain. Prog Neurobiol. 2004;73:1–60. [DOI] [PubMed] [Google Scholar]
- 4.Raspall-Chaure M Chin RF Neville BG, et al. . Outcome of pediatric convulsive status epilepticus: a systematic review. Lancet Neurol. 2006;5:769–779. [DOI] [PubMed] [Google Scholar]
- 5.Shorvon S, Tomson T. Sudden unexpected death in epilepsy. Lancet. 2011;378:2028–2038. [DOI] [PubMed] [Google Scholar]
- 6.Greiner HM Holland K Leach JL, et al. . Nonconvulsive status epilepticus: the encephalopathic pediatric patient. Pediatrics. 2012;129:e748–e755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Agarwal M, Fox SM. Pediatric seizures. Emerg Med Clin North Am. 2013;31:733–754. [DOI] [PubMed] [Google Scholar]
- 8.Chin RF Verhulst L Neville BG, et al. . Inappropriate emergency management of status epilepticus in children contributes to need for intensive care. J Neurol Neurosurg Psychiatry. 2004;75:1584–1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schneider B, Abramo TJ, Albert G. An update on cerebral oxygenation monitoring, an innovative application in cardiac arrest and neurological emergencies. Annu Update Intensive Care Emerg Med. 2015:273–288. [Google Scholar]
- 10.Robertson CS Zager EL Narayan RK, et al. . Clinical evaluation of a portable near-infrared device for detection of traumatic intracranial hematomas. J Neurotrauma. 2010;27:1597–1604. [DOI] [PubMed] [Google Scholar]
- 11.Harris PA Taylor R Thielke R, et al. . Research Electronic Data Capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peng K Pouliot P Lesage F, et al. . Multichannel continuous electroencephalography-functional near-infrared spectroscopy recording of focal seizures and interseizure epileptiform discharges in human epilepsy: a review. Neurophotonics. 2016;3:031402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kane I Abramo TJ Meredith M, et al. . Cerebral oxygen saturation monitoring in pediatric altered mental status patients. Am J Emerg Med. 2014;32:356–362. [DOI] [PubMed] [Google Scholar]
- 14.Al-Rawi P, Kirkpatrick P. Tissue oxygen index: thresholds for cerebral ischemia using near-infrared spectroscopy. Stroke. 2006;37:2720–2725. [DOI] [PubMed] [Google Scholar]
- 15.Abramo TJ Harris ZL Meredith M, et al. . Cerebral oximetry with cerebral blood volume index in detecting pediatric stroke in a pediatric ED. Am J Emerg Med. 2015;33:1622–1629. [DOI] [PubMed] [Google Scholar]
- 16.Moseley BD Britton JW Nelson C, et al. . Periictal cerebral tissue hypoxemia: a potential marker of SUDEP risk. Epilepsia. 2012;53:e208–e211. [DOI] [PubMed] [Google Scholar]
- 17.Roche-Labarbe N Zaaimi B Berquin P, et al. . NIRS-measured oxy- and deoxyhemoglobin changes associated with EEG spike-and-wave discharges in children. Epilepsia. 2008;49:1871–1880. [DOI] [PubMed] [Google Scholar]
- 18.Schwartz TH Hong SB Bagshaw AP, et al. . Preseizure changes in cerebral haemodynamics: review of findings and insights from intracerebral EEG. Epilepsy Res. 2011;97:252–266. [DOI] [PubMed] [Google Scholar]
- 19.Khoa D Nguyen Y Tremblay J, et al. . Noninvasive continuous functional near-infrared spectroscopy combined with electroencephalography recording of frontal lobe seizures. Epilepsia. 2013;54:331–340, 201. [DOI] [PubMed] [Google Scholar]
- 20.Silas R Sehgal A Walker A, et al. . Cerebral oxygenation during subclinical seizures in neonatal hypoxic-ischemic encephalopathy. Eur J Paediatri Neurology. 2012;16:304–307. [DOI] [PubMed] [Google Scholar]
- 21.Slone E Westwood E Dhaliai H, et al. . NIR shows pre-seizure haemodynamic changes in temporal lobe epilepsy. Epileptic Disord. 2012371–378. [DOI] [PubMed] [Google Scholar]
- 22.Brunner E, Domhof S, Langer F. Nonparametric Analysis of Longitudinal Data in Factorial Experiments. New York: Wiley Series in Probability and Statistics; 2002. [Google Scholar]
- 23.Malonek D, Grinvald A. Interactions between electrical activity and cortical microcirculation revealed by imaging spectroscopy: implications for functional brain mapping. Science. 1996;272:551–554. [DOI] [PubMed] [Google Scholar]
- 24.Zhao M Suh M Ma H, et al. . Focal increases in perfusion and decreases in hemoglobin oxygenation precede seizure onset in spontaneous human epilepsy. Epilepsia. 2007;48:2059–2067. [DOI] [PubMed] [Google Scholar]
- 25.Kreisman NR Rosenthal M LaManna JC, et al. . Cerebral oxygenation during recurrent seizures. Adv Neurol. 1983;34:231–239. [PubMed] [Google Scholar]
- 26.Suh M Bahar S Mehta AD, et al. . Temporal dependence in uncoupling of blood volume and oxygenation during interseizure epileptiform events in rat neocortex. J Neuroscience. 2005;25:68–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Adelson PD Nemoto E Scheuer M, et al. . Noninvasive continuous monitoring of cerebral oxygenation periictally using near-infrared spectroscopy: a preliminary report. Epilepsia. 1999;40:1484–1489. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


