Abstract
Clinical examination is critical for the diagnosis and identification of response to treatment. It is fortunate that technologies are continuing to evolve, enabling augmentation of classical clinical examination with noninvasive imaging modalities. This article discusses emerging technologies with a focus on digital photographic imaging, confocal microscopy, optical coherence tomography, and high-frequency ultrasound, as well as several additional developing modalities. The most readily adopted technologies to date include total-body digital photography and dermoscopy, with some practitioners beginning to use confocal microscopy. In this article, applications and limitations are addressed. For a detailed discussion of the principles involved in these technologies, please refer to the first part of this review article.
Keywords: confocal microscopy, dermatology, dermoscopy, digital photographic imaging, fluorescence imaging, high-frequency ultrasound, machine-based learning, multispectral optoacoustic tomography, optical coherence tomography, Raman spectroscopy
Imaging modalities aid in diagnosis and monitoring treatment response for many conditions. The most highly studied applications are in the diagnosis of skin cancers. This article discusses the utility and limitations of the imaging modalities digital photographic imaging, confocal microscopy (CM), optical coherence tomography (OCT), and high-frequency ultrasound (HFUS), as well as a few others (Table I). For a discussion of the principles involved with these technologies, please refer to part 1 of this review.1
Table I.
Summary of advantages, disadvantages, and future directions of emerging technologies
| Technology | Key features | Advantages | Disadvantages | Future directions |
|---|---|---|---|---|
| Total-body digital photography | Enables photographic monitoring of disease progression, developing and changing nevi, surgical site planning, and cosmetic outcomes | Provides objective evidence regarding disease progression (ie, vitiligo, psoriasis); reduced patient anxiety; fewer biopsies; earlier detection of melanoma; encourages rapport with cosmetic patients for treatment monitoring | Can lack standardization of camera angles and lighting; commercial devices (provide standardized images) but can be expensive and have large spatial footprint; lack of insurance coverage; lengthy photo acquisition times | Insurance covering technology; integration of total-body digital photographs with patients’ mobile devices |
| Dermoscopy | Provides magnification of the skin’s surface akin to a 10x microscope objective with polarized and nonpolarized filters | Increased diagnostic accuracy in general; improved biopsy efficiency;earlier skin cancer detection | Requires advanced training | User friendly integration between dermatoscope and mobile devices for longitudinal photographic monitoring; smaller devices; uploading and storing technology integrated directly into device; algorithms to offer diagnostic suggestions for indeterminant lesions |
| MBL | Concept of large image databases teaching software algorithms on how to recognize clinical, dermoscopic, or histopathologic diagnoses | High accuracy differentiating benign from malignant lesions | Learning currently limited by size and quality of image databases available | Increasing image databases will improve software algorithms; MBL integrates into other technologies to provide suggested differentials or to provide confidence interval for benignity |
| CM | CM images en face up to a 30x microscope objective with the highest resolution Reflectance CM utilizes endogenous chromophores (ie, keratin, melanin) and is predominantly done in vivo Fluorescence CM uses exogenous contrast agents (ie, aluminum chloride, acridine orange) and is predominantly performed ex vivo |
Highest cellular resolution; enables noninvasive lesion diagnosis thus limiting biopsies; permits margin mapping for skin cancer surgeries in cosmetically sensitive areas | Requires extensive training;device expense; large spatial footprint; image acquisition time; depth of penetration; anatomical contour can affect image quality; hyperkeratosis, variable skin texture, and unclean skin affect decrease image resolution and depth | Smaller, more affordable devices;increasing image acquisition speeds; software generating 3D and color images (akin to standard histopathology); histopathology processing of Mohs tissue specimens (either in lieu of or in tandem with frozen sections); combination devices to aid in gross morphology; integration of MBL technology to aid in image interpretation |
| Optical coherence tomography | Generates gray-scale images by using interferometry techniques comparing time delays between echoes reflected from tissue structures | Rapid image acquisition; depth of penetration ~1.5 mm | Device expense; lacks cellular detail; overlying anatomy can affect imaging quality; requires advanced training | Smaller, more affordable devices; combination devices to help overcome limitation of cellular detail; integration of MBL technology to aid in image interpretation |
| High-frequency ultrasound | Ultrasound waves reflect off of skin structures with varying echoes that are then perceived by a transducer and converted into a gray-scale image | Rapid image acquisition; depth of penetration up to 6-7 mm | Device expense; low resolution; lack of functional contrast; requires advanced training; operator dependent | Smaller, more affordable devices; identification of tumor margins preoperatively |
| Raman spectroscopy | Near-infrared lasers generate photons that scatter at the same and at different energies at each chemical bond within a structure; changes detected by device and translated into a highly specific graph | High specificity (in vivo>ex vivo) | Not currently available; expensive; slow acquisition speed; potential for weak imaging signals | Improvements in acquisition speed and expense; integration of MBL technology to provide calculated malignancy risk |
| Fluorescence imaging | Visible or ultraviolet light provides energy to tissue fluorophores (endogenous or exogenous) that become excited and then emit energy photons upon relaxation that are detected and translated into images | High resolution (cellular details); can be performed in vivo or ex vivo | Can require long incubation times; might not be effective in identifying higher risk skin cancers | Integration into combination devices to aid in diagnostic and tumor mapping accuracy |
| Multispectral optoacoustic tomography | Ultrasound waves reflect off of endogenous (hemoglobin, melanin) or exogenous (dyes) chromophores at varying speeds that are detected and translated into 3D images | Depth of penetration 1 cm; can create 3D images | Not currently available |
3D, 3 Dimensional; CM, confocal microscopy; MBL, machine-based learning.
While there are many emerging technologies in the literature, total-body digital photography (TBDP) and dermoscopy are the most readily adopted. In order for the other emerging technologies to become more readily accepted in clinical practice, there must be continued research into their clinical utility that ultimately culminates in data regarding their diagnostic accuracy. Aside from clinical data, widespread adoption of emerging techniques is greatly limited by the cost of equipment and the lack of payer reimbursement. With time, as these technologies become better accepted, we are hopeful that these issues will be overcome.
Another important concept to understand at the outset of this discussion is machine-based learning (MBL), which is a technique by which computer algorithms study and learn from databases of clinical, histopathologic, and dermoscopic images. MBL has been studied in the identification of skin cancers.2 Efficacy data has been mixed, though most agree that MBL has the potential to be consistently more accurate than expert clinicians.3,4 Researchers at Stanford University compared MBL with board-certified dermatologists in the differentiation of melanomas from benign nevi and keratinocyte carcinomas from seborrheic keratoses. MBL performed as well as experts and, in certain scenarios, outperformed dermatologists, although it is important to note that the images the machines used lacked the 3-dimensional (3D) clues that clinicians use when making diagnoses.2 While MBL needs refinement, it will likely be a helpful adjunctive diagnostic tool when combined with clinical expertise.5 Future algorithms will utilize software applications for mobile devices to grow photographic databases to increase its accuracy.2 MBL will become integrated into imaging technologies to improve diagnostic accuracy and provide risk stratification, making it an important adjuvant to other emerging imaging technologies.
DIGITAL PHOTOGRAPHIC IMAGING
Clinical applications
Medical photography is frequently used in dermatology. Secure photographs on portable devices can be directly embedded into medical records. Digital images enable the comparison of disease progression, monitoring of nevi and neoplasms, and the creation of surgical plans. Parallel polarized light differentiates papulopustular rosacea from erythematotelangiectatic subtypes and provides longitudinal monitoring of vitiligo6,7 and chronic wounds.8
TBDP has become a cornerstone for nevi mapping and monitoring. This practice reduces patient anxiety and the need for biopsies. TBDP also can detect melanoma earlier.9,10 Usually, 3D photography devices monitor melanocytic nevi.11 Additional reports have shown TBDP used in the monitoring of psoriasis, vitiligo, wounds, infantile hemangiomas, and scars and for surgical or cosmetic planning.7,12-20
Limitations
Photography can lack standardization with respect to positioning and lighting, which can be overcome by using consistent positioning and available commercial devices. 3D photography devices have difficulties capturing and interpreting hair, which decreases the accuracy of conditions involving the face and scalp.21 Commercial devices can have a large spatial footprint, particularly 3D photography devices. Clinicians have different quality devices, and equipment upgrades are expensive.9,22 Most insurance companies do not cover TBDP, and costs to patients can be prohibitive.23 Photo acquisition times can be lengthy.10
Future directions
With the increasing incidence of skin cancers, there is hope that medical insurance will cover TBDP for longitudinal nevi monitoring. Mobile devices have a growing inventory of applications, and software will help patients monitor their lesions at home.24 Patients will ideally have access to their TBDP through a medical photography application on personal devices to encourage monthly selfskin examinations given that most tumors are self-detected.10
DERMOSCOPY
Clinical applications
Dermoscopic imaging is commonly used for a wide range of conditions, with the most frequent application being identification of skin cancers. Dermoscopy has a high sensitivity for skin cancer, leading to improved biopsy efficiency.24,25 Furthermore, clinicians can identify thinner melanomas with dermoscopy than with clinical examination alone.25 Multiple algorithms, such as the 2-step algorithm, ABCD rule, 7-point checklist, Menzies’ method, CASH algorithm, chaos and clues algorithm, and triage amalgamated dermoscopy algorithm, aid in the identification of common dermoscopic lesions.25-29 Wohlner et al provides a comprehensive review of dermoscopic features of skin cancers.25
Limitations
Although dermoscopy is highly sensitive (90%), it is limited by specificity (59%), which remains low even when performed by experts.30 To use dermoscopy effectively, clinicians require extensive training, and few formal resources exist.25
Future directions
Improvements with dermatoscope attachments to mobile devices enable dermatoscopic images to be easily uploaded into patients’ medical records. Dermatoscopes will continue to become smaller. Hopefully, image uploading capabilities will become integrated into these devices. Eventually, dermatoscopes will utilize MBL algorithms to offer diagnostic suggestions.
CONFOCAL MICROSCOPY
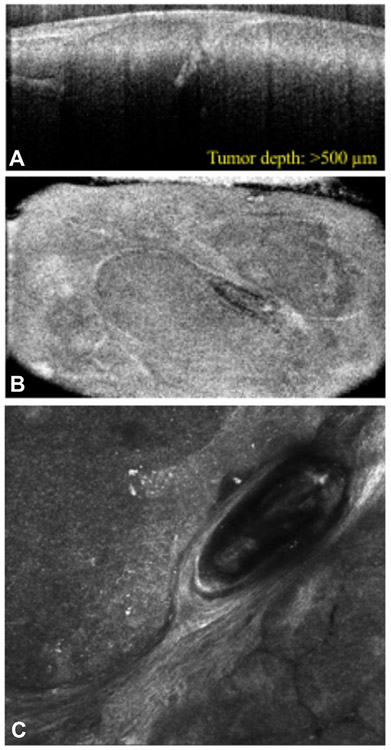
Reflectance CM (RCM) and fluorescence CM (FCM) can identify benign versus malignant lesions in vivo (predominantly RCM) and ex vivo (predominantly FCM) (Fig 1). CM creates black and white high-resolution en face images that extend as deep as the papillary dermis.
Fig 1.
Reflectance confocal microscopy (RCM) and optical coherence tomography (OCT) confirming basal cell carcinoma. RCM in combination with OCT can aid in identification of tumor depth and margins. A, RCM demonstrates tumor islands in the dermis visualized en face. B and C, OCT helps to illustrate the tumor depth visualized orthogonally (B) and en face (C). OCT, Optical coherence tomography; RCM, reflectance confocal microscopy.
Reflectance confocal microscopy
Clinical applications.
RCM can improve diagnosis of basal cell carcinomas (BCCs), which appear as bright tumor islands with elongated cord-like structures and dark cleft-like spaces on RCM.31 Furthermore, validated BCC features distinguishable by RCM have been proposed to differentiate BCC tumor subtypes.32 Keratinocyte malignancies (actinic keratoses, squamous cell carcinoma [SCC] in situ, and SCC) can be challenging to differentiate from each other by RCM, with wide ranges in sensitivities and specificities for these conditions.33 Classic features of SCC include irregular epidermal honeycomb pattern with round bright nuclei in the epidermis (pagetoid spread) and looping vessels in the papillary dermis.34
RCM imaging of melanoma can be performed in vivo and ex vivo. In vivo, classic features include disorganized pattern, focal loss of dermal-epidermal junction, bright round or dendritic cells (pagetoid spread), and irregularly shaped dermal nests.34 RCM has a much higher sensitivity than dermoscopy (84% and 39%, respectively), particularlywhen examining hypopigmented or amelanotic melanoma.34 RCM imaging ex vivo involves using melanin as the chromophore.35 Clinicians can use RCM, typically either handheld or mosaic RCM, to diagnose lentigo maligna and lentigo maligna melanoma.
RCM can be used to determine pre-operative tumor margins for melanocytic and nonmelanoma tumors.34,36-40 RCM visualizes invasion and superficial recurrence and monitors therapeutic interventions for skin cancers.34,41
Fluorescence confocal microscopy
Clinical applications.
Fluorescent staining ex vivo with methylene blue or toluidine blue dyes can be used to identify nonmelanoma skin cancer (NMSC). BCC has a highly fluorescent signal that correlates with tumor islands on histopathology.42 Acridine orange used ex vivo stains nuclear DNA in dermal keratinocytes with increased uptake, and thus brighter fluorescence, in tumor cells (Fig 2).35,43,44 FCM has also been studied in melanoma research.35 This ex vivo technique takes ~20 seconds to stain and then several minutes to image (though imaging time increases with larger tumors).
Fig 2.
Acridine orange staining in fluorescence confocal microscopy. The use of dyes such as acridine orange applied ex vivo can aid in visualization of tumor islands in the dermis. With acridine orange, the tumor islands appear brighter than the surrounding tissue.
Once NMSC has been confirmed by biopsy, patients are referred for Mohs micrographic surgery, which clears margins via frozen histopathology sectioning. BCC features identified by FCM (and staining with acridine orange) strongly correlated with results from Mohs frozen sections.44 In many cases, the time taken to identify tumor margins is decreased in comparison with conventional histopathology (sensitivity 88%-99%, specificity 89%-99%).35,43,45 Ex vivo FCM has been proposed as an adjuvant tool in tumor margin identification of excised Mohs layers.
Limitations of CM
CM requires extensive training to acquire and interpret images.34,35,41-43,46 While RCM might be more helpful in identifying tumors with clear tumor nests, it might miss smaller more infiltrative lesions.35,42,47-49 Furthermore, due to RCM’s small field of view, large NMSC, where tumor nests encompass the entire field, might not be easily identified. CM reaches a depth of 200-300 μm (the level of the papillary dermis) when performed in vivo, so this method could miss deep, recurrent tumors.10,34,35,43,50 Aside from depth, the anatomic contour of tissue can impede image generation,35 although this issue can sometimes be mitigated by using the handheld device. CM devices are expensive and, depending on the lesion (in vivo) or tissue size (ex vivo), can take significant time to scan.34,41,43,50
Future directions of CM
The RCM design is constantly being improved to attain better range of motion with the microscope head, reduce motion artifacts, and increase image acquisition speed. Software is being developed to generate RCM images with pseudocolors to ease interpretation by providing better correlation with standard histopathology.34 With advancements in MBL, software will integrate into CM devices to aid in diagnosis and risk assessment.
OPTICAL COHERENCE TOMOGRAPHY
Clinical applications
OCT has been widely applied to dermatologic conditions. OCT can be used to visualize melanomas and NMSCs for diagnosis and tumor margin delineation.10,51-57 In addition to assessing benignity, OCT can monitor disease progression for inflammatory, infectious, blistering, and vascular lesions; wound healing; and chronologic photoaging.53,58 Skin photo scatter permits a penetration depth of ~1.5 mm (level of reticular dermis).43,59
Limitations
OCT cannot be used to distinguish individual cells, which limits its diagnostic capabilities.43 Anatomic structures, such as scars, can impede visualization of underlying or adjacent features.50 Advanced training is needed for image interpretation, which remains subjective.43,52 OCT devices are expensive.43
Future directions
Of all the technologies discussed, OCT appears to be the one most frequently combined with other techniques. The combination multiphoton tomography (MPT)-OCT device allows visualization of cellular details with MPT and morphology with OCT.60 Because MPT provides a microscopic image and OCT the macroscopic context, it can be challenging to accurately correlate images.60 OCT has also been combined with RCM to identify NMSC borders (Fig 1). This handheld device provides a 3D image by obtaining cross-sectional OCT and en face RCM images.61
HIGH-FREQUENCY ULTRASOUND
Clinical applications
HFUS can be used to determine skin thickness and measure tumor depth, tumor recurrence, and efficacy of therapeutic interventions.62 When studied, HFUS confirmed the dermatologic diagnosis in the majority (82%) of cases and helped revise diagnoses in a number of other cases (17%), indicating the usefulness of this tool.63
The most highly studied application of HFUS is in diagnosis and identification of skin cancer margins. BCC lesions appear as hypoechoic, well-defined masses with irregular shapes62,64 that might occasionally contain hyperechoic spots (ie, corneal cysts, microcalcifications, or apoptotic cells).65 HFUS can be used to diagnose superficial BCCs, rule out infiltrative BCCs, classify the histologic subtype for large (>40 mm2) lesions, and delineate tumor margins.66,67
With HFUS, SCC appears as an irregular hypoechoic mass.65 Compared with BCCs, SCCs often extend deeper and do not have central hyperechoic lesions.64,65 Doppler function in SCC illustrates lesional low-flow vessels.64,65 A thickened epidermal echo with a subepidermal hypoechoic band suggests limitation to the epidermis, distinguishing SCC in situ from SCC.62
Differentiating melanocytic nevi from melanomas represents a diagnostic challenge. With HFUS, nevi and melanomas both appear as well-defined homogenous hypoechoic areas.64 The most useful features for melanomas include tumor thickness, prominent vascularity, irregular shape, and dermal invasion.64,65 HFUS is most helpful in identifying depth greater or less than 1 mm and in detecting satellite (<2 cm), in-transit (>2 cm), or nodal metastases.65
Limitations
With overall low resolution, HFUS is limited in assisting with diagnoses.62,68 HFUS lacks functional contrast,65 creating a challenge when differentiating various hypoechoic tissues (ie, tumor, fat, and inflammatory infiltrates).62,69 Image acquisition and quality are operator-dependent, requiring advanced training.68
Future directions
The main utility of HFUS is identifying preoperative tumor margins and monitoring response to treatment.
RAMAN SPECTROSCOPY
Clinical applications
Raman spectroscopy has been studied in skin cancers.70-72 When performed ex vivo, Raman spectroscopy has a diagnostic accuracy of 92.4% in differentiating benign nevi from BCC and melanoma.71 In vivo, Raman spectroscopy had a sensitivity of 90% and a specificity of 63% for determining benignity.72 A second in vivo study reported 73% accuracy for BCC, 85% for SCC, and 91% for melanoma.73 Raman spectroscopy can also be used to evaluate dermal water content,70,74 photoaging,70,75,76 cutaneous penetration,77 pharmacokinetics,77 and visualization of tattoo pigment78,79 and other foreign bodies.70
Limitations
The main limitation of Raman spectroscopy is acquisition speed,72,80 but this parameter continues to improve with advancing technology.72 Other limitations are weak imaging signals and expense.80
Future directions
Raman spectroscopy combined with MBL will differentiate benign from malignant lesions in real-time with a calculated malignancy risk in indeterminate lesions.80
FLUORESCENCE IMAGING
Clinical applications
Fluorescence imaging occurs through 2 main modalities: quenched activity–based probe imaging and autofluorescence. Fluorescence has been studied in skin cancers, aging, and inflammatory diseases (ie, allergic and irritant contact dermatitis and psoriasis).81
Quenched activity–based probe imaging differentiates NMSC from normal benign tissue (sensitivity 98.8%, specificity 89.4%). Ex vivo, quenched activity–based probe imaging can accurately and efficiently help determine Mohs margins, taking ~15 minutes compared with frozen sections.82
Autofluorescence imaging can be used to identify NMSC, which are more fluorescent than normal tissue because they contain more tryptophan residues, enabling clinicians to make a diagnosis.83 Collagen autofluoresces at a lower intensity in malignant than benign lesions due to induction of collagen crosslinking by tumor enzymes.83
Photodynamic diagnosis also uses fluorescence technology.84 With photodynamic diagnosis, a photosensitizer is applied to the skin, resulting in the fluorescence of malignant cells. Surgeons using photodynamic diagnosis required fewer Mohs stages for SCC, with smaller surgical margins (sensitivity 90%, specificity 80%). In these studies, patients were treated with methyl aminolevulinic acid or aminolevulinic acid with a 3-hour or 6-hour incubation period, respectively.84,85
Limitations
Fluorescence imaging requires long incubation times when performed in vivo; such is the case with photodynamic diagnosis. Photodynamic diagnosis is not effective for identifying high-risk SCCs.84
Future directions
Fluorescence imaging technology has been combined with other techniques, namely polarization-enhanced reflectance and fluorescence imaging.86 This technique combines multispectral polarized light imaging with confocal microscopy. Confocal microscopy aids in the identification of cell types, whereas polarized imaging helps delineate margins.87
FUTURE DIRECTIONS
Multispectral optoacoustic tomography
Multispectral optoacoustic tomography (MSOT) was studied in 3 patients to determine presurgical margins for NMSCs and was found to be consistent with conventional histology. MSOT can also aid in presurgical mapping.88 Beyond NMSC, MSOT has been studied in the classification of psoriatic plaque severity89 and as a noninvasive method of sentinel lymph node biopsy in patients with malignant melanoma.90 MSOT has been combined with fluorescence imaging90 and will likely be combed with other techniques in the future.
Other emerging technologies
Spectrophotometric intracutaneous analysis involves examining skin hemoglobin and melanin content with software algorithms; data are displayed graphically to differentiate benign versus malignant lesions. Currently, this technique is not more effective than dermoscopy but could become more useful with improving algorithms.10,24,91
Electrical impedance spectroscopy can differentiate benign from malignant lesions on the basis of innate electrical differences. Perilesional normal skin is compared with the lesion in question to generate a risk profile. Similar to spectrophotometric intracutaneous analysis, as algorithms improve, electrical impedance spectroscopy might become more commonplace.24,92
CONCLUSIONS
With increasing technologic advancements, new devices and combination imaging technologies will become more readily available. As imaging software becomes more sophisticated, adoption of these technologies will become more palatable. When lesion diagnosis and treatment margins can be accurately assessed noninvasively, there will be rapid acquisition. In the future, it is likely that cutaneous examinations will be aided by MBL to augment clinical acumen, possibly minimize biopsies, and potentially identify malignant lesions earlier.
CAPSULE SUMMARY.
There are emerging technologies in dermatology for use in clinical research, diagnosis, and monitoring therapeutic response. Currently, adjunctive imaging technologies are most frequently used for diagnosis and surgical planning for nonmelanoma and melanoma skin cancer.
With improving technology and usability, imaging devices will become important tools for clinicians.
Acknowledgments
The authors would like to thank Dr Miguel Cordova and Dr Aditi Shau from Memorial Sloan Kettering Cancer Center for contributing the figures for this publication.
Abbreviations used:
- 3D
3-dimensional
- BCC
basal cell carcinoma
- CM
confocal microscopy
- FCM
fluorescence confocal microscopy
- HFUS
high-frequency ultrasound
- MBL
machine-based learning
- MPT
multiphoton tomography
- MSOT
multispectral optoacoustic tomography
- NMSC
nonmelanoma skin cancer
- OCT
optical coherence tomography
- RCM
reflectance confocal microscopy
- SCC
squamous cell carcinoma
- TBDP
total-body digital photography
Footnotes
Conflicts of interest: Dr Schneider has no relevant conflicts to disclose. Dr Kohli has served as a subinvestigator for Estee Lauder, Unigen, Ferndale laboratories, Allergan, Chromaderm, Pfizer, Johnson & Johnson, and Bayer. Dr Hamzavi has served as research investigator for Estee Lauder, Unigen, Ferndale laboratories, Allergan, Bayer, Johnson & Johnson, and Incyte Corporation. Dr Council has served as consultant for MD Outlook and Medline Industries. Dr Rossi has served as consultant for Canfield Scientific Inc. Dr Ozog has served as investigator for MiRagen and Biofrontera, on the advisory board for Allergan, and was on the past medical board for DermOne.
REFERENCES
- 1.Schneider SL, Kohli I, Hamzavi IH, et al. Emerging imaging technologies in dermatology: part 1: basic principles. J Am Acad Derm. 2019;80(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Esteva A, Kuprel B, Novoa RA, et al. Dermatologist-level classification of skin cancer with deep neural networks. Nature. 2017;542(7639):115–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iyatomi H, Oka H, Saito M, et al. Quantitative assessment of tumour extraction from dermoscopy images and evaluation of computer-based extraction methods for an automatic melanoma diagnostic system. Melanoma Res. 2006;16(2):183–190. [DOI] [PubMed] [Google Scholar]
- 4.Marchetti MA, Codella NCF, Dusza SW, et al. Results of the 2016 International Skin Imaging Collaboration International Symposium on Biomedical Imaging challenge: comparison of the accuracy of computer algorithms to dermatologists for the diagnosis of melanoma from dermoscopic images. J Am Acad Dermatol. 2018;78(2):270–277.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jamora MJ, Wainwright BD, Meehan SA, Bystryn JC. Improved identification of potentially dangerous pigmented skin lesions by computerized image analysis. Arch Dermatol. 2003;139(2):195–198. [DOI] [PubMed] [Google Scholar]
- 6.Kwon IH, Choi JE, Seo SH, Kye YC, Ahn HH. Rosacea Subtypes visually and optically distinct when viewed with parallel-polarized imaging technique. Ann Dermatol. 2017;29(2):167–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kohli I, Isedeh P, Al-Jamal M, et al. Three-dimensional imaging of vitiligo. Exp Dermatol. 2015;24(11):879–880. [DOI] [PubMed] [Google Scholar]
- 8.Foltynski P, Ladyzynski P, Ciechanowska A, Migalska-Musial K, Judzewicz G, Sabalinska S. Wound area measurement with digital planimetry: improved accuracy and precision with calibration based on 2 rulers. PLoS One. 2015;10(8):e0134622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rice ZP, Weiss FJ, DeLong LK, Curiel-Lewandrowski C, Chen SC. Utilization and rationale for the implementation of total body (digital) photography as an adjunct screening measure for melanoma. Melanoma Res. 2010;20(5):417–421. [DOI] [PubMed] [Google Scholar]
- 10.Hibler BP, Qi Q, Rossi AM. Current state of imaging in dermatology. Semin Cutan Med Surg. 2016;35(1):2–8. [DOI] [PubMed] [Google Scholar]
- 11.Chung E, Marchetti MA, Scope A, et al. Towards three-dimensional temporal monitoring of naevi: a comparison of methodologies for assessing longitudinal changes in skin surface area around naevi. Br J Dermatol. 2016;175(6):1376–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Canfield. Vectra 3D. https://www.canfieldsci.com/imaging-systems/vectra-wb360-imaging-system/. Accessed March 31, 2018. [Google Scholar]
- 13.Goldman MP, Skover GR, Payonk GS. Three-dimensional imaging techniques in the assessment of facial volume augmentation. J Drugs Dermatol. 2009;8(12):1113–1119. [PubMed] [Google Scholar]
- 14.Stekelenburg CM, Jaspers ME, Niessen FB, et al. In a clinimetric analysis, 3D stereophotogrammetry was found to be reliable and valid for measuring scar volume in clinical research. J Clin Epidemiol. 2015;68(7):782–787. [DOI] [PubMed] [Google Scholar]
- 15.Hermans DJ, Maal TJ, Berge SJ, van der Vleuten CJ. Three-dimensional stereophotogrammetry: a novel method in volumetric measurement of infantile hemangioma. Pediatr Dermatol. 2014;31(1):118–122. [DOI] [PubMed] [Google Scholar]
- 16.Lowe P, Lowe NJ.3D photography and lip filler: a novel assay. J Cosmet Laser Ther. 2007;9(4):237–240. [DOI] [PubMed] [Google Scholar]
- 17.Meier JD, Glasgold RA, Glasgold MJ. 3D photography in the objective analysis of volume augmentation including fat augmentation and dermal fillers. Facial Plast Surg Clin North Am. 2011;19(4):725–735, ix. [DOI] [PubMed] [Google Scholar]
- 18.Ardehali B, Nouraei SA, Van Dam H, Dex E, Wood S, Nduka C. Objective assessment of keloid scars with three-dimensional imaging: quantifying response to intralesional steroid therapy. Plast Reconstr Surg. 2007;119(2):556–561. [DOI] [PubMed] [Google Scholar]
- 19.Kreft S, Kreft M, Resman A, Marko P, Kreft KZ. Computer-aided measurement of psoriatic lesion area in a multicenter clinical trial–comparison to physician’s estimations. J Dermatol Sci. 2006;44(1):21–27. [DOI] [PubMed] [Google Scholar]
- 20.Stockton KA, McMillan CM, Storey KJ, David MC, Kimble RM. 3D photography is as accurate as digital planimetry tracing in determining burn wound area. Burns. 2015;41(1):80–84. [DOI] [PubMed] [Google Scholar]
- 21.Heike CL, Upson K, Stuhaug E, Weinberg SM. 3D digital stereophotogrammetry: a practical guide to facial image acquisition. Head Face Med. 2010;6:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Quigley EA, Tokay BA, Jewell ST, Marchetti MA, Halpern AC. Technology and technique standards for camera-acquired digital dermatologic images: a systematic review. JAMA Dermatol. 2015;151(8):883–890. [DOI] [PubMed] [Google Scholar]
- 23.Berk-Krauss J, Polsky D, Stein JA. Mole mapping for management of pigmented skin lesions. Dermatol Clin. 2017; 35(4):439–445. [DOI] [PubMed] [Google Scholar]
- 24.March J, Hand M, Grossman D. Practical application of new technologies for melanoma diagnosis: part I. Noninvasive approaches. J Am Acad Dermatol. 2015;72(6):929–941. quiz 941-922. [DOI] [PubMed] [Google Scholar]
- 25.Wolner ZJ, Yelamos O, Liopyris K, Rogers T, Marchetti MA, Marghoob AA. Enhancing skin cancer diagnosis with dermoscopy. Dermatol Clin. 2017;35(4):417–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosendahl C, Cameron A, McColl I, Wilkinson D. Dermatoscopy in routine practice - ‘chaos and clues’. Aust Fam Physician. 2012;41(7):482–487. [PubMed] [Google Scholar]
- 27.Argenziano G, Fabbrocini G, Carli P, De Giorgi V, Sammarco E, Delfino M. Epiluminescence microscopy for the diagnosis of doubtful melanocytic skin lesions. Comparison of the ABCD rule of dermatoscopy and a new 7-point checklist based on pattern analysis. Arch Dermatol. 1998;134(12):1563–1570. [DOI] [PubMed] [Google Scholar]
- 28.Henning JS, Dusza SW, Wang SQ, et al. The CASH (color, architecture, symmetry, and homogeneity) algorithm for dermoscopy. J Am Acad Dermatol. 2007;56(1):45–52. [DOI] [PubMed] [Google Scholar]
- 29.Kittler H. The 2-step method and the recognition process in dermoscopy. JAMA Dermatol. 2015;151(9):1037–1038. [DOI] [PubMed] [Google Scholar]
- 30.Bhattacharya A, Young A, Wong A, Stalling S, Wei M, Hadley D. Precision diagnosis of melanoma and other skin lesions from digital images. AMIA Jt Summits Transl Sci Proc. 2017;2017:220–226. [PMC free article] [PubMed] [Google Scholar]
- 31.Ruini C, Hartmann D, Saral S, Krammer S, Ruzicka T, von Braunmuhl T. The invisible basal cell carcinoma: how reflectance confocal microscopy improves the diagnostic accuracy of clinically unclear facial macules and papules. Lasers Med Sci. 2016;31(8):1727–1732. [DOI] [PubMed] [Google Scholar]
- 32.Villarreal-Martinez A, Bennassar A, Gonzalez S, Malvehy J, Puig S. Application of in vivo reflectance confocal microscopy and ex vivo fluorescence confocal microscopy in the most common subtypes of basal cell carcinoma and correlation with histopathology. Br J Dermatol. 2018;178(5):1215–1217. [DOI] [PubMed] [Google Scholar]
- 33.Nguyen KP, Peppelman M, Hoogedoorn L, Van Erp PE, Gerritsen MP. The current role of in vivo reflectance confocal microscopy within the continuum of actinic keratosis and squamous cell carcinoma: a systematic review. Eur J Dermatol. 2016;26(6):549–565. [DOI] [PubMed] [Google Scholar]
- 34.Que SK, Fraga-Braghiroli N, Grant-Kels JM, Rabinovitz HS, Oliviero M, Scope A. Through the looking glass: basics and principles of reflectance confocal microscopy. J Am Acad Dermatol. 2015;73(2):276–284. [DOI] [PubMed] [Google Scholar]
- 35.Gadjiko M, Rossi AM. Ex vivo confocal microscopy: a diagnostic tool for skin malignancies. Cutis. 2017;100(2): 81–83. [PubMed] [Google Scholar]
- 36.Venturini M, Gualdi G, Zanca A, Lorenzi L, Pellacani G, Calzavara-Pinton PG. A new approach for presurgical margin assessment by reflectance confocal microscopy of basal cell carcinoma. Br J Dermatol. 2016;174(2):380–385. [DOI] [PubMed] [Google Scholar]
- 37.Champin J, Perrot JL, Cinotti E, et al. In vivo reflectance confocal microscopy to optimize the spaghetti technique for defining surgical margins of lentigo maligna. Dermatol Surg. 2014;40(3):247–256. [DOI] [PubMed] [Google Scholar]
- 38.Chen CS, Elias M, Busam K, Rajadhyaksha M, Marghoob AA. Multimodal in vivo optical imaging, including confocal microscopy, facilitates presurgical margin mapping for clinically complex lentigo maligna melanoma. Br J Dermatol. 2005;153(5):1031–1036. [DOI] [PubMed] [Google Scholar]
- 39.Yelamos O, Cordova M, Blank N, et al. Correlation of handheld reflectance confocal microscopy with radial video mosaicing for margin mapping of lentigo maligna and lentigo maligna melanoma. JAMA Dermatol. 2017;153(12): 1278–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pan ZY, Lin JR, Cheng TT, Wu JQ, Wu WY. In vivo reflectance confocal microscopy of basal cell carcinoma: feasibility of preoperative mapping of cancer margins. Dermatol Surg. 2012;38(12):1945–1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hibler BP, Yelamos O, Cordova M, et al. Handheld reflectance confocal microscopy to aid in the management of complex facial lentigo maligna. Cutis. 2017;99(5):346–352. [PMC free article] [PubMed] [Google Scholar]
- 42.Al-Arashi MY, Salomatina E, Yaroslavsky AN. Multimodal confocal microscopy for diagnosing nonmelanoma skin cancers. Lasers Surg Med. 2007;39(9):696–705. [DOI] [PubMed] [Google Scholar]
- 43.Que SK. Research techniques made simple: noninvasive imaging technologies for the delineation of basal cell carcinomas. J Invest Dermatol. 2016;136(4):e33–e38. [DOI] [PubMed] [Google Scholar]
- 44.Longo C, Rajadhyaksha M, Ragazzi M, et al. Evaluating ex vivo fluorescence confocal microscopy images of basal cell carcinomas in Mohs excised tissue. Br J Dermatol. 2014; 171(3):561–570. [DOI] [PubMed] [Google Scholar]
- 45.Larson B, Abeytunge S, Seltzer E, Rajadhyaksha M, Nehal K. Detection of skin cancer margins in Mohs excisions with high-speed strip mosaicing confocal microscopy: a feasibility study. Br J Dermatol. 2013;169(4):922–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mu EW, Lewin JM, Stevenson ML, Meehan SA, Carucci JA, Gareau DS. Use of digitally stained multimodal confocal mosaic images to screen for nonmelanoma skin cancer. JAMA Dermatol. 2016;152(12):1335–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chung VQ, Dwyer PJ, Nehal KS, et al. Use of ex vivo confocal scanning laser microscopy during Mohs surgery for nonmelanoma skin cancers. Dermatol Surg. 2004;30(12 Pt 1): 1470–1478. [DOI] [PubMed] [Google Scholar]
- 48.Flores ES, Cordova M, Kose K, et al. Intraoperative imaging during Mohs surgery with reflectance confocal microscopy: initial clinical experience. J Biomed Opt. 2015; 20(6):61103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bennassar A, Vilata A, Puig S, Malvehy J. Ex vivo fluorescence confocal microscopy for fast evaluation of tumour margins during Mohs surgery. Br J Dermatol. 2014;170(2):360–365. [DOI] [PubMed] [Google Scholar]
- 50.Alawi SA, Kuck M, Wahrlich C, et al. Optical coherence tomography for presurgical margin assessment of nonmelanoma skin cancer - a practical approach. Exp Dermatol. 2013;22(8):547–551. [DOI] [PubMed] [Google Scholar]
- 51.Wang KX, Meekings A, Fluhr JW, et al. Optical coherence tomography-based optimization of mohs micrographic surgery of basal cell carcinoma: a pilot study. Dermatol Surg. 2013;39(4):627–633. [DOI] [PubMed] [Google Scholar]
- 52.Chan CS, Rohrer TE. Optical coherence tomography and its role in Mohs micrographic surgery: a case report. Case Rep Dermatol. 2012;4(3):269–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gambichler T, Jaedicke V, Terras S. Optical coherence tomography in dermatology: technical and clinical aspects. Arch Dermatol Res. 2011;303(7):457–473. [DOI] [PubMed] [Google Scholar]
- 54.Hamdoon Z, Jerjes W, Upile T, Hopper C. Optical coherence tomography-guided photodynamic therapy for skin cancer: case study. Photodiagnosis Photodyn Ther. 2011;8(1):49–52. [DOI] [PubMed] [Google Scholar]
- 55.Hussain AA, Themstrup L, Jemec GB. Optical coherence tomography in the diagnosis of basal cell carcinoma. Arch Dermatol Res. 2015;307(1):1–10. [DOI] [PubMed] [Google Scholar]
- 56.Cheng HM, Lo S, Scolyer R, Meekings A, Carlos G, Guitera P. Accuracy of optical coherence tomography for the diagnosis of superficial basal cell carcinoma: a prospective, consecutive, cohort study of 168 cases. Br J Dermatol. 2016;175(6): 1290–1300. [DOI] [PubMed] [Google Scholar]
- 57.Cheng HM, Guitera P. Systematic review of optical coherence tomography usage in the diagnosis and management of basal cell carcinoma. Br J Dermatol. 2015;173(6):1371–1380. [DOI] [PubMed] [Google Scholar]
- 58.Mamalis A, Ho D, Jagdeo J. Optical coherence tomography imaging of normal, chronologically aged, photoaged and photodamaged skin: a systematic review. Dermatol Surg. 2015;41(9):993–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Welzel J Optical coherence tomography in dermatology: a review. Skin Res Technol. 2001;7(1):1–9. [DOI] [PubMed] [Google Scholar]
- 60.Alex A, Weingast J, Weinigel M, et al. Three-dimensional multiphoton/optical coherence tomography for diagnostic applications in dermatology. J Biophotonics. 2013;6(4):352–362. [DOI] [PubMed] [Google Scholar]
- 61.Iftimia N, Yelamos O, Chen CJ, et al. Handheld optical coherence tomography-reflectance confocal microscopy probe for detection of basal cell carcinoma and delineation of margins. J Biomed Opt. 2017;22(7):76006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bhatt KD, Tambe SA, Jerajani HR, Dhurat RS. Utility of high-frequency ultrasonography in the diagnosis of benign and malignant skin tumors. Indian J Dermatol Venereol Leprol. 2017;83(2):162–182. [DOI] [PubMed] [Google Scholar]
- 63.Vidal D, Ruiz-Villaverde R, Alfageme F, et al. Use of high frequency ultrasonography in dermatology departments in Spain. Dermatol Online J. 2016;22(2). [PubMed] [Google Scholar]
- 64.Barcaui Ede O, Carvalho AC, Lopes FP, Pineiro-Maceira J, Barcaui CB. High frequency ultrasound with color Doppler in dermatology. An Bras Dermatol. 2016;91(3):262–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wortsman X Ultrasound in dermatology: why, how, and when? Semin Ultrasound CT MR. 2013;34(3):177–195. [DOI] [PubMed] [Google Scholar]
- 66.Hernandez-Ibanez C, Blazquez-Sanchez N, Aguilar-Bernier M, Funez-Liebana R, Rivas-Ruiz F, de Troya-Martin M. Usefulness of high-frequency ultrasound in the classification of histologic subtypes of primary basal cell carcinoma. Actas Dermosifiliogr. 2017;108(1):42–51. [DOI] [PubMed] [Google Scholar]
- 67.Nassiri-Kashani M, Sadr B, Fanian F, et al. Pre-operative assessment of basal cell carcinoma dimensions using high frequency ultrasonography and its correlation with histopathology. Skin Res Technol. 2013;19(1):e132–e138. [DOI] [PubMed] [Google Scholar]
- 68.Marmur ES, Berkowitz EZ, Fuchs BS, Singer GK, Yoo JY. Use of high-frequency, high-resolution ultrasound before Mohs surgery. Dermatol Surg. 2010;36(6):841–847. [DOI] [PubMed] [Google Scholar]
- 69.Wassef C, Rao BK. Uses of non-invasive imaging in the diagnosis of skin cancer: an overview of the currently available modalities. Int J Dermatol. 2013;52(12):1481–1489. [DOI] [PubMed] [Google Scholar]
- 70.Cinotti E, Labeille B, Perrot JL, Boukenter A, Ouerdane Y, Cambazard F. Characterization of cutaneous foreign bodies by Raman spectroscopy. Skin Res Technol. 2013;19(4):508–509. [DOI] [PubMed] [Google Scholar]
- 71.Bodanese B, Silveira FL, Zangaro RA, Pacheco MT, Pasqualucci CA, Silveira L Jr. Discrimination of basal cell carcinoma and melanoma from normal skin biopsies in vitro through Raman spectroscopy and principal component analysis. Photomed Laser Surg. 2012;30(7):381–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lui H, Zhao J, McLean D, Zeng H. Real-time Raman spectroscopy for in vivo skin cancer diagnosis. Cancer Res. 2012;72(10):2491–2500. [DOI] [PubMed] [Google Scholar]
- 73.Schleusener J, Gluszczynska P, Reble C, et al. In vivo study for the discrimination of cancerous and normal skin using fibre probe-based Raman spectroscopy. Exp Dermatol. 2015; 24(10):767–772. [DOI] [PubMed] [Google Scholar]
- 74.Nakagawa N, Matsumoto M, Sakai S. In vivo measurement of the water content in the dermis by confocal Raman spectroscopy. Skin Res Technol. 2010;16(2):137–141. [DOI] [PubMed] [Google Scholar]
- 75.Gonzalez FJ, Castillo-Martinez C, Martinez-Escaname M, et al. Noninvasive estimation of chronological and photo-induced skin damage using Raman spectroscopy and principal component analysis. Skin Res Technol. 2012; 18(4):442–446. [DOI] [PubMed] [Google Scholar]
- 76.de Vasconcelos Nasser Caetano L, de Oliveira Mendes T, Bagatin E, et al. In vivo confocal Raman spectroscopy for intrinsic aging and photoaging assessment. J Dermatol Sci. 2017. [DOI] [PubMed] [Google Scholar]
- 77.Zhang G, Moore DJ, Sloan KB, Flach CR, Mendelsohn R. Imaging the prodrug-to-drug transformation of a 5-fluorouracil derivative in skin by confocal Raman microscopy. J Invest Dermatol. 2007;127(5):1205–1209. [DOI] [PubMed] [Google Scholar]
- 78.Hutton Carlsen K, Kocks M, Sepehri M, Serup J. Allergic reactions in red tattoos: Raman spectroscopy for ‘fingerprint’ detection of chemical risk spectra in tattooed skin and culprit tattoo inks. Skin Res Technol. 2016;22(4):460–469. [DOI] [PubMed] [Google Scholar]
- 79.Cinotti E, Labeille B, Boukenter A, Ouerdane Y, Cambazard F, Perrot JL. Characterization of coal tattoos by Raman spectroscopy. Skin Res Technol. 2015;21(4):511–512. [DOI] [PubMed] [Google Scholar]
- 80.Zhao J, Zeng H, Kalia S, Lui H. Using Raman spectroscopy to detect and diagnose skin cancer in vivo. Dermatol Clin. 2017; 35(4):495–504. [DOI] [PubMed] [Google Scholar]
- 81.Franco W, Gutierrez-Herrera E, Kollias N, Doukas A. Review of applications of fluorescence excitation spectroscopy to dermatology. Br J Dermatol. 2016;174(3):499–504. [DOI] [PubMed] [Google Scholar]
- 82.Walker E, Mann M, Honda K, et al. Rapid visualization of nonmelanoma skin cancer. J Am Acad Dermatol. 2017;76(2): 209–216.e209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Brancaleon L, Durkin AJ, Tu JH, Menaker G, Fallon JD, Kollias N. In vivo fluorescence spectroscopy of nonmelanoma skin cancer. Photochem Photobiol. 2001;73(2):178–183. [DOI] [PubMed] [Google Scholar]
- 84.Jeon SY, Kim KH, Song KH. Efficacy of photodynamic diagnosis-guided Mohs micrographic surgery in primary squamous cell carcinoma. Dermatol Surg. 2013;39(12):1774–1783. [DOI] [PubMed] [Google Scholar]
- 85.Kamrava SK, Behtaj M, Ghavami Y, et al. Evaluation of diagnostic values of photodynamic diagnosis in identifying the dermal and mucosal squamous cell carcinoma. Photodiagnosis Photodyn Ther. 2012;9(4):293–298. [DOI] [PubMed] [Google Scholar]
- 86.Yaroslavsky AN, Feng X, Neel VA. Optical mapping of nonmelanoma skin cancers-a pilot clinical study. Lasers Surg Med. 2017. [DOI] [PubMed]
- 87.Yaroslavsky AN, Barbosa J, Neel V, DiMarzio C, Anderson RR. Combining multispectral polarized light imaging and confocal microscopy for localization of nonmelanoma skin cancer. J Biomed Opt. 2005;10(1):14011. [DOI] [PubMed] [Google Scholar]
- 88.Chuah SY, Attia AB, Long V, et al. Structural and functional 3D mapping of skin tumours with non-invasive multispectral optoacoustic tomography. Skin Res Technol. 2017;23(2):221–226. [DOI] [PubMed] [Google Scholar]
- 89.Greve TM, Kamp S, Jemec GB. Disease quantification in dermatology: in vivo near-infrared spectroscopy measures correlate strongly with the clinical assessment of psoriasis severity. J Biomed Opt. 2013;18(3):037006. [DOI] [PubMed] [Google Scholar]
- 90.Stoffels I, Morscher S, Helfrich I, et al. Metastatic status of sentinel lymph nodes in melanoma determined noninvasively with multispectral optoacoustic imaging. Sci Transl Med. 2015;7(317):317ra199. [DOI] [PubMed] [Google Scholar]
- 91.Emery JD, Hunter J, Hall PN, Watson AJ, Moncrieff M, Walter FM. Accuracy of SIAscopy for pigmented skin lesions encountered in primary care: development and validation of a new diagnostic algorithm. BMC Dermatol. 2010;10:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Braun RP, Mangana J, Goldinger S, French L, Dummer R, Marghoob AA. Electrical impedance spectroscopy in skin cancer diagnosis. Dermatol Clin. 2017;35(4):489–493. [DOI] [PubMed] [Google Scholar]