The molecular mechanism by which cells take up long-chain fatty acids has been intensely debated for many years. The dispute centers on the question “Does fatty acid uptake depend on transport proteins, or simple diffusion?” Evidence collected in the past years indicates that the transmembrane protein CD36 (SR-B2) serves to bring fatty acids to or from cell membranes while translocation of fatty acids between the leaflets of the lipid bilayer occurs by passive diffusion. A new contribution from Jay et al. (1) published in the Journal of Lipid Research shows results of live cell experiments using fluorescent indicators, supporting the hypothesis that long-chain fatty acids diffuse rapidly across biological membranes. We argue that this additional evidence further integrates opposing models of diffusion and translocation.
Transport of long-chain fatty acids (hereafter referred to as fatty acids) across the plasma membrane of cells is of fundamental importance, because these compounds fulfill a variety of functions including fuel for energy provision, component of membranes, role in signaling pathways, and substrate for posttranslational modification of proteins. Cellular fatty acid uptake rates can be very high, for instance, in the working heart for which fatty acids are the predominant source of ATP production. On the other hand, fatty acids should be handled with care because they may act as detergents and thus unselectively impair various cellular processes. To overcome the aqueous virtual insolubility of fatty acids, their transport in aqueous media, in particular blood plasma and the cellular soluble cytoplasm, is facilitated by their avid binding to specific proteins, i.e., plasma and interstitial albumin and cytoplasmic fatty acid-binding proteins (FABPs), respectively (2). In contrast, the hydrophobic nature of membranes allows the ample presence of fatty acids without the need for specific proteins. Moreover, it is well-documented that in artificial phospholipid bilayer vesicles (no proteins present), fatty acids can easily and rapidly diffuse laterally within a membrane leaflet as well as diffuse between the inner and outer leaflets (designated ‘flip-flop’) (3–5). For this reason, it was commonly believed that for fatty acids to enter cells, the plasma membrane would not present a barrier. However, two developments have caused an active search for membrane-associated proteins possibly involved in cellular fatty acid uptake, namely i) the notion that from a physiological perspective it would be undesirable to have fatty acids entering and leaving cells without control, and ii) in analogy to the cellular uptake of glucose, which is both facilitated and regulated by glucose transporters (GLUTs), membrane proteins may be good candidates to help control the rate of fatty acid movement across the plasma membrane.
Starting in the 1980s and 1990s, a number of putative membrane-associated FABPs have been discovered, of which CD36 [also known as putative fatty acid translocase (FAT), but officially designated as scavenger receptor (SR)-B2] and putative fatty acid transport protein (FATP) are the main examples. FATP was identified as an acyl-CoA synthetase that converts fatty acids into their CoA esters and thus augments cellular fatty acid uptake by so-called metabolic trapping or vectorial acylation (6). CD36 is a widely expressed transmembrane glycoprotein that was found to facilitate fatty acid uptake into several tissues with a high rate of fatty acid utilization such as heart and skeletal muscle, adipose tissue, and liver [reviewed in (7)]. Interestingly, CD36 was shown not only to facilitate but also to regulate fatty acid uptake, with the latter occurring through the subcellular vesicular recycling of CD36 between endosomes and the plasma membrane, a mechanism entirely similar to the regulation of glucose uptake by vesicular recycling of GLUTs. A large number of studies performed in the last two decades by various groups of investigators and addressing several tissues have documented a pivotal physiological role for CD36 in governing cellular fatty acid uptake and utilization (8–10). These studies have employed a variety of model systems including (tissue-specific) CD36 null or overexpressing rodents and various isolated and/or cultured cell models, agents inducing or inhibiting CD36 expression, agents affecting CD36 translocation or internalization, et cetera. Importantly, human studies involving CD36 deficiency or gene mutations clearly support a dynamic role for this protein in cellular lipid metabolism and signaling (11). Furthermore, CD36 could similarly be implicated in dysregulated fatty acid and lipid metabolism in pathophysiological conditions, such as high-fat diet-induced insulin resistance and diabetic cardiomyopathy (7, 12, 13). More recently, the protein structure and membrane topology of CD36 have been disclosed (14).
The molecular mechanism of fatty acid entry into a cell, especially whether and, if so, how CD36 (and/or other membrane proteins) would function therein, has been the subject of ongoing debates. Delineating this mechanism is of interest, as such knowledge will guide more effective therapeutic strategies in case of metabolic disease. In a point-counterpoint discussion published in 2007, Kampf and Kleinfeld (15) and Bonen et al. (16) discussed whether membrane fatty acid transport is mediated by lipids, proteins, or both. They critically discussed the various methodologies (model systems, chemical agents) used to delineate the membrane fatty acid transport process and the methodology-associated limitations, each reaching the conclusion that in order to meet the metabolic demands of certain cells, fatty acid transport must occur through a protein-mediated mechanism. In 2014, Pownall and Moore described the dispute as a “fatty acid war” between “diffusionists” and “translocatists” (17) and argued that activities that create concentration gradients of fatty acids between the extracellular space (high) and the soluble cytoplasm (low) will enhance the diffusive flow of fatty acids into the cell. This could occur either by concentrating fatty acids at the outer leaflet of the cell membrane, assisted, for instance, by proteins (such as CD36) and/or by intracellular metabolic trapping of fatty acids, thus lowering the intracellular fatty acid concentration. In this concept, proteins would be involved only indirectly in the actual transmembrane movement of fatty acids.
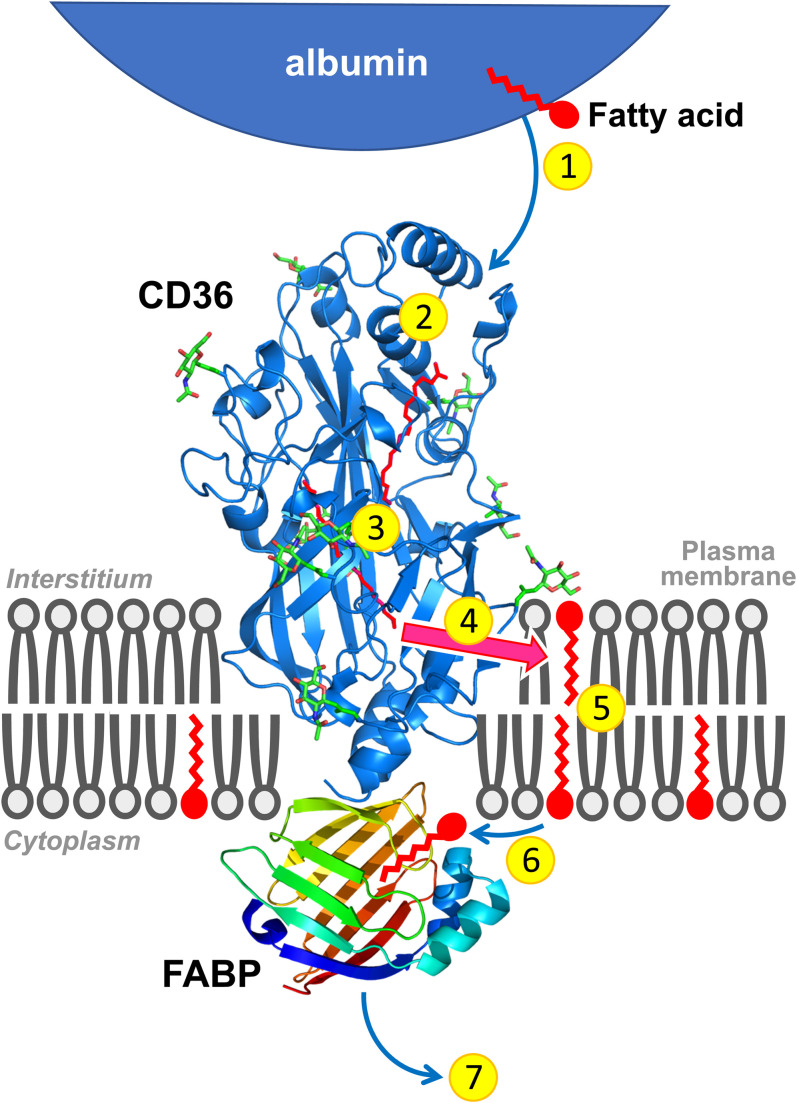
When considering the cellular uptake of fatty acids in more detail, the physical transport can be divided into three steps: i) release of the fatty acid from albumin and entry into the outer leaflet of the lipid bilayer, whereby the hydrocarbon chain intercalates between the chains of the phospholipids while the carboxyl group localizes at the aqueous interface (adsorption), ii) transfer of the fatty acid from the outer to the inner leaflet, whereby the polar carboxyl group moves through the bilayer interior and repositions at the opposite interface (translocation or ’flip-flop’), and iii) movement of the fatty acid into the aqueous phase and its binding to cytoplasmic FABP (desorption). Biophysical studies from various investigators have disclosed that fatty acid adsorption and subsequent ‘flip-flop’ are very fast for virtually all fatty acid types, but that desorption from the membrane will be the rate-limiting step of transmembrane transport (4, 18). The latter step was suggested to be facilitated by the intracellular part of CD36 by providing a docking site for FABP. The corollary is that the mechanistic conundrum would be solved with an integrated involvement of both the rapid movement of single fatty acids within the phospholipid membrane and a facilitating role for membrane-associated proteins in the delivery to as well as desorption from the plasma membrane (Fig. 1). CD36 has been suggested also to be necessary extracellularly to guide the fatty acids through the unstirred water layer and bring them in close contact to membranes (14).
Fig. 1.
Cartoon illustrating the sequential steps involved in the uptake of long-chain fatty acids by cells. 1. Release of fatty acids from (interstitial) albumin. 2. Binding in the hydrophobic cavity of CD36 which can accommodate up to two fatty acids at a time. 3. Guidance of the fatty acid through the CD36 ectodomain interior to pass the unstirred water layer and be exposed to the plasma membrane surface. 4. Exit of the fatty acid from CD36 to the outer leaflet of the phospholipid bilayer. 5. Transmembrane translocation (‘flip-flop’) of single fatty acids. 6. Desorption of fatty acids from the inner leaflet of the phospholipid bilayer and binding to the interior of FABP that is anchored by binding to the intracellular part of CD36. 7. Diffusion into the soluble cytoplasm of the fatty acid-FABP complex toward sites of intracellular fatty acid metabolism. Note that proteins and membranes and their putative mutual interactions are not drawn to scale.
In their recent article, Jay and colleagues (1) report studies with (protein-free) phospholipid vesicles and isolated adipocytes aimed at evaluating whether the presence of CD36 affects the rate of transport of fatty acids across the plasma membrane. For this, they used compounds, in particular sulfo-N-succinimidyl-oleate (SSO), which have previously been used to inhibit putative fatty acid transporters like CD36 (and also were instrumental for the discovery of CD36 as a protein binding fatty acids) (19, 20). The acyl chain moiety of SSO is thought to bind to the putative fatty acid-binding pocket of CD36 (or of any other protein that would bind fatty acids) whereafter the SS group will chemically crosslink the ligand to the protein. Importantly, SSO cannot penetrate biological membranes and does not enter intact cells, but specifically inhibits the bulk of fatty acid uptake into various cell types in a CD36-dependent manner while not affecting octanoate nor glucose uptake (21, 22). Jay and coworkers observed that transmembrane fatty acid transport, as measured using the pH indicator BCECF, is not influenced by CD36, either with or without inhibition by SSO, although marked effects on intracellular fatty acid metabolism were observed. From these studies, the investigators concluded that fatty acids do not require an active protein transporter for their transmembrane movement and that fatty acid uptake is merely governed by a fatty acid metabolism-driven transmembrane gradient (1). However, it should be realized that BCECF records the arrival of protonated fatty acids at the inner leaflet of the membrane (the subsequent release of the proton is monitored) but not the desorption of the fatty acid from the membrane into the vesicular lumen or soluble cytoplasm (5). As a result, their conclusion is valid with respect to the transmembrane movement of fatty acids, but not for the full fatty acid uptake process.
This recent article by Jay et al. (1) was accompanied by a commentary from Henry Pownall (23), who also addresses the dispute on the molecular mechanism of fatty acid transport into cells and reviews a large body of literature on this topic. While in his concluding remark, he does not exclude a role for CD36, he makes a compelling statement for the diffusionist view and argues that interventions aimed at altering the rate of cellular fatty acid uptake should be directed toward targeting intracellular metabolism rather than membrane transport. However, it should be noted that targeting CD36, especially manipulating its presence at the plasma membrane by intervening in the subcellular CD36 trafficking between endosomes and the plasma membrane, has been observed to be an effective strategy to alter fatty acid uptake rates (7, 13). For instance, a study of systematic silencing in cardiomyocytes of vesicle-associated membrane proteins (VAMPs, a family of v-SNARE proteins present in membranes of subcellular transport vesicles) known to regulate vesicular trafficking by fusion with cognate t-SNARE proteins at target membranes, revealed that there is a specific VAMP (VAMP4) dedicated to CD36 trafficking. Silencing of this VAMP simultaneously decreased stimulus-induced CD36 translocation and cellular fatty acid uptake (24). In addition, there is also evidence that once at the plasma membrane, CD36 needs to be “activated” to enable an augmented fatty acid uptake rate (22). The nature of such activation is not yet known (it perhaps comprises the interaction with another protein) but would deserve attention for further study. These observations combined indicate that CD36 does play a pivotal role in the overall process of cellular fatty acid uptake.
Taken together, the debate on the molecular mechanism of cellular (long-chain) fatty acid uptake has become mostly a semantic discussion. After all, investigators agree that once fatty acids are in a membrane, their transmembrane movement occurs by (passive) diffusion without the need for membrane proteins to facilitate the process. However, membrane-associated proteins such as CD36 will accelerate the entry of fatty acids into the membrane by acting as an acceptor for fatty acids to promote the partitioning and their delivery to the outer leaflet of the lipid bilayer, and at the inner side of the membrane will facilitate the desorption of fatty acids and their subsequent binding to FABP (Fig. 1). In this way, the apparently opposing views of diffusionists and translocatists are integrated.
REFERENCES
- 1.Jay A. G., Simard J. R., Huang N., and Hamilton J. A.. 2020. SSO and other putative inhibitors of FA transport across membranes by CD36 disrupt intracellular metabolism, but do not affect FA translocation. J. Lipid Res. 61: 790–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Storch J., and Corsico B.. 2008. The emerging functions and mechanisms of mammalian fatty acid-binding proteins. Annu. Rev. Nutr. 28: 73–95. [DOI] [PubMed] [Google Scholar]
- 3.Hamilton J. A. 1998. Fatty acid transport: difficult or easy? J. Lipid Res. 39: 467–481. [PubMed] [Google Scholar]
- 4.Kleinfeld A. M. 2000. Lipid phase fatty acid flip-flop, is it fast enough for cellular transport? J. Membr. Biol. 175: 79–86. [DOI] [PubMed] [Google Scholar]
- 5.Brunaldi K., Simard J. R., Kamp F., Rewal C., Asawakarn T., O’Shea P., and Hamilton J. A.. 2007. Fluorescence assays for measuring fatty acid binding and transport through membranes. Methods Mol. Biol. 400: 237–255. [DOI] [PubMed] [Google Scholar]
- 6.Kazantzis M., and Stahl A.. 2012. Fatty acid transport proteins, implications in physiology and diseases. Biochim. Biophys. Acta. 1821: 852–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Glatz J. F. C., and Luiken J. J. F. P.. 2018. Thematic Review: Dynamic role of the transmembrane glycoprotein CD36 (SR-B2) in cellular fatty acid uptake and utilization. J. Lipid Res. 59: 1084–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Silverstein R. L., and Febbraio M.. 2009. CD36, a scavenger receptor involved in immunity, metabolism, angiogenesis, and behavior. Sci. Signal. 2: re3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Glatz J. F. C., Luiken J. J. F. P., and Bonen A.. 2010. Membrane fatty acid transporters as regulators of lipid metabolism: implications for metabolic disease. Physiol. Rev. 90: 367–417. [DOI] [PubMed] [Google Scholar]
- 10.Abumrad N. A., and Goldberg I. J.. 2016. CD36 actions in the heart: lipids, calcium, inflammation, repair and more? Biochim. Biophys. Acta. 1861: 1442–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tanaka T., Nakata T., Oka T., Ogawa T., Okamoto F., Kusaka Y., Sohmiya K., Shimamoto K., and Itakura K.. 2001. Defect in human myocardial long-chain fatty acid uptake is caused by FAT/CD36 mutations. J. Lipid Res. 42: 751–759. [PubMed] [Google Scholar]
- 12.Aguer C., Mercier J., Man C. Y., Metz L., Bordenave S., Lambert K., Jean E., Lantier L., Bounoua L., Brun J. F. et al. . 2010. Intramyocellular lipid accumulation is associated with permanent relocation ex vivo and in vitro of fatty acid translocase (FAT)/CD36 in obese patients. Diabetologia. 53: 1151–1163. [DOI] [PubMed] [Google Scholar]
- 13.Glatz J. F. C., Luiken J. J. F. P., and Nabben M.. 2020. CD36 (SR-B2) as a target to treat lipid overload-induced cardiac dysfunction. J. Lipid Atheroscler. 9: 66–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hsieh F. L., Turner L., Bolla J. R., Robinson C. V., Lavstsen T., and Higgins M. K.. 2016. The structural basis for CD36 binding by the malaria parasite. Nat. Commun. 7: 12837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kampf J. P., and Kleinfeld A. M.. 2007. Is membrane transport of FFA mediated by lipid, protein, or both? An unknown protein mediates free fatty acid transport across the adipocyte plasma membrane. Physiology (Bethesda). 22: 7–14. [DOI] [PubMed] [Google Scholar]
- 16.Bonen A., Chabowski A., Luiken J. J. F. P., and Glatz J. F. C.. 2007. Is membrane transport of FFA mediated by lipid, protein, or both? Mechanisms ond regulation of protein-mediated cellular fatty acid uptake: Molecular, biochemical and physiological evidence. Physiology (Bethesda). 22: 15–29. [DOI] [PubMed] [Google Scholar]
- 17.Pownall H., and Moore K.. 2014. Commentary on fatty acid wars: The diffusionists versus the translocatists. Arterioscler. Thromb. Vasc. Biol. 34: e8–e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hamilton J. A. 2007. New insights into the roles of proteins and lipids in membrane transport of fatty acids. Prostaglandins Leukot. Essent. Fatty Acids. 77: 355–361. [DOI] [PubMed] [Google Scholar]
- 19.Harmon C. M., Luce P., Beth A. H., and Abumrad N. A.. 1991. Labeling of adipocyte membranes by sulfo-N-succinimidyl derivatives of long-chain fatty acids: Inhibition of fatty acid transport. J. Membr. Biol. 121: 261–268. [DOI] [PubMed] [Google Scholar]
- 20.Abumrad N. A., El-Maghrabi M. R., Amri E. Z., Lopez E., and Grimaldi P. A.. 1993. Cloning of a rat adipocyte membrane protein implicated in binding or transport of long-chain fatty acids that is induced during preadipocyte differentiation. Homology with human CD36. J. Biol. Chem. 268: 17665–17668. [PubMed] [Google Scholar]
- 21.Coort S. L. M., Willems J., Coumans W. A., van der Vusse G. J., Bonen A., Glatz J. F. C., and Luiken J. J. F. P.. 2002. Sulfo-N-succinimidyl esters of long chain fatty acids specifically inhibit fatty acid translocase (FAT/CD36)-mediated cellular fatty acid uptake. Mol. Cell. Biochem. 239: 213–219. [PubMed] [Google Scholar]
- 22.Angin Y., Steinbusch L. K. M., Simons P. J., Greulich S., Hoebers N. T., Douma K., van Zandvoort M. A., Coumans W. A., Wijnen W., Diamant M. et al. . 2012. CD36 inhibition prevents lipid accumulation and contractile dysfunction in rat cardiomyocytes. Biochem. J. 448: 43–53. [DOI] [PubMed] [Google Scholar]
- 23.Pownall H. J. 2020. Commentary on SSO and other putative inhibitors of FA transport across membranes by CD36 disrupt intracellular metabolism, but do not affect FA translocation. J. Lipid Res. 61: 595–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schwenk R. W., Dirkx E., Coumans W. A., Bonen A., Klip A., Glatz J. F. C., and Luiken J. J. F. P.. 2010. Requirement for distinct vesicle-associated membrane proteins in insulin- and AMP-activated protein kinase (AMPK)-induced translocation of GLUT4 and CD36 in cultured cardiomyocytes. Diabetologia. 53: 2209–2219. [DOI] [PMC free article] [PubMed] [Google Scholar]