Capsule Summary
Combination of IL-6 (non-Type 2 asthma) and FeNO or blood eosinophil count (Type 2 asthma) identified asthma endotypes related to asthma severity, exacerbations, and responsiveness to corticosteroids and potential for response to anti-Type 2 and anti-IL-6 treatment.
Keywords: asthma severity, blood eosinophil count, FeNO, IL-6, corticosteroid responsiveness
To the Editor:
Asthma is a heterogeneous respiratory disease. Eosinophilic inflammation is important in Type 2 early onset more allergic asthma while neutrophilic inflammation or other unknown mechanisms are involved in non-Type 2 asthma. Identification of asthma endotypes linking biological pathways and molecular mechanisms for asthma phenotypes should lead to more precise approaches to characterize and treat asthma.
The NHLBI Severe Asthma Research Program (SARP) has an overall goal to identify clinical and pathophysiologic differences between mild-moderate and severe asthma. SARP1–2 were cross sectional studies and SARP3 was a longitudinal study with higher percentage of severe asthma compared to SARP1–2. In a previous genetic study in SARP1–2, the C allele of rs2228145 (Asp358Ala) in IL6R was associated with higher serum soluble IL-6R (sIL-6R) levels, asthma, and lower lung function.1 Higher levels of plasma interleukin-6 (IL-6) have been correlated with higher BMI, lower lung function, and more frequent asthma exacerbations in the SARP3 and UCSF cohorts.2 Higher fractional exhaled nitric oxide (FeNO) levels correlate with Type 2 asthma and better responses to corticosteroids.3 In this study, we investigated whether IL-6, sIL-6R, FeNO, blood eosinophil count and the combination of FeNO or blood eosinophil count (Type 2 asthma) and IL-6 (non-Type 2 asthma) levels are associated with clinical and cellular phenotypes in the SARP3 and SARP1–2 cohorts (See additional information on methods in the Online Repository). The large comprehensively phenotyped SARP database is ideal to examine relationship between Type 2 eosinophilic and non-Type 2 mechanisms in severe and non-severe asthma.
Adults (age≥12 years) with asthma in SARP3 were older (p<0.0001) and with lower percentage of African Americans (p<0.0001) than SARP1–2 subjects (Table E1). With adjustment of age, sex, BMI, and race, SARP3 subjects had significantly higher percentage of severe asthma and systemic corticosteroid use in last year (p<0.0001), higher percentage of sputum neutrophils (p=0.0002), and lower baseline FEV1/FVC (p<0.0001) than SARP1–2 subjects. SARP3 and SARP1–2 had similar levels of FeNO, blood eosinophil count, serum sIL-6R levels, and plasma or serum IL-6 levels (p>0.05), and thus a merged SARP1–3 dataset was used for analyses.
Serum sIL-6R levels were positively correlated with blood neutrophil counts (p=0.0004) but not significantly correlated with asthma severity or asthma exacerbations with adjustment for age, sex, BMI, and race (Table E2). We confirmed that the C allele of rs2228145 was associated with higher serum sIL-6R levels (p<0.0001). Interestingly, the C allele of rs2228145 was the risk allele for asthma, hay fever, and atopic dermatitis, but it has been reported to be the protective allele for rheumatoid arthritis.4 In summary, sIL-6R levels may not be a predictive biomarker for severe asthma and exacerbations, and thus were not used for further analyses in this study.
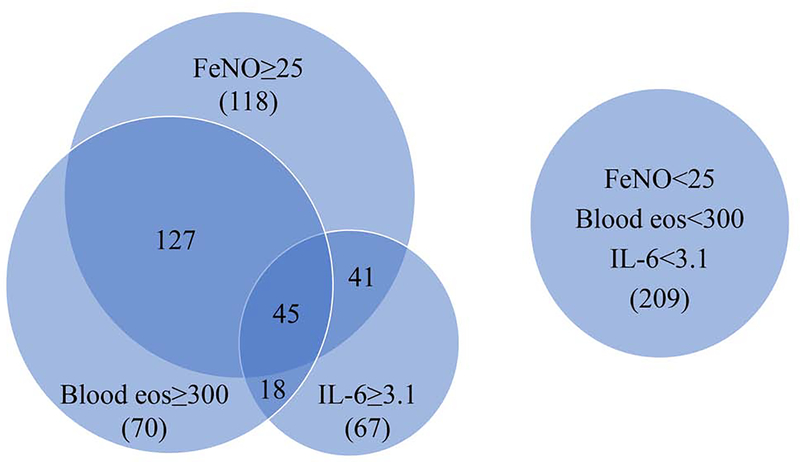
Higher plasma or serum IL-6 levels were significantly correlated with older age (p=0.001), female gender (p=0.01), and higher BMI (p<0.0001) in merged SARP1–3 dataset (Table E3) or with similar trends in SARP1–2 (Table E4) and SARP3 (Table E5). In a recent study, White et al. has reported that plasma IL-6 levels are higher in African Americans than Whites in both control and asthma groups.5 In this study, IL-6 levels were not significantly different in subjects with asthma between African Americans (2.7±4.1 pg/ml; n=230) and Whites (2.6±4.7 pg/ml; n=476) (Table E6). With adjustment for age, sex, BMI, and race, higher IL-6 levels were still significantly correlated with lower baseline % predicted FEV1 (p=0.01), higher blood neutrophil counts (p<0.0001), asthma hospitalizations in the last year (p=0.01), and systemic corticosteroid use in the last year (p<0.0001) in merged SARP1–3 dataset. IL-6 levels were not correlated with cellular characteristics of Type 2 asthma (higher blood eosinophil counts and % sputum eosinophils, total serum IgE levels, and FeNO levels) in merged SARP1–3 dataset (Table E3). Similar trends were observed in SARP1–2 (Table E4) and SARP3 (Table E5). In SARP1–3 subjects (n=695) with IL-6 levels, FeNO levels, and blood eosinophil counts, IL-6 levels and FeNO levels were independent (High IL-6 & High FeNO+Low IL-6 & Low FeNO:High IL-6 &Low FeNO+Low IL-6 & High FeNO=365:330) and IL-6 levels and blood eosinophil counts were also independent (HH+LL:HL+LH=390:305) (Figure 1). IL-6 levels were not significantly correlated with responses to corticosteroid evoked phenotypes6 (change of ACQ6 or % change of FEV1) before and 3 weeks after an intramuscular triamcinolone acetonide injection in SARP3. In summary, IL-6 levels may be a useful biomarker associated with severe asthma and exacerbations. Thus, IL-6 levels are correlated with asthma phenotypes but not Type 2 asthma biomarkers.
Figure 1.
Venn diagram of adults with asthma and IL-6 levels, FeNO levels, and blood eosinophil counts in SARP1–3 (n=695). Subjects were categorized into high IL-6 (≥3.1 pg/ml,) or low IL-6 (<3.1 pg/ml), high FeNO (≥25 ppb) or low FeNO (<25 ppb), and high blood eosinophils (≥300 cells/μl) or low blood eosinophils (<300 cells/μl).
Higher FeNO levels were significantly correlated with younger age (p=0.02) and lower BMI (p=0.0008) (Table E7). With adjustment for age, sex, BMI, and race, higher FeNO levels were still significantly correlated with lower baseline % predicted FEV1 (p=0.002) and FEV1/FVC (p<0.0001), greater bronchodilator reversibility (p<0.0001), asthma hospitalizations in the last year (p=0.05), ICU admissions due to asthma in the last year (p=0.02), and systemic corticosteroid use in the last year (p=0.03). FeNO levels were positively correlated with cellular characteristics of Type 2 asthma (higher blood eosinophil counts, % sputum eosinophils, and total serum IgE levels) (p<0.0001). In SARP1–3 subjects (n=695), FeNO levels were positively correlated with blood eosinophil counts (HH+LL:HL+LH=448:247) (Figure 1). Higher FeNO levels were significantly correlated with greater % change of FEV1 (p=0.0004) and greater decrease of ACQ6 (p=0.01) before and 3 weeks after an intramuscular triamcinolone acetonide injection. With adjustment for age, sex, BMI, and race, higher blood eosinophil count was significantly correlated with lower baseline % predicted FEV1 (p<0.0001) and FEV1/FVC (p<0.0001), greater bronchodilator reversibility (p<0.0001), and higher systemic corticosteroid use in last year (p=0.03) (Table E8). Blood eosinophil count was positively correlated with cellular characteristics of Type 2 asthma (higher % sputum eosinophils, FeNO, and total serum IgE levels) (p<0.0001). Higher blood eosinophil count was significantly correlated with greater % change of FEV1 (p=0.0004) before and 3 weeks after an intramuscular triamcinolone acetonide injection. In summary, FeNO levels and blood eosinophil count may be useful biomarkers for severe asthma and exacerbations. FeNO levels and blood eosinophil count are correlated with asthma phenotypes and corticosteroid evoked phenotypes representing Type 2 asthma biomarkers.
Phenotypes were compared among four groups categorized by the combinations of High-Low IL-6 levels (≥3.1 pg/ml or <3.1 pg/ml) and High-Low FeNO levels (≥25 ppb or < 25ppb) (Table I and Table E9–E11) or High-Low blood eosinophil count (≥300 cells/μl or <300 cells/μl) (Table I and Table E12). Two high IL-6 groups (HL and HH) had higher blood neutrophil counts (p<0.0001), higher BMI (p<0.0001), lower baseline % predicted FEV1 (p=0.01), higher percentage of asthma hospitalizations (p=0.02) and systemic corticosteroid use in last year (p=0.005) than two low IL-6 groups (LL and LH) in merged SARP1–3 dataset (Table E9), and showed similar trend in SARP1–2 (Table E10) and SARP3 (Table E11). Two high FeNO groups (LH and HH) had higher sputum and blood eosinophils and total serum IgE levels (p<0.0001), lower baseline FEVi/FVC (p=0.02), greater bronchodilator reversibility (p=0.004), and greater % changes of FEV1 before and after corticosteroid administration6 (p=0.04) than two low FeNO groups (LL and HL) in merged SARP1–3 dataset (Table E9). Two high blood eosinophil count groups (LH and HH) had higher sputum eosinophils, FeNO, and total serum IgE levels (p<0.0001), lower FEV1/FVC (p=0.01), greater bronchodilator reversibility (p=0.004), and greater % changes of FEV1 before and after corticosteroid administration (p=0.004) than two low blood eosinophil count groups (HL and LL) (Table E12). High FeNO and high blood eosinophil count group (HH) or low FeNO and low blood eosinophil count group (LL) had greater or lower % changes of FEV1 before and after corticosteroid administration or % sputum eosinophils than high FeNO and low blood eosinophil count group (HL) or low FeNO and high blood eosinophil count group (LH), respectively (p<0.0001) (Table E13). Combinations of FeNO and blood eosinophil count appear to be a better predictive biomarker for eosinophilic inflammation than FeNO or blood eosinophil count. In summary, subjects with higher IL-6 levels tend to be more obese with higher blood neutrophil counts, more severe asthma and asthma exacerbations; subjects with higher FeNO levels or blood eosinophil count tend to have Type 2 asthma with eosinophilic inflammation, more severe asthma and asthma exacerbations, and larger improvement after systemic corticosteroid administration. These four groups may reflect four different asthma endotypes.
Table I.
Correlation of IL-6 and FeNO levels or blood eosinophil count with asthma phenotypes in SARP1–3 (partial table).
| IL-6 & FeNO | LL (278)* | LH (249)* | HL (88)* | HH (90)* | Four Groups | |||||
| n | mean ± SD | n | mean ± SD | n | mean ± SD | n | mean ± SD | P value† | P value‡ (Adjusted) | |
| Body mass index (BMI) | 278 | 31 ± 6.9 | 249 | 29 ± 6.9 | 88 | 39 ± 12 | 90 | 35 ± 9.0 | <0.0001 | NA |
| Baseline FEV1 % predicted | 278 | 77 ± 21 | 249 | 76 ± 19 | 88 | 70 ± 21 | 90 | 70 ± 19 | 0.001 | 0.014 |
| Blood neutrophil counts (cells/μl) | 273 | 3974 ±1625 | 247 | 3822 ± 1562 | 86 | 5576 ± 2407 | 87 | 5379 ± 3133 | <0.0001 | <0.0001 |
| Blood eosinophil counts (cells/μl) | 273 | 201 ± 158 | 245 | 339 ± 286 | 85 | 223 ± 262 | 86 | 345 ± 270 | <0.0001 | <0.0001 |
| FeNO (ppb) | 278 | 14 ± 5.4 | 249 | 58± 41 | 88 | 15 ± 5.7 | 90 | 49 ± 35 | <0.0001 | <0.0001 |
| Systemic corticosteroid use in last year (% yes) | 278 | 28 | 249 | 27 | 88 | 45 | 90 | 50 | <0.0001 | 0.0048 |
| % ΔFEV1 (corticosteroid evoked) in SARP3 | 145 | 2.6 ± 13 | 107 | 6.8 ± 16 | 48 | 1.4 ± 15 | 40 | 8.8 ± 18 | 0.0007 | 0.039 |
| IL-6 & Blood eosinophil count | LL (344)** | LH (216)** | HL (113)** | HH (65)** | Four Groups | |||||
| n | mean ± SD | n | mean ± SD | n | mean ± SD | n | mean ± SD | P value† | P value‡ (Adjusted) | |
| Body mass index (BMI) | 344 | 30 ± 7.0 | 215 | 30 ± 7.1 | 113 | 38 ± 11 | 65 | 35 ± 9.3 | <0.0001 | NA |
| Baseline FEV1 % predicted | 344 | 78 ± 20 | 215 | 73 ± 19 | 113 | 71 ± 19 | 65 | 68 ± 20 | <0.0001 | 0.0033 |
| Blood neutrophil counts (cells/μl) | 344 | 3818 ± 1694 | 216 | 4003 ± 1459 | 113 | 5320 ± 2800 | 65 | 5578 ± 2631 | <0.0001 | <0.0001 |
| Blood eosinophil counts (cells/μl) | 344 | 136 ± 70 | 216 | 480 ± 262 | 113 | 141 ± 80 | 65 | 524 ± 305 | <0.0001 | <0.0001 |
| FeNO (ppb) | 327 | 28 ± 29 | 197 | 47 ± 44 | 108 | 27 ± 23 | 63 | 41 ± 38 | <0.0001 | <0.0001 |
| Systemic corticosteroid use in last year (% yes) | 344 | 25 | 215 | 29 | 113 | 44 | 65 | 54 | <0.0001 | 0.002 |
| % ΔFEV1 (corticosteroid evoked) in SARP3 | 164 | 3.3 ± 13 | 92 | 6.2 ± 17 | 55 | 0.4 ± 11 | 33 | 12 ± 21 | 0.008 | 0.004 |
LL: low IL-6 and low FeNO, LH: low IL-6 and high FeNO, HL: high IL-6 and low FeNO, HH: high IL-6 and high FeNO
Kruskal-Wallis test
Generalized linear model adjusted for age, sex, BMI, and race
LL: low IL-6 and low blood eosinophil count, LH: low IL-6 and high blood eosinophil count, HL: high IL-6 and low blood eosinophil count, HH: high IL-6 and high blood eosinophil count. This is a partial table for combination of IL-6 and FeNO levels or IL-6 and blood eosinophil count. Please refer to Table E9 and Table E12 for complete phenotypes tested.
Previous studies have indicated that FeNO is a reliable non-invasive biomarker of eosinophilic inflammation and corticosteroid responses.3 IL-6 binding to membrane IL-6R (classic IL-6 signaling pathway) or sIL-6R (trans-signaling pathway) is involved in host defence against infections, asthma, autoimmune diseases, and cardiovascular diseases.7,8 Combination of sputum eosinophils and neutrophils may identify subjects with more severe asthma symptoms.9 The distribution of IL-6 levels, FeNO levels, and blood eosinophil counts were continuous (Figure E1–E2), indicating that the choice of cutoff values was relatively subjective. Identification of the best cutoff values for these biomarkers is warranted for future categorization of asthma endotypes. Correlation of biomarkers (as quantitative variables) was performed using Spearman rank correlation (Table E14). IL-6 levels were not significantly correlated with Type 2 asthma biomarkers, and thus should reflect a non-Type 2 asthma. IL-6 levels were positively correlated with blood neutrophil count (p<0.0001) but not significantly correlated with % sputum neutrophils, which indicate IL-6 levels may not be a good surrogate for airway neutrophilic inflammation. Type 2 asthma biomarkers (% sputum eosinophils, blood eosinophil count, FeNO, and total serum IgE levels) were positively correlated with each other (p<0.0001), but the correlation was not strong (0.2<correlation coefficient rho<0.5), which may indicate there are more than one Type 2 asthma endotype.
In this study, we showed that combination of IL-6 (representing non-Type 2 asthma) and FeNO or blood eosinophil count (representing Type 2 asthma) may identify different asthma endotypes (Figure E3). In the future, it is important to test measurement consistency, longitudinal changes, and drug effects on these biomarkers. It will be also important to evaluate treatment with IL-6 antibodies to determine their effects on asthma severity phenotypes and control. For example, we can postulate that a combination of anti-Type 2 and anti-IL-6 drugs may be needed for treating severe asthmatics with the HH endotype, while anti-Type 2 or anti-IL-6 therapies may be effective in severe asthmatics with the LH or HL endotypes, respectively. Additional forms of therapies may be more effective for the LL endotype. It will be critical to develop and test potential biomarkers that will predict responsiveness or lack of responsiveness to the biologic therapies, in order to advance stratified therapy in asthma.
Supplementary Material
Acknowledgments
Declaration of all sources of funding: SARP1-2 was supported by NIH grants HL69116, HL69130, HL69149, HL69155, HL69167, HL69170, HL69174, HL69349, UL1RR024992, M01RR018390, M01RR07122, M01RR03186, HL087665, and HL091762. SARP3 was funded by the NHLBI U10 HL109172, HL109168, HL109152, HL109257, HL109046, HL109250, HL109164, and HL109086.
Disclosure of potential conflict of interest:
A. T. Hastie reports grants from NIH and grant support from Genentech during the conduct of the study. W. W. Busse has received consulting fees from AstraZeneca, Genentech, Regeneron, Novartis, Sanofi, and GlaxoSmithKline. M. Castro receives University Grant Funding from NIH, American Lung Association, and PCORI, receives Pharmaceutical Grant Funding from AstraZeneca, Chiesi, Novartis, GSK, and Sanofi-Aventis, serves as a consultant for Genentech, Theravance, VIDA, Teva, Sanofi-Aventis and also a speaker for AstraZeneca, Genentech, GSK, Regeneron, Sanofi, Teva, and receives Royalties from Elsevier. S. E. Wenzel has consulted for AstraZeneca, Genentech, GSK, Sanofi-Aventis in the last 3 yrs, in matters unrelated to the content of this manuscript, participated in multicenter clinical trial for AstraZeneca, GSK, Novartis and Sanofi Aventis in the last 3 yrs, unrelated to this manuscript, and received financial support for the last 2 yrs of SARP from an unrestricted grant from Boehringer-Ingelheim. E. R. Bleecker has performed clinical trials through his employer, Wake Forest School of Medicine and University of Arizona, for AstraZeneca, MedImmune, Boehringer Ingelheim, Genentech, Novartis, Regeneron, and Sanofi Genzyme, and has also served as a paid consultant for ALK-Abello, AstraZeneca, MedImmune, Glaxo Smith Kline, Novartis, Regeneron, Sanofi Genzyme, and TEVA, outside the submitted work. The rest of the authors declare that they have no relevant conflict of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hawkins GA, Robinson MB, Hastie AT, Li X, Li H, Moore WC, et al. The IL6R variation Asp(358)Ala is a potential modifier of lung function in subjects with asthma. J Allergy Clin Immunol. 2012;130(2):510–5 el. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peters MC, McGrath KW, Hawkins GA, Hastie AT, Levy BD, Israel E, et al. Plasma interleukin-6 concentrations, metabolic dysfunction, and asthma severity: a cross-sectional analysis of two cohorts. Lancet Respir Med. 2016;4(7):574–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sandrini A, Taylor DR, Thomas PS, Yates DH. Fractional exhaled nitric oxide in asthma: an update. Respirology. 2010;15(1):57–70. [DOI] [PubMed] [Google Scholar]
- 4.Buniello A, MacArthur JAL, Cerezo M, Harris LW, Hayhurst J, Malangone C, et al. The NHGRI-EBI GWAS Catalog of published genome-wide association studies, targeted arrays and summary statistics 2019. Nucleic Acids Res. 2019;47(D1):D1005–D12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.White SR, Laxman B, Naureckas ET, Hogarth DK, Solway J, Sperling AI, et al. Evidence for an IL-6 high asthma phenotype in asthma patients of African ancestry. J Allergy Clin Immunol. 2019. doi: 10.1016/jjaci.2019.04.007. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Phipatanakul W, Mauger DT, Sorkness RL, Gaffin JM, Holguin F, Woodruff PG, et al. Effects of Age and Disease Severity on Systemic Corticosteroid Responses in Asthma. Am J Respir Crit Care Med. 2017;195(11):1439–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Calabrese LH, Rose-John S. IL-6 biology: implications for clinical targeting in rheumatic disease. Nat Rev Rheumatol. 2014;10(12):720–7. [DOI] [PubMed] [Google Scholar]
- 8.Rose-John S, Winthrop K, Calabrese L. The role of IL-6 in host defence against infections: immunobiology and clinical implications. Nat Rev Rheumatol. 2017;13(7):399–409. [DOI] [PubMed] [Google Scholar]
- 9.Hastie AT, Moore WC, Meyers DA, Vestal PL, Li H, Peters SP, et al. Analyses of asthma severity phenotypes and inflammatory proteins in subjects stratified by sputum granulocytes. J Allergy Clin Immunol. 2010;125(5):1028–36 e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.