Abstract
Background
Opioid dependence carries the highest disease burden of all illicit drugs. Opioid agonist therapy (OAT) is an evidence-based medical intervention that reduces morbidity and mortality. There is limited knowledge on the health-related quality of life (HRQoL) of long-term patients in OAT. This study measures HRQoL and self-perceived health of long-term patients on OAT, compares the scores to a Norwegian reference population, and assesses changes in these scores at 1-year follow up.
Methods
We conducted a nested prospective cohort study among nine OAT outpatient clinics in Norway. 609 OAT patients were included, 245 (40%) followed-up one year later. Data on patient characteristics, HRQoL, and self-perceived health was collected. HRQoL was assessed with the EQ-5D-5L, which measures five dimensions (mobility, self-care, usual activities, pain/discomfort and anxiety/depression) on a five-point Likert scale (from “no problems” to “extreme problems”). An UK value set was applied to calculate index values (from 0 to 1) for the EQ-5D-5L and compare them to a Norwegian reference population. Self-perceived health was measured with EQ-VAS (from 0 to 100).
Results
Mean (standard deviation (SD)) EQ-5D-5L index value at baseline was 0.699 (0.250) and EQ-VAS 57 (22) compared to 0.848 (0.200) and 80(19) for the Norwegian reference population. There were large variations in EQ-5D-5L index values, where 43% had > 0.8 and 5% had < 0.2 at baseline. The lowest EQ-5D-5L index values were observed for female patients, age groups older than 40 years and for methadone users. At follow-up, improvements in HRQoL were observed across almost all dimensions and found significant for mobility and pain/discomfort. Mean (SD) overall index value and EQ-VAS at follow up were 0.729 (0.237) and 59 (22) respectively.
Conclusion
The average HRQoL and self-perceived health of OAT patients is significantly lower than that of the general population, and lower than what has been found among other severe somatic and psychiatric conditions. Around 34% had very good HRQoL, higher than average Norwegian values, and around 5% had extremely poor HRQoL.
Keywords: Health related quality of life, Quality of life, EQ-5D, Opiate substitution therapy, Opioid agonist therapy, Opioid dependence, Epidemiology
Background
Opioid dependence is a severe chronic relapsing disorder consisting of a cluster of physiological, behavioral, and cognitive phenomena [1]. Worldwide, opioid use disorders affect over 16 million people and are responsible for over 120.000 deaths per year [2]. Of all illegal drugs, opioids denote the highest disease burden, have the highest demand for treatment, contribute to substantial increased healthcare costs, and have given rise to a marked increase in opioid related deaths in the last decade [3–5]. People with opioid use disorders suffer not only from a shorter life-span as compared to the general population, but also severe social marginalization and long-term impairments in most aspects of their lives [6]. Research consistently shows that people with opioid use disorders have inferior quality of life (QoL) compared to the general population [7, 8]. This is partly explained by the extensive co-occurrence of substance use disorder and mental disorders, which both seem underdiagnosed and undertreated [9], in addition to high prevalence of somatic disorders such as chronic hepatitis C of almost 50% [10]. Epidemiological studies suggest a prevalence of around 27% for anxiety disorders, 35% for affective disorders, 30% for attention-deficit hyperactivity disorder, and 51% for personality disorders in patients with substance use disorders [11–13]. However, prevalence may be even higher in clinical studies as people with severe problems are more likely to seek help; studies have found prevalence of around 70% for one or more personality disorder [14] and around 66% for childhood trauma among people with substance use disorders [15], and at least one comorbid psychiatric disorder in approximately 80% of patients on opioid agonist therapy (OAT) [16].
Increased focus on, and availability of harm reduction programs, such as OAT have lowered the demand for heroin in Western Europe including Norway [5]. OAT is an evidence-based medical intervention that reduces illicit opioid use, improves patients’ health and reduces crude mortality rates significantly [3, 17–20]. For instance, results of 22 pooled longitudinal cohort studies showed a crude mortality rate for patients on OAT of 0.90 per 100 person years, compared to 1.63 when OAT was ceased and 4.91 for untreated periods [21]. Most research on OAT has emphasized on crude mortality rate, abstinence and retention in treatment, rather than what may be most important for each individual patient; personal wellbeing. In turn, several researchers argued that health related quality of life (HRQoL) should be included as an outcome when evaluating substance use and OAT treatment [22–25]. Thus, to evaluate real life outcome of OAT, changes in objective and self-perceived health, including the individual’s own experience, should be examined. In addition, for more individualized OAT treatment and management, it is important to understand the relationship between clinical and demographic characteristics and HRQoL.
Factors associated with poor HRQoL among OAT patients are older age, female gender, and mental and physical comorbidity [7, 26, 27]. There is building evidence that HRQoL is substantially lower among people with opioid dependence and that HRQoL improves at OAT initiation and during the first few months of treatment [27–30]. However, a recent systematic review suggests there is still limited knowledge regarding HRQoL outcomes in OAT treatment programs and HRQoL outcomes are rarely used [22]. Many of the previous studies are cross-sectional rather than longitudinal designs, offers few participants and with non-validated HRQoL measures for opioid dependence, which make comparisons difficult across opioid dependence and other diseases.
The principal aim of this study is to evaluate the HRQoL and self-perceived health of a large cohort of long-term patients with opioid dependence enrolled in an integrated OAT program in Norway. The HRQoL of OAT patients will also be compared to that of the general population in Norway. Finally, an assessment of changes in HRQoL and self-perceived health at one-year follow-up will be conducted.
Methods
Study design and setting
This study is a nested prospective cohort study linked to the Integrated Treatment of Hepatitis C study (INTRO-HCV) [31]. The observational study recruited participants from May 2017 until January 2020 [31]. HRQoL baseline data was collected at the first OAT health assessment, and follow-up data was collected one-year after baseline for each patient. Trained research nurses, who were not responsible for clinical patient follow-up, collected the research data via structured patient interviews. The data was recorded directly in an electronic data entry system (CheckWare). The study took place in Bergen and Stavanger, which are cities in southwestern parts of Norway with around 280,000 and 130,000 inhabitants each. The target population was individuals with opioid dependence who received OAT treatment and care in all together nine OAT outpatient clinics. The clinics have adopted an integrated treatment and care model where patients are charted on a nearly daily basis by health professionals; including social workers, specialized and general nurses, psychologists, and physicians specialized in addiction medicine. OAT medications include mostly methadone or buprenorphine-based medications, often with directly observed intake [32].
Study sample
The study sample included individuals diagnosed with opioid dependence according to International Classification of Diseases version 10 (ICD-10) [33], currently enrolled in OAT treatment, aged 18 years or older, and have given a written informed consent to participate in the study. Individuals were eligible for inclusion regardless of the type of OAT medication or administration form. Remuneration, of around euro 20, was provided once for the participants upon inclusion to participate in the study. Of the 900 patients invited, a total of 609 (68%) patients completed the EQ-5D-5L questionnaire at baseline, and of those, 245 (40%) were followed up with a follow-up questionnaire approximately 1 year after the first visit. Nineteen patients (2%) were excluded because they did not complete the interview or due to missing data of the EQ-5D-5L instrument The mean time between the first and second annual OAT assessment was 375 days (95% confidence interval (CI): 359–392 days). See Table 1 for details on clinical and demographic characteristics and additional file 1 for flowchart of study sample.
Table 1.
Baseline characteristics of study sample
| Percentages or Mean (SD) | |
|---|---|
| Patients, na | |
| Gender, n = 609 | |
| Male | 71% |
| Female | 29% |
| Age, n = 609 | |
| < 25 | 3% |
| 26–40 | 39% |
| 41–60 | 53% |
| ≥ 61 | 5% |
| Mean age (SD) | 44 (10) |
| Current OAT medication, n = 588 | |
| Methadone | 38% |
| Buprenorphine | 56% |
| Buprenorphine/naloxone | 4% |
| Other | 2% |
| Duration of OAT treatment in years, mean (SD), n = 583 | 7.9 (5.4) |
| Background demographics | |
| Highest level of completed education, n = 588 | |
| Did not complete primary and secondary school | 5% |
| Completed primary and secondary school | 45% |
| High school | 40% |
| Undergraduate education ≤3 years | 8% |
| Postgraduate education ≥3 years | 2% |
| Main source of income, several answers possible, n = 611 | |
| Paid work (full time or part time) | 7% |
| Sick pay or unemployment benefits | 10% |
| Social or disability benefits | 79% |
| Savings or scholarships | 1% |
| Other | 3% |
| Accommodation last 30 days several answers possible, n = 606 | |
| Owned property | 9% |
| Rented property | 68% |
| Temporary property | 7% |
| Prison | 1% |
| Homeless | 1% |
| At friends or family | 12% |
| Other | 2% |
| Living conditions n = 586 | |
| Living alone | 63% |
| Living with others | 37% |
| Children n = 587 | |
| Do not have children | 44% |
| Have children | 56% |
| For those having children < 18 years old, n = 152 | |
| Having children < 18 with visiting rights | 79% |
| Having children < 18, but no visiting rights | 21% |
OAT Opioid agonist therapy, SD Standard deviation,
an = number of respondents, some questions allow multiple answers
Instruments
Health related quality of life: EQ-5D-5L
The EQ-5D-5L instrument is a widely used generic measure of HRQoL [34] and validated for opioid use disorders [35, 36]. It consists of two components. The first descriptive system evaluates health in five dimensions (Mobility, Self-care, Usual activities, Pain/Discomfort, Anxiety/Depression). Each dimension has five levels of response, ranging from no problems, slight problems, moderate problems, severe problems, to extreme problems [37]. The second part of EQ-5D-5L entails a visual analogue scale (VAS) where the respondent rates the self-perceived health from 0 (worst health imaginable) to 100 (best health imaginable) [37]. A systematic review supports the use of the EQ-5D-5L in a broad range of patients [38]. We therefore selected this instrument to assess the HRQoL of patients in OAT and to compare their HRQoL to the general population.
Statistical analysis
Responses to the five HRQoL dimensions are coded as a five-digit code, which represents a numerical description of a health state. The digits have no arithmetic properties and therefore a single summery number (an index value) needs to be arrived by applying a formula with an appropriate value set, which is a representative sample of the general population. The index value then represents how good or bad a health state is according to the preferences of the general population, ranging from 1 (full health) to 0 (dead, with negative values indicating health states worse than death) [37]. In the absence of a Norwegian value set, we applied an EQ-5D-5L value set for UK, i.e. the societal preference weights for the health state, to determine the EQ-5D-5L index values for each health state in the OAT cohort [39]. Summary statistics were derived, including proportions and number of patients for the five EQ-5D-5L dimensions by age, gender and OAT medications. The EQ-VAS score was summarized descriptively by mean, standard deviation (SD), minimum and maximum as the data was not particularly skewed. A paired t-test of means for the 245 patients with two time points was used in the analysis to investigate whether there was any statistical significance in EQ-5D-5L between the measurements. An ANCOVA model for EQ-VAS changes from baseline to the next OAT health assessment was conducted where place and treatment were fixed effects and baseline covariate. If data were missing from more than one dimension participants were excluded. Altogether eight patients missed data on one dimension at baseline but were included in the analyses. There were no missing data from EQ-5D-5L follow-up or EQ-VAS. To estimate the unbiased treatment effects from baseline to follow-up we used an inverse probability weighted method as we had follow-up data for a subgroup. We calculated population weights based on age, gender and how many times OAT medication was collected during a week in a binominal regression model with follow-up values as the dependent variable. More weight was given to cases with valid data, which were associated with highest probability of having missing data, and less weight was given to cases with lowest probability of missing. The mean for the population weights was 1.0 (SD 0.12) in our model. Statistical significance was set at p < 0.05 level. All analyses were made with STATA SE 16.0.
Results
Baseline characteristics of study sample
The patients were predominantly male (71%) with a mean (SD) age of 44 years (10). The age range of the study sample was 23–74 years. Most received buprenorphine-based medication (60%) followed by methadone (38%). Duration in OAT treatment ranged from 0 to 25 years, with a mean (SD) of 7.9 (5.4) years. Results therefore reflects HRQoL of patients that have received OAT over a long period of time. Of the participants, 45% had completed secondary school and 40% completed high school. Almost 80% received either disability pension or social benefits as main source of income. Of the 152 OAT patients with children under 18 years, 21% reported they had no visiting rights (Table 1). The distributions of sociodemographic variables were similar for the baseline- and the follow-up samples. However, the proportion of males increased from 71 to 76% in the follow-up sample and mean (SD) age increased from 44 (10) to 45 (10) years compared to the sample at baseline.
HRQoL of OAT patients at baseline
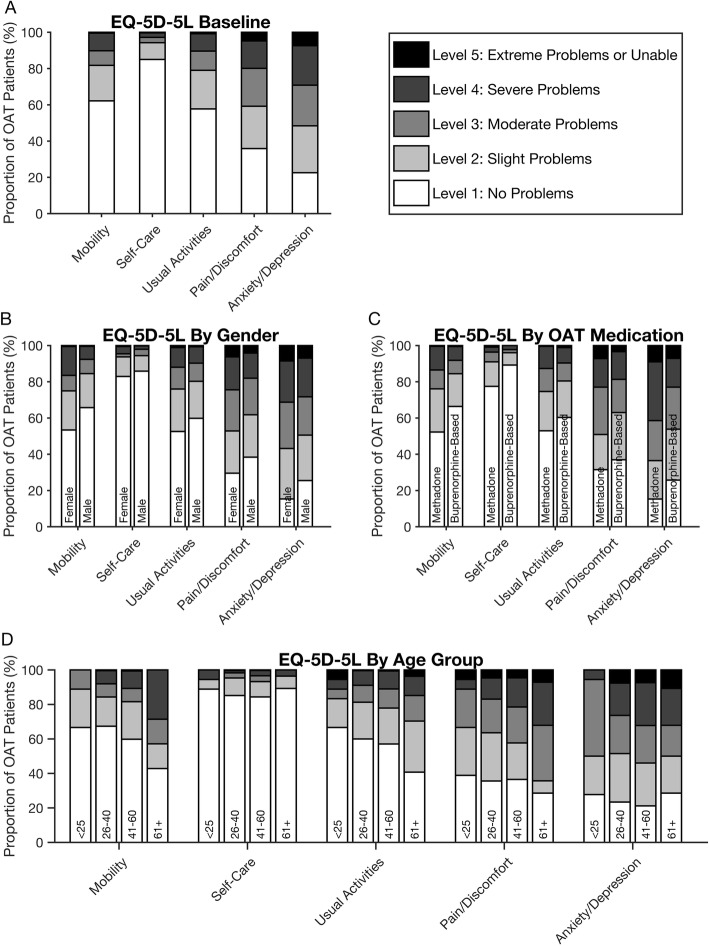
The distributions of unadjusted EQ-5D-5L scores are presented as norm sets according to gender, age groups, and OAT medications (Fig. 1). Overall, mean scores for the five dimensions were 1.7 (95% CI: 1.6–1.8) for mobility, 1.3 (95% CI: 1.2–1.3) for self-care, 1.8 (95% CI: 1.7–1.9) for usual activity, 2.3 (95% CI: 2.2–2.4) for pain/discomfort and 2.7 (95% CI: 2.6–2.8) for anxiety/depression (additional file 2). “No problems” were reported by 62% for mobility, 85% for self-care, 58% for usual activities, 36% for pain/discomfort, and only 23% for anxiety/depression. This means that the majority of patients had no problems with mobility and conducting usual activities and self-care. On the other hand, extreme problems” with pain/discomfort were noted by 5 % and 7 % reported “extreme problems” with anxiety/depression. Under 1 % reported “extreme problems” with mobility, self-care and usual activities. Females and patients receiving methadone treatment reported more problems across all EQ-5D-5L domains compared to males and patients on buprenorphine-based medications, respectively. Patients in the age group 41–60 reported more problems on every domain except pain/discomfort compared to patients under 40 years of age, while patients over 60 years of age reported most problems for mobility and pain/discomfort (additional file 2).
Fig. 1.
Proportion of individuals reporting problems by EQ-5D-5L domain; overall, by age, gender and OAT medication. OAT = opioid agonist therapy. Altogether 609 respondents, 8 patients missed values on one dimension. 11 patients, which did not receive either methadone or buprenorphine-based medications are left out of the illustration but not analysis. Proportions (%) and dimensions (mobility, self-care, usual activities, pain/discomfort and anxiety/depression). 1A: Overall, 1B: Gender 1C: OAT medication 1D: Age groups
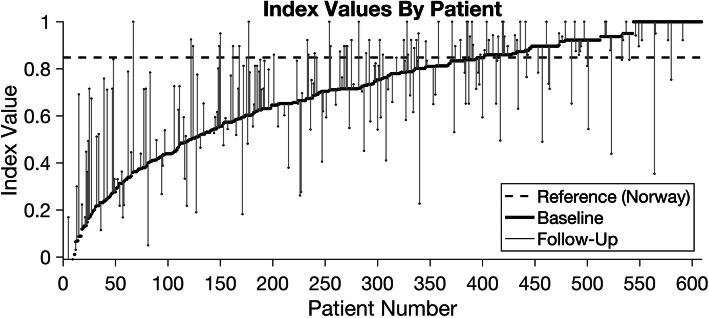
The mean (SD) EQ-5D-5L index value for OAT patients was 0.699 (0.250) at baseline. Forty-three percent had an index value above 0.8, meaning they had “no problems” in the five health domains. Thirty-four percent of the sample even had an index value above 0.848 (Norwegian reference population [40]), meaning their HRQoL was better than that of the Norwegian general population. Around 5 % had an index value below 0.2, meaning they had “extreme problems” in HRQoL. The distribution in baseline EQ-5D-5L index values are shown in the Pen’s Parade (Fig. 2). The parade shows the HRQoL distribution, and is defined as a succession of every OAT patient included, with their height proportional to their EQ-5D-5L index value, from lowest to highest.
Fig. 2.
Pen’s Parade: distribution from lowest to greatest health. Pen’s Parade: The distribution in baseline EQ-5D-5L index values, where 43% had > 0.8 and 5% had < 0.2 at baseline. The dotted line represent the average reported index value of the Norwegian reference population
The mean (SD) EQ-VAS score of OAT patients was 57 (22) for the total sample at baseline, meaning their self-perceived health was considerably lower compared to the Norwegian reference population of 80 (19) [40]. Females reported an EQ-VAS of 56 (23), while 54 (22) for patients aged 41–60, and 51 (23) for patients older than age 60. Patients on methadone reported 53 (22), which was lower EQ-VAS compared to buprenorphine with 58 (22) and buprenorphine-naloxone 65 (21).
Changes in HRQoL of OAT patients at follow up
Altogether 245 (40%) of the 609 patients at baseline were included for the follow-up analyses. As shown in the Pen’s Parade (Fig. 2), individual changes in EQ-5D-5L index values for patients with follow-up data (n = 245) are indicated with vertical lines. For instance, a patient with an index value of 0.563 at baseline and a long vertical line going up to 0.840 at follow-up means this patient reported a significant improvement in HRQoL. A patient with a vertical line going down from baseline shows worsen HRQoL between baseline and follow-up. Patients with no follow-up data (n = 364) or no change at follow-up (n = 26) has no vertical line. Figure 2 also shows that the majority of patients have a lower index value than the Norwegian reference population, meaning worse HRQoL, illustrated by values below the dotted line. However, changes go in both directions and appear substantial for some. This means that patients receiving long-term OAT are at risk of relatively rapid changes in index values in both better and worse directions. Overall, around 54% reported improvement in HRQoL, around 35% reported worse HRQoL while 11% reported no changes at follow up compared to baseline values. The mean (SD) observed change was 0.038 (0.20) with minimum and maximum values of − 0.646 and 0.639, respectively. Females reported a mean (SD) change of 0.056 (0.17) compared to males 0.032 (0.21). Variation in individual EQ-5D-5L index value changes from baseline to follow-up is illustrated in additional file 3.
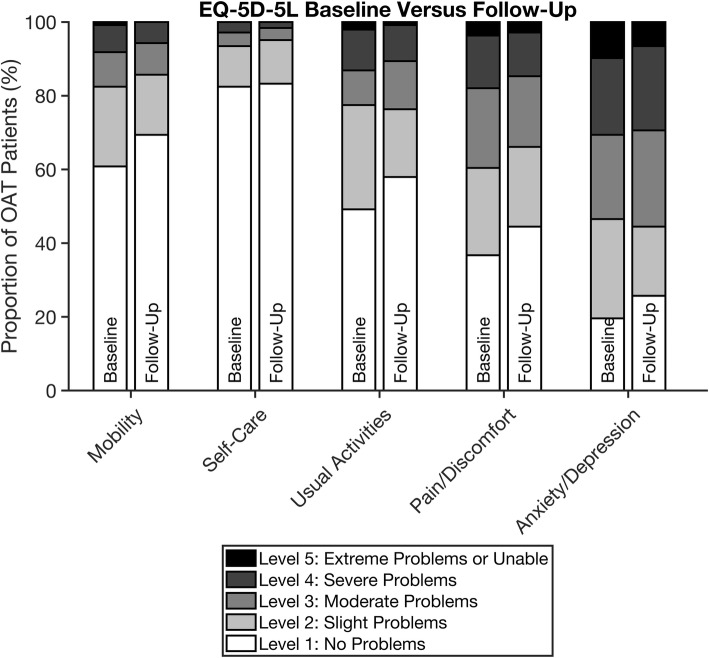
EQ-5D-5L index values improved significantly overall (p = 0.004) and for both genders (m: 0.039, f: 0.016), age group 26–40 (p = 0.002) and buprenorphine-based patients (p = 0.027) as shown in Table 2. The mean (SD) EQ-5D-5L index value was 0.729 (0.237) at follow-up; 49% had an index value above 0.8 while 37% of the sample had an index value above the Norwegian reference population. Around 4 % had an index value below 0.2. Significant improvements in EQ-5D-5L scores were found for mobility (p = 0.008) and pain/discomfort (p = 0.025) (Fig. 3 and Table 3).
Table 2.
OAT patients’ HRQoL and self-perceived health at baseline and follow up as measured by the EQ-5D-5L
| EQ-5D-5L VAS, baseline | EQ-5D-5L VAS, baselinea | EQ-5D-5L VAS, follow-up | EQ-5D-5L Index | EQ-5D-5L Index | EQ-5D-5L Index | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n 609 | n 245 | n 245 | n 609, baseline | n 245, baselinea | n 245, follow-up | |||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Overall | 57 | 22 | 57 | 22 | 59 | 22 | 0.699 | 0.250 | 0.691 | 0.237 | 0.729 | 0.237 |
| Gender | ||||||||||||
| Female | 56 | 23 | 57 | 25 | 57 | 23 | 0.653 | 0.260 | 0.613 | 0.273 | 0.669 | 0.261 |
| Male | 57 | 22 | 57 | 21 | 60 | 22 | 0.718 | 0.243 | 0.716 | 0.220 | 0.748 | 0.226 |
| Age group | ||||||||||||
| < 25 | 58 | 18 | 55 | 19 | 61 | 25 | 0.787 | 0.164 | 0.766 | 0.211 | 0.678 | 0.390 |
| 26–40 | 61 | 22 | 59 | 22 | 60 | 22 | 0.724 | 0.242 | 0.689 | 0.234 | 0.745 | 0.205 |
| 41–60 | 54 | 22 | 55 | 22 | 58 | 22 | 0.684 | 0.253 | 0.686 | 0.238 | 0.716 | 0.253 |
| ≥61 | 51 | 23 | 56 | 16 | 58 | 16 | 0.613 | 0.292 | 0.714 | 0.257 | 0.754 | 0.210 |
| OAT medication | ||||||||||||
| Methadone | 53 | 22 | 54 | 21 | 58 | 23 | 0.636 | 0.260 | 0.657 | 0.228 | 0.686 | 0.236 |
| Buprenorphine | 58 | 22 | 58 | 22 | 60 | 22 | 0.726 | 0.235 | 0.716 | 0.238 | 0.758 | 0.232 |
|
Buprenorphine/ naloxone |
65 | 21 | 59 | 30 | 54 | 26 | 0.775 | 0.242 | 0.558 | 0.352 | 0.737 | 0.335 |
OAT Opioid agonist therapy, SD Standard deviation
Index obtained from Devlin, N., Shah, K., Feng, Y., Mulhern, B. and van Hout, B., 2018. Valuing Health-Related Quality of Life: An EQ-5D-5L Value Set for England. Health Economics
aBaseline values for the 245 patients who were eligible for follow-up analysis
The possible range of scores for EQ-5D-5L (0–1, 0 = dead (scores < 0 is possible), 1 = full health) and EQ-VAS (0–100, 0 = worst health imaginable, 100 = best health imaginable
Statistically significant changes are marked in bold (p < 0.05)
Fig. 3.
EQ-5D-5L domains at baseline versus follow-up. OAT = opioid agonist therapy. Proportions (%) and dimensions (mobility, self-care, usual activities, pain/discomfort and anxiety/depression). Baseline and follow-up for the 245 patients who were eligible for follow-up analysis
Table 3.
Distribution of EQ-5D-5L dimensions at baseline and at follow-up
| Mean time between baseline and follow-up is 375 days (95% CI: 358,6–391.9) | ||||
|---|---|---|---|---|
| Baseline: 609 patients Follow-up: 245 patients | ||||
| Dimension | Baseline | Baselinea | Follow-up | p value |
| Mobility mean (95% CI) | 1.7 (1.6–1.8) | 1.7 (1.5–1.8) | 1.5 (1.4–1.6) | |
| No problems n (%) | 378 (62.1) | 149 (60.8) | 170 (69.4) | 0.0081 |
| Slight problems n (%) | 119 (19.5) | 53 (21.6) | 40 (16.3) | |
| Moderate problems n (%) | 49 (8.1) | 23 (9.4) | 21 (8.6) | |
| Severe problems n (%) | 59 (9.7) | 18 (7.4) | 14 (5.7) | |
| Unable to walk about n (%) | 3 (0.5) | 2 (1.0) | 0 (0) | |
| Self-care mean mean (95% CI) | 1.3 (1.2–1.3) | 1.3 (1.2–1.4) | 1.2 (1.2–1.3) | |
| No problems n (%) | 517 (84.9) | 202 (82.5) | 204 (83.3) | 0.4 |
| Slight problems n (%) | 56 (9.2) | 27 (11.0) | 29 (11.8) | |
| Moderate problems n (%) | 18 (2.9) | 9 (3.7) | 8 (3.3) | |
| Severe problems n (%) | 15 (2.5) | 7 (2.9) | 4 (1.6) | |
| Unable to wash or dress n (%) | 2 (0.2) | 0 (0) | 0 (0) | |
| Usual activities mean (95% CI) | 1.8 (1.7–1.9) | 1.9 (1.8–2.1) | 1.8 (1.6–1.9) | |
| No problems n (%) | 350 (57.5) | 120 (49.0) | 142 (58.0) | 0.1 |
| Slight problems n (%) | 129 (21.1) | 69 (28.2) | 45 (18.4) | |
| Moderate problems n (%) | 64 (10.5) | 23 (9.4) | 32 (13.1) | |
| Severe problems n (%) | 58 (9.5) | 27 (11.0) | 24 (9.8) | |
| Unable to do usual activities n (%) | 5 (0.8) | 5 (2.0) | 2 (0.8) | |
| Pain/discomfort mean (95% CI) | 2.3 (2.2–2.4) | 2.2 (2.1–2.4) | 2.1 (1.9–2.2) | |
| No pain/discomfort n (%) | 218 (35.8) | 90 (36.7) | 109 (44.5) | 0.0245 |
| Slight pain/discomfort n (%) | 142 (23.3) | 58 (23.7) | 53 (21.6) | |
| Moderate pain/discomfort n (%) | 127 (20.9) | 53 (21.6) | 47 (19.2) | |
| Severe pain/discomfort n (%) | 92 (15.1) | 35 (14.3) | 29 (11.8) | |
| Extreme pain/discomfort n (%) | 29 (4.8) | 9 (3.7) | 7 (2.9) | |
| Anxiety/depression mean (95% CI) | 2.7 (2.6–2.8) | 2.7 (2.6–2.9) | 2.7 (2.5–2.8) | |
| Not anxious/depressed n (%) | 137 (22.5) | 48 (19.6) | 63 (25.7) | 0.3 |
| Slightly anxious/depressed n (%) | 157 (25.8) | 66 (26.9) | 46 (18.8) | |
| Moderately anxious/depressed n (%) | 136 (22.3) | 56 (22.9) | 64 (26.1) | |
| Severely anxious/depressed n (%) | 132 (21.7) | 51 (20.8) | 56 (22.9) | |
| Extremely anxious/depressed n (%) | 45 (7.4) | 24 (9.8) | 16 (6.5) | |
CI Confidence interval
P-value: based on paired t-test of means for the 245 patients with two time points
aBaseline values for the 245 patients who were included for follow-up analysis
Significant improvement in self-perceived health (EQ-VAS) were found for males (p = 0.038).
Discussion
This study is one of the first to examine changes in HRQoL in a sample of long-term OAT patients over a one-year follow-up period. Most studies on HRQoL demonstrate improvements in HRQoL upon treatment entry, but data on long-term patients’ HRQoL is scarce. Considerable impairments in HRQoL and self-perceived health (EQ-VAS) were found in many of the OAT patients. However, large variations in EQ-5D-5L index values were found between individuals, both at baseline and at follow-up. Significant improvement in overall HRQoL was observed at one-year follow-up with around half of the OAT patients reported some improvement in HRQoL while around one-third experienced worse HRQoL at one-year follow up, with great individual variations. Males reported significant improvement in their self-perceived health.
Compared to the general Norwegian population, which reported no problems regarding mobility (85%), self-care (98%), usual activities (82%), pain/discomfort (54%) and anxiety/depression (79%) [40], OAT patients reported in average consistently higher percentages of problems across all EQ-5D-5L domains, especially pain/discomfort and anxiety/depression where only 36 and 23% respectively, reported no problems. The mean (SD) EQ-5D-5L index value for the Norwegian reference population 0.848 (0.200) [40] was considerably higher compared to the OAT patients at baseline. Mean (SD) total EQ-VAS for the OAT patients at baseline was considerably lower than the Norwegian reference population who reported overall mean (SD) of 80 (19); females 80 (20) and older age 77 (19) [40].
Our findings are consistent with prior research, such as Strada et. al (2019) study of a large OAT cohort in Germany; found that OAT patients had a lower HRQoL than the general population [41]. Several studies have demonstrated that female OAT patients report worse overall HRQoL compared to males [8, 27]. However it is unclear why that is the case and gender-focused research is urgently needed. Perhaps females are more vulnerable for stigma, traumatizing events or maybe have a poorer function upon entering OAT in the first place. Age is also strongly correlated to poor physical HRQoL [41]. In our sample the mean age was 44, which is consistent with an aging OAT population in Norway [32, 42]. Increased age of OAT patients coupled with poorly reported HRQoL, may place an increased demand for health care services in the future. This raises a debate on how level of OAT and various integrated treatment policies and strategies could better benefit OAT patients. Even if OAT patients treated with methadone reported worse HRQoL than those with buprenorphine, the results should be interpreted carefully. In current Norwegian OAT guidelines buprenorphine is usually recommended as first line substitution medication and considered safer compared to methadone due to its partial antagonistic effect [43]. It is also likely that patients may prefer buprenorphine because it is less sedative than methadone, and that both younger and perhaps more stable patients are dispensed buprenorphine, and as such, results could be highly confounded.
Previous research has revealed that HRQoL improves considerably at OAT treatment initiation and the first few months [44]: however, not much research to date has investigated the long-term effect of OAT on patients’ HRQoL [7, 22, 29]. For instance, one study among patients on methadone maintenance treatment found that QoL increased markedly in the beginning of the observation, but decreased after 6 months [45] while other studies only saw improvements in the beginning of observation [27, 46]. There is therefore the general belief, based on limited data, that once patients are enrolled in OAT, their HRQoL will remain low and does not change substantially anymore. Our study challenges that belief and is among the first to show that changes in HRQoL, including positive changes, are possible. Additionally, our findings also show that many patients had extreme variations in index values from baseline to follow-up, in both positive and negative directions. This suggests that OAT populations are susceptible to severe impairment and also rapid improvements in their HRQoL. Such swift alterations are perhaps less common among other patient groups and future research should examine what causes these changes in HRQoL in long-term OAT patients.
HRQoL in the long-term OAT population was lower in average compared to what is found in other patient groups, such as diabetes type 1 and 2 [47], HIV/AIDS patients [48] and patients with psychiatric comorbidities such as mild to moderate anxiety and depressive disorders, and residual state of bipolar disease [49]. Using the inverse of disability weights (health state valuations) reported in the Global Burden of Disease study (2017), makes comparisons between HRQoL index values and disability weights possible [50]. Research have shown there is a high comorbidity of psychiatric disorders among people with substance use disorders and individuals on OAT [11, 12, 16], while a six-year follow-up study demonstrated that the high psychiatric comorbidity persisted in long-term OAT patients [51]. This may have severe HRQoL impacts and patients with mental disorders may therefore be overrepresented in the lower extreme of the reported index values. Given the wide distribution of severity of disease within the long-term OAT population, treatment needs to be individualized and better adapted to patient functioning and needs. This opts for rethinking and reassessing OAT programs to better facilitate for integrated treatment, which have found to be consistently superior to treatment of substance use and mental disorders with separate treatment plans [52].
Furthermore, as HRQoL profiles of OAT patients are diverse and dynamic this has implications for personalized patient care and the need for regular assessment of HRQoL as an outcome. We need to better understand what drives the extreme and rapid changes in HRQoL in both positive and negative directions among OAT patients. We need to know how to best prevent large drops in index score and how to increase and maintain the increases in index score over time. Additionally, females have worse HRQoL scores compared to men, which indicates that OAT programs should particularly focus on how to improve HRQoL of females and find explanations for why females have lower HRQoL. Similarly, we found that patients older than 40 years have worse HRQoL. This shows that we need to re-examine health care needs of older patients are met and how we can address their needs better. This is particularly important as long-term OAT patients are now aging and we need to plan for aging populations receiving OAT.
A strength of this study is the large sample size of long-term OAT patients at baseline who are receiving the same level of integrated OAT treatment across their respective OAT outpatient clinics in the two cities. However, there are also limitations to our study. Follow-up was conducted on a sub-group of the initial sample. To reduce the selection bias due to the loss-to-follow-up we performed an inverse probability weighted method. In general, it is problematic to compare HRQoL between studies as setting, population, and level of OAT integrated treatment varies and different instruments are being used. This is also the case for comparisons between results based on EQ-5D-3L and EQ-5D-5L, and when different national value sets are being used. Comparative performance across patient groups is driven by differences in the descriptive systems and associated value sets [37] Another weakness is the absence of a Norwegian value set. Both our study and the study of the Norwegian reference population had to use value sets that resembles the Norwegian population, and for this reason the UK value set was chosen. Studies have confirmed that the latter version of EQ-5D significantly increase both reliability and sensitivity and can potentially reduce the possible ceiling effects encountered in the EQ-5D-3L earlier version [53, 54].
Conclusion
We found considerably lower HRQoL among long-term OAT patients in average compared to the general Norwegian reference population. However, this is a heterogeneous population. Around one-third had very good HRQoL, higher than average Norwegian values. Improvements in HRQoL were found over the one-year follow-up across most EQ-5D-5L dimensions with some uncertainties on why this was seen. More research is urgently needed to identify and understand why females and older patients have worse HRQoL and shows there is a need for more gender-and age-specific treatment in OAT programs. The wide variations in HRQoL support more emphasis on individualized treatment and personalized patient care, and the need for regular assessment of HRQoL in OAT programs. Our study is among the first to show that changes in HRQoL, including positive changes, are possible even several years after initiation of treatment. Future research should examine what causes these changes in HRQoL in long-term OAT patients.
Supplementary information
Additional file 1. OAT = opioid agonist therapy. Flow chart of study sample.
Additional file 2. EQ-5D-5L, descriptive health profile at baseline (frequencies and proportions reported by dimensions and level). OAT = opioid agonist therapy, CI = confidence interval. * = total number of respondents. 8 patients who missed data on one dimension were included in the analysis. ** = long acting morphine sulphate and other opioid prescriptions.
Additional file 3. Changes in EQ-5D-5L index value per patient from baseline to follow-up. 609 patients included at baseline, 245 patients at follow-up one year later.
Acknowledgements
Christer Kleppe, data protecting officer, Helse Bergen for his valuable contribution and guidance in data management.
Abbreviations
- HRQoL
Health related quality of life
- INTRO-HCV
Integrated treatment of hepatitis C virus infection
- OAT
Opioid agonist therapy
- QoL
Quality of life
Authors’ contributions
This observational study was led by CFA and KAJ in terms of study design, analyzes, drafting and writing the article. KAJ, JHV and LTF was particularly involved with acquisition of data, analyzes and interpretation. Figures were made by AGL and KAJ. KAJ, LTF, SS, JHV, AGL, SR, KI, JEA, and EML contributed to the conception, writing, and revising the draft(s) critically. All authors have read and approved the version to be published.
Funding
This study is part of the main INTRO-HCV study, which was funded by The Norwegian Research Council (no. 269855) and the Western Norway Regional Health Authority (“Åpen prosjektstøtte) with Department of Addiction Medicine, Haukeland University Hospital as responsible institution. The funders had no role in the study design, data collection and analyzes, decision to publish, nor preparation of any content in the manuscript. Two of the authors, CFA and JHV, are funded from the above research grant, whereas the other authors are funded by their respective affiliations.
Availability of data and materials
The INTRO-HCV study is ongoing and as such the dataset is not publically available. However, parts of the dataset used for EQ-5D-5L used for this publication may be available in an anonymous and shortened version upon contacting the corresponding author: Christer F. Aas: christer.frode.aas@helse-bergen.no
Ethics approval and consent to participate
The study was approved by the Regional committee for medical and health research ethics (no. 2017/51/REK vest). It was conducted in accordance with the Helsinki Declaration and STROBE guidelines All included participants signed a written consent to partake in the study.
Consent for publication
Not applicable. No personal details on any of the participants are reported in the manuscript, tables or figures.
Competing interests
None of the authors have competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Christer Frode Aas, Email: christer.frode.aas@helse-bergen.no.
for the INTRO-HCV Study Group:
Christer Frode Aas, Vibeke Bråthen Buljovcic, Fatemeh Chalabianloo, Jan Tore Daltveit, Silvia Eiken Alpers, Lars T. Fadnes, Trude Fondenes Eriksen, Per Gundersen, Velinda Hille, Kristin Holmelid Håberg, Kjell Arne Johansson, Rafael Alexander Leiva, Siv-Elin Leirvåg Carlsen, Martine Lepsøy Bonnier, Lennart Lorås, Else-Marie Løberg, Mette Hegland Nordbotn, Cathrine Nygård, Maria Olsvold, Christian Ohldieck, Lillian Sivertsen, Hugo Torjussen, Jørn Henrik Vold, Jan-Magnus Økland, Tone Lise Eielsen, Nancy Laura Ortega Maldonado, Ewa Joanna Wilk, Ronny Bjørnestad, Ole Jørgen Lygren, Marianne Cook Pierron, Olav Dalgard, Håvard Midgard, Svetlana Skurtveit, and Peter Vickerman
Supplementary information
Supplementary information accompanies this paper at 10.1186/s13011-020-00309-y.
References
- 1.World Health Organization. Management of substance abuse: Dependence syndrome, ICD-10 Clinical description; 2020. https://www.who.int/substance_abuse/terminology/definition1/en/.
- 2.Chang HY, Kharrazi H, Bodycombe D, Weiner JP, Alexander GC. Healthcare costs and utilization associated with high-risk prescription opioid use: a retrospective cohort study. BMC Med. 2018;16(1):69. doi: 10.1186/s12916-018-1058-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Degenhardt L, Grebely J, Stone J, Hickman M, Vickerman P, Marshall BDL, Bruneau J, Altice FL, Henderson G, Rahimi-Movaghar A, et al. Global patterns of opioid use and dependence: harms to populations, interventions, and future action. Lancet (London, England) 2019;394(10208):1560–1579. doi: 10.1016/S0140-6736(19)32229-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gomes T, Tadrous M, Mamdani MM, Paterson JM, Juurlink DN. The burden of opioid-related mortality in the United States. JAMA Netw Open. 2018;1(2):e180217. doi: 10.1001/jamanetworkopen.2018.0217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.European Monitoring Centre for Drugs and Drug Addiction: European Drug Report. Trends and Developments. In.; 2019.
- 6.Strada L, Vanderplasschen W, Buchholz A, Schulte B, Muller AE, Verthein U, Reimer J. Measuring quality of life in opioid-dependent people: a systematic review of assessment instruments. Qual Life Res. 2017;26(12):3187–3200. doi: 10.1007/s11136-017-1674-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Millson P, Challacombe L, Villeneuve PJ, Strike CJ, Fischer B, Myers T, Shore R, Hopkins S. Determinants of health-related quality of life of opiate users at entry to low-threshold methadone programs. Eur Addict Res. 2006;12(2):74–82. doi: 10.1159/000090426. [DOI] [PubMed] [Google Scholar]
- 8.Griffin ML, Bennett HE, Fitzmaurice GM, Hill KP, Provost SE, Weiss RD. Health-related quality of life among prescription opioid-dependent patients: results from a multi-site study. Am J Addict. 2015;24(4):308–314. doi: 10.1111/ajad.12188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.European Monitoring Centre for Drugs and Drug Addiction: Komorbiditet — samförekomst av narkotikamissbruk och psykisk störning. Ett underskattat tillstånd. In.; 2004.
- 10.Aas CF, Vold JH, Skurtveit S, Odsbu I, Chalabianloo F, Lim AG, Johansson KA, Fadnes LT. Uptake and predictors of direct-acting antiviral treatment for hepatitis C among people receiving opioid agonist therapy in Sweden and Norway: a drug utilization study from 2014 to 2017. Subst Abuse Treat Prev Policy. 2020;15(1):44. doi: 10.1186/s13011-020-00286-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grant BF, Stinson FS, Dawson DA, Chou SP, Dufour MC, Compton W, Pickering RP, Kaplan K. Prevalence and co-occurrence of substance use disorders and independent mood and anxiety disorders: results from the National Epidemiologic Survey on alcohol and related conditions. Arch Gen Psychiatry. 2004;61(8):807–816. doi: 10.1001/archpsyc.61.8.807. [DOI] [PubMed] [Google Scholar]
- 12.Hall W. What have population surveys revealed about substance use disorders and their co-morbidity with other mental disorders? Drug Alcohol Rev. 1996;15(2):157–170. doi: 10.1080/09595239600185811. [DOI] [PubMed] [Google Scholar]
- 13.van Emmerik-van Oortmerssen K, van de Glind G, van den Brink W, Smit F, Crunelle CL, Swets M, Schoevers RA. Prevalence of attention-deficit hyperactivity disorder in substance use disorder patients: a meta-analysis and meta-regression analysis. Drug Alcohol Depend. 2012;122(1–2):11–19. doi: 10.1016/j.drugalcdep.2011.12.007. [DOI] [PubMed] [Google Scholar]
- 14.Burdzovic Andreas J, Lauritzen G, Nordfjaern T. Co-occurrence between mental distress and poly-drug use: a ten year prospective study of patients from substance abuse treatment. Addict Behav. 2015;48:71–78. doi: 10.1016/j.addbeh.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 15.Mørkved N, Winje D, Dovran A, Arefjord K, Johnsen E, Kroken RA, Anda-Ågotnes LG, Thimm JC, Sinkeviciute I, Rettenbacher M, et al. Childhood trauma in schizophrenia spectrum disorders as compared to substance abuse disorders. Psychiatry Res. 2018;261:481–487. doi: 10.1016/j.psychres.2018.01.011. [DOI] [PubMed] [Google Scholar]
- 16.Rosic T, Naji L, Bawor M, Dennis BB, Plater C, Marsh DC, Thabane L, Samaan Z. The impact of comorbid psychiatric disorders on methadone maintenance treatment in opioid use disorder: a prospective cohort study. Neuropsychiatr Dis Treat. 2017;13:1399–1408. doi: 10.2147/NDT.S129480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Connery HS. Medication-assisted treatment of opioid use disorder: review of the evidence and future directions. Harvard Rev Psychiatry. 2015;23(2):63–75. doi: 10.1097/HRP.0000000000000075. [DOI] [PubMed] [Google Scholar]
- 18.Degenhardt L, Bucello C, Mathers B, Briegleb C, Ali H, Hickman M, McLaren J. Mortality among regular or dependent users of heroin and other opioids: a systematic review and meta-analysis of cohort studies. Addiction. 2011;106(1):32–51. doi: 10.1111/j.1360-0443.2010.03140.x. [DOI] [PubMed] [Google Scholar]
- 19.Malta M, Varatharajan T, Russell C, Pang M, Bonato S, Fischer B. Opioid-related treatment, interventions, and outcomes among incarcerated persons: a systematic review. PLoS Med. 2019;16(12):e1003002. doi: 10.1371/journal.pmed.1003002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mathers BM, Degenhardt L, Bucello C, Lemon J, Wiessing L, Hickman M. Mortality among people who inject drugs: a systematic review and meta-analysis. Bull World Health Organ. 2013;91(2):102–123. doi: 10.2471/BLT.12.108282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ma J, Bao YP, Wang RJ, Su MF, Liu MX, Li JQ, Degenhardt L, Farrell M, Blow FC, Ilgen M, et al. Effects of medication-assisted treatment on mortality among opioids users: a systematic review and meta-analysis. Mol Psychiatry. 2019;24(12):1868–1883. doi: 10.1038/s41380-018-0094-5. [DOI] [PubMed] [Google Scholar]
- 22.Bray JW, Aden B, Eggman AA, Hellerstein L, Wittenberg E, Nosyk B, Stribling JC, Schackman BR. Quality of life as an outcome of opioid use disorder treatment: a systematic review. J Subst Abus Treat. 2017;76:88–93. doi: 10.1016/j.jsat.2017.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bullinger M. Assessing health related quality of life in medicine. An overview over concepts, methods and applications in international research. Restor Neurol Neurosci. 2002;20(3–4):93–101. [PubMed] [Google Scholar]
- 24.Muller AE, Skurtveit S, Clausen T. Confirming the factor structure of a generic quality of life instrument among pre-treatment substance use disorder patients. Health Qual Life Outcomes. 2019;17(1):84. doi: 10.1186/s12955-019-1152-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nosyk B, Bray JW, Wittenberg E, Aden B, Eggman AA, Weiss RD, Potter J, Ang A, Hser YI, Ling W, et al. Short term health-related quality of life improvement during opioid agonist treatment. Drug Alcohol Depend. 2015;157:121–128. doi: 10.1016/j.drugalcdep.2015.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lofwall MR, Brooner RK, Bigelow GE, Kindbom K, Strain EC. Characteristics of older opioid maintenance patients. J Subst Abus Treat. 2005;28(3):265–272. doi: 10.1016/j.jsat.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 27.Karow A, Verthein U, Pukrop R, Reimer J, Haasen C, Krausz M, Schafer I. Quality of life profiles and changes in the course of maintenance treatment among 1,015 patients with severe opioid dependence. Subst Use Misuse. 2011;46(6):705–715. doi: 10.3109/10826084.2010.509854. [DOI] [PubMed] [Google Scholar]
- 28.Millson PE, Challacombe L, Villeneuve PJ, Fischer B, Strike CJ, Myers T, Shore R, Hopkins S, Raftis S, Pearson M. Self-perceived health among Canadian opiate users: a comparison to the general population and to other chronic disease populations. Can J Public Health. 2004;95(2):99–103. doi: 10.1007/BF03405775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karow A, Reimer J, Schafer I, Krausz M, Haasen C, Verthein U. Quality of life under maintenance treatment with heroin versus methadone in patients with opioid dependence. Drug Alcohol Depend. 2010;112(3):209–215. doi: 10.1016/j.drugalcdep.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 30.Hayes CJ, Li X, Li C, Shah A, Kathe N, Bhandari NR, Payakachat N. Health-related quality of life among chronic opioid users, nonchronic opioid users, and nonopioid users with chronic noncancer pain. Health Serv Res. 2018;53(5):3329–3349. doi: 10.1111/1475-6773.12836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fadnes LT, Aas CF, Vold JH, Ohldieck C, Leiva RA, Chalabianloo F, Skurtveit S, Lygren OJ, Dalgard O, Vickerman P, et al. Integrated treatment of hepatitis C virus infection among people who inject drugs: study protocol for a randomised controlled trial (INTRO-HCV) BMC Infect Dis. 2019;19(1):943. doi: 10.1186/s12879-019-4598-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Waal H BK, Clausen T, Lillevold PH, and Skeie I.: SERAF Report: Status 2017. MAR 20 years. Status, evaluations and perspectives. In.: The Norwegian Centre for Addiction Research (SERAF); 2018.
- 33.World Health Organization (WHO). Classifications: ICD-10 online versions. World Health Organization (WHO); 2019. [https://www.who.int/classifications/icd/icdonlineversions/en/]. Accessed 13 Mar 2020.
- 34.Rabin R, de Charro F. EQ-5D: a measure of health status from the EuroQol group. Ann Med. 2001;33(5):337–343. doi: 10.3109/07853890109002087. [DOI] [PubMed] [Google Scholar]
- 35.Peak J, Goranitis I, Day E, Copello A, Freemantle N, Frew E. Predicting health-related quality of life (EQ-5D-5 L) and capability wellbeing (ICECAP-A) in the context of opiate dependence using routine clinical outcome measures: CORE-OM, LDQ and TOP. Health Qual Life Outcomes. 2018;16(1):106. doi: 10.1186/s12955-018-0926-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van der Zanden BP, Dijkgraaf MG, Blanken P, de Borgie CA, van Ree JM, van den Brink W. Validity of the EQ-5D as a generic health outcome instrument in a heroin-dependent population. Drug Alcohol Depend. 2006;82(2):111–118. doi: 10.1016/j.drugalcdep.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 37.EuroQoL Research Foundation. EQ-5D-5l User Guide. 2019. [https://euroqol.org/publications/user-guides/]. Accessed 27 Jan 2020.
- 38.Buchholz I, Janssen MF, Kohlmann T, Feng YS. A systematic review of studies comparing the measurement properties of the three-level and five-level versions of the EQ-5D. PharmacoEconomics. 2018;36(6):645–661. doi: 10.1007/s40273-018-0642-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Devlin NJ, Shah KK, Feng Y, Mulhern B, van Hout B. Valuing health-related quality of life: an EQ-5D-5L value set for England. Health Econ. 2018;27(1):7–22. doi: 10.1002/hec.3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stavem K, Augestad LA, Kristiansen IS, Rand K. General population norms for the EQ-5D-3 L in Norway: comparison of postal and web surveys. Health Qual Life Outcomes. 2018;16(1):204. doi: 10.1186/s12955-018-1029-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Strada L, Schmidt CS, Rosenkranz M, Verthein U, Scherbaum N, Reimer J, Schulte B. Factors associated with health-related quality of life in a large national sample of patients receiving opioid substitution treatment in Germany: A cross-sectional study. Subst Abuse Treat Prev Policy. 2019;14(1):2. doi: 10.1186/s13011-018-0187-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Waal HBK, Clausen T, Håseth A, Lillevold PH, Skeie I. SERAF Report: Status 2014, an aging MAR-population? Oslo: The Norwegian Centre for Addiction Research (SERAF); 2015.
- 43.Ministry of Health and Care Services. National guidline for medicaly assisted rehabilitation (MAR) for opioid dependence. Oslo: The Norwegian Ministry of Health and Care Services; 2010.
- 44.Braback M, Bradvik L, Troberg K, Isendahl P, Nilsson S, Hakansson A. Health related quality of life in individuals transferred from a needle exchange program and starting opioid agonist treatment. J Addict. 2018;2018:3025683. doi: 10.1155/2018/3025683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Habrat B, Chmielewska K, Baran-Furga H, Keszycka B, Taracha E. Subjective quality of life in opiate-dependent patients before admission after six months and one-year participation in methadone program. Przeglad lekarski. 2002;59(4–5):351–354. [PubMed] [Google Scholar]
- 46.Ponizovsky AM, Grinshpoon A. Quality of life among heroin users on buprenorphine versus methadone maintenance. Am J Drug Alcohol Abuse. 2007;33(5):631–642. doi: 10.1080/00952990701523698. [DOI] [PubMed] [Google Scholar]
- 47.Solli O, Stavem K, Kristiansen IS. Health-related quality of life in diabetes: the associations of complications with EQ-5D scores. Health Qual Life Outcomes. 2010;8:18. doi: 10.1186/1477-7525-8-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stavem K, Froland SS, Hellum KB. Comparison of preference-based utilities of the 15D, EQ-5D and SF-6D in patients with HIV/AIDS. Qual Life Res. 2005;14(4):971–980. doi: 10.1007/s11136-004-3211-7. [DOI] [PubMed] [Google Scholar]
- 49.Global Burden of Disease Collaborative Network . Global Burden of Disease Study 2017 (GBD 2017) Disability Weights. Seattle, United States: Institute for Health Metrics and Evaluation (IHME), 2018; 2018. [Google Scholar]
- 50.World Health Organization. Health statistics and information systems: Disability weights, discounting and age weighting of DALYs. 2020. [https://www.who.int/healthinfo/global_burden_disease/daly_disability_weight/en/]. Accessed 14 Mar 2020.
- 51.Soyka M, Strehle J, Rehm J, Buhringer G, Wittchen HU. Six-year outcome of opioid maintenance treatment in heroin-dependent patients: results from a naturalistic study in a nationally representative sample. Eur Addict Res. 2017;23(2):97–105. doi: 10.1159/000468518. [DOI] [PubMed] [Google Scholar]
- 52.Kelly TM, Daley DC. Integrated treatment of substance use and psychiatric disorders. Soc Work Public Health. 2013;28(3–4):388–406. doi: 10.1080/19371918.2013.774673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Janssen MF, Bonsel GJ, Luo N. Is EQ-5D-5L better than EQ-5D-3L? A head-to-head comparison of descriptive systems and value sets from seven countries. PharmacoEconomics. 2018;36(6):675–697. doi: 10.1007/s40273-018-0623-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Janssen MF, Birnie E, Haagsma JA, Bonsel GJ. Comparing the standard EQ-5D three-level system with a five-level version. Value Health. 2008;11(2):275–284. doi: 10.1111/j.1524-4733.2007.00230.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. OAT = opioid agonist therapy. Flow chart of study sample.
Additional file 2. EQ-5D-5L, descriptive health profile at baseline (frequencies and proportions reported by dimensions and level). OAT = opioid agonist therapy, CI = confidence interval. * = total number of respondents. 8 patients who missed data on one dimension were included in the analysis. ** = long acting morphine sulphate and other opioid prescriptions.
Additional file 3. Changes in EQ-5D-5L index value per patient from baseline to follow-up. 609 patients included at baseline, 245 patients at follow-up one year later.
Data Availability Statement
The INTRO-HCV study is ongoing and as such the dataset is not publically available. However, parts of the dataset used for EQ-5D-5L used for this publication may be available in an anonymous and shortened version upon contacting the corresponding author: Christer F. Aas: christer.frode.aas@helse-bergen.no