Abstract
Background:
Antenatal corticosteroids reduce morbidity and mortality among preterm neonates. However, the optimal timing of steroid administration with regards to severe neonatal and early childhood morbidity is uncertain.
Objective:
To evaluate the association between the timing of antenatal corticosteroid adminstration and preterm outcomes. We hypothesized that neonates exposed to antenatal corticosteroids 2 to <7 days before delivery would have the lowest risks of neonatal and childhood morbidity.
Study Design:
Secondary analysis of two prospective multicenter studies enriched for spontaneous preterm birth, Genomics and Proteomics Network for Preterm Birth Research (11/2007–1/2011) and Beneficial Effect of Antenatal Magnesium (12/1997–5/2004). We included women with singleton gestations who received antenatal corticosteroids and delivered at 23 0/7–33 6/7 weeks’ gestation. Women who received ≥1 course of corticosteroids were excluded. Neonatal outcomes were compared by the timing of the first dose of antenatal corticosteroids in relation to delivery: <2 days, 2 to <7 days, 7 to <14 days, and ≥14 days. The primary outcome was respiratory distress syndrome. Secondary outcomes included composite neonatal morbidity (death, intraventricular hemorrhage grade III or IV, periventricular leukomalacia, bronchopulmonary dysplasia, or necrotizing enterocolitis), and early childhood morbidity (death or moderate to severe cerebral palsy at age 2). Multivariable logistic regression estimated the association between timing of antenatal corticosteroid administration and study outcomes.
Results:
A total of 2,259 subjects met inclusion criteria: 622 (27.5%) received antenatal corticosteroids <2 days before delivery, 821 (36.3%) 2 to <7 days, 401 (17.8%) 7 to <14 days, and 415 (18.4%) ≥14 days. The majority (78.1%) delivered following idiopathic spontaneous preterm labor or preterm premature rupture of membranes at a mean gestational age of 29.5 +/−2.8 weeks. Neonates exposed to antenatal corticosteroids 2 to <7 days before delivery were the least likely to develop respiratory distress syndrome (51.3%), compared to those receiving antenatal corticosteroids <2 days, 7 to <14 days, and ≥14 days before delivery (62.7%, 55.9%, and 57.6%, respectively, p<0.001). Compared to receipt 2 to <7 days before delivery, there was an increased odds of respiratory distress syndrome with receipt of antenatal corticosteroids <2 days (aOR 2.07, 95%CI 1.61–2.66), 7 to <14 days (aOR 1.40, 95% CI 1.07–1.83), and ≥14 days (aOR 2.34, 95%CI 1.78–3.07). Neonates exposed to antenatal corticosteroids ≥14 days before delivery were at increased odds for severe neonatal morbidity (aOR 1.57, 95%CI 1.12–2.19) and early childhood morbidity (aOR 1.74, 95%CI 1.02–2.95), compared to those exposed 2 to <7 days before delivery. There was no significant association between antenatal corticosteroid receipt <2 days or 7 to <14 days and severe neonatal morbidity or severe childhood morbidity.
Conclusions:
Preterm neonates exposed to antenatal corticosteroids 2 to <7 days before delivery had the lowest odds of respiratory distress syndrome, compared to shorter and longer time intervals between steroid administration and delivery. Antenatal corticosteroid administration ≥14 days before delivery is associated with an increased odds of severe neonatal and childhood morbidity, compared to 2 to <7 days before delivery. These results emphasize the importance of optimally timed antenatal corticosteroids to improve both short- and long-term outcomes.
Keywords: antenatal corticosteroids, childhood morbidity, neonatal morbidity, preterm birth, respiratory distress syndrome
INTRODUCTION
Preterm birth is the leading cause of morbidity and mortality among non-anomalous neonates in the United States.1 Compared with term neonates, neonates born prior to 37 weeks’ gestation are at increased risk for complications such as respiratory distress syndrome (RDS), bronchopulmonary dysplasia, intraventricular hemorrhage, necrotizing enterocolitis and death.2,3
Administration of antenatal corticosteroids prior to preterm delivery is one of the most effective interventions in improving neonatal outcomes.3 Glucocorticoid exposure promotes maturation of various fetal organ systems, including respiratory, gastrointestinal, and central nervous systems. Antenatal corticosteroid administration reduces rates of numerous complications of prematurity, including RDS (RR 0.66, 95% CI 0.59–0.73), intraventricular hemorrhage (RR 0.54, 95% CI 0.43–0.69), necrotizing enterocolitis (RR 0.46, 95% CI 0.29–0.74), and neonatal death (RR 0.69, CI 0.58–0.81).3 For this reason, antenatal corticosteroids are recommended for all women at risk of preterm birth.4
Though previous studies have demonstrated improved respiratory outcomes when antenatal corticosteroids are administered within 7 days of delivery, the evidence for optimal timing of corticosteroid exposure with regards to other severe neonatal morbidities is conflicting.5–7 Additionally, data regarding the effect of antenatal corticosteroid timing on early childhood outcomes is scarce. We hypothesized that neonatal respiratory distress syndrome and severe neonatal morbidity as well as early childhood morbidity vary based on the timing of antenatal corticosteroid exposure in relation to delivery, and that those neonates who are exposed to steroids 2 to <7 days prior to delivery would have the lowest rates of morbidity.
MATERIALS AND METHODS
This was a secondary analysis of two prospective, multi-center studies: the NICHD Genomics and Proteomics Network for Preterm Birth Research (GPN-PBR observational cohort, enrolled 11/2007–1/2011)8 and the NICHD Maternal Fetal Medicine Units Network Beneficial Effects of Antenatal Magnesium Sulfate (BEAM randomized controlled trial, enrolled 12/1997–5/2004) in order to optimize sample size for the primary outcome.9 Results from both studies have been previously reported. Briefly, for the GPN-PBR study, women with a history of a prior documented singleton spontaneous preterm birth between 20.0–36.6 weeks’ gestation were recruited across eight clinical sites from November 2007 through January 2011 and followed prospectively (longitudinal arm). Women delivering preterm <34.0 weeks’ gestation due to spontaneous preterm labor or preterm premature rupture of membranes were also included in the GPN-PBR study (case-control arm).8 For the BEAM study, women at imminent risk for preterm delivery <32 weeks’ gestation were randomized to receive intravenous magnesium sulfate or placebo. Briefly, the main trial found that fetal exposure to MgSO4 did not reduce the pre-specified primary outcome of the combined risk of moderate or severe cerebral palsy or death, but did reduce the rate of moderate-severe cerebral palsy among surviving children (1.9% vs. 3.5%; relative risk, 0.55; 95% CI, 0.32 – 0.95).9
For both studies, the gestational age was determined by a combination of last menstrual period (if available) and ultrasound, using ACOG dating criteria.10 Further, in both studies, research nurses conducted in-person interviews with participants and abstracted additional clinical and demographic data from medical records. Data collected included demographics, medical, social, family, and obstetric histories, obstetric course and complications during the current pregnancy (including intrapartum course, mode of delivery, and neonatal outcomes). With the exception of the study drug provided by the BEAM study, all obstetric management, including decisions regarding whether to administer antenatal corticosteroids and the timing of such administration, was at the discretion of each woman’s primary obstetric provider.
Women carrying non-anomalous, singleton gestations who delivered between 23 0/7 and 33 6/7 weeks’ gestation were included. Women were excluded if they received more than 1 course of antenatal corticosteroids, carried a fetus with major congenital anomalies or aneuploidy, or had unknown timing of antenatal corticosteroid exposure. We chose to evaluate a primary neonatal outcome and a primary childhood outcome. The primary neonatal outcome was respiratory distress syndrome, defined as a clinical diagnosis of respiratory distress (including hyaline membrane disease or respiratory insufficiency of the premature neonate, but not transient tachypnea of the newborn) and oxygen therapy (FiO2 ≥ 0.40) for greater than or equal to 24 hours, or a clinical diagnosis of respiratory distress with death before 24 hours of age. The secondary neonatal outcome was a composite severe neonatal morbidity including death, intraventricular hemorrhage grade III or IV, periventricular leukomalacia, bronchopulmonary dysplasia, or necrotizing enterocolitis prior to hospital discharge. The primary childhood outcome was a composite of death or moderate to severe cerebral palsy at age 2 among those enrolled in the BEAM study.
Study outcomes were compared by timing of antenatal corticosteroids exposure prior to delivery, defined as the interval between the first dose of antenatal corticosteroids and delivery: <2 days (Group 1), 2 to <7 days (Group 2), 7 to <14 days (Group 3), and ≥14 days (Group 4). Demographic and antenatal characteristics were compared using chi-square, Kruskal Wallis and ANOVA as appropriate. Pearson’s correlation was used to evaluate for correlation among characteristics found to be significant in bivariable analysis. Multivariable logistic regression was performed to estimate the association between timing of antenatal corticosteroid administration and the primary outcomes. Antenatal corticosteroid administration 2 to <7 days before delivery served as the referent group. Initial models included factors that were significant at p<0.05 in bivariate analyses but not significantly correlated with one another. Factors with p<0.20 were retained in final regression models. Statistical significance was defined as p<0.05 unless otherwise specified, and all tests were two-tailed. No imputation for missing data was performed. All statistical analyses were performed using Stata.
Institutional Review Board approval and written, informed consent was obtained for both original studies at all participating institutions. This analysis was conducted using de-identified databases and was approved by the IRB at the University of North Carolina-Chapel Hill (#15–2099).
RESULTS
Of 2,631 women enrolled in GPN-PBR and 2,244 women enrolled in the BEAM study, 2,259 women met inclusion criteria for this analysis (Figure 1). Of these, 681 (30.1%) were participants in the GPN-PBR study and 1,578 (69.9%) were enrolled in the BEAM study. Six-hundred and twenty-two (27.5%) were exposed <2 days, 821 (36.3%) for 2 to <7 days, 401 (17.8%) for 7 to <14 days, and 415 (18.4%) for ≥14 days prior to delivery. The majority of women (n=1,764, 78.1%) delivered following preterm premature rupture of membranes (defined as membrane ruptured for at least 72 hours prior to delivery, or preterm prelabor rupture of membranes was the primary reason for antepartum admission). The mean gestational age at delivery was 29.5 +/− 2.8 weeks (Table 1).
Figure 1.
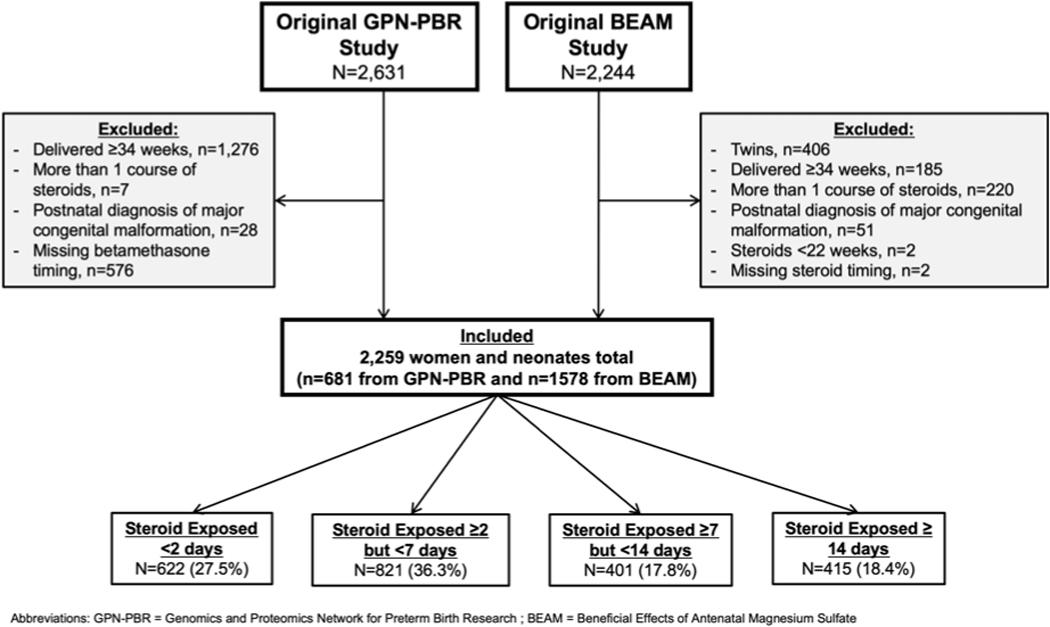
Flow diagram of study cohort
Table 1.
Demographic and antenatal characteristics by timing of antenatal corticosteroid administration
| <2 days (n=622) | 2 to <7 days (n=821) | 7 to <14 days (n=401) | ≥ 14 days (n=415) | p-value | |
|---|---|---|---|---|---|
| Enrolled in Genomics and Proteomics Network for | 352 (56.6) | 174 (21.2) | 73 (18.2) | 82 (19.8) | <0.001 |
| Preterm Birth Research study | |||||
| Maternal age (years) | 25.9 ± 6.1 | 25.0 ± 6.2 | 26.4 ± 5.9 | 26.9 ± 6.2 | <0.001 |
| Nulliparous | 298 (47.9) | 317 (38.6) | 147 (36.7) | 135 (32.5) | <0.001 |
| Black race | 179 (28.8) | 351 (42.8) | 171 (42.6) | 131 (31.6) | <0.001 |
| Less than a high school level education | 408 (65.8) | 522 (64.0) | 281 (70.1) | 300 (72.3) | 0.01 |
| Married | 312 (50.2) | 372 (45.5) | 194 (48.5) | 229 (55.2) | 0.01 |
| Gestational or pre-gestational diabetes mellitus | 18 (2.9) | 36 (4.4) | 9 (2.2) | 22 (5.3) | 0.06 |
| Preterm prelabor rupture of membranes | 356 (57.2) | 670 (81.6) | 363 (90.5) | 375 (90.4) | <0.001 |
| Duration of ruptured membranes (hours) | 3 (0.1, 19) | 79 (33, 122) | 216 (169, 271) | 435 (329, 636) | <0.001 |
| Chorioamnionitis suspected prior to delivery | 56 (9.0) | 140 (17.1) | 88 (22.0) | 67 (16.1) | <0.001 |
| Tobacco use during pregnancy | 135 (21.7) | 232 (28.3) | 100 (24.9) | 110 (26.5) | 0.04 |
| Gestational age at delivery (weeks) | 29.7 ± 2.9 | 29.0 ± 2.7 | 29.2 ± 2.7 | 30.9 ± 2.3 | <0.001 |
| Cesarean delivery | 167 (26.9) | 271 (33.0) | 142 (35.4) | 162 (39.0) | <0.001 |
Data presented as n (%) or mean +/− standard deviation or median (25% IQR, 75% IQR)
Overall, 1,274 neonates were diagnosed with respiratory distress syndrome (56.4%) and 616 (27.3%) with major neonatal morbidity (Table 2). Neonates who were exposed to antenatal corticosteroids at 2 to <7 days before delivery were the least likely to develop respiratory distress syndrome (51.3%), compared to 62.7% of those exposed <2 days before delivery, 55.9% of those exposed 7 to <14 days before delivery and 57.6% of those exposed ≥14 days before delivery (p<0.001). There was also a statistically significant difference in severe neonatal morbidity between groups (Table 2). Of the 1,578 neonates in this cohort whose mothers were originally enrolled in the BEAM study, 1,511 (95.7%) neonates had outcome data available at age 2, and overall 153 (10.1%) had the adverse childhood outcome (moderate or severe cerebral palsy or death). The frequency of childhood morbidity was similar among those exposed to antenatal corticosteroids <2 days, 2 to <7 days, 7 to <14 days, and ≥14 days before delivery (p=0.57).
Table 2.
Neonatal outcomes by timing of antenatal corticosteroid administration
| <2 days (n=622) | 2 to <7 days (n=821) | 7 to <14 days (n=401) | ≥ 14 days (n=415) | p-value | |
|---|---|---|---|---|---|
| Male neonate | 331 (53.2) | 434 (52.9) | 214 (53.4) | 248 (59.8) | 0.11 |
| Birthweight (mean grams, ± SD) | 1486 ± 550 | 1296 ± 475 | 1310 ± 458 | 1585 ± 469 | <0.001 |
| Respiratory distress syndrome | 390 (62.7) | 421 (51.3) | 224 (55.9) | 239 (57.6) | <0.001 |
| Neonatal severe morbidity* | 146 (23.5) | 253 (30.8) | 124 (30.9) | 93 (22.4) | 0.001 |
| Neonatal death | 41 (6.6) | 59 (7.2) | 31 (7.7) | 21 (5.1) | 0.43 |
| Intraventricular hemorrhage grade III / IV | 42 (6.8) | 20 (2.4) | 11 (2.7) | 11 (2.7) | <0.001 |
| Periventricular leukomalacia | 14 (2.2) | 17 (2.1) | 8 (2.0) | 9 (2.2) | 0.99 |
| Bronchopulmonary dysplasia | 67 (10.8) | 146 (17.9) | 67 (16.8) | 41 (10.0) | <0.001 |
| Necrotizing enterocolitis | 31 (5.0) | 80 (9.8) | 45 (11.3) | 33 (8.0) | 0.001 |
| Childhood morbidity† | 65/610 (10.7) | 29/262 (11.1) | 33/316 (10.4) | 26/323 (8.1) | 0.570 |
Data presented as n (%) or mean +/− standard deviation
Composite included death, severe intraventricular hemorrhage grade III or IV, periventricular leukomalacia, bronchopulmonary dysplasia, or necrotizing enterocolitis
Composite included death or moderate to severe cerebral palsy at age 2 among the 1511 women included in this analysis who were originally enrolled in the BEAM study and had longer-term follow-up data available
In initial multivariable logistic regression models, nulliparity, magnesium, spontaneous preterm birth, gestational age at delivery, male neonate, maternal age, black race, chorioamnionitis, and cesarean delivery were included as possible confounding factors. Marital status, maternal education level, and study enrollment were not included as they were correlated with other variables considered above (r ≥ 0.25 and p<0.001 for all). In the final regression model evaluating factors associated with respiratory distress syndrome, we found an increased odds of respiratory distress syndrome in those with steroid receipt <2 days before delivery (aOR 2.07, 95% CI 1.61–2.66), 7 to <14 days before delivery (aOR 1.40, 95% CI 1.07–1.83), and ≥14 days before delivery (aOR 2.34, 95% CI 1.78–3.07), compared to receipt 2 to <7 days before delivery (Table 3). Furthermore, in the final regression models evaluating factors associated with severe neonatal morbidity, we found an increased odds of severe neonatal morbidity when antenatal corticosteroids were received ≥14 days before delivery (aOR 1.57, 95% CI 1.12–2.19), compared to 2 to <7 days before delivery (Table 4). Similarly, antenatal corticosteroid receipt ≥14 days before delivery was associated with an increased odds of severe childhood morbidity (aOR 1.74, 95% CI 1.02–2.95), compared to 2 to <7 days before delivery (Table 5). There was no significant association between antenatal corticosteroid administration < 2 days before delivery or 7 to <14 days before delivery and severe neonatal or childhood morbidity, compared to antenatal corticosteroid administration 2 to <7 days before delivery. In all models, male gender and delivery gestational age were important factors associated with respiratory distress syndrome and the composite severe neonatal and childhood morbidities (Tables 3, 4, and 5).
Table 3.
Multivariable regression of respiratory distress syndrome by timing of antenatal corticosteroid administration
| Respiratory distress syndrome adjusted odds ratio (95% confidence interval) | p-value | |
|---|---|---|
| Timing of antenatal corticosteroid administration | ||
| < 2 days before delivery | 2.07 (1.61–2.66) | <0.001 |
| 2 to <7 days before delivery | 1.0 (referent) | - |
| 7 to <14 days before delivery | 1.40 (1.07–1.83) | 0.015 |
| ≥ 14 days before delivery | 2.34 (1.78–3.07) | <0.001 |
| Gestational age at delivery, per completed week | 0.68 (0.65–0.71) | <0.001 |
| Black race | 0.59 (0.48–0.72) | <0.001 |
| Male | 1.35 (1.12–1.63) | <0.01 |
| Preterm prelabor rupture of membranes | 0.71 (0.56–0.91) | <0.01 |
| Delivered by cesarean section | 1.24 (1.02–1.52) | 0.04 |
| Received magnesium sulfate | 0.85 (0.71–1.03) | 0.09 |
Other factors considered in initial models but removed due to p>0.20 include nulliparity and maternal smoking during pregnancy.
Table 4.
Multivariable regression of severe neonatal morbidity by timing of antenatal corticosteroid administration
| Severe neonatal morbidity* adjusted odds ratio (95% confidence interval) | p-value | |
|---|---|---|
| Timing of antenatal corticosteroid administration | ||
| < 2 days before delivery | 0.80 (0.59–1.07) | 0.135 |
| 2 to <7 days before delivery | 1.0 (referent) | - |
| 7 to <14 days before delivery | 1.15 (0.83–1.58) | 0.404 |
| ≥ 14 days before delivery | 1.57 (1.12–2.19) | 0.009 |
| Gestational age at delivery, per completed week | 0.55 (0.53–0.58) | <0.001 |
| Male gender | 1.31 (1.04–1.65) | 0.022 |
| Nulliparous mother | 1.18 (0.93–1.49) | 0.185 |
| Delivery by cesarean section | 1.23 (0.97–1.56) | 0.092 |
Other factors considered in initial models but removed due to p>0.20 include preterm prelabor rupture of membranes, receipt of magnesium sulfate prior to delivery (for any indication), maternal smoking during pregnancy, black race, and suspected clinical chorioamnionitis.
Composite included death, severe intraventricular hemorrhage grade III or IV, periventricular leukomalacia, bronchopulmonary dysplasia, or necrotizing enterocolitis
Table 5.
Multivariable regression of severe childhood morbidity by timing of antenatal corticosteroid administration (note: limited to participants in BEAM study)
| Severe childhood morbidity* adjusted odds ratio (95% confidence interval) | p-value | |
|---|---|---|
| Timing of antenatal corticosteroid administration | ||
| < 2 days before delivery | 1.14 (0.70–1.89) | 0.595 |
| 2 to <7 days before delivery | 1.0 (referent) | - |
| 7 to <14 days before delivery | 1.10 (0.69–1.77) | 0.690 |
| ≥ 14 days before delivery | 1.74 (1.02–2.95) | 0.042 |
| Gestational age at delivery, per completed week | 0.62 (0.57–0.67) | <0.001 |
| Male gender | 1.74 (1.21–2.52) | 0.003 |
Other factors considered in initial models but removed due to p>0.20 include preterm prelabor rupture of membranes, receipt of magnesium sulfate prior to delivery (for any indication), maternal smoking during pregnancy, nulliparity, black race, and suspected clinical chorioamnionitis.
Severe childhood morbidity included death or moderate to severe cerebral palsy at age 2
COMMENT
Principal findings
In this retrospective cohort study of over 2,000 preterm neonates, we found that neonates who were exposed to antenatal corticosteroids 2 to <7 days prior to delivery had the lowest odds of respiratory distress syndrome compared to both shorter and longer intervals from steroid receipt to delivery. In addition, we found an increased odds of severe neonatal morbidity and severe childhood morbidity among neonates who were exposed to antenatal corticosteroids ≥14 days before delivery, compared to 2 to <7 days before delivery. These results suggest that the ideal timing of antenatal corticosteroids to optimize neonatal short- and long-term outcomes among babies born <34 weeks is 2 to <7 days prior to delivery.
Results of the study in the context of other observations
Our findings confirm and extend those demonstrated in previous retrospective cohort studies.11–13 Compared to infants born at 26 to 34 weeks’ gestation to women who had received antenatal corticosteroids more than 7 days prior to delivery, Peaceman et al demonstrated a lower incidence of need for respiratory support among infants born to women who had received antenatal corticosteroids within 7 days of delivery.11 There was no significant difference, however, in the association between timing of antenatal corticosteroids and necrotizing enterocolitis, intraventricular hemorrhage or mortality.11 One explanation for their negative finding was insufficient power, as a previous meta-analysis had demonstrated increased risk of perinatal mortality when corticosteroids were administered more than 7 days before delivery.7 In a retrospective cohort study of 707 infants born at 22 to 26 weeks’ gestation, the risk of mortality was doubled when the interval from antenatal corticosteroids exposure to delivery was either too short (<24 hours) or too long (>7 days), compared to optimal timing (24 hours – 7 days).14 In contrast to our findings, however, these authors found no association between severe neonatal morbidity and timing of antenatal corticosteroids. It is likely that our study found differences in neonatal morbidity based on duration of antenatal corticosteroid exposure as we evaluated neonates born up to 34 weeks’ gestation, and also included mortality in our composite, as a competing outcome.
In vitro and in vivo animal studies provide biologic plausibility as to why the effect of antenatal corticosteroids appears to be transient. In an in vitro study of human lung cells, corticosteroids were found to increase transcription of surfactant genes with maximum stimulation at 48 hours and return to near control levels by day 8.15 Similarly, an in vivo study of lung function in preterm lambs demonstrated that although the hormonal effects of corticosteroid treatment were transient, some functional responses persisted over the 2 to 7 day interval to delivery.16,17 While many of these studies have been limited by the stability of the cultured fetal lung beyond 7 days, our results and the previously published clinical studies support the transient nature of biochemical changes after corticosteroid administration.
Strengths and weaknesses
Our study has several strengths. These data are from previous prospective studies of preterm birth with rigorous data collection by trained research staff. Our study offers a larger sample size compared to previous retrospective cohort studies, and thus permitted detection of differences in rare adverse neonatal outcomes and also extension to the early childhood period for individuals enrolled in the BEAM study. The findings of our study, however, must be interpreted within the context of the study design. Although we adjusted for many potential confounding factors in multivariable logistic regression including gestational age, race and ethnicity, neonatal sex, preterm premature rupture of membranes and others, we were unable to adjust for parent study enrollment given high degree of correlation with preterm premature rupture of membranes. The combination of data from two different studies, each with distinct aims and protocols and each conducted over different years may have also unintentionally affected our results. We are not able to estimate the relative benefit or risk compared to women who did not receive corticosteroids in this retrospective cohort study, and given that antenatal corticosteroid administration is currently considered to be standard of care for women with anticipated preterm birth it is unlikely that this question could be answered in a randomized clinical trial. Additionally, as we only included women who delivered less than 34 weeks and the majority of women had preterm prelabor rupture of membranes, our results may not be generalizable to all populations.
Conclusions and clinical implications
Nonetheless, our results demonstrate that the optimal timing of antenatal corticosteroid administration is 2 to <7 days before preterm delivery between 23 and 34 weeks’ gestation. Unfortunately, however, as clinicians we acknowledge that it remains challenging to accurately predict the timing of preterm delivery even among those at highest risk.18 Future studies are still needed to better understand when preterm delivery is imminent in order to reduce unnecessary exposure to antenatal corticosteroids while optimizing neonatal outcomes.
IMPLICATIONS AND CONTRIBUTIONS:
A. Why was this study conducted?
Although previous studies have demonstrated improved respiratory outcomes when antenatal corticosteroids are administered within 7 days of delivery, the optimal timing of corticosteroids with regards to other severe neonatal morbidities and early childhood morbidity is unknown. This study was conducted to evaluate the association between timing of antenatal corticosteroids and neonatal and early childhood morbidity.
B. What are the key findings?
Administration of antenatal corticosteroids 2 to <7 days before delivery was associated with the lowest odds of respiratory distress syndrome, compared to shorter and longer intervals. Antenatal corticosteroid administration ≥14 days before delivery was associated with an increased odds of severe neonatal and childhood morbidity.
C. What does this study add to what is already known?
Optimally timed antenatal corticosteroids 2 to 7 days before preterm birth is important for both neonatal and early childhood outcomes.
ACKNOWLEDGEMENT OF FINANCIAL SUPPORT:
This study was funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development Genomic and Proteomic Network for Preterm Birth Research (U01-HD-050062; U01-HD-050078; U01-HD-050080; U01-HD-050088; U01-HD-050094), all authors. This study was also funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development 5K23HD067224 (Dr. Manuck) and R01-MD011609 (Dr. Manuck).
Footnotes
DISCLOSURE STATEMENT: Dr. Sean Esplin holds stock in Sera Prognostics, a private company that was established to create a commercial test to predict preterm birth and other obstetric complications. Dr. Bukowski is an advisor and holds stock in Savran technologies Inc. is a company which developed technology to isolate ultra-rare cells from blood for non-invasive diagnostics. The remaining authors report no conflict of interest.
PRESENTATION: Presented in part at the Society for Maternal Fetal Medicine’s 36th Annual Meeting (Atlanta, GA), February 4, 2016, as a poster presentation, final abstract ID #628.
CONDENSATION: Antenatal corticosteroid administration 2 to <7 days before preterm delivery is associated with the lowest risks for neonatal and early childhood morbidity.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Martin JA, Hamilton BE, Osterman MJK, Driscoll AK, Mathews TJ. Births: Final Data for 2015. Natl Vital Stat Rep. 2017;66(1):1 http://www.ncbi.nlm.nih.gov/pubmed/28135188. Accessed November 13, 2018. [PubMed] [Google Scholar]
- 2.Crowley PA. Antenatal corticosteroid therapy: A meta-analysis of the randomized trials, 1972 to 1994. Am J Obstet Gynecol. 1995;173(1):322–335. doi: 10.1016/0002-9378(95)90222-8 [DOI] [PubMed] [Google Scholar]
- 3.Roberts D, Brown J, Medley N, Dalziel SR. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev. 2017;3:CD004454. doi: 10.1002/14651858.CD004454.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Committee Opinion No. 713: Antenatal Corticosteroid Therapy for Fetal Maturation. Obstet Gynecol. 2017;130(2):e102–e109. doi: 10.1097/AOG.0000000000002237 [DOI] [PubMed] [Google Scholar]
- 5.Sehdev HM, Abbasi S, Robertson P, et al. The effects of the time interval from antenatal corticosteroid exposure to delivery on neonatal outcome of very low birth weight infants. Am J Obstet Gynecol. 2004;191(4):1409–1413. doi: 10.1016/j.ajog.2004.06.055 [DOI] [PubMed] [Google Scholar]
- 6.Vermillion S, Soper D, Newman R. Is betamethasone effective longer than 7 days after treatment? Obs Gynecol. 2001;94(4):491–493. [DOI] [PubMed] [Google Scholar]
- 7.McLaughlin KJ, Crowther CA, Walker N, Harding JE. Effects of a single course of corticosteroids given more than 7 days before birth: a systematic review. Aust N Z J Obstet Gynaecol. 2003;43(2):101–106. http://www.ncbi.nlm.nih.gov/pubmed/14712961. Accessed June 12, 2019. [DOI] [PubMed] [Google Scholar]
- 8.Esplin MS, Merrell K, Goldenberg R, et al. Proteomic identification of serum peptides predicting subsequent spontaneous preterm birth. Am J Obstet Gynecol. 2011;204(5):391.e1–391.e8. doi: 10.1016/j.ajog.2010.09.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rouse DJ, Hirtz DG, Thom E, et al. A Randomized, Controlled Trial of Magnesium Sulfate for the Prevention of Cerebral Palsy. N Engl J Med. 2008;359(9):895–905. doi: 10.1056/NEJMoa0801187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Committee Opinion No 700: Methods for Estimating the Due Date. Obstet Gynecol. 2017;129(5):e150–e154. doi: 10.1097/AOG.0000000000002046 [DOI] [PubMed] [Google Scholar]
- 11.Peaceman AM, Bajaj K, Kumar P, Grobman WA. The interval between a single course of antenatal steroids and delivery and its association with neonatal outcomes. Am J Obstet Gynecol. 2005;193(3):1165–1169. doi: 10.1016/j.ajog.2005.06.050 [DOI] [PubMed] [Google Scholar]
- 12.Ring AM, Garland JS, Stafeil BR, Carr MH, Peckman GS, Pircon RA. The effect of a prolonged time interval between antenatal corticosteroid administration and delivery on outcomes in preterm neonates: a cohort study. Am J Obstet Gynecol. 2007;196(5):457.e1–6. doi: 10.1016/j.ajog.2006.12.018 [DOI] [PubMed] [Google Scholar]
- 13.Wilms FF, Vis JY, Pattinaja DAPM, et al. Relationship between the time interval from antenatal corticosteroid administration until preterm birth and the occurrence of respiratory morbidity. Am J Obstet Gynecol. 2011;205(1):49.e1–49.e7. doi: 10.1016/j.ajog.2011.03.035 [DOI] [PubMed] [Google Scholar]
- 14.Norberg H, Kowalski J, Maršál K, Norman M. Timing of antenatal corticosteroid administration and survival in extremely preterm infants: a national population-based cohort study. BJOG An Int J Obstet Gynaecol. 2017;124(10):1567–1574. doi: 10.1111/1471-0528.14545 [DOI] [PubMed] [Google Scholar]
- 15.Vidaeff AC, Ramin SM, Gilstrap LC, Alcorn JL. In vitro quantification of dexamethasone-induced surfactant protein B expression in human lung cells. J Matern Fetal Neonatal Med. 2004;15(3):155–159. doi: 10.1080/14767050410001668248 [DOI] [PubMed] [Google Scholar]
- 16.Ikegami M, Polk DH, Jobe AH, et al. Effect of interval from fetal corticosteriod treatment to delivery on postnatal lung function of preterm lambs. J Appl Physiol. 1996;80(2):591–597. doi: 10.1152/jappl.1996.80.2.591 [DOI] [PubMed] [Google Scholar]
- 17.Ikegami M, Polk D, Jobe A. Minimum interval from fetal betamethasone treatment to postnatal lung responses in preterm lambs. Am J Obstet Gynecol. 1996;174(5):1408–1413. http://www.ncbi.nlm.nih.gov/pubmed/9065104. Accessed June 12, 2019. [DOI] [PubMed] [Google Scholar]
- 18.Adams TM, Kinzler WL, Chavez MR, Vintzileos AM. The timing of administration of antenatal corticosteroids in women with indicated preterm birth. Am J Obstet Gynecol. 2015;212(5):645.e1–645.e4. doi: 10.1016/j.ajog.2014.11.021 [DOI] [PubMed] [Google Scholar]


