Abstract
Photoreceptor loss is the final common endpoint in most retinopathies that lead to irreversible blindness, and there are no effective treatments to restore vision1,2. Chemical reprogramming of fibroblasts offers an opportunity to reverse vision loss; however, the generation of sensory neuronal subtypes such as photoreceptors remains a challenge. Here we report that the administration of a set of five small molecules can chemically induce the transformation of fibroblasts into rod photoreceptor-like cells. The transplantation of these chemically induced photoreceptor-like cells (CiPCs) into the subretinal space of rod degeneration mice (homozygous for rd1, also known as Pde6b) leads to partial restoration of the pupil reflex and visual function. We show that mitonuclear communication is a key determining factor for the reprogramming of fibroblasts into CiPCs. Specifically, treatment with these five compounds leads to the translocation of AXIN2 to the mitochondria, which results in the production of reactive oxygen species, the activation of NF-κB and the upregulation of Ascl1. We anticipate that CiPCs could have therapeutic potential for restoring vision.
Introduction:
Many retinopathies—such as age-related macular degeneration, diabetic retinopathy and retinitis pigmentosa—ultimately result in the loss of retinal neurons, which leads to irreversible vision loss1,2. Stem-cell therapy, using embryonic stem cells or induced pluripotent stem cells, is a promising strategy to replace lost retinal cells and improve vision3,4. However, protocols for the derivation of candidate replacement cells are cumbersome and time consuming, presenting a challenge for their use in clinical therapy5–8.
Direct reprogramming—using ectopic transcription factors and chemicals—bypasses the requirement for pluripotent cells and has resulted in the generation of neurons, astrocytes and cardiomyocytes; however, the pharmacological conversion of photoreceptors using this method has not been realized9–13. ASCL1, a powerful proneural transcription factor, has been reported to reprogram glial cells into photoreceptors14–16. An improved mechanistic understanding of direct reprogramming may lead to the generation of new cell types.
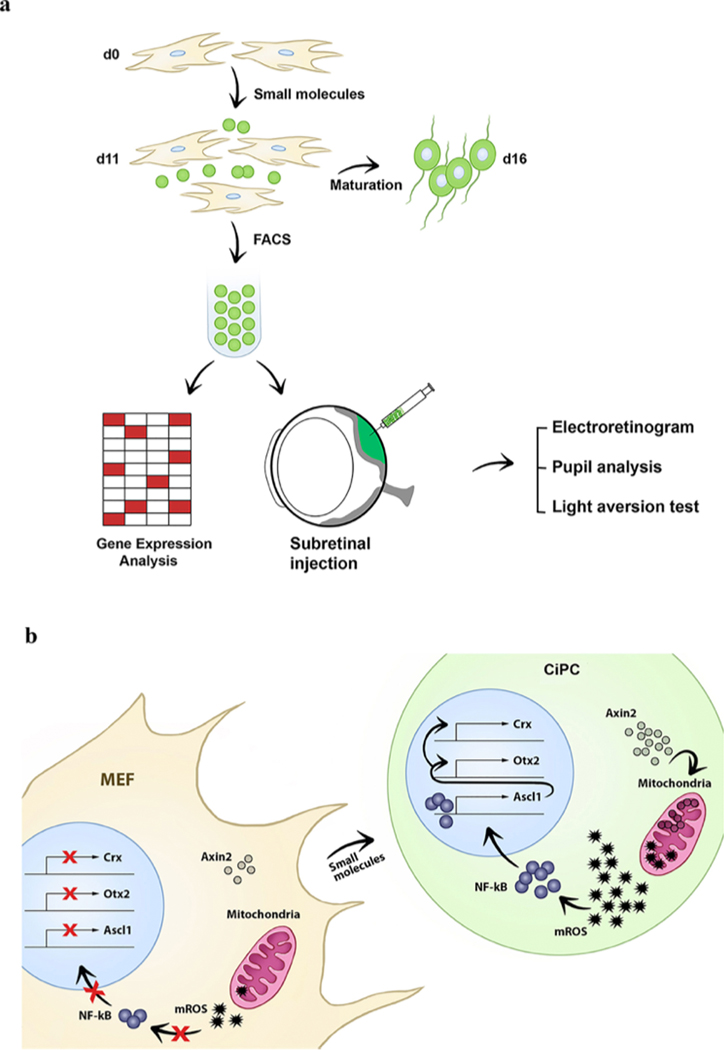
Here we identify a set of five small molecules that can induce fibroblasts to become functional CiPCs without the need for pluripotent cells or viral transcription factors. We demonstrate that CiPCs restore pupil reflex and vision when transplanted into the subretinal space of rd1 mice, a mouse model of retinal degeneration. Moreover, our mechanistic analysis reveals an AXIN2–NF-κB–ASCL1 pathway that promotes retinal lineage during reprogramming and identifies mitochondria as a signaling hub in the orchestration of cell fate conversion.
Results:
A set of five compounds transforms fibroblasts to CiPCs
To generate CiPCs, we used mouse embryonic fibroblasts (MEFs) derived from a transgenic Nrl–GFP mouse, in which the Nrl promoter drives expression of eGFP specifically in rod photoreceptors17,18. We began by testing an established combination of four small moleculesvalproic acid (V), CHIR99021 (a GSK3 inhibitor) (C), RepSox (R) and forskolin (F), together denoted VCRF—that is known to convert fibroblasts into neurons, but only a few of the resultant cells were Nrl–GFP positive12. Various small molecules and culture conditions were attempted (Supplementary Table 5), and we found that the Wnt/β-catenin pathway inhibitor IWR1 (I)—in combination with VCRF and STR (Sonic hedgehog, taurine and retinoic acid)—was able to substantially improve the efficiency of conversion of MEFs into Nrl–GFP + cells (Fig. 1a, b). STR was added on day 8 of reprogramming, to promote and support the formation of photoreceptors after photoreceptor-specifying transcription factors—such as RORβ, ASCL1, PIAS3—were upregulated 14,19–22 (Fig. 1l, Supplementary Tables 1, 2). Similarly, human adult dermal fibroblasts (HADF), and human fetal lung fibroblasts transduced with a Nrl–DsRed reporter, were also converted into CiPCs (Fig. 1f–h, j, Extended Data Fig. 1a, b, Supplementary Fig. 2, Supplementary Table 6). We next tested the small molecules individually and in various combinations, and observed that they failed to generate as many Nrl–GFP + cells (Extended Data Fig. 1c, d). We conclude that all five compounds in combination can efficiently convert fibroblasts into NRL-expressing CiPCs.
Figure 1. Conversion of fibroblasts and the molecular characterization of CiPCs.
(a) Protocol for the reprogramming of mouse fibroblasts into CiPCs. MC, medium change; PIM, photoreceptor induction medium; PDM, photoreceptor differentiation medium. (b) Images of CiPCs expressing Nrl–GFP on day 11 and day 16. Scale bars 14.4 μm. (c) Images of CiPCs expressing CRX on day 11. Scale bars 14.4 μm. (d) FACS purification of reprogrammed Nrl–GFP + CiPCs (0.2%). (e) PCR with reverse transcription reveals the expression of the indicated photoreceptor-specific genes in mouse. For gel source data, see Supplementary Fig. 2a, b. (f) Protocol for the reprogramming of HADFs into CiPCs. (g) Quantitative PCR (qPCR) analysis (fold change compared with HADF) CiPCs after reprogramming from HADF, showing increased expression of photoreceptor-specific genes. Data are presented as mean ± s.e.m. of n = 3 independently treated biological replicates. (h) Micrograph of NRL-stained CiPCs after conversion from HADF. Scale bars 33 μm. (i) Principal component analysis for all RNA-seq samples (n = 3 samples for each). (j) Images of CiPCs after conversion from HADF at day 10. Rcvrn, recoverin; Rho, rhodopsin.Scale bars 14.4 μm. (k) Heat map of RNA-seq data for the indicated photoreceptor genes. RI, reprogramming intermediate. (l) Heat map of RNA-seq data for the expression of the indicated genes that express retinal transcription factors during CiPC conversion. Experiments in b, h, i were repeated three times with similar results and the experiments in c, d, were repeated twice with similar results. CPM, counts per million.
Next, Nrl–GFP + CiPCs were purified by fluorescence-activated cell sorting (FACS) and subject to transcriptomic analysis, which revealed the expression of early retinal neuronal markers (CHX10 and OTX2) and photoreceptor markers (CRX and NRL) (Fig. 1d, e, Supplementary Table 3). Immunostaining analysis of CiPCs on day 11 enabled the visualization of CRX and CHX10 (Fig. 1c, Extended Data Fig. 1c, Supplementary Table 4). Molecular analysis of reprogrammed human CiPCs revealed the expression of photoreceptor-specific markers such as CRX, rhodopsin and recoverin (Fig. 1g–i, Extended Data Fig. 1c, d, Supplementary Fig. 2).
CiPCs express photoreceptor genes
Transcriptome profiling demonstrated that the transcriptome of Nrl–GFP + CiPCs resembles that of native rods, which were used as a positive control23. Heat map analysis revealed the expression of rod-specific genes in CiPCs (Fig. 1i–l, Extended Data Fig. 9a, b), although progenitor-specific genes of other retinal cells—such as cone cells, ganglion cells and Müller glial cells—were expressed at very low levels (Extended Data Fig. 2b). Moreover, fibroblast-specific genes were found to be downregulated in CiPCs and in reprogramming intermediates (Extended Data Fig. 9c). Expression of retinal transcription factors such as Otx2, Nrl and Crx—was also evident in CiPCs (Fig. 1k). Notably, photoreceptor-specific transcription factors—such as Rorb, Ascl1, Pias3, Thrb and Rxrg—were upregulated in reprogramming intermediates21,24,25 (Fig. 1l). These results indicate that CiPCs have a similar gene expression profile to that of their native counterparts.
Because MEFs are composed of a heterogeneous cell population, we performed lineage tracing with Fsp1-Cre; Ai9 MEFs (after FACS purification) and confirmed that CiPCs can originate from fibroblasts (Extended Data Fig. 1e–h). Staining with 5-bromodeoxyuridine (BrdU) during reprogramming to CiPCs indicated that approximately 95% of Nrl–GFP + cells were negative for BrdU, suggesting that no intermediate proliferative stage exists (Extended Data Fig. 2a).
CiPC transplantation in rd1 mice
To test whether CiPCs can activate existing retinal circuitry and restore visual function, FACS-purified Nrl–GFP + CiPCs were transplanted into the subretinal space of 14 rd1 eyes, a mouse model of retinal degeneration (Extended Data Fig. 2c). Recently, pupillary light reflex has been reported as a robust method to measure photoreceptor function after cell transplantation26. Pupillary constriction under low light levels is critically dependent upon the function of rod photoreceptors. Six out of 14 (43%) rd1 eyes demonstrated improved pupil response under low-light conditions three and four weeks after transplantation (Fig. 2a, b). None of the mice demonstrated pupil constriction at two weeks, which served as a baseline (internal negative control) for longitudinal comparison and reduced the likelihood that a pre-existing photoreceptor or alternative pupillary pathway—such as the melanopsin pathway—is responsible for the observed pupillary restoration (Fig. 2b). To assess the restoration of visual function, the six pupil-responsive mice underwent light-aversion testing. The light-aversion test provides an option for mice to spend time in either a dark space or a lit space. Mice have an innate tendency to avoid lit spaces; as such, those with visual function favor dark spaces27. To test rod vision in these pupil-reflex-positive mice, we allowed the mice to adapt to dark conditions and then performed the test using illumination conditions of 50 lx. rd1 mice injected with CiPCs were found to spend significantly more time in the dark space compared with those injected with PBS control (Fig. 2c). None of the CiPC-injected mice without improved pupil responses demonstrated a dark preference. We also performed a modified optomotor test with neutral density filters; this modification was necessary in order to prevent rod saturation and to test rod-mediated vision. An additional benefit of testing at low light intensities is that the possibility of activating any residual cones is substantially reduced. One of the six CiPC-injected mice that demonstrated improved pupil responses and dark preference also showed improvements in visual acuity and contrast sensitivity (Extended Data Fig. 3e, f). These results provide a proof-of-concept that CiPCs can restore visual function in rd1 mice.
Figure 2. Functional analysis of CiPCs in a mouse model of retinal degeneration.

(a) Representative images from the pupil analysis. CiPC-injected eyes 3 months after transplantation. (b) Illuminance response curves for pupillary constriction (n = 6). The stimulus was a 20-s light exposure and the data are fitted with a sigmoidal function. Arrows show the recovery of pupil function. The results presented are for pupil-responsive mice only. Statistical significance was assessed using a two-tailed Student’s t-test. WT, wild type. (c) The time spent by mice in the dark area of the light-aversion test. Wild-type C57BL/6 mice were used for comparison. Statistical significance was assessed using a one-way ANOVA. Data are presented as mean ± s.e.m. (d) Top left, integration and survival of GFP + CiPCs in the retina of a rd1 mouse 3 months after transplantation. Scale bar, 20 μm. Top right, a magnified view of the top left image. Scale bar, 10 μm. Bottom, additional images of the integration of GFP + CiPCs. Bottom left, 40× magnification; bottom right, z-section. Scale bars: left, 10 μm; right, 20 μm. GCL, ganglion cell layer; INL, inner nuclear layer. Experiments in a and d were repeated twice with similar results and n denotes the number of biologically independent mice.
We next sought to rule out the possibility that CiPCs primarily function to reduce the degeneration of host photoreceptors. We transplanted Nrl–GFP + CiPCs into the subretinal space of rd1 mice on day 31—a later time point at which there is near complete photoreceptor degeneration and electroretinogram (ERG) signals are extinguished28. Long-term retinal function was analyzed by pupillary light reflex and ERG (Extended Data Fig. 2c). Approximately three months after transplantation (at postnatal day (P)128), we recorded a 30%–40% increase in pupillary constriction in response to a low light stimulus (50 lx) in three of the six eyes into which CiPCs had been transplanted (Extended Data Figs. 2c and 3d). Full-field ERG analysis demonstrated an improvement of the scotopic a-wave in three out of six eyes into which CiPCs had been transplanted at P45; this improvement was lost at and after P59 (Extended Data Fig. 3a–c). One eye showed improvement in the scotopic b-wave at P45 (Extended Data Fig. 2d).
The long-term survival of transplanted CiPCs and their synaptic connections to inner retinal neurons were assessed by immunofluorescence. Three months after CiPC transplantation, we identified GFP+, GFP+ recoverin + or GFP+ rhodopsin + CiPCs in the outer aspect of the inner nuclear layer in eyes that demonstrated restoration of the pupillary light reflex (Fig. 2d, Extended Data Figs. 4a, b, 8d). We quantified CiPC survival and found a strong correlation between pupil constriction and cell survival (Extended Data Fig. 8e). Specifically, eyes with improved pupil response and light/dark response had an average of 58 CiPCs per section, compared with 8 CiPCs per section in non-responders (Extended Data Fig. 8f). Moreover, CiPCs were found to have synaptic terminals that express the rod ribbon-synapse protein ribeye and the synaptic vesicle protein synaptophysin. These synaptic terminals are in close proximity to rod bipolar cells (expressing bipolar marker PKC-α), which is essential for transmitting a light signal into the inner retina (Extended Data Fig. 4c–e). Taken together, these results suggest that some of the transplanted CiPCs survive, function and connect with the inner retinal neurons of rd1 mice.
NF-κB induces ASCL1 during conversion to CiPCs
To explore the mechanism of reprogramming to CiPCs, we identified candidate transcription factors from RNA sequencing (RNA-seq) analysis. We detected the expression of Ascl1 on day 5, which continued to increase until day 8 (Fig. 1l, Fig. 3a). ASCL1, a proneural transcription factor, is capable of converting fibroblasts into neurons11,29. Furthermore, ASCL1 is reported to be transiently expressed in photoreceptor precursors and can reprogram Müller glia into rod photoreceptor-like cells14,15,30. Taken together, we proposed that the Ascl1 transcriptional network has a central role in reprogramming fibroblasts to CiPCs. To test this hypothesis, we reprogrammed Ascl1-depleted (Fig. 3b) Nrl–GFP MEFs with the set of five compounds (VCRFI). We noted a 70%–80% reduction in the generation of CiPCs, which suggests that ASCL1 has an important role in the reprogramming of fibroblasts to CiPCs (Fig. 3c).
Figure 3. The mROS–NF-κB–ASCL1 signalling axis determines the reprogramming of fibroblasts to CiPCs.
(a) Ascl1 transcript expression during reprogramming of MEFs to CiPCs, analysed by qPCR using the 2-ΔΔCT method. RI, reprogramming intermediates, R, reprogrammed cells. (b) Ascl1 qPCR analysis in Ascl1 depleted MEFs, using short hairpin RNA (shRNA). (c), Number of GFP + cells after reprogramming to CiPCs from Ascl1-knockdown (KD) and wild-type Nrl–GFP MEFs. (d) NF-κB-luciferase activity during reprogramming of MEFs to CiPCs. LPS, lipopolysaccharide. (e) rVista sequence alignment of human ASCL1 and mouse Ascl1 genes show highly conserved NF-κB-binding sites (red vertical bar) downstream of the 3′UTR region. (f) ChIP assay shows binding of NF-κB at downstream loci of Ascl1. (g) Accumulation of mROS in CiPCs converted from Nrl–GFP MEFs on day 11. Scale bar 33 μm. (h) Fluorometric analysis of mROS generation during reprogramming of MEFs to CiPCs. AntiA, antimycin A; RFU, relative fluorescence units. (i) Quantification of Nrl–GFP + cells on day 11 after depletion (by MitoTEMPO treatment) or generation (by Tfam knockdown) of mROS. SM, small molecules. In a–d, f, h, i, data are presented as mean ± s.e.m. of n = 3 independently treated biological replicates. Statistical significance was assessed by two-tailed Student’s t-test.
To determine the mechanism of ASCL1 induction, we investigated potential upstream regulators of ASCL1. NF-κB is a rapidly acting primary transcription factor that is known to have a role in neural stem-cell differentiation and embryonic neurogenesis31. We therefore postulated that NF-κB is an upstream regulator of ASCL1 induction. A luciferase assay demonstrated that NF-κB activation began on day 5 and reached a maximum at day 11 (Fig. 3d). These results suggested the involvement of NF-κB, and so we next explored whether NF-κB induces the expression of ASCL1. Bioinformatics analysis (using rVista) identified a putative binding site for NF-κB near the Ascl1 loci (Fig. 3e), and a chromatin immunoprecipitation (ChIP) assay confirmed the binding of NF-κB at this locus (Fig. 3f). Furthermore, transient transfection analysis with a luciferase reporter gene confirmed that NF-κB positively regulates Ascl1 during reprogramming to CiPCs. NF-κB depletion in MEFs that were subsequently reprogrammed to CiPCs resulted in reduced expression of Ascl1 and fewer CiPCs overall, which further confirms the pathway (Extended Data Fig. 5a–c). Overexpression of Ascl1 alone in MEFs was not sufficient for reprogramming to CiPCs (Extended Data Fig. 5f–h). These results indicate that treatment with our set of five compounds leads to the activation of NF-κB, which in turn binds to the regulatory regions of Ascl1 and controls its expression. Notably, analysis of CiPCs using an assay for transposase-accessible chromatin with sequencing (ATAC–seq) revealed the presence of open chromatins at the upstream regions of the Ascl1 gene in reprogramming intermediates. Homer analysis revealed the enrichment of Ascl1 and NF-κB-binding motifs in both intermediates and reprogrammed cells, which may be important for the binding of regulatory transcription factors and for their expression (Extended Data Fig. 9f, Supplementary Table 7).
Reactive oxygen species activate NF-κB
To investigate the mechanism by which NF-κB activation is induced by the combination of five small molecules, we considered known inducers of NF-κB—such as TNFα, lipopolysaccharide, ionizing radiation and mitochondrial reactive oxygen species (mROS)—as possible candidates32,33. mROS have been reported to induce nuclear gene expression through the activation of NF-κB32. We therefore postulated that mROS generated by treatment with the five compounds may activate NF-κB.
We observed an evident increase in mROS accumulation, beginning on day 8 in reprogramming intermediates (Fig. 3g, h). To determine the importance of mROS, we used the antioxidant MitoTEMPO, an mROS scavenger, and observed a reduction in reprogramming to CiPCs (Fig. 3i). Depletion of mitochondrial transcription factor A (TFAM) has been reported to lead to the generation of mROS34. Our data show that withdrawal of the compound IWR1 reduces reprogramming efficiency and mROS generation (Fig. 3i, Extended Data Fig. 6f). We considered the possibility that TFAM-depleted MEFs may have increased reprogramming potential in the absence of IWR1. Consistent with this hypothesis, we found increased reprogramming to CiPCs and increased mROS generation in TFAM-depleted MEFs in the absence of IWR1 (Fig. 3i, Extended Data Fig. 6e, f). Furthermore, exogenous ROS or TNFα (a NF-κB inducer) did not have a significant effect on CiPC conversion efficiency, and excessive ROS had a negative effect (Extended Data Fig. 5d, e). These data indicate that mROS has an important role in the reprogramming of fibroblasts to CiPCs.
To determine whether the activation of NF-κB is dependent on mROS, we added MitoTEMPO on day 3 of reprogramming and observed significantly decreased activity in the luciferase assay (Extended Data Fig. 6a). Additionally, we found that the extent of binding of NF-κB near the Ascl1 loci was reduced upon treatment with MitoTEMPO (Extended Data Fig. 6g). Taken together, these results demonstrate that mitochondrial ROS activates NF-κB, which controls the expression of Ascl1 by binding its regulatory region.
The five compounds promote Axin2 mitolocalization
To identify the mechanism by which mROS is generated during reprogramming to CiPCs, we considered two reports that demonstrate the stabilization of AXIN2 by treatment with CHIR and IWR1 35 and the translocation of AXIN2 to mitochondria upon treatment with XAV939 (an IWR1 analogue)36. We proposed that treatment with the group of five compounds—which includes IWR1 and CHIR—induces the stabilization of AXIN2 and its subsequent translocation to mitochondria; mitochondria-targeted AXIN2 then generates mROS, which in turn activates NF-κB. To test the hypothesis, the expression of AXIN2 was examined during the reprogramming to CiPCs. We discovered that AXIN2 in reprogramming intermediates and in day 11 non-purified CiPCs is more stabilized compared with that in starting MEFs (Fig. 4a). Subsequently, we found that stabilized AXIN2 translocates to the mitochondria of CiPCs, as evidenced by its co-localization with mitochondria (Fig. 4b, c, Extended Data Fig. 7a, b). Axin2-depleted MEFs demonstrate reduced reprogramming to CiPCs and reduced mROS generation (Fig. 4d–f, Extended Data Fig. 6d). For further confirmation we measured NF-κB activation and Ascl1 expression in Axin2-depleted reprogramming intermediates. We detected reduced Ascl1 expression and decreased NF-κB activity (Fig. 4g, Extended Data Fig. 6b). Taken together, these results indicate that mitochondria-targeted AXIN2 induces increased mROS generation in CiPCs and activates the downstream NF-κB–ASCL1 pathway to promote a lineage switch to a photoreceptor fate. Moreover, mitochondrial analysis of CiPCs revealed low basal mitochondrial respiration, low ATP turnover, low reserve capacity and high glycolysis rates; this is indicative of an immature mitochondrial state, which may be linked to the generation of mROS (Extended Data Fig. 7c–f).
Figure 4. Mitochondria-translocated AXIN2 causes mROS generation and the reprogramming of fibroblasts to CiPCs.
(a) Western blot demonstrating AXIN2 expression during reprogramming of MEFs to CiPCs. For gel source data, see Supplementary Fig. 1 (c-d). (b) No co-localization of AXIN2 (green) with mitochondria (MitoTracker) was observed in MEFs. Scale bar, 13.4 μm. (c) AXIN2 (red) localization in mitochondria (green) of GFP + CiPCs (purple pseudocolour). Scale bar, 2 μm. (d) Western blot demonstrating reduced levels of AXIN2 after knockdown of its encoding gene, Axin2, by shRNA. For gel source data, see Supplementary Fig. 1g, h. In a–c, experiments were repeated twice; in d, the experiment was performed once. (e) Quantification of Nrl–GFP+ cells after conversion of Axin2-depleted MEFs to CiPCs on day 11. (f)Reduced mROS generation in Axin2-depleted cells during reprogramming to CiPCs, as assessed by fluorimetry. AFU, arbitrary fluorescence units. (g) qPCR analysis shows that knockdown of Axin2 is associated with reduced expression of Ascl1 in reprogramming intermediates on day 8 and CiPCs on day 11. Scr., scramble. In (e–g), data are presented as mean ± s.e.m. of n = 3 independently treated biological replicates. Statistical significance was assessed by two-tailed Student’s t-test.
Discussion
Here we report that a combination of five small molecules can convert fibroblasts into functional CiPCs that are capable of partially restoring pupil reflex and visual function in a mouse model of retinal degeneration (Extended Data Fig. 10a). Gene expression profiling reveals that CiPCs are similar to their in vivo rod counterpart, and CiPC conversion recapitulates the ontogeny of photoreceptor genesis—as indicated by the upregulation of Thrb, Rorb and Pias3, among others21,24. The occurrence of open chromatin regions near photoreceptor loci and the enrichment of photoreceptor-specifying transcription-factor-binding motifs during reprogramming to CiPCs further validate these results37,38 (Extended Data Fig. 9f, Supplementary Table 7). Subretinal transplantation of rod-like CiPCs into rd1 mice led to long-term improvement in pupillary light reflex and the restoration of normal visual behavior in the light aversion test. In our study, 6 out of 14 mice (43%) demonstrated an improved pupillary response. The strong correlation between pupil constriction and cell survival may explain why some eyes showed an improvement and others did not (Extended Data Fig. 8e).
Material transfer and cell fusion are mechanisms that can explain improvement in visual function; however, these mechanisms have not been reported in the late stages of retinal degeneration, during which viable host photoreceptors are required in order for improvement to occur26,39. These mechanisms are of most concern in photoreceptor loss-of-function models—such as Gnat1—in which the photoreceptors have limited or no degeneration and retinal morphology and structure are preserved. To avoid the possibility of donor–host cell fusion and material transfer, we chose to transplant CiPCs into rd1 mice at a time point (postnatal day (P)31) at which no rod photoreceptors exist28,40. Although rod loss is almost complete at P31, we examined the possibility that existing cones or bipolar cells could participate in CiPC material transfer or cell fusion; however, we found that these are unlikely to have a major role (Extended Data Figs. 4d, 8c).
Our mechanistic studies reveal that mROS-mediated NF-κB activation directly regulates the expression of Ascl1 and the reprogramming of fibroblasts into CiPCs. Mitochondrial signaling is reported to have a role in cellular homeostasis and neuronal function41–43. To our knowledge, this report is the first to demonstrate that mitochondria-to-nucleus signaling acts as a mediator for direct chemical reprogramming (Extended Data Fig. 10b). Moreover, mitochondria translocated AXIN2 probably generates mROS, although the mechanism is currently unknown. Induction of the reprogramming of fibroblasts to CiPCs by our group of five compounds reveals a new function of the mitochondria that may provide valuable knowledge for the generation of other cell types.
Although CiPCs have therapeutic potential, a lack of proliferation—as is the case for native photoreceptors—and low conversion efficiency are the main impediments for a translational application. We anticipate that optimization of our current protocol may be beneficial for obtaining large numbers of CiPCs. For example, temporal modulation of IWR1 in the protocol resulted in a significant increase in the conversion of HADF to CiPCs (Fig. 1f, Extended Data Fig. 8d, right). Overall, CiPCs are a promising cell-replacement candidate and may lead to a scalable therapy for vision restoration.
Methods
Generation of chemically induced photoreceptor-like cells
All small molecules were diluted in DMSO or DMEM according to the manufacturer’s instructions. Approximately 50,000–80,000 MEFs (passage 2) were seeded into each well of a 12-well plate (0.1% gelatin-coated overnight) and cultured overnight. On day 1, the medium was replaced with photoreceptor induction medium (PIM; Supplementary Tables 1, 2) containing V (0.5 mM), C (4.8 μM), R (2 μM) and F (10 μM). On day 3, fresh PIM containing V, C, R, F plus I (10 μM) were added into each well. The medium was then replaced with PIM containing VCRFI on days 4–7 depending on cell appearance. S (3 nM), T (100 μM) and R (1 μM) were added on day 8 with fresh medium containing all the small molecules mentioned above. On day 10–11, GFP + cells were collected and analyzed for gene expression. For CiPC maturation, PIM was replaced with photoreceptor differentiation medium with the small molecules VCRF and cultured up to day 15–16 (Supplementary Tables 1, 2). CiPC survival decreases considerably after day 11 with only a few mature cells obtained at day 15–16. For human adult dermal fibroblast (HADF) reprogramming, cells were seeded (to 95 to 100% confluence) in IMR90 medium. On day 1, PIM containing VCRFI (at the same concentrations as stated above) was added to the wells. PIM containing VCRFISTR was added on day 5. NRL staining was performed between day 7–10. We noted more autofluorescent cells after fixation and these cells were not considered as reprogrammed. The medium and all small molecules were replenished daily throughout the conversion period depending upon appearance. Human Fetal Lung Fibroblasts (HFL1) conversion to CiPCs was similar to mouse protocol, except that 5 μM IWR1 was added on day 2. The small molecules S, T and R, as well as brain-derived neurotrophic factor, glial cell line-derived neurotrophic factor and neurotrophin-3 were added on day 5. On day 6–8, DsRed+ cells were collected for analysis.
Mouse models and MEF isolation and lineage tracing
All animal studies and animal care were performed in accordance with relevant guidelines and regulations and approved by the Institutional Animal Care and Use Committee at the University of North Texas Health Science Center. Nrl–GFP reporter mice were a gift from A. Swaroop (National Eye Institute, NEI) and were used to generate MEFs. Nrl–GFP cells were not identified in either starting fibroblast culture44. rtTa mice (Jackson Laboratory, 006965) were crossed with Nrl–GFP mice for unrelated experiments. Embryos from Fsp1-Cre mice (Jackson Laboratory, stock# 012641) crossed with R26-LSL-tdTomato mice (Jackson Laboratory, stock# 007909) were used for lineage tracing. Embryos were examined for tdTomato expression before isolation of MEFs. td-Tomato + cells obtained from FACS were used for reprogramming to CiPCs according to the protocol described above, and CiPCs were stained with anti-NRL antibody according to the method detailed below. CBA/J (rd1) mice (stock# 000656) and C57BL/6 mice (stock# 000664) were purchased from the Jackson Laboratory.
Subretinal injection of CiPCs into rd1 mice
After conversion, GFP + CiPCs were sorted by FACS from multiple 10-cm dishes and resuspended in IMR90 medium. For subretinal injection, mice (P31 and P24) were first anaesthetized by intramuscular injection of 85 mg kg −1 ketamine and 14 mg kg −1 xylazine. A 30-gauge blunt-end needle attached to a 10 ml Nanofil syringe (World Precision Instruments) was inserted into the puncture site (0.5–1 mm under the limbus line) with visualization aided by the use of a surgical microscope (Leica). CiPCs (80,000; 1.5–2 μl per eye) or saline (1.5–2 μl per eye) were delivered into the subretinal space in the superior temporal quadrant. After injection, the needle was left in place for a few seconds to reduce reflux and enable maximal cell release before being slowly withdrawn. The eyelid was returned to its original position and a drop of Triple Antibiotic (Equate, Walmart) ointment was applied. Mice were warmed on a 37 °C bed until fully awake.
Pupillometry
Similar to a previously published protocol26, dark-adapted mice were used to capture images under infrared illumination. CiPC-transplanted eyes that showed ERG improvement were exposed to low-irradiance white light through a light guide from a 100-W goose arc lamp at a range of intensities. For the illuminance curve experiments, dark-adapted mice were subjected to a series of light exposures (with increasing illuminance) for 10s on weeks 2–4 after transplantation. A complete intensity series was performed in one eye before retesting the other eye at similar intensities. A gap of at least 2 min was maintained in between measurements. Pupil constriction was imaged in the contralateral, non-transplanted eyes. An infrared light-emitting diode was used throughout the experiment for background illumination. An infrared camera (Sony, DCR-HC96) was used to acquire images. The pupil area for each eye was measured before and after light exposure with ImageJ software (National Institutes of Health). For each mouse, the change in pupil constriction was represented by the difference between the pupil area measured in the dark and in the light.
Quantitative PCR and PCR with reverse transcription
Total RNA was extracted using a kit (Zymo Research, Cat# R1050) according to the manufacturer’s instructions. RNA (1 μg) was converted to cDNA using a High Capacity cDNA Reverse Transcription kit (Applied Biosystems, 4368814). Isolated RNAs were treated with DNAase-I before c-DNA synthesis. A thermal cycler from Applied Biosystems and OneStep Plus real-time PCR were used for amplification. qPCR was performed using Fast SYBR Green Master Mix (Applied Biosystems, 4385612). Results were normalized with glyceraldehyde 3-phosphate dehydrogenase or hypoxanthine-guanine phosphoribosyltransferase and fold change was calculated using the 2 −ΔΔCT method. A list of primers is provided in Supplementary Table 3.
Transcriptome analysis Quantification
RNA-seq data analysis was performed at the gene level as previously45 with Ensembl data release 87. The gene level count matrix was then TMM-normalized (TMM, trimmed mean of M values) using the edgeR (v3.18.1) package in the R (v3.4.0) programming environment, as previously described46.
Gene expression clustering and heat maps Gene expression clustering was performed on selected genes using the affinity propagation algorithm by using negative distance as the similarity for ordering the genes in each set before drawing the heat maps. The heat map function was developed in-house and is available upon request.
ATAC–seq: Cells were washed twice with fresh medium and 1:100 volume of 100XDNase buffer (250 mM MgCl 2 and 50 mM CaCl in H2 O) and 1:100 volume of 100X DNAaseI solution (Worthington, LS002006, resuspended in HBSS at 20,000 units per ml) was added to the medium. Cells were incubated in DNase at 37 °C for 30 min in a tissue culture incubator. After washing the medium with PBS, cells were then trypsinized, centrifuged (800g for 5 min) and resuspended in 500 μl of growth medium containing 5% DMSO in a slow cooling chamber. Approximately 100,000 cells were then shipped to Active Motif to perform ATAC–seq and bioinformatics analysis.
Immunohistochemistry, subcellular fractionation and immunoblotting
Paraformaldehyde (4%) was used to fix enucleated eyes. Cryo-embedded eyes were then sectioned with a thickness of 14 μm. Fixed eye sections were analysed with primary and secondary antibodies listed in Supplementary Table 4. DAPI (0.1%) was used to stain the nucleus in mounting medium. Images were taken using a Zeiss LSM510 confocal/Leica DMi8 florescence microscope. Subcellular fractionation was performed following a kit protocol from Thermo Fisher Scientific (Cat# 89874). For immunoblotting, total protein was extracted with commercially available lysis buffer (Thermo Scientific, Cat# 89900) and then concentration of proteins was measured using a BCA protein assay kit (Thermo Scientific, Cat# 23227). An equivalent amount of proteins was loaded into each well, immunoblotted and antibody-stained using a standard procedure (Supplementary Table 4). SuperSignal West Femto maximum sensitivity substrate (Thermo Scientific, Cat# 34094) was used to develop the subcellular fractionation western blot.
Electroretinogram
After a minimum dark-adaptation period of 12 h, mice were anaesthetized by intraperitoneal injection of 85 mg kg−1 ketamine and 14 mg kg−1 xylazine. Preparation was performed under a dim red light (<50 lux). ERG analyses were performed using an Espion system (Diagnosys). For the assessment of scotopic response, a stimulus intensity of 40 cd·s m−2 was presented to the dark-adapted dilated eyes. The amplitude of the scotopic a-wave was then measured from the pre-stimulus baseline to the a-wave trough. The amplitude of the b-wave was then measured from the trough of the a-wave to the crest of the b-wave. A total of 15 repeated flashes and measurements were averaged to produce the final waveform. The amplitude of the photopic b-wave was then measured from the trough of the a-wave to the crest of the b-wave. At the beginning of the day, the response of wild-type C57BL/6 mice (aged over P21, n ≥ 2) was recorded and quantified to ensure proper device calibration.
Light-aversion test
The visual discrimination (light/dark) test was conducted in an apparatus that consists of black opaque (100%) acrylic test chambers (30.48 × 15.24 × 30.48 cm (length, width, height)). This chamber was further divided into equal-sized compartments (15.24 × 15.24 × 30.48 cm) by the addition of an insert, to create a dividing wall in the center. Further, to create light and dark zones, one compartment was illuminated with dim ambient light (50 ± 1.5 lx) and the other compartment was kept dark (~ 0.1 lx). The light and dark compartments were connected by an opening (5 × 5 cm). The position of the mouse within the apparatus was recorded using a photocell-based system (Model 71-CPPX, Omnitech). The acrylic chambers were housed separately in sound-attenuating chambers (Model 71-ECC, Omnitech). Ambient noise within the chambers was 64 dB and testing took place under dim illumination.
Mice were maintained in the testing room overnight (about 12 h) in dark conditions in their home cage with free access to food and water. Each mouse was allowed to habituate to the testing apparatus (both sides) for 10 min while in the dark. After habituation, one side of the apparatus was illuminated with an ambient light at around 50 lx and the mouse was allowed to roam freely between each compartment for 5 min. The time spent in the dark and light compartments was recorded by a photocell-based system.
Optomotor task
The testing apparatus was a chamber (39 × 39 × 32.5 cm) with mirrored floors and ceilings. Attached to each of the four walls was a 20-inch computer monitor facing inwards. In the center of the chamber was a platform (7-cm diameter) that was elevated approximately 15 cm from the floor. When a mouse was placed on the platform, a video camera positioned in the ceiling of the apparatus enabled the behavior of the mouse to be clearly visible during testing. A computer program was used to project visual stimuli (vertical gratings) onto the monitors (OptoMotry, Cerebral Mechanics). The gratings were rotated at 12° s−1, producing the appearance of a virtual rotating cylinder. The moving gratings elicited a tracking behavior and the visual acuity threshold and contrast sensitivity was determined for each eye rotating in a clockwise direction (when testing the left eye) or in the anticlockwise direction (when testing the right eye). Light levels were lowered to 50 lx and 65 lx by using neutral density filters placed on the screens. Before any testing took place, the mice were dark-adapted in their home cages with free access to food and water.
Visual acuity
A mouse was placed on top of the platform and allowed to acclimatize for a short period of time. Testing began when the mouse was no longer actively moving around. The visual acuity threshold was determined with contrast set at 100% and a grating of low spatial frequency (0.042 cycles per degree) as previously described in a recent report. When tracking behavior was observed, the same stimuli were rotated in the anticlockwise direction (thus effectively testing the right eye). A staircase method of determining acuity threshold was implemented. A series of gratings of increasingly higher spatial frequencies were presented (rotating in one, then in the alternative direction) as long as the mouse indicated that it could detect the grating movements. When the mouse ceased to respond to a particular spatial frequency, a lower frequency grating was presented; when the mouse responded to a frequency, the frequency was increased. The acuity threshold was set as the highest spatial frequency to which the animal responded.
Contrast-sensitivity function
A contrast threshold was measured for six spatial frequencies (0.031, 0.064, 0.092, 0.103, 0.192, 0.272 cycles per degree). The initial contrast was set at 100% for each of the above spatial frequencies. Contrast was lowered until the mouse ceased to respond to the particular grating. The lowest contrast that elicited a response was assessed for each of the six spatial frequencies, and a contrast-sensitivity function was calculated with the formula 100/C, where C is the lowest contrast that elicits a response at a particular frequency. This data transform means that when a mouse can see at a very low contrast setting, the sensitivity number will be large and will indicate better visual performance at a particular spatial frequency.
Immunofluorescence and laser scanning confocal microscopy
For confocal microscopy, converted and sorted cells were seeded on a chambered cover glass coated with 0.2% gelatin (Nunc). For mROS detection, cells were stained with MitoTracker Red (500 nM; Molecular Probes, M7512) for 30 min, washed, and used for downstream applications. For antibody staining, cells were fixed with 4% paraformaldehyde for 15 min and permeabilized with 0.25% Triton. Cells were then stained with primary antibody overnight at 4°C. After incubation with the appropriate secondary antibody, images were captured using a Zeiss LSM 510 confocal microscope. Data analysis and 3D reconstruction were performed with the assistance of ZEN lite software. Alexa Fluor 633-, 549- and 488-tagged secondary antibodies were used. For counting Nrl–GFP + CiPCs, ten 20× visual fields were selected randomly. The list of primary antibodies is provided in Supplementary Table 4.
Measurement of mitochondrial ROS and TNF, H2O2 treatment during reprogramming
Mitochondrial ROS was detected and quantified using a published protocol47. In brief, CiPCs, Axin2-depleted MEFs or reprogramming intermediates were incubated with MitoSOX Red (500 nM; Molecular Probes, M36008) for 30 min. Cells were then washed twice with 1× PBS and fluorescence was monitored with a microplate reader set to an excitation wavelength of 510 nm (excitation bandwidth, 10 nm) and an emission wavelength of 595 nm (emission bandwidth, 35 nm). mROS generation was also imaged with a fluorescence microscope and quantified with Leica Application Suite X Software. The region of interest was outlined for each cell on the image after background subtraction. The average intensity within each region of interest was measured and exported to an Excel spreadsheet. The average change in fluorescence was calculated for each type of cell. There were at least three replicates for each condition.
For H2O2 production during reprogramming, d-galactose (0.5 mM) and galactose oxidase (0.015, 0.05, 0.1, 1 U ml−1) were added on day 4 of reprogramming along with all the small molecules48. On day 10–11, the number of CiPCS was quantified under the microscope (20 fields were randomly selected for counting). For TNFα treatment, cells were treated with three different concentrations of TNFα (20 ng ml−1, 50 ng ml−1, 100 ng ml−1) on day 8 and the number of cells was counted on day 11.
Measurement of oxygen consumption rate and extracellular acidification rate by Seahorse assay
About 25,000 CiPCs in 100 μl medium with all small molecules were seeded in XF24 cell culture plates. Blank medium was added to the appropriate wells for background correction. Plates were then placed in an incubator at 37 °C with 5% CO 2 for 6 h. This step enables the cells to adhere on the surface of the plate. Next, 150 μl growth medium containing all small molecules was added to each well followed by incubation overnight at 37 °C and 5% CO2. The following morning, the experiment was performed in a Seahorse Extracellular Flux Analyzer according to the manufacturer’s instruction at the Metabolic Phenotyping Core facility, University of Texas South Western Medical Center.
FACS
For FACS, CiPCs were trypsinized (0.25%) and passed through a 40-μm Nylon cell strainer (Fisher Scientific, Cat# 08–777-1) and suspended in PBS containing 3% bovine serum. Initiating MEFs were used as a negative control. Cells were then sorted in a Beckton-Dickinson LSRII Flow cytometer at the Flow Cytometry core facility, University of Texas Southwestern Medical Center and a Sony SH800 cell sorter at Flow Cytometry core facility, University of North Texas Health Science Center. Sorted cells were collected in IMR90 medium, centrifuged, and processed for RNA extraction and other downstream applications.
RNAi and generation of shRNA transduced MEFs
Lentiviral doxycycline-inducible shRNA constructs for Axin2 (GE Dharmacon, 12006), Tfam (GE Dharmacon, 21780) and RelA (Sigma, TRCN00023583) were purchased for lentivirus preparation. Lentiviral supernatants were collected for 4 days and concentrated using lenti-X concentrator (Clonetech, Cat# 631231). An aliquot of concentrated lentivirus was then used to transduce P0 Nrl–GFP MEFs. For Ascl1 knockdown experiments, control (scramble) shRNA and lentiviral shRNA (a gift from J. Johnson, UT-Southwestern Medical Center; cloned into PLKO.1 (Addgene)) constructs were transduced in MEFs. All lentiviral-transduced cells were selected for 3d in the presence of puromycin (1 μg ml−1). Drug-selected cells were then used for chemical conversion and shRNA induction (Tfam and Axin2) was performed between day 4 and day 8 with doxycycline. Finally, CiPCs were quantified on day 11.
Western blotting
Whole-cell lysates were prepared as follows: cells were washed twice with ice-cold PBS, then lysed in RIPA buffer (Thermo Fisher Scientific) with 1X protease and phosphatase inhibitor cocktail (Thermo Fisher Scientific) on ice for 15–20 min. The mitochondrial and cytosolic fractions were prepared using a commercial kit (Thermo Fisher Scientific, Cat# 89874). Protein concentration was measured using a Pierce BCA protein assay kit (Thermo Fisher Scientific), and optical density at 562 nm was measured using a Biotek Synergy 2 Microplate Reader (BioTek Instruments). The NuPAGE protein gel system was used to separate cell lysates by electrophoresis. Blots were incubated in appropriate primary antibodies overnight and incubated with western HRP substrate (Millipore) or SuperSignal West Femto Maximum Sensitivity Substrate (34094). Images were acquired using the Innotech FluorChem HD2 imaging system (Alpha Innotech).
ChIP and amplification
Real-time PCR-based quantitative ChIP analysis was performed using a ChIP Assay Kit according to the manufacturer’s instructions (EMD Millipore, Cat# 17–295). In brief, 20,000 cells were cross-linked with formaldehyde (to a final concentration of 1%) for 10 min at room temperature with gentle agitation. Cell sonication was performed such that the size of the chromatin would be between 300–500 bp. After pre-clearance with protein A agarose, chromatins were used for immunoprecipitation with specific antibodies targeted to NF-κB-p65. Immunoprecipitated chromatins were amplified with a Whole-Genome Amplification Kit (Sigma, WGA2). Amplified products were identified in agarose gel. Primers were designed to amplify 60–100 bp amplicons and were based on sequences from the Ensembl Genome Browser for mouse. Products were amplified with Fast SYBR Green Master Mix in a 20-μl reaction. The amount of product was determined relative to a standard curve of input chromatin. Dissociation curves showed a single product for the amplicons. Primers for ChIP analysis are detailed in Supplementary Table 3.
BrdU labelling and staining
BrdU (1 μM) was added on day 3 of reprogramming, and conversion was continued until day 11. CiPCs were washed twice with 1× PBS and immunofluorescence was performed according to the published procedure (https://www.abcam.com/protocols/brdu-staining-protocol).
Preparation of Nrl–DsRed promoter reporter cells
To prepare the Nrl–DsRed promoter reporter, we digested out the promoter and reporter fragment from a commercially available vector, pNrl–DsRed (Addgene, 13764), using restriction enzymes. These fragments were then cloned into a gateway entry vector pENTR2B (Thermo Fisher Scientific, A10463). Positive clones were then shuffled into a destination vector, pLentiX1 Zeo DEST (Addgene, 17299). The final product was then used for lentivirus preparation. Human fetal lung fibroblasts (HFL1; ATCC, CCL153) were transduced with the lentivirus and selected with Zeocin (200 μg ml−1; InvivoGen, ant-zn-1) for 8 days.
Statistical analysis
All data are presented as mean ± s.e.m. Statistical significance was determined using Student’s t-test and one-way ANOVA using GraphPad Prism Software (GraphPad Software); P values are indicated in the figures.
Extended Data
Extended Data Figure 1.
Preparation of Nrl–DsRed lentiviral reporter construct and lineage tracing. (a) DNA gel shows cloned Nrl and DsRed fragments digested out from the pENTR2B vector. This experiment was performed once. (b) Expression of Nrl–DsRed in human CiPCs on day 8. (c) The number of Nrl–GFP + cells upon subtraction of the indicated small molecules from the mixture. (d) The number of Nrl–GFP + cells after conversion (on day 11) upon treatment with each small molecule alone. (e) Scheme showing the generation of the mouse model for lineage tracing. (f) Flow sorting of FSP1CretdTomato + MEFs. Left, scatter plot from FACS; middle, MEFs before cell sorting; right, MEFs after cell sorting. Scale bars, 25 μm. This experiment was performed once. (g) Cells expressing NRL are FSP1–tdTomato-positive on day 11, suggesting that they originate from fibroblasts. (h) Percentage of cells that are positive for both NRL and tdTomato, compared with those that are just positive for NRL. In c, d, h data are presented as mean ± s.e.m. of n = 3 independently treated wells. Statistical significance was assessed using a two-tailed Student’s t-test.
Extended Data Figure 2.
BrdU staining, transcriptome analysis and functional testing of CiPCs. (a) Images of BrdU-stained CiPCs on day 11. Scale bars, 10 μm. (b) Heat map analysis of RNA-seq data for the indicated cell-type-specific genes. (c) Timeline for subretinal transplantation of CiPCs and functional analysis. (d) Scotopic b-wave after transplantation of converted CiPCs. n denotes the number of biologically independent animals.
Extended Data Figure 3.
Functional analysis CiPCs in retinal degeneration mice (rd1). (a-c) Analysis for the scotopic a-wave of ERG after transplantation of CiPCs at day 45 (a), day 59 (b) and day 77 (c). (d) Scatter plot of pupil constriction measurements for CiPC-injected rd1 mice taken three months after transplantation. (e-f) Measurement of visual acuity (e) and contrast sensitivity (f) for CiPC-injected mice that demonstrated improved pupil response after transplantation and control mice. OR, optokinetic response. For the CiPC-injected rd1 experiment, repeated values are plotted (for n = 1 mouse). In (a-e) n denotes biologically independent animals and the experiment in (f) was performed twice.
Extended Data Figure 4.
Transplanted CiPCs express photoreceptor-specific markers and connected to the inner retina three months after transplantation. (a) Expression of recoverin (Recov, red) in CiPCs (green). (b) Expression of rhodopsin (Rho, red) in GFP-expressing CiPCs. (c) Expression of rod ribbon-synapse protein ribeye (red) in transplanted CiPCs (green). The bottom panel shows a magnified version of the image (arrowhead). (d) Close apposition of transplanted CiPCs (green) with PKC-positive (red) rod bipolar cells (white arrowhead). (e) Expression of synaptic marker protein synaptophysin (red, arrowhead) in transplanted CiPCs (green) (co-localization, yellow). (d-e) Left, images at 20× magnification; right, images at higher digital magnification. In (a–e), experiments were repeated twice with similar results. Scale bars, 10 μm.
Extended Data Figure 5.
Effect of NF-κB knockdown, H2 O 2, TNFα and the overexpression of Ascl1 on the reprogramming of fibroblasts to CiPCs. (a) qPCR analysis following shRNA-mediated knockdown (kd) of RelA (which encodes NF-κB) in MEFs. (b) qPCR analysis of Ascl1 expression after the reprogramming of RelA-depleted MEFs on day 11. (c) Number of Nrl–GFP cells after the conversion of RelA-knockdown MEFs on day 11. (d) Effect of different concentrations of H2O2, generated by galactose and galactose oxidase (GAO), on the reprogramming of fibroblasts to CiPCs. (e) Effect of cytokine (TNF, 50 ng ml−1) on CiPC conversion. (f) qPCR analysis showing the overexpression (OE) of Ascl1 in MEFs. (g) Phase contrast micrographs of MEFs overexpressing Ascl1 before and after reprogramming in inducing medium. No Nrl–GFP + cells were evident up to day 15. (h) Fluorometric measurement of mROS in Ascl1-overexpressing cells on day 8 of reprogramming. Data are presented as mean ± s.e.m. of n = 3 independently treated wells. Statistical significance was assessed using a two-tailed Student’s t-test.
Extended Data Figure 6.
NF-κB–luciferase activity and generation of mROS during the reprogramming of fibroblasts to CiPCs. (a) A luciferase activity assay reveals decreased activation of NF-κB upon treatment with MitoTEMPO and increased NF-κB activity upon depletion of TFAM on day 11 of reprogramming to CiPCs. (b) Luciferase activity shows decreased NF-κB activation in Axin2-knockdown MEFs on day 8 and day 11 of reprogramming. SM, small molecules. (c) MEFs stained with MitoSOX showed low levels of mROS production (scale bar, 33 μm). (d) Plot of mROS accumulation on day 8, as visualized using fluorescence microscopy after MitoSOX staining, in wild-type (WT) Nrl–GFP MEFs treated with all compounds (top), Axin2-knockdown Nrl–GFP MEFs treated with all compounds (middle) and control Nrl–GFP MEFs (bottom). (e) qPCR analysis following Tfam depletion in MEFs using shRNA. (f) Fluorometric measurement of mROS (after MitoSOX staining) in MitoTEMPO-treated and TFAM-knockdown cells on day 11. (g) ChIP assay shows reduced binding of activated NF-κB near the Ascl1 locus upon treatment with MitoSOX. In (a-b, e–g) data are presented as mean ± s.e.m. of n = 3 independently treated wells. Statistical significance was assessed using a two-tailed Student’s t-test.
Extended Data Figure 7.
Mitochondrial localization of AXIN2 in converted cells and a mito-stress test for CiPCs. (a) Western blot after subcellular fractionation of CiPCs showed that AXIN2 was localized in the mitochondria. C, cytoplasm; M, mitochondria. For gel source data, see Supplementary Fig. 1e-f. (b) Micrograph of AXIN2 (red)- and GFP (green)-stained CiPCs after z-stack and 3D reconstruction, also showing that AXIN2 localizes in the mitochondria. In a, b experiments were performed once. (c) CiPCs were found to have a reduced oxygen consumption rate (OCR) compared with MEFs and day-8 reprogramming intermediates, as measured using a Seahorse assay. Oligo, oligomycin; FCCP, carbonyl cyanide-4-phenylhydrazone. (d) The indicated mitochondrial parameters of CiPCs, quantified in terms of OCR using a Seahorse assay, compared with those of MEFs and day-8 reprogramming intermediates. (e) The extracellular acidification rate (ECAR) of CiPCs, MEFs and day-8 reprogramming intermediates was measured using a Seahorse assay. (f) The indicated mitochondrial parameters of CiPCs, quantified in terms of ECAR using a Seahorse assay, compared with those of MEFs and day-8 reprogramming intermediates. In c–f, data are presented as mean ± s.e.m. of n = 3 independently treated wells.
Extended Data Figure 8.
Conversion of human fibroblasts into CiPCs and in vivo functional testing of mouse CiPCs. (a) qPCR analysis shows expression of the indicated photoreceptor-specific genes in CiPCs. (b) Fluorescence microscopy image demonstrates the expression of NRL in CiPCs. a, b, HADF from patients with retinitis pigmentosa. This experiment was performed three times with similar results. (c) Confocal microscopy image of the retina of an rd1 mouse, immunostained with GFP (red) and PNA (peanutagglutinin, green), three months after transplantation of CiPCs. (d) Left, confocal microscopy image of a retinal section showing GFP-positive CiPCs three months after transplantation. This experiment was repeated twice with similar results. Right, conversion of human HFL1s (original protocol, see Methods) and a modified protocol using HADFs (Fig. 1f) to CiPCs. (e) The correlation between the number of transplanted CiPCs and the extent of pupil constriction. n = 6 eyes classified as pupil responders and n = 8 eyes classified as pupil non-responders. (f) CiPC survival in the eyes of rd1 mice classified as pupil responders and pupil non-responders. The n values denote the number of eyes. In a, d, e, data are presented as mean ± s.e.m. of n = 3 independently treated wells.
Extended Data Figure 9.
Gene expression and chromatin analysis of CiPCs. (a) Pairwise gene expression comparison of RNA-seq data demonstrates that neuronal markers are increased, glial markers are not activated, and fibroblast genes are silenced. Numbers on both axes represent logarithmic base 2-transformed counts per million values for each gene. (b) Heat map analysis (from RNA-seq data) of photoreceptor genes along with other neuronal and fibroblast genes. (c) Fibroblast-specific gene expression as determined by RNA-seq during reprogramming. The expression of Col1a1, Col2a1, Thy1, Ctgf, S100a4 (also known as Fsp1) and Slc17a5 is reduced in reprogramming intermediates as well as in CiPCs. (d) Pie charts of results of ATAC–seq analysis show increased open chromatin corresponding to the proximal promoter region in reprogramming intermediates. (e) Principal component analysis of ATAC–seq data shows open chromatin regions for the indicated samples. (f) ATAC–seq analysis shows opening of specific chromatin regions for the indicated photoreceptor-specific loci (Ascl1, Rho, Gngt1 and Thrb). All data are representative of three independently treated samples.
Extended Data Figure 10.
Schematic of the transplantation study and the mechanism of reprogramming fibroblasts to CiPCs. (a) Schematic of reprogramming to CiPCs and functional analysis. (b) Schematic of the mechanism of reprogramming fibroblasts to CiPCs.
Supplementary Material
Acknowledgements
S.H.C. is supported by the Nancy Lee and Perry R. Bass Endowment, Foundation Fighting Blindness, and NEI awards EY021171 and EY025667. A.S. is supported by NEI Intramural Research Program (ZIAEY000450, ZIAEY000474 and ZIAEY000546). S.M. and S.B. are supported by NEI awards EY025905 and EY025717. T.M. is supported by 2T32AG020494-16A1. We thank A. Quiambao for performing the in vivo rodent injection studies, A. Ganguly for pupillometry assistance, the Histology Research Core at the University of North Carolina at Chapel Hill, and L. Gieser of NNRL, NEI for performing RNA-seq.
References:
- 1.Wright AF, Chakarova CF, Abd El-Aziz MM & Bhattacharya SS Photoreceptor degeneration: genetic and mechanistic dissection of a complex trait. Nat. Rev. Genet 11, 273–284 (2010). [DOI] [PubMed] [Google Scholar]
- 2.Bramall AN, Wright AF, Jacobson SG & McInnes RR The genomic, biochemical, and cellular responses of the retina in inherited photoreceptor degenerations and prospects for the treatment of these disorders. Annu. Rev. Neurosci 33, 441–472 (2010). [DOI] [PubMed] [Google Scholar]
- 3.Schwartz SD et al. Human embryonic-stem cell derived retinal pigment eputhellium in patients with age-related macular degeneration and Stagardt’s macular dystrophy: follow up of two open-label phase 1/2 studies. Lancet 385, 509–516 (2015). [DOI] [PubMed] [Google Scholar]
- 4.Dyer MA An eye on retinal recovery. Nature 540, 350–351 (2016). [DOI] [PubMed] [Google Scholar]
- 5.Mellough CB, Sernagor E, Moreno-Gimeno I, Steel DH & Lako M Efficient stage-specific differentiation of human pluripotent stem cells toward retinal photoreceptor cells. Stem Cells 30, 673–686 (2012). [DOI] [PubMed] [Google Scholar]
- 6.Zhong X et al. Generation of three-dimensional retinal tissue with functional photoreceptors from human iPSCs. Nat. Commun 5, 4047 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gamm DM & Wright LS From embryonic stem cells to mature photoreceptors. Nat. Biotechnol 31, 712–713 (2013). [DOI] [PubMed] [Google Scholar]
- 8.Völkner M et al. Retinal organoids from pluripotent stem cells efficiently recapitulate retinogenesis. Stem Cell Reports 6, 525–538 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fu Y et al. Direct reprogramming of mouse fibroblasts into cardiomyocytes with chemical cocktails. Cell Res 25, 1013–1024 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tian E et al. Small-molecule-based lineage reprogramming creates functional astrocytes. Cell Rep. 16, 781–792 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vierbuchen T et al. Direct conversion of fibroblasts to functional neurons by defined factors. Nature 463, 1035–1041 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Babos K & Ichida JK Small molecules take a big step by converting fibroblasts into neurons. Cell Stem Cell 17, 127–129 (2015). [DOI] [PubMed] [Google Scholar]
- 13.Zhang L et al. Small molecules efficiently reprogram human astroglial cells into functional neurons. Cell Stem Cell 17, 735–747 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ueki Y et al. Transgenic expression of the proneural transcription factor Ascl1 in Müller glia stimulates retinal regeneration in young mice. Proc. Natl Acad. Sci. USA 112, 13717–13722 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jorstad NL et al. Stimulation of functional neuronal regeneration from Müller glia in adult mice. Nature 548, 103–107 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pollak J et al. ASCL1 reprograms mouse Müller glia into neurogenic retinal progenitors. Development 140, 2619–2631 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Akimoto M et al. Targeting of GFP to newborn rods by Nrl promoter and temporal expression profiling of flow-sorted photoreceptors. Proc. Natl Acad. Sci. USA 103, 3890–3895 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim JW et al. NRL-regulated transcriptome dynamics of developing rod photoreceptors. Cell Rep. 17, 2460–2473 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Osakada F et al. Toward the generation of rod and cone photoreceptors from mouse, monkey and human embryonic stem cells. Nat. Biotechnol 26, 215–224 (2008). [DOI] [PubMed] [Google Scholar]
- 20.Khanna H et al. Retinoic acid regulates the expression of photoreceptor transcription factor NRL. J. Biol. Chem 281, 27327–27334 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Onishi A et al. Pias3-dependent SUMOylation directs rod photoreceptor development. Neuron 61, 234–246 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fu Y et al. Feedback induction of a photoreceptor-specific isoform of retinoid-related orphan nuclear receptor β by the rod transcription factor NRL. J. Biol. Chem 289, 32469–32480 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim JW et al. Recruitment of rod photoreceptors from short-wavelength-sensitive cones during the evolution of nocturnal vision in mammals. Dev. Cell 37, 520–532 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jia L et al. Retinoid-related orphan nuclear receptor ROR is an early-acting factor in rod photoreceptor development. Proc. Natl Acad. Sci. USA 106, 17534–17539 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brzezinski JA IV, Kim EJ, Johnson JE & Reh TA Ascl1 expression defines a subpopulation of lineage-restricted progenitors in the mammalian retina. Development 138, 3519–3531 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhu J, Cifuentes H, Reynolds J & Lamba DA Immunosuppression via loss of IL2rγ enhances long-term functional integration of hESC-derived photoreceptors in the mouse retina. Cell Stem Cell 20, 374–384.e5 (2017). [DOI] [PubMed] [Google Scholar]
- 27.Lin B, Koizumi A, Tanaka N, Panda S & Masland RH Restoration of visual function in retinal degeneration mice by ectopic expression of melanopsin. Proc. Natl Acad. Sci. USA 105, 16009–16014 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nishiguchi KM et al. Gene therapy restores vision in rd1 mice after removal of a confounding mutation in Gpr179. Nat. Commun 6, 6006 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pang ZP et al. Induction of human neuronal cells by defined transcription factors. Nature 476, 220–223 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Swaroop A, Kim D & Forrest D Transcriptional regulation of photoreceptor development and homeostasis in the mammalian retina. Nat. Rev. Neurosci 11, 563–576 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang Y et al. Nuclear factor kappa B signaling initiates early differentiation of neural stem cells. Stem Cells 30, 510–524 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Formentini L, Sánchez-Aragó M, Sánchez-Cenizo L & Cuezva JM The mitochondrial ATPase inhibitory factor 1 triggers a ROS-mediated retrograde prosurvival and proliferative response. Mol. Cell 45, 731–742 (2012). [DOI] [PubMed] [Google Scholar]
- 33.Andreakos E et al. Distinct pathways of LPS-induced NF-kappa B activation and cytokine production in human myeloid and nonmyeloid cells defined by selective utilization of MyD88 and Mal/TIRAP. Blood 103, 2229–2237 (2004). [DOI] [PubMed] [Google Scholar]
- 34.Vernochet C et al. Adipose-specific deletion of TFAM increases mitochondrial oxidation and protects mice against obesity and insulin resistance. Cell Metab. 16, 765–776 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim H et al. Modulation of β-catenin function maintains mouse epiblast stem cell and human embryonic stem cell self-renewal. Nat. Commun 4, 2403 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shin JH, Kim HW, Rhyu IJ & Kee SH Axin is expressed in mitochondria and suppresses mitochondrial ATP synthesis in HeLa cells. Exp. Cell Res 340, 12–21 (2016). [DOI] [PubMed] [Google Scholar]
- 37.Rehemtulla A et al. The basic motif-leucine zipper transcription factor Nrl can positively regulate rhodopsin gene expression. Proc. Natl Acad. Sci. USA 93, 191–195 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morrow EM, Furukawa T, Lee JE & Cepko CL NeuroD regulates multiple functions in the developing neural retina in rodent. Development 126, 23–36 (1999). [DOI] [PubMed] [Google Scholar]
- 39.Ortin-Martinez A et al. A reinterpretation of cell transplantation: GFP transfer from donor to host photoreceptors. Stem Cells 35, 932–939 (2017). [DOI] [PubMed] [Google Scholar]
- 40.Singh MS et al. Reversal of end-stage retinal degeneration and restoration of visual function by photoreceptor transplantation. Proc. Natl Acad. Sci. USA 110, 1101–1106 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhou G, Meng S, Li Y, Ghebre YT & Cooke JP Optimal ROS signaling is critical for nuclear reprogramming. Cell Rep. 15, 919–925 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shadel GS & Horvath TL Mitochondrial ROS signaling in organismal homeostasis. Cell 163, 560–569 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cagin U, Duncan OF, Gatt AP, Dionne MS, Sweeney ST & Bateman JM Mitochondrial retrograde signaling regulates neuronal function. Proc. Natl Acad. Sci. USA 112, E6000–E6009 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jozefczuk J, Drews K & Adjaye J Preparation of mouse embryonic fibroblast cells suitable for culturing human embryonic and induced pluripotent stem cells. J. Vis. Exp 3854 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen HY et al. Three-dimensional retinal organoids from mouse pluripotent stem cells mimic in vivo development with enhanced stratification and rod photoreceptor differentiation. Mol. Vis 22, 1077–1094 (2016). [PMC free article] [PubMed] [Google Scholar]
- 46.Kaya KD et al. Transcriptome-based molecular staging of human stem cell-derived retinal organoids uncovers accelerated photoreceptor differentiation by 9-cis retinal. Mol. Vis 25, 663–678 (2019). [PMC free article] [PubMed] [Google Scholar]
- 47.Wojtala A et al. Methods to monitor ROS production by fluorescence microscopy and fluorometry. Methods Enzymol. 542, 243–262 (2014). [DOI] [PubMed] [Google Scholar]
- 48.Tormos KV et al. Mitochondrial complex III ROS regulate adipocyte differentiation. Cell Metab. 14, 537–544 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.