Abstract
Development of brain therapeutics is significantly hampered by the presence of the blood-brain barrier (BBB). Classical transwell models are able to recapitulate many important aspects of drug transport across the BBB, but are not completely predictive of in vivo brain uptake. Species differences further complicate translation of experimental therapeutics from the benchtop to the clinic. Human BBB models offer some solutions to this problem, and by increasing device complexity both in terms of multicellularity, flow and physical architecture, physiological models of the BBB have been developed that can more faithfully model different aspects of transport and homeostasis BBB. Using these models, it may be possible to improve the predictive capacity in benchmarking candidate therapeutics, and to identify new druggable targets by studying multicellular interactions.
Introduction
It is well established that the brain is a difficult target for drug development, with a high frequency of preclinical and clinical failures compared with other organs [1]. This is due in large part to the presence of the blood-brain barrier (BBB), formed by brain endothelial cells (BECs), that strictly controls transport into the brain [2]. While BECs form the principal barrier, interactions with other cells of the neurovascular unit (NVU), including astrocytes and pericytes, are crucial to establish and regulate barrier properties in BECs [3]. As a result, the BBB limits the brain uptake of the vast majority of small molecules and biologics [2]. Therefore, a major obstacle in the brain drug development process is the successful transitioning of a therapy from an in vitro model, to pre-clinical in vivo models, and subsequently to human clinical trials [4, 5]. A more physiologically accurate in vitro model that, in turn, more faithfully recapitulates the properties of the human BBB would be helpful in the translation of experimental medicines to human patients. As such, the development of new and improved BBB models has been a major push for researchers in the field.
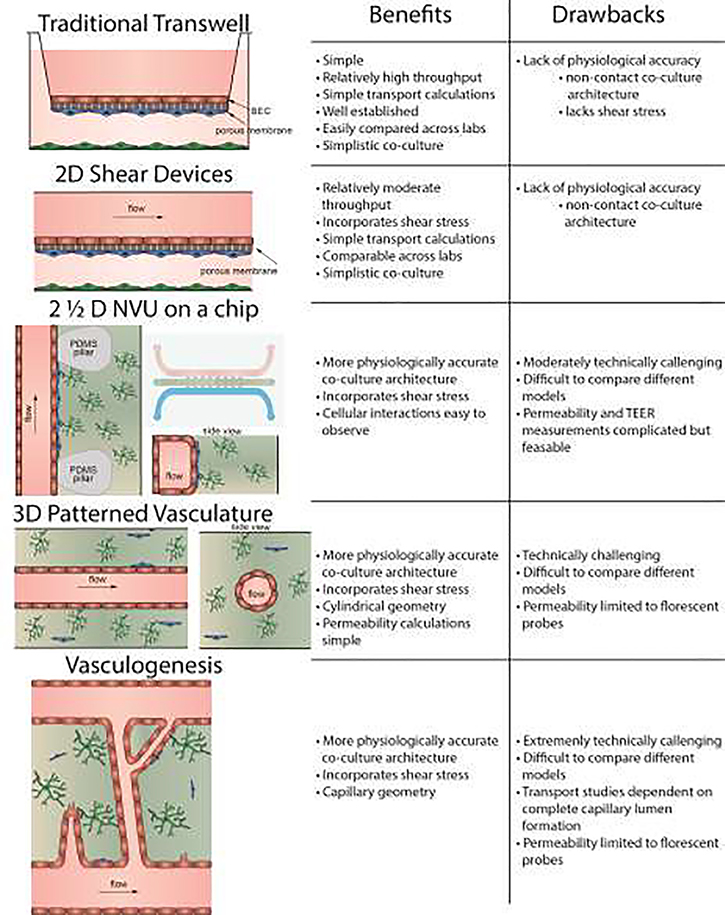
To date, in vitro BBB modeling has largely been focused on two compartment transwell models, where BECs are grown as a monolayer on a porous membrane often with astrocytes, pericytes, or other neural cells grown in the basolateral compartment (Figure 1). These facile models allow for moderate throughput, can be composed entirely of human cells, and can in some instances be predictive of in vivo permeability [6]. There are, however, a number of physiological cues that may increase the predictive quality of BBB models including shear flow, NVU cell contact, growth medium composition, and vascular geometry. Inclusion of such model refinements has been shown to increase efflux pump activity and expression of junctional markers, and improve responses to vascular stress [7–9]. Increasing physiological accuracy also likely increases the predictive potential of BBB models, improving the chance of downstream success in drug development.
Figure 1.
Schematic illustration of the categories of in vitro BBB models described in this review, and a comparison of some of their advantages and disadvantages.
Increased physiological accuracy in BBB models goes hand in hand with increased technical complexity. While there are many layers of complexity that can be built into an in vitro model, each must be carefully considered, and not all will be required of any given application. In this review, we will focus on these BBB model alternatives to the traditional transwell and their application in drug delivery and design. While cell sourcing and the effects of BEC co-culture with various NVU cell types are important considerations for the development of such models, these topics have been extensively reviewed elsewhere and will not be the focus here [3]. Instead, this review will spotlight the effects that physiological cues such as shear stress, direct contact co-culture architectures, and vascular geometry have on BBB model quality. We will consider four categories of in vitro BBB models organized in order of increasing physiological accuracy: 2D shear devices, 2½ D NVU on a chip models, 3D patterned vasculature models, and vasculogenesis models (Figure 1). As described below, each of these models has specific advantages and disadvantages compared with the traditional transwell model, and can oftentimes offer differing insights into the mechanisms of transport and homeostasis at the BBB.
2D devices incorporating shear
BBB models incorporating fluid shear forces often comprise microfluidic devices having endothelial cells cultured in 2D, with a secondary compartment often containing supporting cell types (Figure 1). These devices have the most in common with the traditional transwell model. Commonly, these chips are composed of a porous membrane sandwiched between polymeric, PDMS channels and bonded to a glass slide. BECs are then cultured on top of the porous membrane, and supporting cells such as astrocytes and pericytes are grown in the bottom chamber, similar to the set-up of a standard transwell. Flow is typically connected to the upper chamber to apply fluid shear stress to the BECs, but may also be connected to the basolateral chamber depending on device design. In addition to having fluid shear stress, these models differ from the traditional transwell in that they are more customizable, and have been designed so that they can be more readily imaged. An additional benefit of these devices is that shear flow over the endothelial cells prevents the formation of an unstirred boundary layer directly over the endothelial cells, while this is often combated in traditional transwells by placing the plate on rotator, it is an easily overlooked step and can result in inaccuracies in measured transport. These devices are relatively common and easily accessible, with commercially available chip options that are easily adapted to multiple cell sources, requiring minimal specialized techniques or equipment [10–12]. Despite the relatively simple geometry, the incorporation of shear in microfluidic devices can be an important upgrade over the standard transwell model. For instance, the transcriptome and proteome of BECs is modified under shear stress with the expression of BBB-relevant ion channels and transporters being upregulated under shear [8]. Shear has also been shown to increase passive barrier characteristics of BECs through elevated transendothelial electrical resistance (TEER) and decreased small molecule permeability, increase efflux transporter function, and improve tight junction morphology [13, 14]. Thus, shear can play an important role in evaluating transport at the BBB.
2D devices incorporating shear are commonly used in low to moderate throughput screening applications (2–10 compounds of interest) that both require shear and the fundamental BBB characteristics of classical transwells [15, 16]. Jeong and colleagues have developed a platform of an array of 4×4 microfluidic channels on a chip that could potentially allow for the investigation of four different molecules or concentrations, with four replicates on the same chip. Outputs include TEER and measuring transport of the molecule(s) of interest [15]. Consequently, these models have often been employed to measure solute permeability, barrier integrity, and cytotoxicity, with similar assay structures to the traditional transwell model. The impact of NVU cell co-culture, media composition, inflammation, and other physical parameters such as cell morphology or turnover can also be readily measured in 2D shear devices [17, 18]. 2D BBB chips can also be linked to other chips modeling another physiological system creating a multi-tissue device. For example, sunitinib, a chemotherapeutic, was introduced to the apical side of a BBB chip and the basolateral compartment was connected to another organ on a chip modeling the brain parenchyma containing glioma cells. The cytotoxic effects of sunitinib on the glioma cells were assessed, before finally passing into a third compartment where samples were collected for mass spectrometry and drug concentration analyzed [16]. This example demonstrates that these 2D devices can be multifunctional, modeling multiple stages of delivery and distribution simultaneously.
One of the major advantages of the 2D microfluidic technology is the ability to more readily measure nanoparticle permeability as nanoparticles often get trapped in the filter or along the side walls of the transwell [19]. Using the 2D setup with shear minimizes nonspecific nanoparticle binding, and facilitates the measurement of the nanoparticle localization rather than measuring exact concentrations; thereby, allowing more mechanistic understanding of the transport and trafficking of nanoparticles than one could achieve from a transwell device [7, 20, 21]. For example, using a sheared 2D device with BECs cultured on a porous membrane with pericytes and astrocytes in the basolateral compartment, Ahn and colleagues were able leverage the in vitro BBB model to help interpret an in vivo study and determined that the receptor-mediated SR-B1 protein mediated both receptor-mediated transcytosis of the nanoparticles through the BECs, and subsequent uptake in astrocytes and pericytes [20]. This type of experiment is greatly enhanced by the ability of the researcher to use confocal microscopy to localize the particles in 2D microfluidic devices and this would be much more difficult in a standard transwell or in vivo experiment. Similarly, 2D shear devices can also be utilized to observe immune cell trafficking, as this phenomenon is known to be greatly impacted by flow. In fact, using live cell microscopy, the path of individual T cells can be tracked as they undergo transcytosis across an in vitro BBB [22].
2D shear devices allow for many of the same assays that can be performed in the traditional transwell assay, but the addition of shear allows further insight into transport mechanisms that are influenced by shear, and can be more readily imaged with live-cell microscopy. They are however lower throughput than the traditional transwell, and while they are starting to be more commonly deployed as a standard research tool, there is still little standardization and platform designs vary widely (Table 1).
Table 1:
2D shear
| Cell Sources | Key Features and Findings | Media Composition | Shear Stress | Culture Support | Reference |
|---|---|---|---|---|---|
| • iPSC derived BECs • Primary rat astrocytes |
• Oscillatory shear stress used • Enabled the study of metabolites due to smaller media volume/cell ratio |
EC growth medium | 0 – 1.8 dyne cm−2 | Polycarbonate membrane 0.4 μm pore size coated with collagen IV and fibronectin | [17] |
| • Human immortalized BECs • Primary human Astrocytes and pericytes |
• BEC phenotype shifted with co-culture • Nanoparticles localized after injection into chip |
EC growth medium | 4 dyne cm−2 | Polycarbonate membrane 8 μm pore size Fibronectin coated | [20] |
| • Immortalized murine BECs | • Measured transport of nanoparticles across the membrane | EC growth medium | 0.15 dynes/cm2 | Polyester filter 3 μm pores | [21] |
| • Human primary BECs | • Showed the effect of electrical pulses on permeability | EC growth medium | 18% variation across parallel channels | 0.4 μm pore polyester membrane coated with fibronectin | [18] |
| • Immortalized murine BECs | • Shear increased permeability and claudin 5 expression • Visualized localization of nanoparticles with confocal |
EC growth medium | 1 and 6 dynes/cm2 | Polycarbonate membrane 1 μm pore size coated with collagen IV and fibronectin | [7] |
| • Immortalized human BECs • Primary human astrocytes |
• Studied patient specific astrocytes • Measured T-cell migration |
Growth medium | Polycarbonate membrane 3 μm pore size collagen 1 coated | [22] | |
| • Immortalized human BECs • Glioma |
• Platform designed for drug screens • Measured permeability, cytotoxicity, and treatment potential of drug on one chip |
Growth medium | 10 μL min−1 | Porous polycarbonate membrane | [16] |
| • Immortalized human BECs • Primary rat pericytes and astrocytes |
• Adapted for different organs • Measured permeability and TEER in a chip |
EC growth medium supplemented with hydrocortisone, insulin, transferrin, and sodium selenite | 0.15 dyne | PET membrane 0.45 μm pore size | [10] |
| • Primary mouse BECs and astrocytes | • 4×4 chip enabled technical replicates on a single chip • Measured TEER and histamine response |
EC growth medium | 1 – 30 dyne cm−2 | Polycarbonate membrane 0.4 μm pore size Fibronectin or Matrigel coated | [15] |
| • Immortalized murine BECs and pericytes • Primary murine astrocytes |
• Measured TEER, permeability, efflux | EC growth medium | Polyester membrane 0.4 μm pore size Fibronectin coated | [11] | |
| • BECs, astrocytes, neurons, and microglia | • Microbiota, gut, and brain chips coupled • Introduced model |
Introduction of model, no details given | [12] | ||
| • Porcine primary BECs • Rat primary astrocytes |
• Shear stress increased TEER andefflux | EC growth medium | 0 – 110 × 10−6 dyne cm−2 | Porous membrane | [13] |
2½ D NVU on a chip
The increase in complexity from 2D shear devices to the 2 ½ D NVU on a chip is significant. Where 2D shear devices contain BECs separated from any supporting cell types by a membrane, the 2 ½ D NVU systems often contain a 3D extracellular matrix (ECM) compartment with supporting cell types, commonly astrocytes and pericytes, and a rectangular BEC compartment separated by PDMS pillars [23] (Figure 1). These devices are called 2 ½ D as the geometry of the cultured BECs is still functionally monolayers, with the BECs forming a rectangular channel of flat 2 D monolayers along the surface of the ECM and surrounding PDMS device. NVU on a chip models are becoming increasingly common, but are currently even less standardized than the aforementioned 2D sheared devices. NVU on a chip models require a greater degree of technical expertise and access to specialized equipment for device fabrication, although commercial devices are becoming more widely available [24, 25]. As a result, the 2 ½ D NVU models are largely in the proof-of-concept stage, and published studies have fittingly focused primarily on model development and initial validation (Table 2).
Table 2:
2 ½ D NVU on a chip
| Cell Sources | Key Features and Findings | Media Composition | Shear Stress | Culture Support | Reference |
|---|---|---|---|---|---|
| • iPSC derived BECs • Primary human astrocytes and pericytes |
• Incorporated hypoxia in differentiation of BECs • Measured transcytosis of angiopep-2 quantum-dots and anti-TfR antibody |
EC growth medium | 60 – 100 μL hr−1 | PET membranes 0.4 μm pore size Coated with collagen IV and fibronectin | [29] |
| • HUVEC • Human primary astrocytes |
• BEC channel separated from ECM compartment by PDMS pillars | EC growth medium | 0.089 dyne cm−2 | 2.5 mg mL−1 collagen 1 gel | [23] |
| • Endothelial cells • Astrocytes • Glioma |
• Astrocytes cultured in hydrogel interface with vascular channel • Brain metastasis modeled, both invasion from vasculature to ECM and reverse with growth of glioma in ECM |
EC growth medium | 0.1 dyne cm−2 | 6 mg mL−1 collagen 1 | [26] |
| • Primary human BECs, astrocytes, and pericytes • Neural stem cell derived neurons |
• 2 parallel BEC compartments with parenchyma/CSF compartment • Protein expression in BEC and CSF coupled chips • Metabolites measured and mapped |
Growth medium | 1 μL min−1 | Laminin coated polycarbonate | [28] |
| • HUVEC • Rat primary astrocytes |
• Rectangular channel of ECs in a ring surrounding a disk of cancer cells/astrocytes • Inclusion of tumor cells increased BEC permeability |
EC growth medium | 1.9 × 10−3 dyne cm−2 | Matrigel or fibronectin coated device | [25] |
| • Immortalized human BECs • Primary human astrocytes |
• Commercial device • Nanoparticle size and shape impacted permeability |
EC growth medium | 5 μL min−1 | Fibronectin coated device | [27] |
| • HUVEC and immortalized human BECs • Rat primary neurons and astrocytes |
• BEC channel adjacent to astrocyte compartment • Astrocyte compartment adjacent to neuronal compartment • Measured functional permeability using glutamate transport and neuronal response |
EC growth medium | No shear | Collagen I 7 2.5 mg mL−1, and collagen I coating | [32] |
| • Immortalized human BECs, pericytes, and astrocytes | • Commercially available plate style device • ECM separating two BEC rectangular channels |
EC growth medium | Rocked between + 7° and − 7° over 8 minutes | 4 mg mL−1 collagen I | [24] |
| • iPSC derived neural cultures and BECs • primary human astrocytes and pericytes |
• Rectangular EC channel • Permeability of dextran, IgG, albumin, transferrin, and efflux activity • Barrier disruption in response to inflammatory cytokines • RNA-seq differences between BECs with and without shear |
EC growth medium or whole blood | 0.01 – 5 dyne cm−2 | PDMS membrane 7 μm pore size coated with laminin, collagen IV, and fibronectin | [14] |
One potentially significant advantage is the lack of a BEC-supporting membrane between compartments enabling the direct contact, cellular interactions between BECs and other NVU cells. This distinct advantage is enabling for experiments that require NVU cell contact or dynamic cellular behavior such as migration, metastasis [26], or nanoparticle trafficking studies [27]. As one example, using NVU on a chip models, cancer cells may either be introduced into the endothelial flow channel, or incorporated in the ECM, thus allowing both extravasation of a metastatic tumor as well as the growth and invasion of a primary tumor to be studied [26]. In this way, Xu and colleagues leveraged time lapse microscopy to follow cancer cell fate after introducing chemotherapeutics into the endothelial flow channel to compare efficacy of different chemotherapeutics on cancer cells that had extravasated into the ECM [26]. As another example of the very broad applications one could envision for an NVU on a chip model, Maoz and colleagues used a three compartment NVU chip, with a BEC compartment where compounds of interest are added, an ECM parenchyma/cerebral spinal fluid (CSF) compartment containing NVU cell types, and finally a second BEC compartment to model clearance of the compounds of interest to study both the penetration and clearance of molecules in the brain. Using this setup, they were able to investigate the metabolic communication between the NVU and surrounding parenchymal neurons after methamphetamine administration, and found that the incorporation of the parenchymal/CSF compartment significantly altered the proteome of the BECs, pericytes, astrocytes, and neuronal cells composing the NVU. These approaches can easily be applied to other molecules of interest, helping to distinguish the NVU and parenchymal contributions to drug metabolism [28].
The applications of the 2 ½ D NVU chips has been focused predominately on transport of small molecules, antibodies, and nanoparticles [29]. However, TEER and permeability measurements are complicated by the geometry of the chip. Since TEER measures the of the flux of ions across a monolayer of BECs, it is critical that these measurements are normalized by the surface area of the monolayer itself, or one cannot compare values across different models [30]. Additionally, it is more complicated when studying molecular transport across the BECs as the molecules enter the gel phase ECM compartment leading to sampling difficulty. For this reason, permeability of tracer molecules are often used, and fluorescent tracer permeability can be measured in nearly all platforms and can be a proxy measurement of endothelial integrity [31]. Researchers have often instead employed an “effective permeability”, and rather than focusing on the precise drug concentration that crosses the BECs, a downstream effect of the molecule is monitored. For example, using a model comprised of a rectangular channel of BECs, a first ECM compartment containing astrocytes, and a second ECM compartment containing neurons, the control of glutamate transport across BECs was evaluated by monitoring whether BEC presence would reduce neuronal calcium signaling when compared to a control lacking BECs [32]. However, when using “effective permeability” measures, it is important to consider the experimental timescales as there will always be some paracellular leakage through the BEC monolayer and at the edge of the fabricated devices, and thus, proper controls must be included.
Since NVU on a chip devices are currently in the early stages of development and fairly custom in their design, the learning curve can be substantial. Their distinct advantage is the more physiologically relevant cellular interactions and the maintenance of shear stress established in the 2D models, allowing for unique applications investigating more complex cellular interactions that can be readily imaged with live-cell microscopy. However, this category of models is very diverse, and to date, each device has been designed for a custom application.
3D patterned vascular geometry
While most 2 ½ D systems employ a rectangular or square channel, the next category of devices begins to additionally factor in BBB geometry by patterning cylindrical BEC vessels inside an ECM hydrogel (Figure 1). Substrate curvature is known to impact the phenotype of cells cultured in vitro, from lipid composition, to cell morphology, and polarization of key proteins [33], and this has also been observed for endothelial cells from other organs [34]. Thus, curvature is also an important biological variable to be considering when modeling the BBB.
The diameter of these patterned vessels range anywhere from 100 – 800 μm. They can be patterned with wires or needles or alternatively can be created using viscous fingering, a technique where a less viscous fluid is used to displace the more viscous hydrogel excising a roughly cylindrical channel. Often, astrocytes or pericytes are included in the ECM hydrogel surrounding the vessel, but patterning of these cells for vascular proximity is difficult, as they must be included in the hydrogel before gelation, and ultimately they may not end up closely associated with the BECs forming the channel [35]. In some models, in order to maintain a confluent monolayer of BECs adhered to the ECM, the surrounding ECM is crosslinked which also precludes the ability to include other cell types [36]. In some embodiments, a large fiber is used to pattern the vessels, and since it is not removed, the fiber can inhibit BEC interactions with the co-cultured cells in the surrounding ECM [37]. Overall, these devices require a higher level of technical expertise and are less well established than either of the aforementioned models.
As with the 2 ½ D NVU chips, the geometry of these devices makes TEER and permeability more difficult to measure and compare across different platforms. However, as the geometry of these devices is often pre-defined by the template used to make the vessels, permeability of a tracer molecule is commonly reported as it is relatively simple to model radial diffusion of molecules from a cylindrical source. Tracer permeability has thus far been limited to fluorescent markers that can be quantified in the ECM gel phase using confocal microscopy, as much like the 21/2 D NVU on a chip models, it is difficult to collect and quantitate the compounds that make it across the BECs to the surrounding ECM compartment [36, 38, 39]. In contrast, measuring TEER is technically difficult and rarely done in this format.
One of the major advantages of patterned vasculature devices is the ease of imaging and capability of capturing live cell events including cellular interactions, small fluorescent molecule transport, or nanoparticle trafficking. Using a microscope objective and time lapse microscopy while focusing of the BECs on the bottom of a 3D patterned vessel, cellular morphology, cell division and apoptosis can be monitored and quantified [36]. Additional assays well matched to these devices include the study of inflammation and immune activation [37, 40, 41]. For example, TNF-α, or other inflammatory cytokines can be added into the vessel lumen and the effect on white blood cell adhesion[36], permeability [37, 40], and protein expression [41] at the BBB can be observed. Using a gravity driven flow set up, Yu and colleagues used primary rat cells to construct a 300 – 400 μm diameter vessel. By measuring TEER and permeability of 40 kDa dextran they monitored barrier integrity and demonstrated that the vessel became leakier with the addition of TNF-α, an effect which could be prevented with BEC treatment with dexamethasone. They determined that the effects of dexamethasone were due to increased expression of occludin and decrease in release of IL-6 and CINC1 in response to TNF-α treatment [40]. Another emerging application for 3D patterned BBB models is their use as a model of transient BBB disruption by inflammation or hyperosmolarity, which could ultimately provide more physiologically relevant BBB models that could have utility in identifying molecules or pathways that could be used to reverse pathologic BBB disruption. As one example, BEC disruption in cylindrically patterned 3D models has been accomplished through the use of mannitol, which has been shown to induce transient barrier break down [36]. Performing these types of experiment in 3D patterned vessels offers addition insight into the disruption. For instance, using live cell microscopy, changes in vascular phenotype can be visualized including the formation of vacuoles or formation of transient focal leaks.
3D patterned vascular models are very much in their infancy, with the majority of studies focusing on simply establishing patent BEC vessels, and evaluating their properties. Having BECs in a defined cylindrical geometry, they offer the unique advantage of studying molecules diffusing radially from a source with well-defined geometry. An additional advantage is the ability to directly image and monitor the BECs as different molecules are applied to them. However, the diameter of the patterned vessels is significantly larger than the microvasculature in the brain, and direct contact co-culture is difficult or lacking in some of these models. Additionally, there is no standardization of models, with widely ranging vascular diameters and ECM composition based on the sourcing of BEC cells, and throughput of these models is very low (Table 3).
Table 3:
3D Patterned Vasculature
| Cell Sources | Key Features and Findings | Media Composition | Shear Stress | Culture Support | Reference |
|---|---|---|---|---|---|
| • Rat primary BECs, astrocytes, and pericytes | • Gravity driven flow • Measured BECs response to TNF-α treatment |
EC growth medium with 20% FBS | 50 – 100 μL hr−1 | Collagen I gel 300 – 400 μm diameter vessel | [40] |
| • Human immortalized BECs • Human primary astrocytes |
• Hollow PVDF fibers • Phalloidin stained actin filaments aligned with shear • VEGF, TNF-α, and verapamil increased permeability of tracer |
EC growth medium | 0, 0.77, 3, 17 dyne cm−2 | PDVF fibers 0.1 μm pore size | [37] |
| • Human primary BECs, astrocytes, and pericytes | • Large 500 – 700 μm vessels • Incorporation of astrocytes and pericytes decreased tracer permeability • 3D culture increased ability to respond to excitatory stimuli |
EC growth medium | 1 dyne cm−2 | 5 mg mL−1 collagen I gel | [41] |
| • Immortalized human BECs • Primary human astrocytes |
• Permeability and TEER measured in vessels • Measured the effect of mechanical deformation of gel on vascular integrity |
EC growth medium | 0.7 dyne cm−2 | 5 mg mL−1 collagen I, 1 mg mL−1 Matrigel, 2 mg mL−1 hyaluronan | [35] |
| • hPSC derived BECs | • Determined the role of ECM composition and stiffness in the formation of vessels | EC growth medium | 1 dyne cm−2 | Crosslinked 7 mg mL−1 collagen I gels coated with collagen IV and fibronectin | [38] |
| • hPSC derived BMECs | • Measured permeability and efflux activity • Recorded cell proliferation • Displayed transient disruption of BECs due to hyperosmolarity |
EC growth medium | 4 dyne cm−2 | Crosslinked 7 mg mL−1 collagen I gels coated with collagen IV and fibronectin | [36] |
| • hPSC derived BMECs • HUVEC |
• Permeability was stable over 2 weeks • Permeability decreased with shear stress |
EC growth medium | 100 μL min−1 | Gelatin and microbial transglutaminase coated with collagen IV and fibronectin | [39] |
3D vasculogenesis
Although patterned vascular models more accurately represent the cylindrical geometry of blood vessels, they have a lower limit of 30 – 50 μm diameter vessels, as patterning and seeding the resultant pattern at smaller diameters is not feasible. Thus, an argument could be made that the model may not be able to recapitulate all aspects of the brain microvasculature given the capillaries and post-capillary venules have diameters <30μm. The main strategy being employed to examine smaller vessel-type structures formed by BECs is based on in vitro vasculogenesis generated either by invasion of tip cells from a BEC monolayer into a compatible hydrogel, or self-assembly of BECs seeded within a hydrogel (Figure 1). Importantly, these devices require optimization of ECM composition and compliance to enable EC invasion and ECM degradation while maintaining structural stability required for long term culture and perfusion of nutrients. The hydrogel also needs to accommodate supporting cells, such as pericytes, within the gel to support the de novo vasculature [42]. For example, Uwamori and colleagues showed that the formation of neurites in a fibrin hyaluronan hydrogel gel was required before the hydrogel would be sufficiently stable to allow for vasculogenesis without the gel collapsing and being degraded too rapidly by BECs and mesenchymal stem cells [43].
Vasculogenesis models as still very much in their infancy in BBB modeling. Due to the complexity of these models and the difficulty of working with many BEC lines, they often incorporate endothelial cells from other tissues that are more angiogenic [44, 45]. There are however models that have been able to successfully integrate BECs into vasculogenesis models, but thus far the studies have been limited to the establishment and characterization of the models. We include the models here as they have potentially interesting applications in drug delivery and discovery (Table 4).
Table 4:
Vasculogenesis
| Cell Sources | Key Features and Findings | Media Composition | Culture Support | Reference |
|---|---|---|---|---|
| • iPSC derived generic ECs • Primary human astrocytes and pericytes |
• Permeability was feasible but complicated | EC growth medium | 3 mg mL−1 Fibrin gel | [45] |
| • HUVEC • Human lung fibroblasts • Primary E17 cortical rat neurites |
• Employed 3 separate channels using 3 separate medium compartments to maintain individual cell lines | EC growth medium | 2.5 mg mL−1 Fibrin gel | [44] |
| • HUVEC and primary human BECs • Primary human placental pericytes, lung fibroblasts, and astrocytes |
• Astrocytes and pericytes were determined to be important for vasculogenesis • Co-culture decreased permeability • ECM composition mediated cellular interaction |
EC growth medium | 2.5 mg mL−1 fibrin gel | [42] |
| • Neural stem cells hPSC derived • Human primary BECs • Human mesenchymal stem cells |
• Investigated neurite and capillary sprout interactions • ECM composition and cell seeding timing was critical |
EC growth medium | 2 mg mL−1 fibrin, 2 mg mL−1 hyaluronan, 2, 4, or 8 mg mL−1 Matrigel | [43] |
One example of well-developed BEC microvessels in a vasculogenesis platform was reported by Lee and colleagues. A mixture of BECs and pericytes was seeded along the surface of a fibrin gel seeded with astrocytes, and an additional compartment containing fibroblasts was present on the chip to assist in creating a chemotactic gradient to support angiogenesis. After 7 days, they were able to observe 30 μm diameter vessels with complete lumens spanning the fibrin gel and they were able to probe these vessels for permeability to 10 and 70 kDa dextrans and confirm the functionality of efflux transporters using Calcein-AM and P-gp/BCRP inhibitor elacridar [42].
One area of interest, particularly in the area of vasculogenesis, is the recent advancement of brain organoids. Vascularizing brain organoids is critical both for their growth and for the development of multicellular architecture. Recently, there have been advancements in vascularization of brain organoids with the incorporation of endothelial cells into the organoids [46, 47]. Although the overall vascular architecture is not controlled like the 3D vasculogenesis models described above, applying the lessons learned from organoid vascularization to the development of microfluidic based chips may prove beneficial.
Vasculogenesis models, despite their technical difficulty, are unique in their abilities; vasculogenesis is the only method that has been used to model capillary-sized vasculature. As these models are further developed, they will likely be able to provide important insight into transport mechanisms at the capillary level. However, it will be difficult to achieve direct measures of permeability in these models as a result of limited perfusion and complex ill-defined geometry of the de novo vasculature.
Summary
Here we have outlined the benefits of different BBB model systems, 2D shear stress devices, 2 ½ D NVU models, 3D patterned vessels, and vasculogenesis models. These devices range in technical complexity and physiological accuracy. 2D shear stress devices allow for many of the same experiments as the traditional transwell, but with the addition of shear stress. 2 ½ D NVU models focus on direct contact co-culture architecture, enabling the study of more complex cellular interactions while also including the shear stress introduced in the 2D chips. 3D patterned vessels start to consider the important role of curvature on endothelial cell phenotype and its role in transport across the BBB, returning the focus to the endothelial cells. Vasculogenesis models are almost a combination of the NVU on a chip and the patterned vessel, forming capillary diameter vessels within a soft hydrogel containing supporting NVU cells. Each of these models has specific advantages and applications best suited for their use and a summary of each of the models mentioned and their specific applications and components can be found in Tables 1–4. Here, we have summarized just a few of the considerations that are key to BBB modeling, and ultimately, additional areas of model refinement will likely include media composition (blood-relevance) [14], oxygen content (hypoxia) [29] and recirculation of culture medium (growth factor and metabolite concentrations).
The purpose of this review was not to suggest that the traditional transwell model is outdated or lacking, or to suggest that the most physiologically accurate and technically complex model of the BBB is required to model every aspect of drug delivery to the brain. Instead, we suggest that the most important part of selecting an in vitro BBB model is to determine what BBB attributes are necessary to accurately model for a specific application. For example, what level of shear stress is required for the model- is a high shear necessary (e.g. capillary shear)? If so, a 2D device is likely the best avenue. If shear is not really the most important aspect- Would a more moderate shear be sufficient (e.g. venous shear)? Such vetting of the more complex models is critical to conserve resources while still meeting experimental objectives. More complex does not always equate to the better model for the application as described throughout the review.
The use of in vitro BBB models for drug delivery and design experiments is not a new one. Outside of the BBB field, researchers have developed organ on a chip, or whole body on a chip model systems with the goal of being able to model all aspects of delivery and distribution of a therapeutic within the body. They have proven be a valuable tool for drug delivery and discovery in peripheral organs [48]. These models allow the complex interconnected nature of the organ system in the human body to be considered, and the incorporation of a BBB on a chip into such a system could lead to valuable insights into human pharmacodynamics. As of yet such systems typically focus on incorporating brain chips modeling the parenchyma [49], missing the important role of the BBB in protecting the brain. However, it is clear that there are labs working toward this goal, individually constructing and optimizing separate organs on a chip for incorporation into a larger system.
As BBB knowledge increases, development of more accurate and more complex in vitro systems will continue, as will the difficulty of selecting the appropriate model. One of the areas that seems likely to expand is the development of disease models of the BBB, particularly in the area of neurodegenerative diseases [50, 51]. Improved disease modeling is important for both drug discovery and delivery. Another important consideration is the standardization of BBB models. Currently, the lack of standardization makes comparison between models difficult, further complicating the progression of a potential therapeutic from benchtop to clinic. Improving our ability to model the BBB with physiological accuracy in relatively simple in vitro models is critical for the development of new brain therapies. There is no one size fits all in vitro BBB model, and it is still unclear which components of an in vitro BBB model are absolutely critical to recapitulate BBB function. Thus, as our knowledge of the in vivo BBB increases, we need to continue to advance the phenotype and reproducibility of in vitro BBB models.
Acknowledgements
The authors acknowledge financial support from the NIH NINDS R01NS099158, and support for MK through the NHGRI training grant to the Genomic Sciences Training Program 5T32HG002760.
Footnotes
Conflict of interest statement
Nothing declared.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gribkoff VK and Kaczmarek LK, The need for new approaches in CNS drug discovery: why drugs have failed, and what can be done to improve outcomes. Neuropharmacology, 2017. 120: p. 11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abbott NJ, et al. , Structure and function of the blood–brain barrier. Neurobiology of disease, 2010. 37(1): p. 13–25. [DOI] [PubMed] [Google Scholar]
- 3.Gastfriend BD, Palecek SP, and Shusta EV, Modeling the blood–brain barrier: beyond the endothelial cells. Current opinion in biomedical engineering, 2018. 5: p. 6–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lajoie JM and Shusta EV, Targeting receptor-mediated transport for delivery of biologics across the blood-brain barrier. Annual review of pharmacology and toxicology, 2015. 55: p. 613–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stanimirovic DB, et al. , Blood–brain barrier models: in vitro to in vivo translation in preclinical development of CNS-targeting biotherapeutics. Expert opinion on drug discovery, 2015. 10(2): p. 141–155. [DOI] [PubMed] [Google Scholar]
- 6.Heymans M, et al. , Mimicking brain tissue binding in an in vitro model of the blood-brain barrier illustrates differences between in vitro and in vivo methods for assessing the rate of brain penetration. European Journal of Pharmaceutics and Biopharmaceutics, 2018. 127: p. 453–461. [DOI] [PubMed] [Google Scholar]
- 7.Papademetriou I, et al. , Effect of flow on targeting and penetration of angiopep-decorated nanoparticles in a microfluidic model blood-brain barrier. PloS one, 2018. 13(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cucullo L, et al. , The role of shear stress in Blood-Brain Barrier endothelial physiology. BMC neuroscience, 2011. 12(1): p. 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rochfort KD and Cummins PM, In Vitro Cell Models of the Human Blood-Brain Barrier: Demonstrating the Beneficial Influence of Shear Stress on Brain Microvascular Endothelial Cell Phenotype, in Blood-Brain Barrier. 2019, Humana Press: New York, NY: p. 71–98. [Google Scholar]
- 10.Walter FR, et al. , A versatile lab-on-a-chip tool for modeling biological barriers. Sensors and Actuators B: Chemical, 2016. 222: p. 1209–1219. [Google Scholar]
- 11.Wang JD, et al. , Organization of endothelial cells, pericytes, and astrocytes into a 3D microfluidic in vitro model of the blood–brain Barrier. Molecular pharmaceutics, 2016. 13(3): p. 895–906. [DOI] [PubMed] [Google Scholar]
- 12.Raimondi MT, Albani D, and Giordano C, An Organ-On-A-Chip Engineered Platform to Study the Microbiota–Gut–Brain Axis in Neurodegeneration. Trends in molecular medicine, 2019. 25(9): p. 737–740. [DOI] [PubMed] [Google Scholar]
- 13.Elbakary B and Badhan RK, A dynamic perfusion based blood-brain barrier model for cytotoxicity testing and drug permeation. Scientific Reports, 2020. 10(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vatine GD, et al. , Human iPSC-derived blood-brain barrier chips enable disease modeling and personalized medicine applications. Cell stem cell, 2019. 24(6): p. 995–1005. e6. [DOI] [PubMed] [Google Scholar]
- 15.Jeong S, et al. , A three-dimensional arrayed microfluidic blood–brain barrier model with integrated electrical sensor array. IEEE Transactions on Biomedical Engineering, 2017. 65(2): p. 431–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shao X, et al. , Development of a blood-brain barrier model in a membrane-based microchip for characterization of drug permeability and cytotoxicity for drug screening. Analytica chimica acta, 2016. 934: p. 186–193. [DOI] [PubMed] [Google Scholar]
- 17.Wang YI, Abaci HE, and Shuler ML, Microfluidic blood–brain barrier model provides in vivo‐like barrier properties for drug permeability screening. Biotechnology and bioengineering, 2017. 114(1): p. 184–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bonakdar M, Graybill P, and Davalos R, A microfluidic model of the blood–brain barrier to study permeabilization by pulsed electric fields. RSC advances, 2017. 7(68): p. 42811–42818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Jong E, et al. , A filter-free blood-brain barrier model to quantitatively study transendothelial delivery of nanoparticles by fluorescence spectroscopy. Journal of Controlled Release, 2018. 289: p. 14–22. [DOI] [PubMed] [Google Scholar]
- 20.Ahn SI, et al. , Microengineered human blood–brain barrier platform for understanding nanoparticle transport mechanisms. Nature Communications, 2020. 11(1): p. 1–12.**The authors combine experiments in a 2D sheared device with human immortalized BMECs cultured on a porous membrane over human primary astrocytes and pericytes with in vivo experiments. Using confocal microscopy the authors were able to determine the target receptor and method of endocytosis in BECs used by a nanoparticulate delivery system.
- 21.Falanga AP, et al. , Shuttle‐mediated nanoparticle transport across an in vitro brain endothelium under flow conditions. Biotechnology and bioengineering, 2017. 114(5): p. 1087–1095. [DOI] [PubMed] [Google Scholar]
- 22.Lauranzano E, et al. , A Microfluidic Human Model of Blood–Brain Barrier Employing Primary Human Astrocytes. Advanced Biosystems, 2019. 3(7): p. 1800335. [DOI] [PubMed] [Google Scholar]
- 23.Nguyen PQH, et al. , Hybrid elastomer–plastic microfluidic device as a convenient model for mimicking the blood–brain barrier in vitro. Biomedical microdevices, 2019. 21(4): p. 90. [DOI] [PubMed] [Google Scholar]
- 24.Wevers NR, et al. , A perfused human blood–brain barrier on-a-chip for high-throughput assessment of barrier function and antibody transport. Fluids and Barriers of the CNS, 2018. 15(1): p. 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Terrell-Hall TB, et al. , Permeability across a novel microfluidic blood-tumor barrier model. Fluids and Barriers of the CNS, 2017. 14(1): p. 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu H, et al. , A dynamic in vivo-like organotypic blood-brain barrier model to probe metastatic brain tumors. Scientific reports, 2016. 6: p. 36670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nowak M, et al. , Size, Shape, and Flexibility Influence Nanoparticle Transport Across Brain Endothelium Under Flow. Bioengineering & Translational Medicine, 2019. 5(2): p. e10153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maoz BM, et al. , A linked organ-on-chip model of the human neurovascular unit reveals the metabolic coupling of endothelial and neuronal cells. Nature biotechnology, 2018. 36(9): p. 865–874.* The authors developed a multi-compartment NVU on a chip model, with vascular compartments bookending a parenchymal CSF compartment. This model is able to capture the majority of the process of drug delivery, distribution, and clearance within the brain and to measure the metabolic coupling of the CSF and BBB compartments.
- 29.Park T-E, et al. , Hypoxia-enhanced Blood-Brain Barrier Chip recapitulates human barrier function and shuttling of drugs and antibodies. Nature communications, 2019. 10(1): p. 2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Elbrecht DH, Long CJ, and Hickman JJ, Transepithelial/endothelial Electrical Resistance (TEER) theory and ap-plications for microfluidic body-on-a-chip devices. tc, 2016. 1(3): p. 46–52. [Google Scholar]
- 31.DeStefano JG, et al. , Benchmarking in vitro tissue-engineered blood–brain barrier models. Fluids and Barriers of the CNS, 2018. 15(1): p. 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Adriani G, et al. , A 3D neurovascular microfluidic model consisting of neurons, astrocytes and cerebral endothelial cells as a blood–brain barrier. Lab on a Chip, 2017. 17(3): p. 448–459. [DOI] [PubMed] [Google Scholar]
- 33.Baptista D, et al. , Overlooked? Underestimated? Effects of substrate curvature on cell behavior. Trends in biotechnology, 2019. 37(8): p. 838–854. [DOI] [PubMed] [Google Scholar]
- 34.Fiddes LK, et al. , A circular cross-section PDMS microfluidics system for replication of cardiovascular flow conditions. Biomaterials, 2010. 31(13): p. 3459–3464. [DOI] [PubMed] [Google Scholar]
- 35.Partyka PP, et al. , Mechanical stress regulates transport in a compliant 3D model of the blood-brain barrier. Biomaterials, 2017. 115: p. 30–39. [DOI] [PubMed] [Google Scholar]
- 36.Linville RM, et al. , Human iPSC-derived blood-brain barrier microvessels: Validation of barrier function and endothelial cell behavior. Biomaterials, 2019. 190: p. 24–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moya ML, et al. , A Reconfigurable In Vitro Model for Studying the Blood–Brain Barrier. Annals of biomedical engineering, 2020. 48(2): p. 780–793. [DOI] [PubMed] [Google Scholar]
- 38.Katt ME, et al. , Functional brain-specific microvessels from iPSC-derived human brain microvascular endothelial cells: the role of matrix composition on monolayer formation. Fluids and Barriers of the CNS, 2018. 15(15): p. 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Faley SL, et al. , iPSC-Derived Brain Endothelium Exhibits Stable, Long-Term Barrier Function in Perfused Hydrogel Scaffolds. Stem cell reports, 2019. 12(3): p. 474–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yu F, et al. , A pump‐free tricellular blood‐brain barrier on‐a‐chip model to understand barrier property and evaluate drug response. Biotechnology and Bioengineering, 2019. 117(4).* Viscous fingering was used to pattern a 300 – 400 μm diameter vessel, seeded with primary rat BECs, embedded in a collagen gel encasing primary rat pericytes and astrocytes. The authors use a simple gravity driven flow system with a paper flow resistor to regulate flow rate.
- 41.Herland A, et al. , Distinct contributions of astrocytes and pericytes to neuroinflammation identified in a 3D human blood-brain barrier on a chip. PLoS One, 2016. 11(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee S, et al. , 3D brain angiogenesis model to reconstitute functional human blood–brain barrier in vitro. Biotechnology and bioengineering, 2019. 117(3).** The authors developed an angiogenesis model with BECs and pericytes cultured on the surface of a hydrogel seeded with astrocytes. Fibroblasts were used to develop a chemotactic gradient to direct BEC angiogenesis. Incorporation of NVU cells decreased the BEC permeability and increased P-gp activity.
- 43.Uwamori H, et al. , Integration of neurogenesis and angiogenesis models for constructing a neurovascular tissue. Scientific reports, 2017. 7: p. 17349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bang S, et al. , A low permeability microfluidic blood-brain barrier platform with direct contact between perfusable vascular network and astrocytes. Scientific reports, 2017. 7(1): p. 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Campisi M, et al. , 3D self-organized microvascular model of the human blood-brain barrier with endothelial cells, pericytes and astrocytes. Biomaterials, 2018. 180: p. 117–129.* Using iPSC derived ECs in an ECM with astrocytes and pericytes between two EC rectangular channels, this study develops a vasculogenesis model with a network of capillaries forming between the two channels. The ECs generate capillaries with lumens and develop a more BEC like phenotype with co-culture of NVU cells.
- 46.Pham MT, et al. , Generation of human vascularized brain organoids. Neuroreport, 2018. 29(7): p. 588–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Çakir B, et al. , Engineering of human brain organoids with a functional vascular-like system. Nature methods, 2019. 16: p. 1169–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Esch MB and Mahler GJ, Body-on-a-chip systems: Design, fabrication, and applications, in Microfluidic Cell Culture Systems. 2019, Elsevier; p. 323–350. [Google Scholar]
- 49.Mahler GJ, et al. , Body-on-a-chip systems for animal-free toxicity testing. Alternatives to Laboratory Animals, 2016. 44(5): p. 469–478. [DOI] [PubMed] [Google Scholar]
- 50.Ferreira L, What human blood-brain barrier models can tell us about BBB function and drug discovery? Expert opinion on drug discovery, 2019. 14(11): p. 1113–1123. [DOI] [PubMed] [Google Scholar]
- 51.Katt ME, et al. , The role of mutations associated with familial neurodegenerative disorders on blood–brain barrier function in an iPSC model. Fluids and Barriers of the CNS, 2019. 16(1): p. 20. [DOI] [PMC free article] [PubMed] [Google Scholar]