Abstract
Context-
The highly invasive properties demonstrated by Head and Neck Squamous Cell Carcinoma (HNSCC) is often associated with locoregional recurrence and lymph node metastasis in patients, and is a key factor leading to an expected 5-year survival rate of approximately 50% for patients with advanced disease. It is important to understand the features and mediators of HNSCC invasion so that new treatment approaches can be developed.
Objective-
To provide an overview of the characteristics, mediators and mechanisms of HNSCC invasion.
Data Sources-
A literature review of peer-reviewed articles in PubMed on HNSCC invasion.
Conclusions-
Histological features of HNSCC tumors can help predict prognosis and influence clinical treatment decisions. Cell surface receptors, signaling pathways, proteases, invadopodia function, epithelial-mesenchymal transition, microRNAs, and tumor microenvironment are all involved in the regulation of the invasive behavior of HNSCC cells. Identifying effective HNSCC invasion inhibitors has the potential to improve outcomes for patients by reducing the rate of spread and increasing responsiveness to chemoradiation.
The clinical relevance of invasion
The ability of tumors to invade into surrounding tissues is a “hallmark of cancer”1 and is arguably the property that most greatly impacts morbidity and mortality. The head and neck region is very complex, with a dense organization of fascial planes, bony and cartilaginous scaffolding, and abundant neurovascular structures. On one hand, this complex anatomy poses several barriers for an invasive tumor to overcome. On the other hand, these structures, when breached, can provide insidious pathways for cancer cells to travel, cause damage, and evade treatment. From a clinical perspective, levels of invasion impact prognosis and are therefore considered both in the current cancer staging system and in the decision-making process for determining treatment.
Head and neck squamous cell carcinoma (HNSCC) is an epithelial cancer, arising from the mucosa of the upper aerodigestive tract. Therefore, an invasive cancer, by definition, must first invade the basement membrane of the native epithelium. This property is achieved during the process of carcinogenesis as multiple genetic insults are accumulated and in turn the acquisition of invasive capabilities allows invasion through the basement membrane. This primary event of invasion differentiates carcinoma in situ from invasive carcinoma. In oral tongue cancer, as this layer of connective tissue is breached, the invading tumor encounters the underlying tongue musculature. Depth of invasion (DOI) for these tumors is directly associated with patient outcome, and as the depth approaches 2–4 mm of invasion, the rate of lymph node metastasis increases greatly and prognosis worsens2–4. Therefore, elective neck dissection is recommended for oral tongue cancers with a depth of invasion of more than 2–4 mm, even when no clinical evidence of lymph node spread exists. A modified staging system has been developed which integrates DOI into the T category of the currently accepted American Joint Committee on Cancer (AJCC) TNM staging3,5. DOI was significantly associated with disease-specific survival in oral squamous cell carcinoma (OSCC)3.
In addition to depth, analysis of histological parameters in resected tumors of patients has indicated that invasion of tumor cells into the normal host tissue can predict patient outcome. The invasive front of an invading tumor has been shown to be an important indicator of patient prognosis2,6–10. Bryne et al. developed an invasive front grading system (IFG) of tumors that incorporated four parameters: the pattern of invasion, the degree of keratinization, nuclear polymorphism, and host response (inflammatory cell infiltration)9,10. The scores for each parameter were combined to yield a total malignancy score for the whole tumor. The worst part of the tumor (most invasive) was used for the assessment. A higher malignancy score meant a poorer prognosis. Sandu et al. used the invasive front grading system described by Bryne et al. and found that patients with a higher IFG score had significantly lower disease-free survival2. Brandwein-Gensler et al. found the histological parameter, the pattern of invasion, to predict patient outcome in OSCC patients as part of their risk assessment model6,8. The worst pattern of invasion was significantly associated with overall survival, as well as local recurrence6,8. Li et al. demonstrated that this risk model predicted local recurrence and disease-specific survival in an independent study for patients with low-stage OSCC7.
Bone and cartilage are ensheathed in a dense layer of connective tissue. Therefore, the mandible has a natural barrier to tumor invasion. If a mandible cancer is able to breach the mandibular periosteum and invade the bony cortex, the relatively uninhibited spread of tumor in the bone marrow space can lead to extensive involvement and ill-defined tumor margins with poor prognosis11–14. The presence of mandibular invasion places a tumor in an advanced primary stage (T4a) and overall stage (IV) according to the AJCC staging system11. Similar to invasion of OSCCs into surrounding normal tissue, the histological pattern of invasion of OSCC into bone can also be assessed and correlated with outcome. Invasion into bone displaying a broad pushing front and a sharp interface between tumor and bone is categorized as an erosive pattern. Projections of tumor cells along an irregular front with residual bone islands within the tumor are categorized as an infiltrative pattern11–14. A retrospective study of patients with mandibular invasion by OSCC found that patients with an infiltrative pattern of invasion into the bone had a four-fold increased risk of death with disease compared to the patients who displayed erosive pattern of invasion13. The infiltrative pattern of invasion into bone tissue is associated with more aggressive tumor behavior, increased likelihood of positive margins, recurrence, death with disease, and shorter disease-free survival11–13. Tumors with mandibular spread are generally treated aggressively, most often with segmental mandible resection, followed by postoperative radiation or chemoradiation. Similarly, the laryngeal cartilage poses a barrier to tumor spread. A cancer of the larynx that exhibits frank cartilage destruction is deemed a T4/stage IV cancer by AJCC criteria. If extensive cartilage invasion is present, often an organ-sparing approach with chemoradiation is not feasible, and laryngectomy with postoperative adjuvant therapy is the treatment of choice.
Another feature that HNSCC can exhibit is a propensity for invasion into neural structures. The head and neck region is populated by a rich network of innervation. The hypoglossal nerve extensively innervates the fine musculature of the tongue, while the lingual nerve provides a network for tongue sensation, and carries distal fibers from the chorda tympani to provide special sensory taste fibers. The mandible carries the V3 branch of the trigeminal nerve, which innervates the teeth and transits to the mental foramen and provides sensation to the buccal mucosa of the lower lip and the skin of the chin. Similarly, the pharynx and larynx are innervated by vagus nerve fibers and branches of the glossopharyngeal nerve. When tumor cells invade perineurium and nerve sheaths, the cranial nerves can essentially provide a direct route of spread toward the base of the skull and intracranial extension. This is particularly evident for adenoid cystic carcinoma of the salivary gland, which has a very high affinity for perineural spread. HNSCC also commonly exhibits perineural invasion, and this feature has been cited as a harbinger for an increased rate of locoregional recurrence and poor outcome. Brandwein-Gensler et al. found that perineural invasion (PNI) of small and large nerves was associated with reduced overall survival, and PNI of large nerves was specifically associated with local recurrence6. De Matos et al. applied the same criteria6,8 to independently assess another set of OSCC cases and found a significant positive correlation between PNI and POI, as well as between POI and tumor thickness15, or depth of invasion (DOI)3. High levels of nerve growth factor have been found to be correlated with PNI and worse survival16. When perineural spread is identified after surgical resection, this feature can influence the decision to administer postoperative adjuvant treatment. Clinicians have evaluated PNI as an indicator in patients with early stage (I and II) OSCC tumors to determine treatment strategies, but PNI and lymphovascular invasion (LVI) were not found to be significant risk factors for disease-free or overall survival17.
The clinical significance of invasive properties is not limited to local disease. It has been well established that in regional lymph node metastases, invasion outside of the lymph node capsule is associated with poor outcome18,19. Two large randomized clinical trials demonstrated that extracapsular spread of lymph node disease identified after surgical resection is an indication for maximized treatment with postoperative radiation and concurrent cisplatin20.
The strategies that cancer cells use to achieve all of the invasive properties highlighted above likely require genetic and molecular alterations that are highly complex. The details of how these cancers acquire the ability to invade normal, organized, anatomic structures are being revealed. An improved understanding of the molecular events that lead to these invasive phenotypes may provide insight that could improve both prognostication and treatment. The following sections summarize what is known about the mechanisms of invasion of head and neck cancer cells.
Cell surface receptors shown to influence invasive behavior in HNSCC
The best characterized receptor for stimulation of HNSCC invasion is the epidermal growth factor receptor (EGFR, Figure 1)21–26. Epidermal growth factor (EGF) is present in saliva at concentrations around 1 ng/ml27–29. These concentrations30,31 or higher32–39 are able to stimulate migration and invasion in oral carcinoma lines in vitro, as well as in vivo 40. Other EGFR ligands besides EGF have been shown to stimulate invasion, including transforming growth factor alpha (TGFA), betacellulin (BTC), heparin binding EGF-like growth factor (HBEGF) and amphiregulin (AREG)32. Conversely inhibition of the EGFR has been shown to block invasion in response to EGF32,37,41–43. Intriguingly, the EGFR also mediates invasion induced by some other receptors, notably G protein coupled receptors (GPCRs)44 for lysophosphatidic acid (LPA)45,46, bradykinin (BK), prostaglandin E2 (PGE2)47 and gastrin-releasing peptide (GRP)48. In some cases, the mechanism of cross-activation of EGFR by GPCRs has been identified as the release of EGFR ligands. EGFR ligands are initially produced as cell surface transmembrane proteins49 which can then be released from their transmembrane tethers by members of a disintegrin and metalloproteinase (ADAM) family of proteases50. ADAM17 mediates the stimulated release of TGFA by BK and PGE247. GRP stimulates the release of both AREG and TGFA48. In the case of GRP, EGFR ligand release involves a pathway in which Src activates phosphatidylinositol 3-kinase (PI3K), leading to phosphoinositide dependent kinase 1 (PDK1) activation and phosphorylation of ADAM1751. Estrogen receptor activation can also trigger EGFR ligand release30. In addition, autocrine release of AREG, HBEGF, BTC and TGFA has been shown to occur during serum starvation48,52–55, indicating that autocrine stimulation of EGFR can contribute to basal invasion capability.
Figure 1. Signaling pathways in HNSCC invasion involving the EGFR.
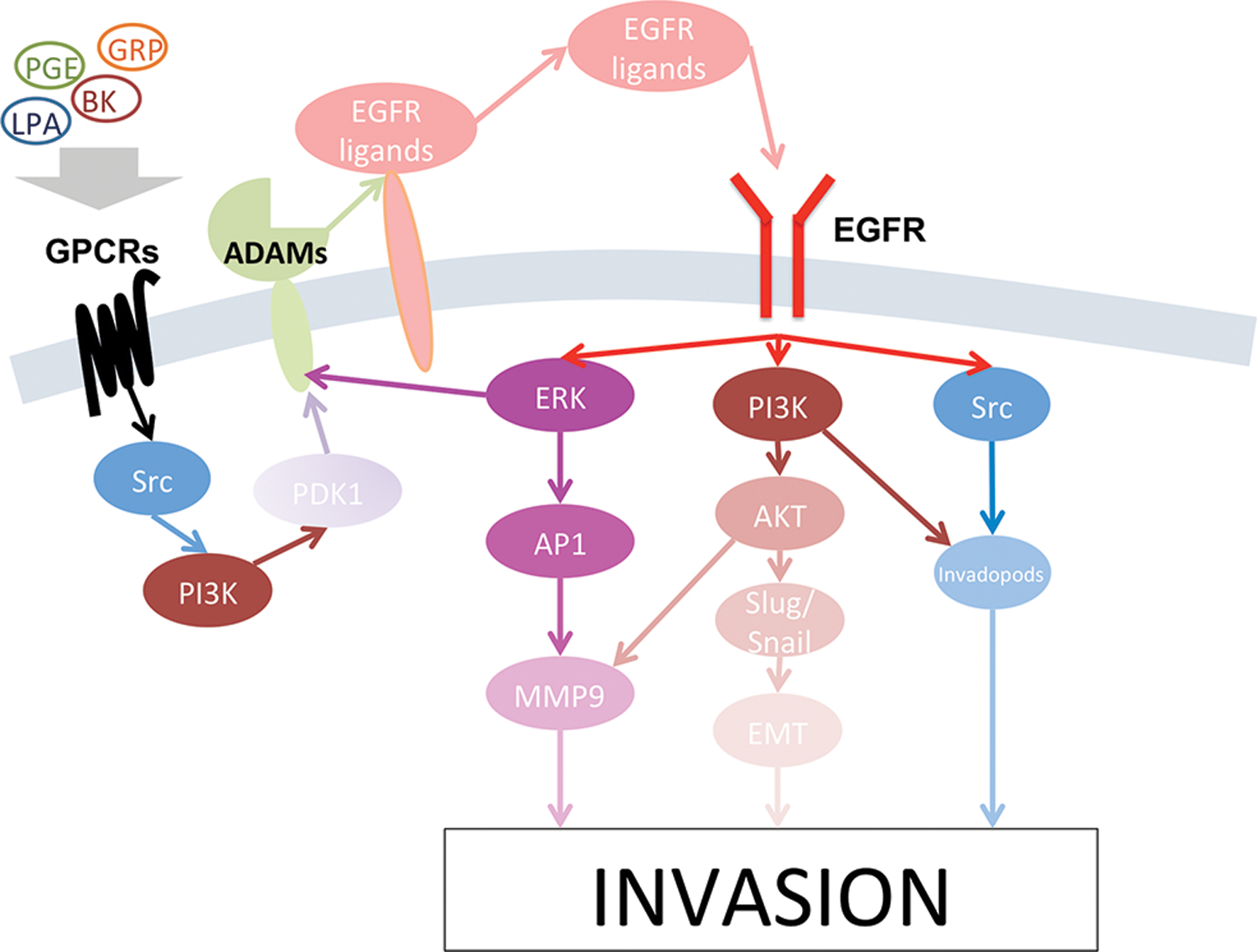
Epidermal growth factor receptor (EGFR), activated by EGFR ligands, can activate ERK/AP1, PI3K/Akt, or Src activity, which all lead to increased invasion in head and neck cancer. EGFR can be cross-activated by GCPRs, whose downstream signaling leads to the release of EGFR ligands from their transmembrane precursors through activity by the ADAM family of proteases.
A number of other receptors that can regulate HNSCC invasion have been identified. Other GPCRs that stimulate invasion with ERK and MMP9 activation include chemokine receptors CXCR1 and CXCR256,57 and CXCR458,59. Interleukin 6 (IL6) receptors can activate the ERK and c-Jun N-terminal kinase (JNK) pathways60,61. Toll-like receptors (TLRs) have also been shown to stimulate invasion with activation of the NFKB pathway62,63. NFKB signaling can synergize with the ERK/AP1 pathways to stimulate invasion64,65. Wnt-5B knockdown suppresses MMP10 production and invasion indicating that Wnt signaling pathways can also contribute to invasion66,67. Transforming growth factor beta (TGFB) can be produced by tumor-associated fibroblasts68 and induce NFKB activation69 and SMAD–dependent MMP production70 as well as EMT factors such as Snail and Slug71–76. Invasion is stimulated by hypoxia in a Notch-dependent fashion 77. Inhibition of Notch can also suppress MMP production78.
Signaling pathways involved in HNSCC invasion
Several signaling pathways have been shown to be important for invasion of HNSCC. Inhibition of ERK results in reduced invasion 37,41,79. An important ERK target is AP1 80, which can increase transcription of matrix metalloproteases (MMPs), such as MMP979,81,82. PI3K activity is also important for invasion37,79,83,84. Inhibition of PI3K also results in reduced MMP9 expression37,79. Additional contributions of PI3K to invasion can occur through AKT to stimulate Slug and Snail expression for enhancing EMT85,86, or phosphorylation of ezrin87 and yes-associated protein (YAP)88. Phospholipase C (PLC) gamma-1 has been shown to be important in EGFR induced invasion in some cases79,89, potentially in a complex with c-Src35. Src family members can contribute to invasion either through enhanced release of receptor ligands as described above or through stimulation of invadopod formation, which is described in more detail below48,55,90–94. A number of HNSCC cell lines have been shown to have constitutively high levels of Rac1 activity, and inhibition of Rac activity results in reduced invasion capability95. In most cases the increased Rac1 activity correlated with increased tyrosine phosphorylation of the Rac GEF Vav2 induced by EGFR, but an alternative pathway utilized Ras activation. The role of the Janus kinase (JAK)-Signal Transducer and Activator of Transcription (STAT) pathway in HNSCC invasion is complex. STAT3 has been shown to be important for ligand-independent invasion induced by the EGFR vIII isoform 96, but JAK2-STAT5a signaling is important for erythropoietin (EPO) induced invasion but not EGF-induced invasion96. STAT3 activity may generally enhance invasion capability through suppression of PTEN 97 and E-cadherin98.
Proteases implicated in HNSCC invasion
A key distinction between migration and invasion is the ability to degrade extracellular matrix barriers in the latter activity. Thus expression of proteases, especially matrix metalloproteases (MMPs), has been shown to be important for HNSCC invasion in a number of cases99. The most commonly identified MMP has been MMP9. Its activity is increased by EGFR32,37,42,54,100–102 and integrins75, while its inhibition reduces invasion81,103,104. MMP9 can degrade type IV collagen105–107, a key constituent of the basement membrane, and thus has the potential to play an important role in enabling HNSCC cells to become frankly invasive. Analysis of OSCC cases by immunohistochemistry revealed that expression of MMPs corresponded to areas with loss of collagen alpha IV chain. The enzymatic activity of MMPs was assessed by zymography and was found to be enhanced in higher grade OSCC and along the advancing tumor front108. MMP9 is used as a marker of invasive OSCC in many studies and the extent of its expression is related to the infiltration pattern of SCC at the invasive front109. In vitro invasion assays that utilize Matrigel, which is derived from basement membranes, often show a dependence on MMP9 activity. However, MMP9 can cleave other proteins exposed to the extracellular milieu as well, activating ligands such as TGFB and chemokines as well as cleaving cell surface receptors110. In addition, MMP9 may be involved in cleavage of E-cadherin, resulting in reduced cell-cell adhesion and increased invasion37.
MMP2 is another MMP that has been implicated in HNSCC invasion103,104. While MMP2 and MMP9 are secreted MMPs, the transmembrane protease MMP14 (MT1-MMP) also plays a significant role. MMP14, together with TIMP2111,112, is important in the activation of secreted proteases such as MMP2113, and suppression of MMP14 reduces overall matrix degradation activity114 and invasion43. Another protein that enhances protease activity and is important for invasion is extracellular matrix metalloprotease inducer (EMMPRIN)100,115–117. Although the mechanism is unclear, EMMPRIN homophilic binding induces the expression of a number of proteases, including MMPs, cathepsin B, and urokinase plasminogen activator receptor (uPAR) 100,115,117,118. Other proteases that have been shown to contribute to HNSCC invasion include MMP1066, MMP1380, matriptase119, fibroblast activation protein120, and uPA/uPAR117,121–124, as well as the glycosidase heparanase125.
The roles of adhesion in HNSCC invasion
Cell-cell adhesion mediated by E-cadherin is generally found to inhibit invasion26,126. Induction of cell-cell junctions can inhibit invasion127. Reduction in E-cadherin expression can occur through methylation of the promoter, and demethylating agents, which reverse this, can enhance invasion128. Inhibition of E-cadherin function also enhances invasion129. However, the inhibitory effect of E-cadherin may be overcome by expression of other invasion-inducing proteins130,131. Desmosomes are another cell-cell adhesion structure, which can suppress invasion132. However, overexpression of certain adhesion proteins such as claudin 1133 and desmoglein 3134 can enhance invasion.
Although degradation of the extracellular matrix, especially the basement membrane, is a key property of invasive tumors, interaction with the extracellular matrix is also important for invasion and prognosis135–138. Knockdown of the integrin beta 1 subunit (ITGB1) using siRNAs can reduce MMP2 activity and invasive capability139, mimicking the ability of miR-124, which has been shown to target ITGB1 and reduce invasion140. The integrin alpha v beta 6 (IAVB6) is upregulated in wound healing, and inhibition of AVB6 also inhibits invasion141–143. Integrin AVB6 is important in inducing protease expression142,144. Conversely, expression of specific ECM proteins such as collagen I145, collagen XVI146,147, or laminin148 can enhance invasion. Binding of integrins to these matrix molecules triggers a signaling cascade including integrin linked kinase (ILK)149, talin92,150, focal adhesion kinase (FAK)151–153, and Src family members90,154 that then link to the MAP kinase and PI3 kinase pathways for stimulation of MMPs82,145,149,154. It is unclear why both growth factor signaling and adhesion signaling are needed for invasion – the connection between adhesion and growth factor signaling in invadopod formation is an active area of investigation155–159.
MicroRNAs that regulate HNSCC invasion
MicroRNAs (miRNAs) are a class of gene expression regulators that often have altered expression in various human cancers, including HNSCC160–165. MiRNAs have emerged as having key roles in diverse cellular processes, including cancer cell proliferation, migration, invasion and metastasis160,161,166,167. Dicer, a key enzyme involved in siRNA and miRNA function, was reduced in expression in tongue SCC lines, and reduction of Dicer led to increased cell proliferation and invasion168. Consistent with these observations, the majority of the miRNAs that have been found to affect invasion in HNSCC are inhibitory (see Table 1).
Table 1:
MicroRNAs (miRNAs) That Affect Invasion in Head and Neck Squamous Cell Carcinoma
| miRNA | Effect on invasion | Target | Function | Reference |
|---|---|---|---|---|
| hsa-miR-1 | Reduction | TAGLN2 | Cytoskeleton | 178 |
| hsa-miR-107 | Reduction | PRKCE | Signaling | 174 |
| hsa-miR-29a,b,c | Reduction | LAMC2, ITGA6, MMP2, SP1 | Adhesion, Proteolysis, Gene expression | 148,176,188 |
| hsa-miR-99a | Reduction | IGF1R | Signaling | 171 |
| hsa-miR-126 | Reduction | EGFL7, VEGF, FGF2 | Signaling | 169 |
| hsa-miR-133a | Reduction | CAV1, MSN, ARPC5 | Cytoskeleton | 179,180,302 |
| hsa-miR-138 | Reduction | RHOC, ROCK2, VIM, ZEB2, EZH2 |
Cytoskeleton EMT |
212,213,184,185 |
| hsa-miR-140–5p | Reduction | ADAM10 | Proteolysis | 177 |
| hsa-miR-181a | Reduction | TWIST1 | EMT | 187 |
| hsa-miR-218 | Reduction | LAMB3 | Adhesion | 175 |
| hsa-miR-363 | Reduction | PDPN | Cytoskeleton | 181 |
| hsa-miR-375 | Reduction | MTDH | Gene expression | 189–193 |
| hsa-miR-491–5p | Reduction | GIT1 | Adhesion | 303 |
| hsa-miR-639 | Reduction | FOXC1 | Gene expression, EMT | 194 |
| hsa-miR-21 | Increase | DKK2, PDCD4 | Signaling, Protein Synthesis | 97,170,195 |
| hsa-miR-134 | Increase | WWOX | Cell death | 304 |
| hsa-miR-155 | Increase | SOCS1 | Signaling | 173 |
| hsa-miR-193b | Increase | NF1 | Signaling | 172 |
| hsa-miR-504 | Increase | FOXP1 | Gene expression | 194 |
| hsa-miR-222 | Mixed | BBC3, SOD2 | Cell death/ROS | 196,197 |
Abbreviations: EMT – epithelial to mesenchymal transition, ROS – reactive oxygen species
The targets of the miRNAs that have been identified thus far can be categorized according to a number of functions involved in invasion. A large number of miRNAs target proteins associated with signal transduction including ligands, receptors, and intracellular regulators. MiR-126 was found to downregulate protein levels of ligands epidermal growth factor-like domain 7 (EGFLD7), vascular endothelial growth factor (VEGF), and basic fibroblast growth factor (FGF2)169. Dickkopf 2 (DKK2), a target of miR-21, promotes cell invasion by antagonizing Wnt/beta-catenin signaling170. MiR-99a was found to be a metastasis suppressor in OSCC by regulating expression of the insulin-like growth factor 1 receptor (IGF1R)171. The regulation of neurofibromyin 1 (NF1) by miR-193b impaired cell migration and invasion via inhibiting Erk1/2 phosphorylation172. MiR-155 promotes the migration and invasion of laryngeal SCC. Knockdown of miR-155 in LSCC cells suppressed invasion by negatively regulating suppressor of cytokine signaling 1 (SOC1), which allowed increased STAT3 signaling173. HNSCC cells overexpressing miR-107 had reduced cell invasion in vitro and tumor growth in vivo resulting from the targeting of protein kinase C epsilon (PRKCE) 174.
Many adhesion proteins are also targets of miRNAs. Laminin beta 3 (LAMB3), a component of laminin-332 in the extracellular matrix, is targeted by miR-218175. MiR-29s (miR-29a, miR-29b and miR-29c) target another component of laminin-322, laminin gamma 2 (LAMC2)148. MiR-29s also target adhesion molecule integrin alpha 6 (ITGA6)148. MiR-29a was found to upregulate the ECM protease MMP2176. ADAM10, another protease, is a target of miR-140–5p177. There is also a significant group of miRNAs whose targets are associated with cell protrusions and the cytoskeleton, including transgelin 2 (TAGLN2), targeted by miR-1178, moesin (MSN) and actin-related protein 2/3 complex subunit 5 (ARPC5), targeted by miR-133a179,180, and podoplanin (PDPN) targeted by miR-363181. The cytoskeleton proteins, Ras homology family member C (RHOC) and Rho-associated coiled-coil containing protein kinase (ROCK2) are targeted by miR-138, which also targets the EMT-associated protein vimentin (VIM)182–185. Other miRNA targets associated with EMT in terms of general gene expression are twist basic helix-loop-helix transcription factor 1 (TWIST1) and forkhead box C1 (FOXC1), targeted by miR-181a and miR-639186,187.
Transcription factors that may regulate genes associated with motility and invasion are also targets of miRNAs. MiR-29b suppresses Sp1 expression, which impaired OSCC invasion via reduced Akt activation188. MiR-375 targets metadherin (MTDH)189–193 and miR-504 targets forkhead box 1 (FOXP1)194, also important for invasion. Finally, there is a small group of targets, whose direct function in invasion are unclear, such as programmed cell death 4 (PDCD4)195 targeted by miR-21, BCL2 binding component 3 (BBC3)196 and manganese superoxide dismutase 2 (SOD2)197 targeted by miR-222.
Invadopodia as mediators of HNSCC invasion
Invadopodia (Figure 2) are specialized actin-rich structures that mediate extracellular matrix (ECM) proteolysis and are thought to play a key role in cell invasion198–201. An important component and marker of invadopodia is cortactin. HNSCC cells that have chromosome 11q13 amplification and subsequently overexpress cortactin have elevated binding and activity of Arp2/3 complex together with increased HNSCC cell motility and invasion202. The EGFR inhibitor, gefitinib, impairs motility in HNSCC cells with the degree of inhibition positively correlated with the level of cortactin in the cells202. High levels of cortactin reduce EGFR down-regulation and extend ERK activation203. Cortactin is important for both invadopodium assembly and ECM degradation204. Cortactin was shown to promote MMP2 and MMP9 secretion, as well as the cell surface levels of MT1-MMP in invadopodia of HNSCC cells204. Figure 3 shows an example of an in vitro assay to assess invadopodial matrix degradation, with cortactin and Tks5 as markers for invadopodia.
Figure 2. Proteins that contribute to invadopodium structure and function.
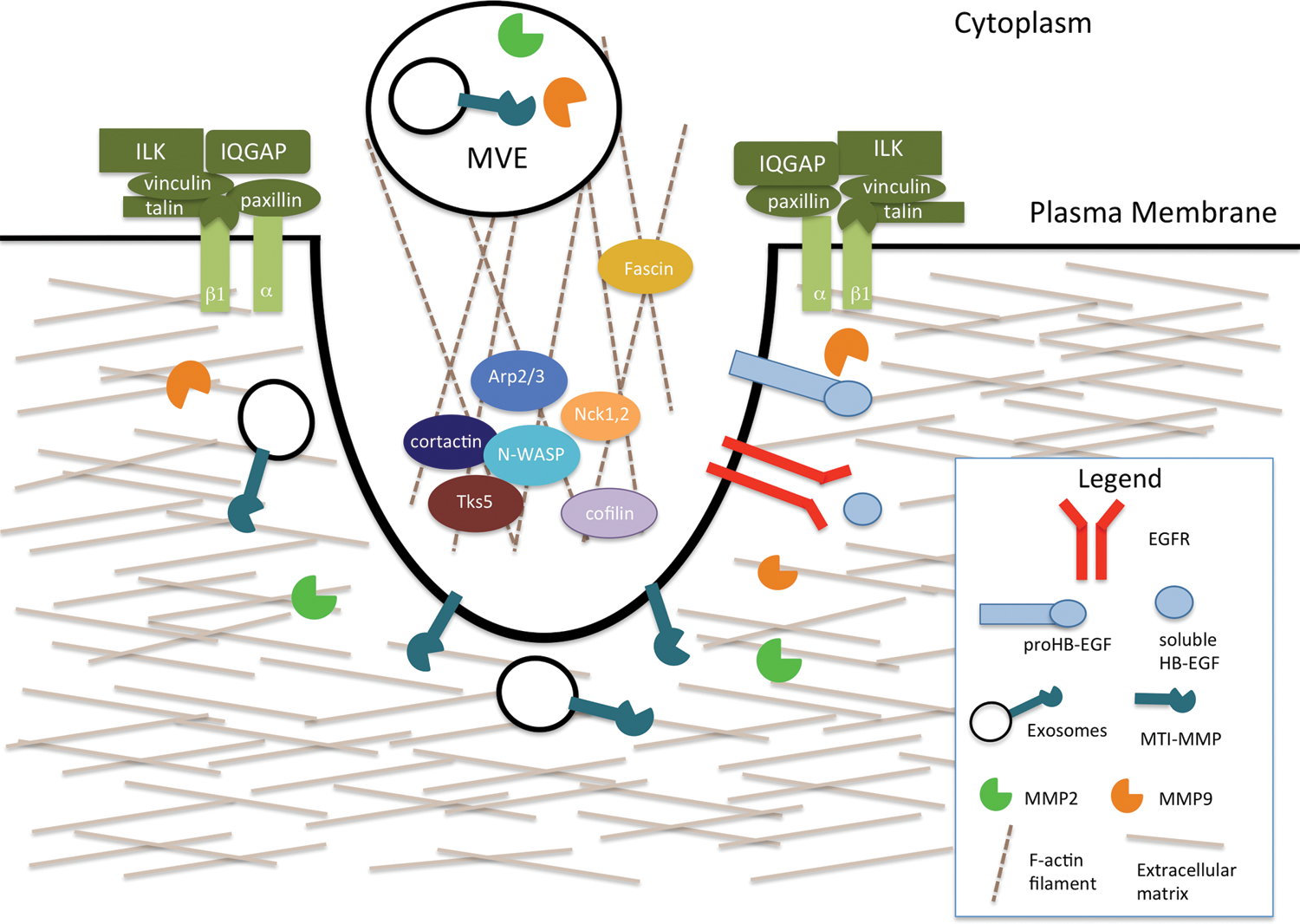
Invadopodia are actin-rich structures that specialize in mediating the degradation of the ECM. Their formation can be induced by EGFR signaling. The key components of the core structure are cortactin, Tks5, N-WASP, Arp2/3, Nck-1 and −2, and cofilin. Adhesion rings, made up of adhesion proteins such as integrins and ILKs, are important for the formation and stabilization of invadopodia. Proteases, such as MMP2 and MMP9, are secreted at invadopodia. MT1-MMP can be transported by exosomes, which are secreted from multi-vesicular endosomes.
Figure 3. Invadopodial matrix degradation assay.
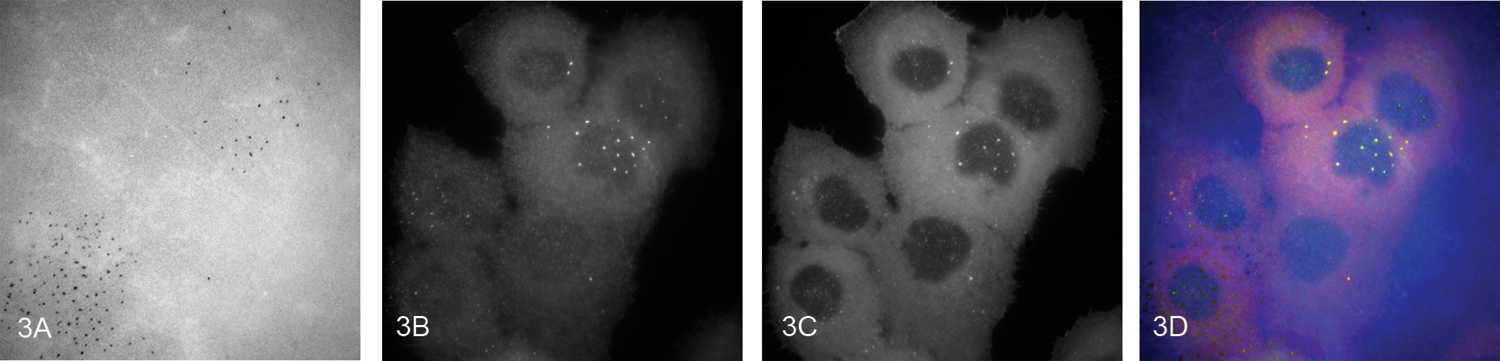
UMSCC1 cells, a human oral cavity squamous cell carcinoma cell line, were plated onto an Alexa Fluor-405 labeled gelatin matrix. After 4 hours the cells were fixed and stained for cortactin and Tks5, two invadopodia markers. Representative images of the degraded fluorescent matrix (A) Tks5 (B) and cortactin (C) and the merged (D) (cortactin: red, Tks5: green, matrix: blue) staining at 60X magnification. The merged image shows colocalization of Tks5- and cortactin-rich structures associated with degradation holes in the Alexa Fluor-405 matrix.
Src is an important regulator of invadopodium formation and activity. Saracatinib, a Src inhibitor, inhibited Src activation and phosphorylation of FAK, p130 CAS and cortactin in HNSCC cells along with reduction of invadopodia formation, ECM degradation, and MMP9 secretion91. Intriguingly, although constitutively active Src induces invadopodium formation, wild-type Src expression is also needed in HNSCC cells for invadopodium-associated matrix degradation205. Abelson (Abl) kinase activity diminishes invadopodial ECM degradation in HNSCC cells: the inhibition of Abl and Arg by imatinib, an Abl family inhibitor, led to EGFR activation through increased HB-EGF production and shedding, and as a result of increased EGFR signaling, Src and ERK activity induced tyrosine and serine phosphorylation of cortactin55. These findings differ from a proposed model of EGFR-invadopodia signaling in breast cancer where elevated EGFR signaling induces Src activation, which subsequently leads to activation of Abl/Arg and tyrosine phosphorylation of cortactin by Abl/Arg206. In addition to the EGFR207, transforming growth factor beta receptor 1 (TGFBR1) can induce invadopodium formation through stimulation of insulin-like growth factor II mRNA binding protein 3 (IMP3) and podoplanin (PDPN), both of which are important for invadopodium formation, ECM degradation and MMP9 activity73,208.
PI3K activity also regulates the formation and activity of invadopodia209,210. It has been shown in breast cancer cell lines that PI3K signaling induced formation of invadopodia, and specifically the p100 alpha catalytic subunit of PI3K was responsible for the effect210. In HNSCC cells, the mechanism of PI3K signaling differs from the breast cancer model209. LY294002, a PI3K inhibitor, suppressed invadopodia numbers and ECM degradation, as well as focal adhesion numbers and size. Stimulation of invadopodia by PI3K may be in part due to production of PI(3,4)P2 from PIP3 through the action of SH2-containing inositol 5’-phosphatase (SHIP2)209. Expression levels of formin homology domain protein 1 (FHOD1), an actin nucleating protein, are dependent upon PI3K211. Silencing FHOD1 reduced cell migration, invasion, invadopodium formation and invadopodium-mediated ECM degradation211. Protein kinase C alpha (PKCA) can provide feedback negative regulation of cells with mutant PI3K, although in cells with wild-type PI3K, PKCA appears to stimulate invadopodium formation.209
Adhesion signaling has also been shown to be important for invadopodium formation and maturation. Adhesions rings form around invadopodia and are recruited after invadopodium formation114. Integrin activity and ILK are needed for adhesion ring formation, MT1-MMP accumulation in invadopodia and invadopodia-associated ECM degradation114. MT1-MMP is necessary for ECM degradation, but not for the adhesion ring formation. Invadopodia are key docking sites for multivesicular endosomes (MVEs) containing smaller secreted vesicles termed exosomes, and are secretion sites for exosomes212. Exosomes were also shown to promote invadopodia formation, enable the exocytosis of MT1-MMP at invadopodia and stimulate invadopodia-associated matrix degradation212.
Epithelial to mesenchymal transition, cancer stem cells, and invasion
Epithelial to mesenchymal transition (EMT) is a highly regulated process in which epithelial cells acquire a mesenchymal morphology through coordinated changes in gene and protein expression that lead to decreased cell adhesion and cell polarity, resulting in a more invasive phenotype26,213–219. Common protein indicators of EMT are decreased expression of E-cadherin, and increased expression of N-cadherin and vimentin. The expression of these genes is controlled by transcription factors including Snail, ZEB, and Twist. There are also changes in cellular proteins such as integrins and alpha-smooth muscle actin, as well as ECM proteins, such as collagen, laminin, and fibronectin149,213–216,220–223. HNSCC cells that undergo EMT have been shown to be more invasive57,77,152,215,217,221–224. The loss of E-cadherin associated with EMT has shown to be an indicator of poor prognosis in HNSCC26.
EMT can induce the generation of a sub-population of cells that have the potential for tumor initiation, called cancer stem cells (CSCs)225,226. CSCs exhibit the properties of self-renewal, potential to behave as tumor progenitor cells, and the ability to differentiate 223,225–227. CSCs are slow-dividing, and therefore often more resistant to chemotherapy225–227. CSCs are found in invasive fronts, and in many tumors are near the blood vessels217,223,225–227. EMT can generate cells within tumors that have stem-like properties216,219,223,228. In head and neck cancer, Twist, a regulator of EMT, was found to induce expression of Bmi-1, a regulator of stemness219,228. Twist and Bmi-1 can act together to repress expression of E-cadherin and p16INK4a. Higher expression of Twist and Bmi-1 has been correlated with lower E-cadherin and p16INK4a, and was associated with poor prognosis219.
There are a number of common molecules and pathways involved in invasion, EMT, and stemness. TGF beta regulates transcription of EMT transcription factors via SMADs229. CD44, a cell surface glycoprotein that binds hyaluronic acid, is involved in cell-cell crosstalk, cell adhesion, and migration, and interacts with c-Met and EGFR227,230. EGF can induce EMT in HNSCC cell lines through PI3K/Akt signaling223. Expression of Bmi-1, ALDH, and CD44 also increases with EGF treatment, indicating an acquisition of stem-like properties. Notch signaling can induce EMT under hypoxic conditions, with increased expression of Notch pathway molecules and Snail, decreased expression of E-cadherin, and an increase in invasiveness77. In addition to inducing EMT and increased invasiveness, Snail has also been shown to induce stem-like properties in OSCC224. Adhesion-related signaling molecules that are important for EMT and invasion include ILK149 and FAK152. In terms of proteases, membrane type 1 matrix metalloproteinase (MT1-MMP/MMP14) has been shown to induce EMT and stem-like properties in OSCC as well as invasion, in parallel with increased levels of Twist and ZEB221. However, there may be subtypes of cancer stem cells, which differ in their invasive ability.231
One aspect of invasion that needs more examination in HNSCC is collective invasion, in which cell-cell contacts may be maintained during invasion. Three-dimensional reconstructions of the invasive front of 14 oral tongue SCCs reveal that most invasive tumor cells are in multicellular structures of variable size232, suggesting the presence of cell-cell contacts. There is an ongoing debate regarding the relative importance of single cell and collective invasion for patient prognosis. One possibility is that a partial EMT could maintain cell-cell contacts but stimulate invasive properties sufficient to generate a more aggressive tumor that results in a worse prognosis233.
Tumor microenvironment and invasion
Cancer associated fibroblasts (CAFs) are the major stromal cells present in the microenvironment of HNSCC, and these tend to be myofibroblasts showing increased expression of alpha-smooth muscle actin234–237. Myofibroblasts and CAFs have been shown to enhance HNSCC invasion in vitro in a variety of assays238–247. Treatments that can enhance the ability of fibroblasts to stimulate tumor cell invasion include irradiation182 and reactive oxygen species (ROS)183,248,249, as well as lifestyle-correlated factors such as cigarette smoke250 and areca nut extract251. Fibroblasts have been shown to stimulate tumor cell invasion through secretion of a number of factors, including chemokine (C-C motif) ligand 2 (CCL2)183,252, CCL7253, stromal cell-derived factor-1 (SDF1)254, TGFB73,248,249,255,256, IL33257, MMP2145,249–251,258,259, EGFR ligands260, and hepatocyte growth factor (HGF)254,261–265. CAFs can also produce extracellular matrix molecules that enhance invasion, such as thrombospondin-1266 and fibronectin267. The mechanism of stimulation of tumor cell invasion by CAFs can include induction of EMT245,268.
In a number of studies, a paracrine interaction between tumor cells and fibroblasts has been shown to stimulate invasion. For example, CCL2 from fibroblasts can stimulate invasion and ROS production by tumor cells, which in turn stimulates fibroblast senescence and CCL2 production from the fibroblasts183. Galectin production by tumor cells can also stimulate CCL2 production from fibroblasts252. IL1 from OSCC stimulates CCL7, HGF and TGFB production by fibroblasts73,253,265, while endothelin stimulates ADAM17 mediated release of EGFR ligands246,260. Interestingly, there is a report of TGFB from tumor cells inducing HGF production by fibroblasts as well264.
Other features of the tumor microenvironment have also been identified as stimulating HNSCC invasion, although not as thoroughly as CAFs. The perivascular niche has been associated with cancer stem cells and invasion227. Endothelial cells can secrete EGF to induce EMT and invasion223. Tumor associated endothelial cells have been shown to stimulate invasion of HNSCC cells in vitro through the secretion of IL8269 and CXCL1270. These chemokines are produced and secreted upon stimulation of endothelial cells by VEGF via BCL2 upregulation269,271. Macrophages have been shown to stimulate Axl-mediated invasion272 and perineural invasion through production of GDNF273. However, macrophages did not enhance invasion in response to EGF in an in vivo invasion assay40. Hypoxia in the tumor microenvironment can induce Notch and EMT factors, such as Slug and Snail, to increase invasion77,274. MMP9 can be induced by hypoxia to enhance invasion in an NHE1-dependent fashion275. Hypoxia-inducible factor (HIF) 2 alpha leads to EGFR activation276, while HIF 1 alpha increases integrin alpha 5 and fibronectin277 in invasion. Of concern for treatments utilizing erythropoietin (EPO) to mitigate chemotherapy side effects, hypoxia can induce the EPO receptor in tumor cells, enabling EPO to stimulate tumor cell invasion278.
Genomic changes in invasion-associated genes
DNA changes such as mutations and copy number variations (CNVs) at the genomic level have been implicated in cancer progression279. Higher numbers of CNVs in the genome are associated with the development of cancer, and more CNVs are accumulated with tumor progression279. It has been shown in HNSCC that disease-specific survival and recurrence can be predicted by CNVs280. We used HNSCC TCGA data downloaded from the UCSC Cancer Browser to evaluate the genes discussed in this review281. The 25 genes with the highest copy number variation or number of mutations in HNSCC discussed here are presented in Table 2. PIK3CA is top in copy number increase and second in mutations, with EGFR and RAC1 also being high in both mutations and copy number increases, emphasizing the potential importance of these genes in HNSCC .
Table 2:
Ranking of the 25 Genes With the Greatest Number of Mutations or Copy Number Variation (CNV) in Head and Neck Squamous Cell Carcinoma
| Mutation Number Rank | Copy Number Variation Rank | |||||
|---|---|---|---|---|---|---|
| Gene | CNV | Muts | Gene | CNV | Muts | |
| CDKN2A | −0.77299 | 66 | PIK3CA | 0.919765 | 64 | |
| PIK3CA | 0.919765 | 64 | CLDN1 | 0.896282 | 0 | |
| NOTCH1 | 0.166341 | 59 | PTK2 | 0.827789 | 4 | |
| EGFR | 0.485323 | 14 | MTDH | 0.704501 | 2 | |
| NOTCH3 | −0.04892 | 14 | PIK3CB | 0.696673 | 5 | |
| NOTCH2 | −0.03327 | 13 | SNAI2 | 0.614481 | 1 | |
| FN1 | −0.20744 | 13 | CTTN | 0.571429 | 4 | |
| TLN1 | 0.009785 | 12 | EGFR | 0.485323 | 14 | |
| ITGB1 | −0.18395 | 10 | GDNF | 0.401174 | 1 | |
| RAC1 | 0.373777 | 9 | RAC1 | 0.373777 | 9 | |
| NF1 | 0.060665 | 9 | SRC | 0.362035 | 1 | |
| ZEB2 | 0.050881 | 9 | TWIST1 | 0.358121 | 1 | |
| PIK3CG | 0.23092 | 8 | IGF2BP3 | 0.34638 | 1 | |
| HGF | 0.203523 | 8 | SNAI1 | 0.320939 | 0 | |
| ZEB1 | −0.18591 | 8 | MMP9 | 0.318982 | 0 | |
| TLR4 | 0.242661 | 7 | PLCG1 | 0.313112 | 5 | |
| ABL2 | 0.195695 | 7 | EPO | 0.293542 | 2 | |
| IGF1R | 0.023483 | 7 | WNT5B | 0.25636 | 1 | |
| COL1A1 | 0.107632 | 6 | LPAR5 | 0.25636 | 0 | |
| ROCK2 | 0.099804 | 6 | TLR4 | 0.242661 | 7 | |
| COL16A1 | −0.03523 | 6 | ABL1 | 0.238748 | 0 | |
| AXL | −0.04501 | 6 | LPAR1 | 0.236791 | 2 | |
| THBS1 | −0.08023 | 6 | FOSL1 | 0.236791 | 0 | |
| EGF | −0.20548 | 6 | PIK3CG | 0.23092 | 8 | |
| PIK3R1 | −0.34442 | 6 | VAV2 | 0.23092 | 1 | |
Conclusions
Studies of invasion have identified a number of possible applications in the treatment of HNSCC. The most straightforward approach is to develop specific inhibitors targeted to molecules that are identified as being important in invasion. In particular, proteases such as MMP2, MMP9 and MMP14 are commonly identified as being important for invasion, and thus could be targeted for therapeutic development. However, there has been extensive development of broad spectrum MMP inhibitors with very limited success282. Inhibition of the beneficial roles of MMPs resulted in unacceptable side effects283, and thus there is now caution in considering therapeutics targeting MMPs, with the assumption that such treatments likely need to be brief in order to avoid side effects caused by longer term treatments284. A possible use for brief treatments could be in counteracting the effects of radiation. Radiation has been found to induce migration and invasion285, and treatment with invasion inhibitors during radiation treatment could then potentially enhance its efficacy286,287. Inhibitors targeting EGFR288, Na+/H+ exchanger 1 (NHE1)275, Src91 and microtubules289, can inhibit invasion at concentrations which are not toxic to cells and could be useful in counteracting radiation-induced spread.
For longer term treatments, potentially lower toxicity compounds for inhibiting invasion have been identified whose targets are unknown. These include extracts from herbs, green tea and other natural sources41,101,290–300. Such compounds could constrain further tumor spread and extend patient survival without directly killing tumor cells. Limiting tumor spread could develop additional benefits due to the consequences of keeping tumor cells clustered together. As noted above, there is an intimate relationship between invasion, EMT, and stem cell properties. It is possible that by inhibiting invasion of HNSCC cells, there is partial reversion of EMT and loss of stemness, which could result in greater sensitivity to cytotoxic treatments. Compounds have been identified which on their own inhibit invasion and EMT without affecting viability292 but then sensitize HNSCC cells to cisplatin.292,301 Identification of invasion inhibitors which can enhance sensitivity to chemoradiation while suppressing the spread of tumor cells would provide a valuable addition to the therapy options of head and neck cancer patients.
References
- 1.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. March 4 2011;DOI: 10.1016/j.cell.2011.02.013; 144(5):646–674. [DOI] [PubMed] [Google Scholar]
- 2.Sandu K, Nisa L, Monnier P, Simon C, Andrejevic-Blant S, Bron L. Clinicobiological progression and prognosis of oral squamous cell carcinoma in relation to the tumor invasive front: impact on prognosis. Acta oto-laryngologica. April 2014;DOI: 10.3109/00016489.2013.849818; 134(4):416–424. [DOI] [PubMed] [Google Scholar]
- 3.Ebrahimi A, Gil Z, Amit M, et al. Primary Tumor Staging for Oral Cancer and a Proposed Modification Incorporating Depth of Invasion: An International Multicenter Retrospective Study. JAMA otolaryngology-- head & neck surgery. July 30 2014;DOI: 10.1001/jamaoto.2014.1548. [DOI] [PubMed]
- 4.Spiro RH, Huvos AG, Wong GY, Spiro JD, Gnecco CA, Strong EW. Predictive value of tumor thickness in squamous carcinoma confined to the tongue and floor of the mouth. American journal of surgery. October 1986; 152(4):345–350. [DOI] [PubMed] [Google Scholar]
- 5.Edge S, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC Cancer Staging Manual. Seventh ed. New York: Springer; 2011. [Google Scholar]
- 6.Brandwein-Gensler M, Teixeira MS, Lewis CM, et al. Oral squamous cell carcinoma: histologic risk assessment, but not margin status, is strongly predictive of local disease-free and overall survival. The American journal of surgical pathology. February 2005; 29(2):167–178. [DOI] [PubMed] [Google Scholar]
- 7.Li Y, Bai S, Carroll W, et al. Validation of the risk model: high-risk classification and tumor pattern of invasion predict outcome for patients with low-stage oral cavity squamous cell carcinoma. Head and neck pathology. September 2013;DOI: 10.1007/s12105-012-0412-1; 7(3):211–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brandwein-Gensler M, Smith RV, Wang B, et al. Validation of the histologic risk model in a new cohort of patients with head and neck squamous cell carcinoma. The American journal of surgical pathology. May 2010;DOI: 10.1097/PAS.0b013e3181d95c37; 34(5):676–688. [DOI] [PubMed] [Google Scholar]
- 9.Bryne M Is the invasive front of an oral carcinoma the most important area for prognostication? Oral diseases. June 1998; 4(2):70–77. [DOI] [PubMed] [Google Scholar]
- 10.Bryne M, Boysen M, Alfsen CG, et al. The invasive front of carcinomas. The most important area for tumour prognosis? Anticancer research. Nov-Dec 1998; 18(6B):4757–4764. [PubMed] [Google Scholar]
- 11.Jimi E, Shin M, Furuta H, Tada Y, Kusukawa J. The RANKL/RANK system as a therapeutic target for bone invasion by oral squamous cell carcinoma (Review). International journal of oncology. March 2013;DOI: 10.3892/ijo.2013.1794; 42(3):803–809. [DOI] [PubMed] [Google Scholar]
- 12.Jimi E, Furuta H, Matsuo K, Tominaga K, Takahashi T, Nakanishi O. The cellular and molecular mechanisms of bone invasion by oral squamous cell carcinoma. Oral diseases. July 2011;DOI: 10.1111/j.1601-0825.2010.01781.x; 17(5):462–468. [DOI] [PubMed] [Google Scholar]
- 13.Wong RJ, Keel SB, Glynn RJ, Varvares MA. Histological pattern of mandibular invasion by oral squamous cell carcinoma. The Laryngoscope. January 2000;DOI: 10.1097/00005537-200001000-00013; 110(1):65–72. [DOI] [PubMed] [Google Scholar]
- 14.Quan J, Johnson NW, Zhou G, Parsons PG, Boyle GM, Gao J. Potential molecular targets for inhibiting bone invasion by oral squamous cell carcinoma: a review of mechanisms. Cancer metastasis reviews. June 2012;DOI: 10.1007/s10555-011-9335-7; 31(1–2):209–219. [DOI] [PubMed] [Google Scholar]
- 15.de Matos FR, Lima E, Queiroz LM, da Silveira EJ. Analysis of inflammatory infiltrate, perineural invasion, and risk score can indicate concurrent metastasis in squamous cell carcinoma of the tongue. Journal of oral and maxillofacial surgery : official journal of the American Association of Oral and Maxillofacial Surgeons. July 2012;DOI: 10.1016/j.joms.2011.08.023; 70(7):1703–1710. [DOI] [PubMed] [Google Scholar]
- 16.Shen WR, Wang YP, Chang JY, Yu SY, Chen HM, Chiang CP. Perineural invasion and expression of nerve growth factor can predict the progression and prognosis of oral tongue squamous cell carcinoma. Journal of oral pathology & medicine : official publication of the International Association of Oral Pathologists and the American Academy of Oral Pathology. April 2014; 43(4):258–264. [DOI] [PubMed] [Google Scholar]
- 17.Chen TC, Wang CP, Ko JY, et al. The impact of perineural invasion and/or lymphovascular invasion on the survival of early-stage oral squamous cell carcinoma patients. Annals of surgical oncology. July 2013;DOI: 10.1245/s10434-013-2870-4; 20(7):2388–2395. [DOI] [PubMed] [Google Scholar]
- 18.Bernier J, Domenge C, Ozsahin M, et al. Postoperative irradiation with or without concomitant chemotherapy for locally advanced head and neck cancer. The New England journal of medicine. May 6 2004;DOI: 10.1056/NEJMoa032641; 350(19):1945–1952. [DOI] [PubMed] [Google Scholar]
- 19.Cooper JS, Pajak TF, Forastiere AA, et al. Postoperative concurrent radiotherapy and chemotherapy for high-risk squamous-cell carcinoma of the head and neck. The New England journal of medicine. May 6 2004;DOI: 10.1056/NEJMoa032646; 350(19):1937–1944. [DOI] [PubMed] [Google Scholar]
- 20.Bernier J, Cooper JS, Pajak TF, et al. Defining risk levels in locally advanced head and neck cancers: a comparative analysis of concurrent postoperative radiation plus chemotherapy trials of the EORTC (#22931) and RTOG (# 9501). Head & neck. October 2005;DOI: 10.1002/hed.20279; 27(10):843–850. [DOI] [PubMed] [Google Scholar]
- 21.Silva SD, Alaoui-Jamali MA, Hier M, Soares FA, Graner E, Kowalski LP. Cooverexpression of ERBB1 and ERBB4 receptors predicts poor clinical outcome in pN+ oral squamous cell carcinoma with extranodal spread. Clinical & experimental metastasis. March 2014;DOI: 10.1007/s10585-013-9629-y; 31(3):307–316. [DOI] [PubMed] [Google Scholar]
- 22.Kalyankrishna S, Grandis JR. Epidermal growth factor receptor biology in head and neck cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. June 10 2006;DOI: 10.1200/jco.2005.04.8306; 24(17):2666–2672. [DOI] [PubMed] [Google Scholar]
- 23.Lim SC. Expression of c-erbB receptors, MMPs and VEGF in head and neck squamous cell carcinoma. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie. October 2005; 59 Suppl 2:S366–369. [DOI] [PubMed] [Google Scholar]
- 24.Rogers SJ, Harrington KJ, Rhys-Evans P, P OC, Eccles SA. Biological significance of c-erbB family oncogenes in head and neck cancer. Cancer metastasis reviews. January 2005;DOI: 10.1007/s10555-005-5047-1; 24(1):47–69. [DOI] [PubMed] [Google Scholar]
- 25.Leemans CR, Braakhuis BJ, Brakenhoff RH. The molecular biology of head and neck cancer. Nature reviews. Cancer. January 2011;DOI: 10.1038/nrc2982; 11(1):9–22. [DOI] [PubMed] [Google Scholar]
- 26.Pectasides E, Rampias T, Sasaki C, et al. Markers of epithelial to mesenchymal transition in association with survival in head and neck squamous cell carcinoma (HNSCC). PloS one. 2014;DOI: 10.1371/journal.pone.0094273; 9(4):e94273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thesleff I, Viinikka L, Saxen L, Lehtonen E, Perheentupa J. The parotid gland is the main source of human salivary epidermal growth factor. Life sciences. 1988; 43(1):13–18. [DOI] [PubMed] [Google Scholar]
- 28.Dagogo-Jack S Epidermal growth factor EGF in human saliva: effect of age, sex, race, pregnancy and sialogogue. Scandinavian journal of gastroenterology. Supplement. 1986; 124:47–54. [DOI] [PubMed] [Google Scholar]
- 29.Ino M, Ushiro K, Ino C, Yamashita T, Kumazawa T. Kinetics of epidermal growth factor in saliva. Acta oto-laryngologica. Supplementum. 1993; 500:126–130. [DOI] [PubMed] [Google Scholar]
- 30.Egloff AM, Rothstein ME, Seethala R, Siegfried JM, Grandis JR, Stabile LP. Cross-talk between estrogen receptor and epidermal growth factor receptor in head and neck squamous cell carcinoma. Clinical cancer research : an official journal of the American Association for Cancer Research. November 1 2009;DOI: 10.1158/1078-0432.ccr-09-0862; 15(21):6529–6540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ohshima M, Sato M, Ishikawa M, Maeno M, Otsuka K. Physiologic levels of epidermal growth factor in saliva stimulate cell migration of an oral epithelial cell line, HO-1-N-1. European journal of oral sciences. April 2002; 110(2):130–136. [DOI] [PubMed] [Google Scholar]
- 32.P O-c, Modjtahedi H, Rhys-Evans P, Court WJ, Box GM, Eccles SA. Epidermal growth factor-like ligands differentially up-regulate matrix metalloproteinase 9 in head and neck squamous carcinoma cells. Cancer research. February 15 2000; 60(4):1121–1128. [PubMed] [Google Scholar]
- 33.Shiratsuchi T, Ishibashi H, Shirasuna K. Inhibition of epidermal growth factor-induced invasion by dexamethasone and AP-1 decoy in human squamous cell carcinoma cell lines. Journal of cellular physiology. December 2002;DOI: 10.1002/jcp.10181; 193(3):340–348. [DOI] [PubMed] [Google Scholar]
- 34.Yeudall WA, Miyazaki H, Ensley JF, Cardinali M, Gutkind JS, Patel V. Uncoupling of epidermal growth factor-dependent proliferation and invasion in a model of squamous carcinoma progression. Oral oncology. August 2005;DOI: 10.1016/j.oraloncology.2005.03.004; 41(7):698–708. [DOI] [PubMed] [Google Scholar]
- 35.Nozawa H, Howell G, Suzuki S, et al. Combined inhibition of PLC{gamma}-1 and c-Src abrogates epidermal growth factor receptor-mediated head and neck squamous cell carcinoma invasion. Clinical cancer research : an official journal of the American Association for Cancer Research. July 1 2008;DOI: 10.1158/1078-0432.CCR-07-4857; 14(13):4336–4344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ohnishi Y, Lieger O, Attygalla M, Iizuka T, Kakudo K. Effects of epidermal growth factor on the invasion activity of the oral cancer cell lines HSC3 and SAS. Oral oncology. December 2008;DOI: 10.1016/j.oraloncology.2008.02.015; 44(12):1155–1159. [DOI] [PubMed] [Google Scholar]
- 37.Zuo JH, Zhu W, Li MY, et al. Activation of EGFR promotes squamous carcinoma SCC10A cell migration and invasion via inducing EMT-like phenotype change and MMP-9-mediated degradation of E-cadherin. Journal of cellular biochemistry. September 2011;DOI: 10.1002/jcb.23175; 112(9):2508–2517. [DOI] [PubMed] [Google Scholar]
- 38.Lin MC, Huang MJ, Liu CH, Yang TL, Huang MC. GALNT2 enhances migration and invasion of oral squamous cell carcinoma by regulating EGFR glycosylation and activity. Oral oncology. May 2014;DOI: 10.1016/j.oraloncology.2014.02.003; 50(5):478–484. [DOI] [PubMed] [Google Scholar]
- 39.Ohnishi Y, Watanabe M, Yasui H, Kakudo K. Effects of epidermal growth factor on the invasive activity and cytoskeleton of oral squamous cell carcinoma cell lines. Oncology letters. May 2014;DOI: 10.3892/ol.2014.1946; 7(5):1439–1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smirnova T, Adomako A, Locker J, Van Rooijen N, Prystowsky MB, Segall JE. In vivo invasion of head and neck squamous cell carcinoma cells does not require macrophages. The American journal of pathology. June 2011;DOI: 10.1016/j.ajpath.2011.02.030; 178(6):2857–2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sun Q, Prasad R, Rosenthal E, Katiyar SK. Grape seed proanthocyanidins inhibit the invasive potential of head and neck cutaneous squamous cell carcinoma cells by targeting EGFR expression and epithelial-to-mesenchymal transition. BMC complementary and alternative medicine. 2011;DOI: 10.1186/1472-6882-11-134; 11:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee EJ, Whang JH, Jeon NK, Kim J. The epidermal growth factor receptor tyrosine kinase inhibitor ZD1839 (Iressa) suppresses proliferation and invasion of human oral squamous carcinoma cells via p53 independent and MMP, uPAR dependent mechanism. Annals of the New York Academy of Sciences. January 2007;DOI: 10.1196/annals.1397.015; 1095:113–128. [DOI] [PubMed] [Google Scholar]
- 43.Oku N, Sasabe E, Ueta E, Yamamoto T, Osaki T. Tight junction protein claudin-1 enhances the invasive activity of oral squamous cell carcinoma cells by promoting cleavage of laminin-5 gamma2 chain via matrix metalloproteinase (MMP)-2 and membrane-type MMP-1. Cancer research. May 15 2006;DOI: 10.1158/0008-5472.CAN-05-4478; 66(10):5251–5257. [DOI] [PubMed] [Google Scholar]
- 44.Liebmann C EGF receptor activation by GPCRs: an universal pathway reveals different versions. Molecular and cellular endocrinology. January 15 2011;DOI: 10.1016/j.mce.2010.04.008; 331(2):222–231. [DOI] [PubMed] [Google Scholar]
- 45.Brusevold IJ, Tveteraas IH, Aasrum M, Odegard J, Sandnes DL, Christoffersen T. Role of LPAR3, PKC and EGFR in LPA-induced cell migration in oral squamous carcinoma cells. BMC cancer. 2014;DOI: 10.1186/1471-2407-14-432; 14:432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gschwind A, Prenzel N, Ullrich A. Lysophosphatidic acid-induced squamous cell carcinoma cell proliferation and motility involves epidermal growth factor receptor signal transactivation. Cancer research. November 1 2002; 62(21):6329–6336. [PubMed] [Google Scholar]
- 47.Thomas SM, Bhola NE, Zhang Q, et al. Cross-talk between G protein-coupled receptor and epidermal growth factor receptor signaling pathways contributes to growth and invasion of head and neck squamous cell carcinoma. Cancer research. December 15 2006;DOI: 10.1158/0008-5472.can-06-2876; 66(24):11831–11839. [DOI] [PubMed] [Google Scholar]
- 48.Zhang Q, Thomas SM, Xi S, et al. SRC family kinases mediate epidermal growth factor receptor ligand cleavage, proliferation, and invasion of head and neck cancer cells. Cancer research. September 1 2004;DOI: 10.1158/0008-5472.can-04-0504; 64(17):6166–6173. [DOI] [PubMed] [Google Scholar]
- 49.Schneider MR, Wolf E. The epidermal growth factor receptor ligands at a glance. Journal of cellular physiology. March 2009;DOI: 10.1002/jcp.21635; 218(3):460–466. [DOI] [PubMed] [Google Scholar]
- 50.Kataoka H EGFR ligands and their signaling scissors, ADAMs, as new molecular targets for anticancer treatments. Journal of dermatological science. December 2009;DOI: 10.1016/j.jdermsci.2009.10.002; 56(3):148–153. [DOI] [PubMed] [Google Scholar]
- 51.Zhang Q, Thomas SM, Lui VW, et al. Phosphorylation of TNF-alpha converting enzyme by gastrin-releasing peptide induces amphiregulin release and EGF receptor activation. Proceedings of the National Academy of Sciences of the United States of America. May 2 2006;DOI: 10.1073/pnas.0509719103; 103(18):6901–6906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.P OC, Rhys-Evans P, Eccles S. A synthetic matrix metalloproteinase inhibitor prevents squamous carcinoma cell proliferation by interfering with epidermal growth factor receptor autocrine loops. International journal of cancer. Journal international du cancer. August 10 2002;DOI: 10.1002/ijc.10531; 100(5):527–533. [DOI] [PubMed] [Google Scholar]
- 53.Holz C, Niehr F, Boyko M, et al. Epithelial-mesenchymal-transition induced by EGFR activation interferes with cell migration and response to irradiation and cetuximab in head and neck cancer cells. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology. October 2011;DOI: 10.1016/j.radonc.2011.05.042; 101(1):158–164. [DOI] [PubMed] [Google Scholar]
- 54.Ohnishi Y, Inoue H, Furukawa M, Kakudo K, Nozaki M. Heparin-binding epidermal growth factor-like growth factor is a potent regulator of invasion activity in oral squamous cell carcinoma. Oncology reports. April 2012;DOI: 10.3892/or.2011.1616; 27(4):954–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hayes KE, Walk EL, Ammer AG, Kelley LC, Martin KH, Weed SA. Ableson kinases negatively regulate invadopodia function and invasion in head and neck squamous cell carcinoma by inhibiting an HB-EGF autocrine loop. Oncogene. October 2013;DOI: 10.1038/onc.2012.513; 32(40):4766–4777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Khurram SA, Bingle L, McCabe BM, Farthing PM, Whawell SA. The chemokine receptors CXCR1 and CXCR2 regulate oral cancer cell behaviour. Journal of oral pathology & medicine : official publication of the International Association of Oral Pathologists and the American Academy of Oral Pathology. June 26 2014;DOI: 10.1111/jop.12191; 43(9):667–674. [DOI] [PubMed] [Google Scholar]
- 57.Qian Y, Wang Y, Li DS, et al. The chemokine receptor-CXCR2 plays a critical role in the invasion and metastases of oral squamous cell carcinoma in vitro and in vivo. Journal of oral pathology & medicine : official publication of the International Association of Oral Pathologists and the American Academy of Oral Pathology. October 2014;DOI: 10.1111/jop.12189; 43(9):658–666. [DOI] [PubMed] [Google Scholar]
- 58.Yu T, Wu Y, Helman JI, Wen Y, Wang C, Li L. CXCR4 promotes oral squamous cell carcinoma migration and invasion through inducing expression of MMP-9 and MMP-13 via the ERK signaling pathway. Molecular cancer research : MCR. February 2011;DOI: 10.1158/1541-7786.MCR-10-0386; 9(2):161–172. [DOI] [PubMed] [Google Scholar]
- 59.Watanabe H, Iwase M, Ohashi M, Nagumo M. Role of interleukin-8 secreted from human oral squamous cell carcinoma cell lines. Oral oncology. October 2002; 38(7):670–679. [DOI] [PubMed] [Google Scholar]
- 60.Kanazawa T, Nishino H, Hasegawa M, et al. Interleukin-6 directly influences proliferation and invasion potential of head and neck cancer cells. European archives of oto-rhino-laryngology : official journal of the European Federation of Oto-Rhino-Laryngological Societies. July 2007;DOI: 10.1007/s00405-007-0264-6; 264(7):815–821. [DOI] [PubMed] [Google Scholar]
- 61.Chuang JY, Huang YL, Yen WL, Chiang IP, Tsai MH, Tang CH. Syk/JNK/AP-1 signaling pathway mediates interleukin-6-promoted cell migration in oral squamous cell carcinoma. International journal of molecular sciences. 2014;DOI: 10.3390/ijms15010545; 15(1):545–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ren G, Hu J, Wang R, et al. Rapamycin inhibits Toll-like receptor 4-induced pro-oncogenic function in head and neck squamous cell carcinoma. Oncology reports. June 2014;DOI: 10.3892/or.2014.3134; 31(6):2804–2810. [DOI] [PubMed] [Google Scholar]
- 63.Johnson J, Shi Z, Liu Y, Stack MS. Inhibitors of NF-kappaB reverse cellular invasion and target gene upregulation in an experimental model of aggressive oral squamous cell carcinoma. Oral oncology. May 2014;DOI: 10.1016/j.oraloncology.2014.02.004; 50(5):468–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ruan M, Zhang Z, Li S, et al. Activation of Toll-like receptor-9 promotes cellular migration via up-regulating MMP-2 expression in oral squamous cell carcinoma. PloS one. 2014;DOI: 10.1371/journal.pone.0092748; 9(3):e92748. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 65.Nottingham LK, Yan CH, Yang X, et al. Aberrant IKKalpha and IKKbeta cooperatively activate NF-kappaB and induce EGFR/AP1 signaling to promote survival and migration of head and neck cancer. Oncogene. February 27 2014;DOI: 10.1038/onc.2013.49; 33(9):1135–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Deraz EM, Kudo Y, Yoshida M, et al. MMP-10/stromelysin-2 promotes invasion of head and neck cancer. PloS one. 2011;DOI: 10.1371/journal.pone.0025438; 6(10):e25438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Takeshita A, Iwai S, Morita Y, Niki-Yonekawa A, Hamada M, Yura Y. Wnt5b promotes the cell motility essential for metastasis of oral squamous cell carcinoma through active Cdc42 and RhoA. International journal of oncology. January 2014;DOI: 10.3892/ijo.2013.2172; 44(1):59–68. [DOI] [PubMed] [Google Scholar]
- 68.Elahi M, Rakhshan V, Ghasemian NT, Moshref M. Prognostic value of transforming growth factor beta 1 [TGF-beta1] and matrix metalloproteinase 9 [MMP-9] in oral squamous cell carcinoma. Biomarkers : biochemical indicators of exposure, response, and susceptibility to chemicals. February 2012;DOI: 10.3109/1354750X.2011.635804; 17(1):21–27. [DOI] [PubMed] [Google Scholar]
- 69.Freudlsperger C, Bian Y, Contag Wise S, et al. TGF-beta and NF-kappaB signal pathway cross-talk is mediated through TAK1 and SMAD7 in a subset of head and neck cancers. Oncogene. March 21 2013;DOI: 10.1038/onc.2012.171; 32(12):1549–1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Leivonen SK, Ala-Aho R, Koli K, Grenman R, Peltonen J, Kahari VM. Activation of Smad signaling enhances collagenase-3 (MMP-13) expression and invasion of head and neck squamous carcinoma cells. Oncogene. April 27 2006;DOI: 10.1038/sj.onc.1209291; 25(18):2588–2600. [DOI] [PubMed] [Google Scholar]
- 71.Sun L, Diamond ME, Ottaviano AJ, Joseph MJ, Ananthanarayan V, Munshi HG. Transforming growth factor-beta 1 promotes matrix metalloproteinase-9-mediated oral cancer invasion through snail expression. Molecular cancer research : MCR. January 2008;DOI: 10.1158/1541-7786.MCR-07-0208; 6(1):10–20. [DOI] [PubMed] [Google Scholar]
- 72.Quan J, Elhousiny M, Johnson NW, Gao J. Transforming growth factor-beta1 treatment of oral cancer induces epithelial-mesenchymal transition and promotes bone invasion via enhanced activity of osteoclasts. Clinical & experimental metastasis. June 2013;DOI: 10.1007/s10585-013-9570-0; 30(5):659–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hwang YS, Xianglan Z, Park KK, Chung WY. Functional invadopodia formation through stabilization of the PDPN transcript by IMP-3 and cancer-stromal crosstalk for PDPN expression. Carcinogenesis. November 2012;DOI: 10.1093/carcin/bgs258; 33(11):2135–2146. [DOI] [PubMed] [Google Scholar]
- 74.Munshi HG, Wu YI, Mukhopadhyay S, et al. Differential regulation of membrane type 1-matrix metalloproteinase activity by ERK 1/2- and p38 MAPK-modulated tissue inhibitor of metalloproteinases 2 expression controls transforming growth factor-beta1-induced pericellular collagenolysis. The Journal of biological chemistry. September 10 2004;DOI: 10.1074/jbc.M404958200; 279(37):39042–39050. [DOI] [PubMed] [Google Scholar]
- 75.Dang D, Yang Y, Li X, et al. Matrix metalloproteinases and TGFbeta1 modulate oral tumor cell matrix. Biochemical and biophysical research communications. April 9 2004;DOI: 10.1016/j.bbrc.2004.02.143; 316(3):937–942. [DOI] [PubMed] [Google Scholar]
- 76.Takayama S, Hatori M, Kurihara Y, Kinugasa Y, Shirota T, Shintani S. Inhibition of TGF-beta1 suppresses motility and invasiveness of oral squamous cell carcinoma cell lines via modulation of integrins and down-regulation of matrix-metalloproteinases. Oncology reports. January 2009; 21(1):205–210. [PubMed] [Google Scholar]
- 77.Ishida T, Hijioka H, Kume K, Miyawaki A, Nakamura N. Notch signaling induces EMT in OSCC cell lines in a hypoxic environment. Oncology letters. November 2013;DOI: 10.3892/ol.2013.1549; 6(5):1201–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yu B, Wei J, Qian X, Lei D, Ma Q, Liu Y. Notch1 signaling pathway participates in cancer invasion by regulating MMPs in lingual squamous cell carcinoma. Oncology reports. February 2012;DOI: 10.3892/or.2011.1492; 27(2):547–552. [DOI] [PubMed] [Google Scholar]
- 79.P Oc, Wongkajornsilp A, Rhys-Evans PH, Eccles SA. Signaling pathways required for matrix metalloproteinase-9 induction by betacellulin in head-and-neck squamous carcinoma cells. International journal of cancer. Journal international du cancer. August 20 2004;DOI: 10.1002/ijc.20228; 111(2):174–183. [DOI] [PubMed] [Google Scholar]
- 80.Chuang JY, Tsai CF, Chang SW, et al. Glial cell line-derived neurotrophic factor induces cell migration in human oral squamous cell carcinoma. Oral oncology. December 2013;DOI: 10.1016/j.oraloncology.2013.08.009; 49(12):1103–1112. [DOI] [PubMed] [Google Scholar]
- 81.Nair RR, Avila H, Ma X, et al. A novel high-throughput screening system identifies a small molecule repressive for matrix metalloproteinase-9 expression. Molecular pharmacology. March 2008;DOI: 10.1124/mol.107.042606; 73(3):919–929. [DOI] [PubMed] [Google Scholar]
- 82.Bedal KB, Grassel S, Oefner PJ, Reinders J, Reichert TE, Bauer R. Collagen XVI induces expression of MMP9 via modulation of AP-1 transcription factors and facilitates invasion of oral squamous cell carcinoma. PloS one. 2014;DOI: 10.1371/journal.pone.0086777; 9(1):e86777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chen Y, Hou Q, Yan W, et al. PIK3CA is critical for the proliferation, invasiveness, and drug resistance of human tongue carcinoma cells. Oncology research. 2011; 19(12):563–571. [DOI] [PubMed] [Google Scholar]
- 84.Murugan AK, Hong NT, Fukui Y, Munirajan AK, Tsuchida N. Oncogenic mutations of the PIK3CA gene in head and neck squamous cell carcinomas. International journal of oncology. January 2008; 32(1):101–111. [PubMed] [Google Scholar]
- 85.Okui G, Tobiume K, Rizqiawan A, et al. AKT primes snail-induced EMT concomitantly with the collective migration of squamous cell carcinoma cells. Journal of cellular biochemistry. September 2013;DOI: 10.1002/jcb.24545; 114(9):2039–2049. [DOI] [PubMed] [Google Scholar]
- 86.Hong KO, Kim JH, Hong JS, et al. Inhibition of Akt activity induces the mesenchymal-to-epithelial reverting transition with restoring E-cadherin expression in KB and KOSCC-25B oral squamous cell carcinoma cells. Journal of experimental & clinical cancer research : CR. 2009;DOI: 10.1186/1756-9966-28-28. [DOI] [PMC free article] [PubMed]
- 87.Wang Y, Lin Z, Sun L, et al. Akt/Ezrin Tyr353/NF-kappaB pathway regulates EGF-induced EMT and metastasis in tongue squamous cell carcinoma. British journal of cancer. February 4 2014;DOI: 10.1038/bjc.2013.770; 110(3):695–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ehsanian R, Brown M, Lu H, et al. YAP dysregulation by phosphorylation or DeltaNp63-mediated gene repression promotes proliferation, survival and migration in head and neck cancer subsets. Oncogene. November 18 2010;DOI: 10.1038/onc.2010.339; 29(46):6160–6171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Thomas SM, Coppelli FM, Wells A, et al. Epidermal growth factor receptor-stimulated activation of phospholipase Cgamma-1 promotes invasion of head and neck squamous cell carcinoma. Cancer research. September 1 2003; 63(17):5629–5635. [PubMed] [Google Scholar]
- 90.Johnson FM, Saigal B, Talpaz M, Donato NJ. Dasatinib (BMS-354825) tyrosine kinase inhibitor suppresses invasion and induces cell cycle arrest and apoptosis of head and neck squamous cell carcinoma and non-small cell lung cancer cells. Clinical cancer research : an official journal of the American Association for Cancer Research. October 1 2005;DOI: 10.1158/1078-0432.ccr-05-0757; 11(19 Pt 1):6924–6932. [DOI] [PubMed] [Google Scholar]
- 91.Ammer AG, Kelley LC, Hayes KE, et al. Saracatinib Impairs Head and Neck Squamous Cell Carcinoma Invasion by Disrupting Invadopodia Function. Journal of cancer science & therapy. November 30 2009;DOI: 10.4172/1948-5956.1000009; 1(2):52–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sansing HA, Sarkeshik A, Yates JR, et al. Integrin alphabeta1, alphavbeta, alpha6beta effectors p130Cas, Src and talin regulate carcinoma invasion and chemoresistance. Biochemical and biophysical research communications. March 11 2011;DOI: 10.1016/j.bbrc.2011.01.109; 406(2):171–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhang H, Su L, Muller S, et al. Restoration of caveolin-1 expression suppresses growth and metastasis of head and neck squamous cell carcinoma. British journal of cancer. November 18 2008;DOI: 10.1038/sj.bjc.6604735; 99(10):1684–1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wheeler SE, Morariu EM, Bednash JS, et al. Lyn kinase mediates cell motility and tumor growth in EGFRvIII-expressing head and neck cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. May 15 2012;DOI: 10.1158/1078-0432.ccr-11-2486; 18(10):2850–2860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Patel V, Rosenfeldt HM, Lyons R, et al. Persistent activation of Rac1 in squamous carcinomas of the head and neck: evidence for an EGFR/Vav2 signaling axis involved in cell invasion. Carcinogenesis. June 2007;DOI: 10.1093/carcin/bgm008; 28(6):1145–1152. [DOI] [PubMed] [Google Scholar]
- 96.Wheeler SE, Suzuki S, Thomas SM, et al. Epidermal growth factor receptor variant III mediates head and neck cancer cell invasion via STAT3 activation. Oncogene. September 16 2010;DOI: 10.1038/onc.2009.279; 29(37):5135–5145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhou X, Ren Y, Liu A, et al. STAT3 inhibitor WP1066 attenuates miRNA-21 to suppress human oral squamous cell carcinoma growth in vitro and in vivo. Oncology reports. May 2014;DOI: 10.3892/or.2014.3114; 31(5):2173–2180. [DOI] [PubMed] [Google Scholar]
- 98.Zhao Y, Zhang J, Xia H, et al. Stat3 is involved in the motility, metastasis and prognosis in lingual squamous cell carcinoma. Cell biochemistry and function. June 2012;DOI: 10.1002/cbf.2810; 30(4):340–346. [DOI] [PubMed] [Google Scholar]
- 99.Rosenthal EL, Matrisian LM. Matrix metalloproteases in head and neck cancer. Head & neck. July 2006;DOI: 10.1002/hed.20365; 28(7):639–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Suzuki S, Ishikawa K. Combined inhibition of EMMPRIN and epidermal growth factor receptor prevents the growth and migration of head and neck squamous cell carcinoma cells. International journal of oncology. March 2014;DOI: 10.3892/ijo.2013.2238; 44(3):912–917. [DOI] [PubMed] [Google Scholar]
- 101.Chen HJ, Lin CM, Lee CY, et al. Phenethyl isothiocyanate suppresses EGF-stimulated SAS human oral squamous carcinoma cell invasion by targeting EGF receptor signaling. International journal of oncology. August 2013;DOI: 10.3892/ijo.2013.1977; 43(2):629–637. [DOI] [PubMed] [Google Scholar]
- 102.P OC, Rhys-Evans P, Modjtahedi H, Court W, Box G, Eccles S. Overexpression of epidermal growth factor receptor in human head and neck squamous carcinoma cell lines correlates with matrix metalloproteinase-9 expression and in vitro invasion. International journal of cancer. Journal international du cancer. May 1 2000; 86(3):307–317. [DOI] [PubMed] [Google Scholar]
- 103.Mitra RS, Goto M, Lee JS, et al. Rap1GAP promotes invasion via induction of matrix metalloproteinase 9 secretion, which is associated with poor survival in low N-stage squamous cell carcinoma. Cancer research. May 15 2008;DOI: 10.1158/0008-5472.can-07-2755; 68(10):3959–3969. [DOI] [PubMed] [Google Scholar]
- 104.Van Tubergen EA, Banerjee R, Liu M, et al. Inactivation or loss of TTP promotes invasion in head and neck cancer via transcript stabilization and secretion of MMP9, MMP2, and IL-6. Clinical cancer research : an official journal of the American Association for Cancer Research. March 1 2013;DOI: 10.1158/1078-0432.ccr-12-2927; 19(5):1169–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Stetler-Stevenson WG. Type IV collagenases in tumor invasion and metastasis. Cancer metastasis reviews. December 1990; 9(4):289–303. [DOI] [PubMed] [Google Scholar]
- 106.Deryugina EI, Quigley JP. Matrix metalloproteinases and tumor metastasis. Cancer metastasis reviews. March 2006;DOI: 10.1007/s10555-006-7886-9; 25(1):9–34. [DOI] [PubMed] [Google Scholar]
- 107.Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nature reviews. Cancer. March 2002;DOI: 10.1038/nrc745; 2(3):161–174. [DOI] [PubMed] [Google Scholar]
- 108.Tamamura R, Nagatsuka H, Siar CH, et al. Comparative analysis of basal lamina type IV collagen alpha chains, matrix metalloproteinases-2 and −9 expressions in oral dysplasia and invasive carcinoma. Acta histochemica. March 2013;DOI: 10.1016/j.acthis.2012.05.001; 115(2):113–119. [DOI] [PubMed] [Google Scholar]
- 109.Mohtasham N, Babakoohi S, Shiva A, et al. Immunohistochemical study of p53, Ki-67, MMP-2 and MMP-9 expression at invasive front of squamous cell and verrucous carcinoma in oral cavity. Pathology, research and practice. February 15 2013;DOI: 10.1016/j.prp.2012.11.002; 209(2):110–114. [DOI] [PubMed] [Google Scholar]
- 110.Bauvois B New facets of matrix metalloproteinases MMP-2 and MMP-9 as cell surface transducers: outside-in signaling and relationship to tumor progression. Biochimica et biophysica acta. January 2012;DOI: 10.1016/j.bbcan.2011.10.001; 1825(1):29–36. [DOI] [PubMed] [Google Scholar]
- 111.Perez-Sayans Garcia M, Suarez-Penaranda JM, Gayoso-Diz P, Barros-Angueira F, Gandara-Rey JM, Garcia-Garcia A. Tissue inhibitor of metalloproteinases in oral squamous cell carcinomas - a therapeutic target? Cancer letters. October 1 2012;DOI: 10.1016/j.canlet.2012.03.040; 323(1):11–19. [DOI] [PubMed] [Google Scholar]
- 112.Singh RD, Haridas N, Patel JB, et al. Matrix metalloproteinases and their inhibitors: correlation with invasion and metastasis in oral cancer. Indian journal of clinical biochemistry : IJCB. July 2010;DOI: 10.1007/s12291-010-0060-8; 25(3):250–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Munshi HG, Wu YI, Ariztia EV, Stack MS. Calcium regulation of matrix metalloproteinase-mediated migration in oral squamous cell carcinoma cells. The Journal of biological chemistry. November 1 2002;DOI: 10.1074/jbc.M207695200; 277(44):41480–41488. [DOI] [PubMed] [Google Scholar]
- 114.Branch KM, Hoshino D, Weaver AM. Adhesion rings surround invadopodia and promote maturation. Biology open. August 15 2012;DOI: 10.1242/bio.20121867; 1(8):711–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Huang Z, Tan N, Guo W, et al. Overexpression of EMMPRIN isoform 2 is associated with head and neck cancer metastasis. PloS one. 2014;DOI: 10.1371/journal.pone.0091596; 9(4):e91596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sweeny L, Liu Z, Bush BD, Hartman Y, Zhou T, Rosenthal EL. CD147 and AGR2 expression promote cellular proliferation and metastasis of head and neck squamous cell carcinoma. Experimental cell research. August 15 2012;DOI: 10.1016/j.yexcr.2012.04.022; 318(14):1788–1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lescaille G, Menashi S, Cavelier-Balloy B, et al. EMMPRIN/CD147 up-regulates urokinase-type plasminogen activator: implications in oral tumor progression. BMC cancer. 2012;DOI: 10.1186/1471-2407-12-115; 12:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Dang D, Atakilit A, Ramos DM. EMMPRIN modulates migration and deposition of TN-C in oral squamous carcinoma. Anticancer research. Jul-Aug 2008; 28(4B):2049–2054. [PubMed] [Google Scholar]
- 119.Cheng MF, Huang MS, Lin CS, et al. Expression of matriptase correlates with tumour progression and clinical prognosis in oral squamous cell carcinoma. Histopathology. July 2014;DOI: 10.1111/his.12361; 65(1):24–34. [DOI] [PubMed] [Google Scholar]
- 120.Wang H, Wu Q, Liu Z, et al. Downregulation of FAP suppresses cell proliferation and metastasis through PTEN/PI3K/AKT and Ras-ERK signaling in oral squamous cell carcinoma. Cell death & disease. 2014;DOI: 10.1038/cddis.2014.122; 5:e1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Liang X, Yang X, Tang Y, et al. RNAi-mediated downregulation of urokinase plasminogen activator receptor inhibits proliferation, adhesion, migration and invasion in oral cancer cells. Oral oncology. December 2008;DOI: 10.1016/j.oraloncology.2008.03.004; 44(12):1172–1180. [DOI] [PubMed] [Google Scholar]
- 122.Ghosh S, Johnson JJ, Sen R, et al. Functional relevance of urinary-type plasminogen activator receptor-alpha3beta1 integrin association in proteinase regulatory pathways. The Journal of biological chemistry. May 12 2006;DOI: 10.1074/jbc.M508526200; 281(19):13021–13029. [DOI] [PubMed] [Google Scholar]
- 123.Nozaki S, Endo Y, Nakahara H, et al. Inhibition of invasion and metastasis in oral cancer by targeting urokinase-type plasminogen activator receptor. Oral oncology. November 2005;DOI: 10.1016/j.oraloncology.2005.05.013; 41(10):971–977. [DOI] [PubMed] [Google Scholar]
- 124.Albo D, Tuszynski GP. Thrombospondin-1 up-regulates tumor cell invasion through the urokinase plasminogen activator receptor in head and neck cancer cells. The Journal of surgical research. July 2004;DOI: 10.1016/j.jss.2004.03.007; 120(1):21–26. [DOI] [PubMed] [Google Scholar]
- 125.Chen J, Zheng D, Shen J, et al. Heparanase is involved in the proliferation and invasion of nasopharyngeal carcinoma cells. Oncology reports. May 2013;DOI: 10.3892/or.2013.2325; 29(5):1888–1894. [DOI] [PubMed] [Google Scholar]
- 126.Sasaya K, Sudo H, Maeda G, Kawashiri S, Imai K. Concomitant loss of p120-catenin and beta-catenin membrane expression and oral carcinoma progression with E-cadherin reduction. PloS one. 2013;DOI: 10.1371/journal.pone.0069777; 8(8):e69777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Munshi HG, Ghosh S, Mukhopadhyay S, et al. Proteinase suppression by E-cadherin-mediated cell-cell attachment in premalignant oral keratinocytes. The Journal of biological chemistry. October 11 2002;DOI: 10.1074/jbc.M202384200; 277(41):38159–38167. [DOI] [PubMed] [Google Scholar]
- 128.Nakayama S, Sasaki A, Mese H, Alcalde RE, Tsuji T, Matsumura T. The E-cadherin gene is silenced by CpG methylation in human oral squamous cell carcinomas. International journal of cancer. Journal international du cancer. September 1 2001; 93(5):667–673. [DOI] [PubMed] [Google Scholar]
- 129.Doki Y, Shiozaki H, Tahara H, et al. Correlation between E-cadherin expression and invasiveness in vitro in a human esophageal cancer cell line. Cancer research. July 15 1993; 53(14):3421–3426. [PubMed] [Google Scholar]
- 130.Wang HC, Chiang WF, Huang HH, Shen YY, Chiang HC. Src-homology 2 domain-containing tyrosine phosphatase 2 promotes oral cancer invasion and metastasis. BMC cancer. 2014;DOI: 10.1186/1471-2407-14-442; 14:442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Margulis A, Zhang W, Alt-Holland A, Crawford HC, Fusenig NE, Garlick JA. E-cadherin suppression accelerates squamous cell carcinoma progression in three-dimensional, human tissue constructs. Cancer research. March 1 2005;DOI: 10.1158/0008-5472.CAN-04-3399; 65(5):1783–1791. [DOI] [PubMed] [Google Scholar]
- 132.Sobolik-Delmaire T, Katafiasz D, Keim SA, Mahoney MG, Wahl JK, 3rd. Decreased plakophilin-1 expression promotes increased motility in head and neck squamous cell carcinoma cells. Cell communication & adhesion. Mar-Jun 2007;DOI: 10.1080/15419060701463082; 14(2–3):99–109. [DOI] [PubMed] [Google Scholar]
- 133.Dos Reis PP, Bharadwaj RR, Machado J, et al. Claudin 1 overexpression increases invasion and is associated with aggressive histological features in oral squamous cell carcinoma. Cancer. December 1 2008;DOI: 10.1002/cncr.23934; 113(11):3169–3180. [DOI] [PubMed] [Google Scholar]
- 134.Chen YJ, Chang JT, Lee L, et al. DSG3 is overexpressed in head neck cancer and is a potential molecular target for inhibition of oncogenesis. Oncogene. January 18 2007;DOI: 10.1038/sj.onc.1209802; 26(3):467–476. [DOI] [PubMed] [Google Scholar]
- 135.Theocharis S, Klijanienko J, Giaginis C, Alexandrou P, Patsouris E, Sastre-Garau X. FAK and Src expression in mobile tongue squamous cell carcinoma: associations with clinicopathological parameters and patients survival. Journal of cancer research and clinical oncology. August 2012;DOI: 10.1007/s00432-012-1215-1; 138(8):1369–1377. [DOI] [PubMed] [Google Scholar]
- 136.Zhao D, Tang XF, Yang K, Liu JY, Ma XR. Over-expression of integrin-linked kinase correlates with aberrant expression of Snail, E-cadherin and N-cadherin in oral squamous cell carcinoma: implications in tumor progression and metastasis. Clinical & experimental metastasis. December 2012;DOI: 10.1007/s10585-012-9485-1; 29(8):957–969. [DOI] [PubMed] [Google Scholar]
- 137.Kramer RH, Shen X, Zhou H. Tumor cell invasion and survival in head and neck cancer. Cancer metastasis reviews. January 2005;DOI: 10.1007/s10555-005-5046-2; 24(1):35–45. [DOI] [PubMed] [Google Scholar]
- 138.Thomas GJ, Nystrom ML, Marshall JF. Alphavbeta6 integrin in wound healing and cancer of the oral cavity. Journal of oral pathology & medicine : official publication of the International Association of Oral Pathologists and the American Academy of Oral Pathology. January 2006;DOI: 10.1111/j.1600-0714.2005.00374.x; 35(1):1–10. [DOI] [PubMed] [Google Scholar]
- 139.Wang D, Muller S, Amin AR, et al. The pivotal role of integrin beta1 in metastasis of head and neck squamous cell carcinoma. Clinical cancer research : an official journal of the American Association for Cancer Research. September 1 2012;DOI: 10.1158/1078-0432.ccr-11-3127; 18(17):4589–4599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hunt S, Jones AV, Hinsley EE, Whawell SA, Lambert DW. MicroRNA-124 suppresses oral squamous cell carcinoma motility by targeting ITGB1. FEBS letters. January 3 2011;DOI: 10.1016/j.febslet.2010.11.038; 585(1):187–192. [DOI] [PubMed] [Google Scholar]
- 141.Xue H, Atakilit A, Zhu W, Li X, Ramos DM, Pytela R. Role of the alpha(v)beta6 integrin in human oral squamous cell carcinoma growth in vivo and in vitro. Biochemical and biophysical research communications. November 2 2001;DOI: 10.1006/bbrc.2001.5813; 288(3):610–618. [DOI] [PubMed] [Google Scholar]
- 142.Morgan MR, Jazayeri M, Ramsay AG, et al. Psoriasin (S100A7) associates with integrin beta6 subunit and is required for alphavbeta6-dependent carcinoma cell invasion. Oncogene. March 24 2011;DOI: 10.1038/onc.2010.535; 30(12):1422–1435. [DOI] [PubMed] [Google Scholar]
- 143.Ramos DM, But M, Regezi J, et al. Expression of integrin beta 6 enhances invasive behavior in oral squamous cell carcinoma. Matrix biology : journal of the International Society for Matrix Biology. April 2002; 21(3):297–307. [DOI] [PubMed] [Google Scholar]
- 144.Scott KA, Arnott CH, Robinson SC, et al. TNF-alpha regulates epithelial expression of MMP-9 and integrin alphavbeta6 during tumour promotion. A role for TNF-alpha in keratinocyte migration? Oncogene. September 9 2004;DOI: 10.1038/sj.onc.1207915; 23(41):6954–6966. [DOI] [PubMed] [Google Scholar]
- 145.Koontongkaew S, Amornphimoltham P, Monthanpisut P, Saensuk T, Leelakriangsak M. Fibroblasts and extracellular matrix differently modulate MMP activation by primary and metastatic head and neck cancer cells. Medical oncology. June 2012;DOI: 10.1007/s12032-011-9871-6; 29(2):690–703. [DOI] [PubMed] [Google Scholar]
- 146.Ratzinger S, Grassel S, Dowejko A, Reichert TE, Bauer RJ. Induction of type XVI collagen expression facilitates proliferation of oral cancer cells. Matrix biology : journal of the International Society for Matrix Biology. March 2011;DOI: 10.1016/j.matbio.2011.01.001; 30(2):118–125. [DOI] [PubMed] [Google Scholar]
- 147.Grassel S, Bauer RJ. Collagen XVI in health and disease. Matrix biology : journal of the International Society for Matrix Biology. March 11 2013;DOI: 10.1016/j.matbio.2012.11.001; 32(2):64–73. [DOI] [PubMed] [Google Scholar]
- 148.Kinoshita T, Nohata N, Hanazawa T, et al. Tumour-suppressive microRNA-29s inhibit cancer cell migration and invasion by targeting laminin-integrin signalling in head and neck squamous cell carcinoma. British journal of cancer. November 12 2013;DOI: 10.1038/bjc.2013.607; 109(10):2636–2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Xing Y, Qi J, Deng S, Wang C, Zhang L, Chen J. Small interfering RNA targeting ILK inhibits metastasis in human tongue cancer cells through repression of epithelial-to-mesenchymal transition. Experimental cell research. August 1 2013;DOI: 10.1016/j.yexcr.2013.05.014; 319(13):2058–2072. [DOI] [PubMed] [Google Scholar]
- 150.Lai MT, Hua CH, Tsai MH, et al. Talin-1 overexpression defines high risk for aggressive oral squamous cell carcinoma and promotes cancer metastasis. The Journal of pathology. July 2011;DOI: 10.1002/path.2867; 224(3):367–376. [DOI] [PubMed] [Google Scholar]
- 151.Canel M, Secades P, Garzon-Arango M, et al. Involvement of focal adhesion kinase in cellular invasion of head and neck squamous cell carcinomas via regulation of MMP-2 expression. British journal of cancer. April 8 2008;DOI: 10.1038/sj.bjc.6604286; 98(7):1274–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Xiao W, Jiang M, Li H, Li C, Su R, Huang K. Knockdown of FAK inhibits the invasion and metastasis of Tca8113 cells in vitro. Molecular medicine reports. August 2013;DOI: 10.3892/mmr.2013.1555; 8(2):703–707. [DOI] [PubMed] [Google Scholar]
- 153.Kurio N, Shimo T, Fukazawa T, et al. Anti-tumor effect of a novel FAK inhibitor TAE226 against human oral squamous cell carcinoma. Oral oncology. November 2012;DOI: 10.1016/j.oraloncology.2012.05.019; 48(11):1159–1170. [DOI] [PubMed] [Google Scholar]
- 154.Li X, Yang Y, Hu Y, et al. Alphavbeta6-Fyn signaling promotes oral cancer progression. The Journal of biological chemistry. October 24 2003;DOI: 10.1074/jbc.M306274200; 278(43):41646–41653. [DOI] [PubMed] [Google Scholar]
- 155.Hoshino D, Branch KM, Weaver AM. Signaling inputs to invadopodia and podosomes. Journal of cell science. July 15 2013;DOI: 10.1242/jcs.079475; 126(Pt 14):2979–2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Beaty BT, Condeelis J. Digging a little deeper: The stages of invadopodium formation and maturation. European journal of cell biology. July 21 2014;DOI: 10.1016/j.ejcb.2014.07.003. [DOI] [PMC free article] [PubMed]
- 157.Gould CM, Courtneidge SA. Regulation of invadopodia by the tumor microenvironment. Cell adhesion & migration. May-Jun 2014; 8(3):226–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Revach OY, Geiger B. The interplay between the proteolytic, invasive, and adhesive domains of invadopodia and their roles in cancer invasion. Cell adhesion & migration. May-Jun 2013; 8(3):215–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Boateng LR, Huttenlocher A. Spatiotemporal regulation of Src and its substrates at invadosomes. European journal of cell biology. Nov-Dec 2012;DOI: 10.1016/j.ejcb.2012.06.003; 91(11–12):878–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Farazi TA, Hoell JI, Morozov P, Tuschl T. MicroRNAs in human cancer. Advances in experimental medicine and biology. 2013;DOI: 10.1007/978-94-007-5590-1_1; 774:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Lee YS, Dutta A. MicroRNAs in cancer. Annual review of pathology. 2009;DOI: 10.1146/annurev.pathol.4.110807.092222; 4:199–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Nohata N, Hanazawa T, Kinoshita T, Okamoto Y, Seki N. MicroRNAs function as tumor suppressors or oncogenes: aberrant expression of microRNAs in head and neck squamous cell carcinoma. Auris, nasus, larynx. April 2013;DOI: 10.1016/j.anl.2012.07.001; 40(2):143–149. [DOI] [PubMed] [Google Scholar]
- 163.Janiszewska J, Szaumkessel M, Szyfter K. microRNAs are important players in head and neck carcinoma: a review. Critical reviews in oncology/hematology. December 2013;DOI: 10.1016/j.critrevonc.2013.07.012; 88(3):716–728. [DOI] [PubMed] [Google Scholar]
- 164.Chen D, Cabay RJ, Jin Y, et al. MicroRNA Deregulations in Head and Neck Squamous Cell Carcinomas. Journal of oral & maxillofacial research. 2013;DOI: 10.5037/jomr.2013.4102; 4(1):e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.John K, Wu J, Lee BW, Farah CS. MicroRNAs in Head and Neck Cancer. International journal of dentistry. 2013;DOI: 10.1155/2013/650218; 2013:650218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Cheng Q, Yi B, Wang A, Jiang X. Exploring and exploiting the fundamental role of microRNAs in tumor pathogenesis. OncoTargets and therapy. 2013;DOI: 10.2147/OTT.S52730; 6:1675–1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Babu JM, Prathibha R, Jijith VS, Hariharan R, Pillai MR. A miR-centric view of head and neck cancers. Biochimica et biophysica acta. August 2011;DOI: 10.1016/j.bbcan.2011.04.003; 1816(1):67–72. [DOI] [PubMed] [Google Scholar]
- 168.Zeng S, Yang J, Zhao J, et al. Silencing Dicer expression enhances cellular proliferative and invasive capacities in human tongue squamous cell carcinoma. Oncology reports. February 2014;DOI: 10.3892/or.2013.2903; 31(2):867–873. [DOI] [PubMed] [Google Scholar]
- 169.Yang X, Wu H, Ling T. Suppressive effect of microRNA-126 on oral squamous cell carcinoma in vitro. Molecular medicine reports. July 2014;DOI: 10.3892/mmr.2014.2171; 10(1):125–130. [DOI] [PubMed] [Google Scholar]
- 170.Kawakita A, Yanamoto S, Yamada SI, et al. MicroRNA-21 Promotes Oral Cancer Invasion via the Wnt/beta-Catenin Pathway by Targeting DKK2. Pathology oncology research : POR. September 3 2013;DOI: 10.1007/s12253-013-9689-y. [DOI] [PubMed]
- 171.Yen YC, Shiah SG, Chu HC, et al. Reciprocal regulation of microRNA-99a and insulin-like growth factor I receptor signaling in oral squamous cell carcinoma cells. Molecular cancer. 2014;DOI: 10.1186/1476-4598-13-6; 13:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Lenarduzzi M, Hui AB, Alajez NM, et al. MicroRNA-193b enhances tumor progression via down regulation of neurofibromin 1. PloS one. 2013;DOI: 10.1371/journal.pone.0053765; 8(1):e53765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Zhao XD, Zhang W, Liang HJ, Ji WY. Overexpression of miR −155 promotes proliferation and invasion of human laryngeal squamous cell carcinoma via targeting SOCS1 and STAT3. PloS one. 2013;DOI: 10.1371/journal.pone.0056395; 8(2):e56395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Datta J, Smith A, Lang JC, et al. microRNA-107 functions as a candidate tumor-suppressor gene in head and neck squamous cell carcinoma by downregulation of protein kinase Cvarepsilon. Oncogene. September 6 2012;DOI: 10.1038/onc.2011.565; 31(36):4045–4053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Kinoshita T, Hanazawa T, Nohata N, et al. Tumor suppressive microRNA-218 inhibits cancer cell migration and invasion through targeting laminin-332 in head and neck squamous cell carcinoma. Oncotarget. November 2012; 3(11):1386–1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Lu L, Xue X, Lan J, et al. MicroRNA-29a upregulates MMP2 in oral squamous cell carcinoma to promote cancer invasion and anti-apoptosis. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie. February 2014;DOI: 10.1016/j.biopha.2013.10.005; 68(1):13–19. [DOI] [PubMed] [Google Scholar]
- 177.Kai Y, Peng W, Ling W, Jiebing H, Zhuan B. Reciprocal effects between microRNA-140–5p and ADAM10 suppress migration and invasion of human tongue cancer cells. Biochemical and biophysical research communications. June 6 2014;DOI: 10.1016/j.bbrc.2014.02.032; 448(3):308–314. [DOI] [PubMed] [Google Scholar]
- 178.Nohata N, Sone Y, Hanazawa T, et al. miR-1 as a tumor suppressive microRNA targeting TAGLN2 in head and neck squamous cell carcinoma. Oncotarget. Jan-Feb 2011; 2(1–2):29–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Kinoshita T, Nohata N, Fuse M, et al. Tumor suppressive microRNA-133a regulates novel targets: moesin contributes to cancer cell proliferation and invasion in head and neck squamous cell carcinoma. Biochemical and biophysical research communications. February 10 2012;DOI: 10.1016/j.bbrc.2012.01.030; 418(2):378–383. [DOI] [PubMed] [Google Scholar]
- 180.Kinoshita T, Nohata N, Watanabe-Takano H, et al. Actin-related protein 2/3 complex subunit 5 (ARPC5) contributes to cell migration and invasion and is directly regulated by tumor-suppressive microRNA-133a in head and neck squamous cell carcinoma. International journal of oncology. June 2012;DOI: 10.3892/ijo.2012.1390; 40(6):1770–1778. [DOI] [PubMed] [Google Scholar]
- 181.Sun Q, Zhang J, Cao W, et al. Dysregulated miR-363 affects head and neck cancer invasion and metastasis by targeting podoplanin. The international journal of biochemistry & cell biology. March 2013;DOI: 10.1016/j.biocel.2012.12.004; 45(3):513–520. [DOI] [PubMed] [Google Scholar]
- 182.Kamochi N, Nakashima M, Aoki S, et al. Irradiated fibroblast-induced bystander effects on invasive growth of squamous cell carcinoma under cancer-stromal cell interaction. Cancer science. December 2008;DOI: 10.1111/j.1349-7006.2008.00978.x; 99(12):2417–2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Li X, Xu Q, Wu Y, et al. A CCL2/ROS autoregulation loop is critical for cancer-associated fibroblasts-enhanced tumor growth of oral squamous cell carcinoma. Carcinogenesis. June 2014;DOI: 10.1093/carcin/bgu046; 35(6):1362–1370. [DOI] [PubMed] [Google Scholar]
- 184.Islam M, Datta J, Lang JC, Teknos TN. Down regulation of RhoC by microRNA-138 results in de-activation of FAK, Src and Erk1/2 signaling pathway in head and neck squamous cell carcinoma. Oral oncology. May 2014;DOI: 10.1016/j.oraloncology.2014.01.014; 50(5):448–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Liu X, Wang C, Chen Z, et al. MicroRNA-138 suppresses epithelial-mesenchymal transition in squamous cell carcinoma cell lines. The Biochemical journal. November 15 2011;DOI: 10.1042/BJ20111006; 440(1):23–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Lin Z, Sun L, Chen W, et al. miR-639 regulates transforming growth factor beta-induced epithelial–mesenchymal transition in human tongue cancer cells by targeting FOXC1. Cancer science. 2014;DOI: 10.1111/cas.12499; 105(10):1288–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Liu M, Wang J, Huang H, Hou J, Zhang B, Wang A. miR-181a-Twist1 pathway in the chemoresistance of tongue squamous cell carcinoma. Biochemical and biophysical research communications. November 15 2013;DOI: 10.1016/j.bbrc.2013.10.051; 441(2):364–370. [DOI] [PubMed] [Google Scholar]
- 188.Jia LF, Huang YP, Zheng YF, et al. miR-29b suppresses proliferation, migration, and invasion of tongue squamous cell carcinoma through PTEN-AKT signaling pathway by targeting Sp1. Oral oncology. August 7 2014;DOI: 10.1016/j.oraloncology.2014.07.010. [DOI] [PubMed]
- 189.Hui AB, Bruce JP, Alajez NM, et al. Significance of dysregulated metadherin and microRNA-375 in head and neck cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. December 15 2011;DOI: 10.1158/1078-0432.CCR-11-2102; 17(24):7539–7550. [DOI] [PubMed] [Google Scholar]
- 190.Hui AB, Lenarduzzi M, Krushel T, et al. Comprehensive MicroRNA profiling for head and neck squamous cell carcinomas. Clinical cancer research : an official journal of the American Association for Cancer Research. February 15 2010;DOI: 10.1158/1078-0432.CCR-09-2166; 16(4):1129–1139. [DOI] [PubMed] [Google Scholar]
- 191.Nohata N, Hanazawa T, Kikkawa N, et al. Tumor suppressive microRNA-375 regulates oncogene AEG-1/MTDH in head and neck squamous cell carcinoma (HNSCC). Journal of human genetics. August 2011;DOI: 10.1038/jhg.2011.66; 56(8):595–601. [DOI] [PubMed] [Google Scholar]
- 192.Harris T, Jimenez L, Kawachi N, et al. Low-level expression of miR-375 correlates with poor outcome and metastasis while altering the invasive properties of head and neck squamous cell carcinomas. The American journal of pathology. March 2012;DOI: 10.1016/j.ajpath.2011.12.004; 180(3):917–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Chen Z, Jin Y, Yu D, et al. Down-regulation of the microRNA-99 family members in head and neck squamous cell carcinoma. Oral oncology. August 2012;DOI: 10.1016/j.oraloncology.2012.02.020; 48(8):686–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Yang MH, Lin BR, Chang CH, et al. Connective tissue growth factor modulates oral squamous cell carcinoma invasion by activating a miR-504/FOXP1 signalling. Oncogene. May 10 2012;DOI: 10.1038/onc.2011.423; 31(19):2401–2411. [DOI] [PubMed] [Google Scholar]
- 195.Reis PP, Tomenson M, Cervigne NK, et al. Programmed cell death 4 loss increases tumor cell invasion and is regulated by miR-21 in oral squamous cell carcinoma. Molecular cancer. 2010;DOI: 10.1186/1476-4598-9-238; 9:238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Jiang F, Zhao W, Zhou L, Zhang L, Liu Z, Yu D. miR-222 regulates the cell biological behavior of oral squamous cell carcinoma by targeting PUMA. Oncology reports. March 2014;DOI: 10.3892/or.2014.2985; 31(3):1255–1262. [DOI] [PubMed] [Google Scholar]
- 197.Liu X, Yu J, Jiang L, et al. MicroRNA-222 regulates cell invasion by targeting matrix metalloproteinase 1 (MMP1) and manganese superoxide dismutase 2 (SOD2) in tongue squamous cell carcinoma cell lines. Cancer genomics & proteomics. May-Jun 2009; 6(3):131–139. [PMC free article] [PubMed] [Google Scholar]
- 198.Parekh A, Weaver AM. Regulation of cancer invasiveness by the physical extracellular matrix environment. Cell adhesion & migration. Jul-Sep 2009; 3(3):288–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Bravo-Cordero JJ, Hodgson L, Condeelis J. Directed cell invasion and migration during metastasis. Current opinion in cell biology. April 2012;DOI: 10.1016/j.ceb.2011.12.004; 24(2):277–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Weaver AM. Invadopodia: specialized cell structures for cancer invasion. Clinical & experimental metastasis. 2006;DOI: 10.1007/s10585-006-9014-1; 23(2):97–105. [DOI] [PubMed] [Google Scholar]
- 201.Artym VV, Yamada KM, Mueller SC. ECM degradation assays for analyzing local cell invasion. Methods in molecular biology. 2009;DOI: 10.1007/978-1-59745-413-1_15; 522:211–219. [DOI] [PubMed] [Google Scholar]
- 202.Rothschild BL, Shim AH, Ammer AG, et al. Cortactin overexpression regulates actin-related protein 2/3 complex activity, motility, and invasion in carcinomas with chromosome 11q13 amplification. Cancer research. August 15 2006;DOI: 10.1158/0008-5472.CAN-05-4490; 66(16):8017–8025. [DOI] [PubMed] [Google Scholar]
- 203.Timpson P, Lynch DK, Schramek D, Walker F, Daly RJ. Cortactin overexpression inhibits ligand-induced down-regulation of the epidermal growth factor receptor. Cancer research. April 15 2005;DOI: 10.1158/0008-5472.CAN-04-2118; 65(8):3273–3280. [DOI] [PubMed] [Google Scholar]
- 204.Clark ES, Whigham AS, Yarbrough WG, Weaver AM. Cortactin is an essential regulator of matrix metalloproteinase secretion and extracellular matrix degradation in invadopodia. Cancer research. May 1 2007;DOI: 10.1158/0008-5472.CAN-06-3928; 67(9):4227–4235. [DOI] [PubMed] [Google Scholar]
- 205.Kelley LC, Ammer AG, Hayes KE, et al. Oncogenic Src requires a wild-type counterpart to regulate invadopodia maturation. Journal of cell science. November 15 2010;DOI: 10.1242/jcs.075200; 123(Pt 22):3923–3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Mader CC, Oser M, Magalhaes MA, et al. An EGFR-Src-Arg-cortactin pathway mediates functional maturation of invadopodia and breast cancer cell invasion. Cancer research. March 1 2011;DOI: 10.1158/0008-5472.CAN-10-1432; 71(5):1730–1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Hwang YS, Park KK, Chung WY. Invadopodia formation in oral squamous cell carcinoma: the role of epidermal growth factor receptor signalling. Archives of oral biology. April 2012;DOI: 10.1016/j.archoralbio.2011.08.019; 57(4):335–343. [DOI] [PubMed] [Google Scholar]
- 208.Hwang YS, Park KK, Cha IH, Kim J, Chung WY. Role of insulin-like growth factor-II mRNA-binding protein-3 in invadopodia formation and the growth of oral squamous cell carcinoma in athymic nude mice. Head & neck. September 2012;DOI: 10.1002/hed.21929; 34(9):1329–1339. [DOI] [PubMed] [Google Scholar]
- 209.Hoshino D, Jourquin J, Emmons SW, et al. Network analysis of the focal adhesion to invadopodia transition identifies a PI3K-PKCalpha invasive signaling axis. Science signaling. 2012;DOI: 10.1126/scisignal.2002964; 5(241):ra66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Yamaguchi H, Yoshida S, Muroi E, et al. Phosphoinositide 3-kinase signaling pathway mediated by p110alpha regulates invadopodia formation. The Journal of cell biology. June 27 2011;DOI: 10.1083/jcb.201009126; 193(7):1275–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Gardberg M, Kaipio K, Lehtinen L, et al. FHOD1, a formin upregulated in epithelial-mesenchymal transition, participates in cancer cell migration and invasion. PloS one. 2013;DOI: 10.1371/journal.pone.0074923; 8(9):e74923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Hoshino D, Kirkbride KC, Costello K, et al. Exosome secretion is enhanced by invadopodia and drives invasive behavior. Cell reports. December 12 2013;DOI: 10.1016/j.celrep.2013.10.050; 5(5):1159–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Scanlon CS, Van Tubergen EA, Inglehart RC, D’Silva NJ. Biomarkers of epithelial-mesenchymal transition in squamous cell carcinoma. Journal of dental research. February 2013;DOI: 10.1177/0022034512467352; 92(2):114–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Smith A, Teknos TN, Pan Q. Epithelial to mesenchymal transition in head and neck squamous cell carcinoma. Oral oncology. April 2013;DOI: 10.1016/j.oraloncology.2012.10.009; 49(4):287–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Barrette K, Van Kelst S, Wouters J, et al. Epithelial-mesenchymal transition during invasion of cutaneous squamous cell carcinoma is paralleled by AKT activation. The British journal of dermatology. March 14 2014;DOI: 10.1111/bjd.12967. [DOI] [PubMed]
- 216.Mani SA, Guo W, Liao MJ, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. May 16 2008;DOI: 10.1016/j.cell.2008.03.027; 133(4):704–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Zhang Z, Filho MS, Nor JE. The biology of head and neck cancer stem cells. Oral oncology. January 2012;DOI: 10.1016/j.oraloncology.2011.10.004; 48(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Chang JY, Wright JM, Svoboda KK. Signal transduction pathways involved in epithelial-mesenchymal transition in oral cancer compared with other cancers. Cells, tissues, organs. 2007;DOI: 10.1159/000101301; 185(1–3):40–47. [DOI] [PubMed] [Google Scholar]
- 219.Yang MH, Hsu DS, Wang HW, et al. Bmi1 is essential in Twist1-induced epithelial-mesenchymal transition. Nature cell biology. October 2010;DOI: 10.1038/ncb2099; 12(10):982–992. [DOI] [PubMed] [Google Scholar]
- 220.Yang N, Hui L, Wang Y, Yang H, Jiang X. SOX2 promotes the migration and invasion of laryngeal cancer cells by induction of MMP-2 via the PI3K/Akt/mTOR pathway. Oncology reports. June 2014;DOI: 10.3892/or.2014.3120; 31(6):2651–2659. [DOI] [PubMed] [Google Scholar]
- 221.Yang CC, Zhu LF, Xu XH, Ning TY, Ye JH, Liu LK. Membrane Type 1 Matrix Metalloproteinase induces an epithelial to mesenchymal transition and cancer stem cell-like properties in SCC9 cells. BMC cancer. 2013;DOI: 10.1186/1471-2407-13-171; 13:171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Higashikawa K, Yoneda S, Tobiume K, Taki M, Shigeishi H, Kamata N. Snail-induced down-regulation of DeltaNp63alpha acquires invasive phenotype of human squamous cell carcinoma. Cancer research. October 1 2007;DOI: 10.1158/0008-5472.CAN-07-0932; 67(19):9207–9213. [DOI] [PubMed] [Google Scholar]
- 223.Zhang Z, Dong Z, Lauxen IS, Filho MS, Nor JE. Endothelial cell-secreted EGF induces epithelial to mesenchymal transition and endows head and neck cancer cells with stem-like phenotype. Cancer research. May 15 2014;DOI: 10.1158/0008-5472.CAN-13-2032; 74(10):2869–2881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Zhu LF, Hu Y, Yang CC, et al. Snail overexpression induces an epithelial to mesenchymal transition and cancer stem cell-like properties in SCC9 cells. Laboratory investigation; a journal of technical methods and pathology. May 2012;DOI: 10.1038/labinvest.2012.8; 92(5):744–752. [DOI] [PubMed] [Google Scholar]
- 225.Krishnamurthy S, Nor JE. Head and neck cancer stem cells. Journal of dental research. April 2012;DOI: 10.1177/0022034511423393; 91(4):334–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Krishnamurthy S, Dong Z, Vodopyanov D, et al. Endothelial cell-initiated signaling promotes the survival and self-renewal of cancer stem cells. Cancer research. December 1 2010;DOI: 10.1158/0008-5472.CAN-10-1712; 70(23):9969–9978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Ritchie KE, Nor JE. Perivascular stem cell niche in head and neck cancer. Cancer letters. September 10 2013;DOI: 10.1016/j.canlet.2012.07.025; 338(1):41–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Wu KJ, Yang MH. Epithelial-mesenchymal transition and cancer stemness: the Twist1-Bmi1 connection. Bioscience reports. December 2011;DOI: 10.1042/BSR20100114; 31(6):449–455. [DOI] [PubMed] [Google Scholar]
- 229.Lamouille S, Connolly E, Smyth JW, Akhurst RJ, Derynck R. TGF-beta-induced activation of mTOR complex 2 drives epithelial-mesenchymal transition and cell invasion. Journal of cell science. March 1 2012;DOI: 10.1242/jcs.095299; 125(Pt 5):1259–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Judd NP, Winkler AE, Murillo-Sauca O, et al. ERK1/2 regulation of CD44 modulates oral cancer aggressiveness. Cancer research. January 1 2012;DOI: 10.1158/0008-5472.CAN-11-1831; 72(1):365–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Biddle A, Liang X, Gammon L, et al. Cancer stem cells in squamous cell carcinoma switch between two distinct phenotypes that are preferentially migratory or proliferative. Cancer research. August 1 2011;DOI: 10.1158/0008-5472.CAN-11-1059; 71(15):5317–5326. [DOI] [PubMed] [Google Scholar]
- 232.Kudo T, Shimazu Y, Yagishita H, et al. Three-dimensional reconstruction of oral tongue squamous cell carcinoma at invasion front. International journal of dentistry. 2013;DOI: 10.1155/2013/482765; 2013:482765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Friedl P, Locker J, Sahai E, Segall JE. Classifying collective cancer cell invasion. Nature cell biology. August 2012;DOI: 10.1038/ncb2548; 14(8):777–783. [DOI] [PubMed] [Google Scholar]
- 234.Salo T, Vered M, Bello IO, et al. Insights into the role of components of the tumor microenvironment in oral carcinoma call for new therapeutic approaches. Experimental cell research. July 15 2014;DOI: 10.1016/j.yexcr.2013.12.029; 325(2):58–64. [DOI] [PubMed] [Google Scholar]
- 235.Curry JM, Sprandio J, Cognetti D, et al. Tumor microenvironment in head and neck squamous cell carcinoma. Seminars in oncology. April 2014;DOI: 10.1053/j.seminoncol.2014.03.003; 41(2):217–234. [DOI] [PubMed] [Google Scholar]
- 236.Koontongkaew S The tumor microenvironment contribution to development, growth, invasion and metastasis of head and neck squamous cell carcinomas. Journal of Cancer. 2013;DOI: 10.7150/jca.5112; 4(1):66–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Rivera C, Venegas B. Histological and molecular aspects of oral squamous cell carcinoma (Review). Oncology letters. July 2014;DOI: 10.3892/ol.2014.2103; 8(1):7–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Wheeler SE, Shi H, Lin F, et al. Enhancement of head and neck squamous cell carcinoma proliferation, invasion, and metastasis by tumor-associated fibroblasts in preclinical models. Head & neck. March 2014;DOI: 10.1002/hed.23312; 36(3):385–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Matsumoto K, Horikoshi M, Rikimaru K, Enomoto S. A study of an in vitro model for invasion of oral squamous cell carcinoma. Journal of oral pathology & medicine : official publication of the International Association of Oral Pathologists and the American Academy of Oral Pathology. October 1989; 18(9):498–501. [DOI] [PubMed] [Google Scholar]
- 240.Berndt A, Hyckel P, Konneker A, Katenkamp D, Kosmehl H. Oral squamous cell carcinoma invasion is associated with a laminin-5 matrix re-organization but independent of basement membrane and hemidesmosome formation. clues from an in vitro invasion model. Invasion & metastasis. 1997; 17(5):251–258. [PubMed] [Google Scholar]
- 241.Satoh S, Toda S, Inokuchi A, Sugihara H. A new in vitro model for analyzing the biological behavior of well-differentiated squamous cell carcinoma. Pathology, research and practice. 2005;DOI: 10.1016/j.prp.2004.09.015; 201(1):27–35. [DOI] [PubMed] [Google Scholar]
- 242.Nystrom ML, Thomas GJ, Stone M, Mackenzie IC, Hart IR, Marshall JF. Development of a quantitative method to analyse tumour cell invasion in organotypic culture. The Journal of pathology. March 2005;DOI: 10.1002/path.1716; 205(4):468–475. [DOI] [PubMed] [Google Scholar]
- 243.Sobral LM, Bufalino A, Lopes MA, Graner E, Salo T, Coletta RD. Myofibroblasts in the stroma of oral cancer promote tumorigenesis via secretion of activin A. Oral oncology. September 2011;DOI: 10.1016/j.oraloncology.2011.06.011; 47(9):840–846. [DOI] [PubMed] [Google Scholar]
- 244.Berndt A, Buttner R, Guhne S, et al. Effects of activated fibroblasts on phenotype modulation, EGFR signalling and cell cycle regulation in OSCC cells. Experimental cell research. April 1 2014;DOI: 10.1016/j.yexcr.2013.12.024; 322(2):402–414. [DOI] [PubMed] [Google Scholar]
- 245.Zhou B, Chen WL, Wang YY, et al. A role for cancer-associated fibroblasts in inducing the epithelial-to-mesenchymal transition in human tongue squamous cell carcinoma. Journal of oral pathology & medicine : official publication of the International Association of Oral Pathologists and the American Academy of Oral Pathology. September 2014;DOI: 10.1111/jop.12172; 43(8):585–592. [DOI] [PubMed] [Google Scholar]
- 246.Hinsley EE, Kumar S, Hunter KD, Whawell SA, Lambert DW. Endothelin-1 stimulates oral fibroblasts to promote oral cancer invasion. Life sciences. October 15 2012;DOI: 10.1016/j.lfs.2012.04.001; 91(13–14):557–561. [DOI] [PubMed] [Google Scholar]
- 247.Lengyel E, Gum R, Juarez J, et al. Induction of M(r) 92,000 type IV collagenase expression in a squamous cell carcinoma cell line by fibroblasts. Cancer research. February 15 1995; 55(4):963–967. [PubMed] [Google Scholar]
- 248.Hassona Y, Cirillo N, Lim KP, et al. Progression of genotype-specific oral cancer leads to senescence of cancer-associated fibroblasts and is mediated by oxidative stress and TGF-beta. Carcinogenesis. June 2013;DOI: 10.1093/carcin/bgt035; 34(6):1286–1295. [DOI] [PubMed] [Google Scholar]
- 249.Hassona Y, Cirillo N, Heesom K, Parkinson EK, Prime SS. Senescent cancer-associated fibroblasts secrete active MMP-2 that promotes keratinocyte dis-cohesion and invasion. British journal of cancer. September 9 2014;DOI: 10.1038/bjc.2014.438; 111(6):1230–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Pal A, Melling G, Hinsley EE, et al. Cigarette smoke condensate promotes pro-tumourigenic stromal-epithelial interactions by suppressing miR-145. Journal of oral pathology & medicine : official publication of the International Association of Oral Pathologists and the American Academy of Oral Pathology. April 2013;DOI: 10.1111/jop.12017; 42(4):309–314. [DOI] [PubMed] [Google Scholar]
- 251.Lu HH, Liu CJ, Liu TY, Kao SY, Lin SC, Chang KW. Areca-treated fibroblasts enhance tumorigenesis of oral epithelial cells. Journal of dental research. November 2008; 87(11):1069–1074. [DOI] [PubMed] [Google Scholar]
- 252.Wu MH, Hong HC, Hong TM, Chiang WF, Jin YT, Chen YL. Targeting galectin-1 in carcinoma-associated fibroblasts inhibits oral squamous cell carcinoma metastasis by downregulating MCP-1/CCL2 expression. Clinical cancer research : an official journal of the American Association for Cancer Research. March 15 2011;DOI: 10.1158/1078-0432.CCR-10-1824; 17(6):1306–1316. [DOI] [PubMed] [Google Scholar]
- 253.Jung DW, Che ZM, Kim J, et al. Tumor-stromal crosstalk in invasion of oral squamous cell carcinoma: a pivotal role of CCL7. International journal of cancer. Journal international du cancer. July 15 2010;DOI: 10.1002/ijc.25060; 127(2):332–344. [DOI] [PubMed] [Google Scholar]
- 254.Daly AJ, McIlreavey L, Irwin CR. Regulation of HGF and SDF-1 expression by oral fibroblasts--implications for invasion of oral cancer. Oral oncology. July 2008;DOI: 10.1016/j.oraloncology.2007.08.012; 44(7):646–651. [DOI] [PubMed] [Google Scholar]
- 255.Costea DE, Hills A, Osman AH, et al. Identification of two distinct carcinoma-associated fibroblast subtypes with differential tumor-promoting abilities in oral squamous cell carcinoma. Cancer research. July 1 2013;DOI: 10.1158/0008-5472.CAN-12-4150; 73(13):3888–3901. [DOI] [PubMed] [Google Scholar]
- 256.Hwang YS, Park KK, Chung WY. Stromal transforming growth factor-beta 1 is crucial for reinforcing the invasive potential of low invasive cancer. Archives of oral biology. July 2014;DOI: 10.1016/j.archoralbio.2014.03.017; 59(7):687–694. [DOI] [PubMed] [Google Scholar]
- 257.Chen SF, Nieh S, Jao SW, et al. The paracrine effect of cancer-associated fibroblast-induced interleukin-33 regulates the invasiveness of head and neck squamous cell carcinoma. The Journal of pathology. October 2013;DOI: 10.1002/path.4226; 231(2):180–189. [DOI] [PubMed] [Google Scholar]
- 258.Kurihara Y, Hatori M, Ando Y, et al. Inhibition of cyclooxygenase-2 suppresses the invasiveness of oral squamous cell carcinoma cell lines via down-regulation of matrix metalloproteinase-2 production and activation. Clinical & experimental metastasis. 2009;DOI: 10.1007/s10585-009-9241-3; 26(5):425–432. [DOI] [PubMed] [Google Scholar]
- 259.Sobral LM, Zecchin KG, Nascimento de Aquino S, Lopes MA, Graner E, Coletta RD. Isolation and characterization of myofibroblast cell lines from oral squamous cell carcinoma. Oncology reports. April 2011;DOI: 10.3892/or.2011.1161; 25(4):1013–1020. [DOI] [PubMed] [Google Scholar]
- 260.Hinsley EE, Hunt S, Hunter KD, Whawell SA, Lambert DW. Endothelin-1 stimulates motility of head and neck squamous carcinoma cells by promoting stromal-epithelial interactions. International journal of cancer. Journal international du cancer. January 1 2012;DOI: 10.1002/ijc.25968; 130(1):40–47. [DOI] [PubMed] [Google Scholar]
- 261.Matsumoto K, Matsumoto K, Nakamura T, Kramer RH. Hepatocyte growth factor/scatter factor induces tyrosine phosphorylation of focal adhesion kinase (p125FAK) and promotes migration and invasion by oral squamous cell carcinoma cells. The Journal of biological chemistry. December 16 1994; 269(50):31807–31813. [PubMed] [Google Scholar]
- 262.Knowles LM, Stabile LP, Egloff AM, et al. HGF and c-Met participate in paracrine tumorigenic pathways in head and neck squamous cell cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. June 1 2009;DOI: 10.1158/1078-0432.CCR-08-3252; 15(11):3740–3750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Uchida D, Kawamata H, Omotehara F, et al. Role of HGF/c-met system in invasion and metastasis of oral squamous cell carcinoma cells in vitro and its clinical significance. International journal of cancer. Journal international du cancer. August 15 2001; 93(4):489–496. [DOI] [PubMed] [Google Scholar]
- 264.Lewis MP, Lygoe KA, Nystrom ML, et al. Tumour-derived TGF-beta1 modulates myofibroblast differentiation and promotes HGF/SF-dependent invasion of squamous carcinoma cells. British journal of cancer. February 23 2004;DOI: 10.1038/sj.bjc.6601611; 90(4):822–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Hasina R, Matsumoto K, Matsumoto-Taniura N, Kato I, Sakuda M, Nakamura T. Autocrine and paracrine motility factors and their involvement in invasiveness in a human oral carcinoma cell line. British journal of cancer. August 1999;DOI: 10.1038/sj.bjc.6690587; 80(11):1708–1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Hayashido Y, Nakashima M, Urabe K, et al. Role of stromal thrombospondin-1 in motility and proteolytic activity of oral squamous cell carcinoma cells. International journal of molecular medicine. October 2003; 12(4):447–452. [PubMed] [Google Scholar]
- 267.Liu H, Chen B, Zardi L, Ramos DM. Soluble fibronectin promotes migration of oral squamous-cell carcinoma cells. International journal of cancer. Journal international du cancer. October 5 1998; 78(2):261–267. [DOI] [PubMed] [Google Scholar]
- 268.Fu L, Zhang C, Zhang LY, et al. Wnt2 secreted by tumour fibroblasts promotes tumour progression in oesophageal cancer by activation of the Wnt/beta-catenin signalling pathway. Gut. December 2011;DOI: 10.1136/gut.2011.241638; 60(12):1635–1643. [DOI] [PubMed] [Google Scholar]
- 269.Kumar P, Ning Y, Polverini PJ. Endothelial cells expressing Bcl-2 promotes tumor metastasis by enhancing tumor angiogenesis, blood vessel leakiness and tumor invasion. Laboratory investigation; a journal of technical methods and pathology. July 2008;DOI: 10.1038/labinvest.2008.46; 88(7):740–749. [DOI] [PubMed] [Google Scholar]
- 270.Warner KA, Miyazawa M, Cordeiro MM, et al. Endothelial cells enhance tumor cell invasion through a crosstalk mediated by CXC chemokine signaling. Neoplasia. February 2008; 10(2):131–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Kumar P, Miller AI, Polverini PJ. p38 MAPK Mediates γ-Irradiation-induced Endothelial Cell Apoptosis, and Vascular Endothelial Growth Factor Protects Endothelial Cells through the Phosphoinositide 3-Kinase-Akt-Bcl-2 Pathway. Journal of Biological Chemistry. October 8, 2004. 2004;DOI: 10.1074/jbc.M405777200; 279(41):43352–43360. [DOI] [PubMed] [Google Scholar]
- 272.Lee CH, Liu SY, Chou KC, et al. Tumor-associated macrophages promote oral cancer progression through activation of the Axl signaling pathway. Annals of surgical oncology. March 2014;DOI: 10.1245/s10434-013-3400-0; 21(3):1031–1037. [DOI] [PubMed] [Google Scholar]
- 273.Cavel O, Shomron O, Shabtay A, et al. Endoneurial macrophages induce perineural invasion of pancreatic cancer cells by secretion of GDNF and activation of RET tyrosine kinase receptor. Cancer research. November 15 2012;DOI: 10.1158/0008-5472.CAN-12-0764; 72(22):5733–5743. [DOI] [PubMed] [Google Scholar]
- 274.Zhang J, Cheng Q, Zhou Y, Wang Y, Chen X. Slug is a key mediator of hypoxia induced cadherin switch in HNSCC: correlations with poor prognosis. Oral oncology. November 2013;DOI: 10.1016/j.oraloncology.2013.08.003; 49(11):1043–1050. [DOI] [PubMed] [Google Scholar]
- 275.Lv C, Yang X, Yu B, Ma Q, Liu B, Liu Y. Blocking the Na+/H+ exchanger 1 with cariporide (HOE642) reduces the hypoxia-induced invasion of human tongue squamous cell carcinoma. International journal of oral and maxillofacial surgery. October 2012;DOI: 10.1016/j.ijom.2012.03.001; 41(10):1206–1210. [DOI] [PubMed] [Google Scholar]
- 276.Wang X, Schneider A. HIF-2alpha-mediated activation of the epidermal growth factor receptor potentiates head and neck cancer cell migration in response to hypoxia. Carcinogenesis. July 2010;DOI: 10.1093/carcin/bgq078; 31(7):1202–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Ryu MH, Park HM, Chung J, Lee CH, Park HR. Hypoxia-inducible factor-1alpha mediates oral squamous cell carcinoma invasion via upregulation of alpha5 integrin and fibronectin. Biochemical and biophysical research communications. February 26 2010;DOI: 10.1016/j.bbrc.2010.01.060; 393(1):11–15. [DOI] [PubMed] [Google Scholar]
- 278.Mohyeldin A, Lu H, Dalgard C, et al. Erythropoietin signaling promotes invasiveness of human head and neck squamous cell carcinoma. Neoplasia. May 2005; 7(5):537–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Shlien A, Malkin D. Copy number variations and cancer. Genome medicine. 2009;DOI: 10.1186/gm62; 1(6):62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Chen Y, Chen C. DNA copy number variation and loss of heterozygosity in relation to recurrence of and survival from head and neck squamous cell carcinoma: a review. Head & neck. October 2008;DOI: 10.1002/hed.20861; 30(10):1361–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Goldman M, Craft B, Swatloski T, et al. The UCSC Cancer Genomics Browser: update 2015. Nucleic acids research. November 11 2014;DOI: 10.1093/nar/gku1073. [DOI] [PMC free article] [PubMed]
- 282.Coussens LM, Fingleton B, Matrisian LM. Matrix metalloproteinase inhibitors and cancer: trials and tribulations. Science. March 29 2002;DOI: 10.1126/science.1067100; 295(5564):2387–2392. [DOI] [PubMed] [Google Scholar]
- 283.Chien MH, Lin CW, Cheng CW, Wen YC, Yang SF. Matrix metalloproteinase-2 as a target for head and neck cancer therapy. Expert opinion on therapeutic targets. February 2013;DOI: 10.1517/14728222.2013.740012; 17(2):203–216. [DOI] [PubMed] [Google Scholar]
- 284.Dufour A, Overall CM. Missing the target: matrix metalloproteinase antitargets in inflammation and cancer. Trends in pharmacological sciences. April 2013;DOI: 10.1016/j.tips.2013.02.004; 34(4):233–242. [DOI] [PubMed] [Google Scholar]
- 285.Moncharmont C, Levy A, Guy JB, et al. Radiation-enhanced cell migration/invasion process: A review. Critical reviews in oncology/hematology. May 22 2014;DOI: 10.1016/j.critrevonc.2014.05.006. [DOI] [PubMed]
- 286.Pickhard AC, Margraf J, Knopf A, et al. Inhibition of radiation induced migration of human head and neck squamous cell carcinoma cells by blocking of EGF receptor pathways. BMC cancer. 2011;DOI: 10.1186/1471-2407-11-388; 11:388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Beck C, Piontek G, Haug A, et al. The kallikrein-kinin-system in head and neck squamous cell carcinoma (HNSCC) and its role in tumour survival, invasion, migration and response to radiotherapy. Oral oncology. December 2012;DOI: 10.1016/j.oraloncology.2012.06.001; 48(12):1208–1219. [DOI] [PubMed] [Google Scholar]
- 288.Yang C, Yan J, Yuan G, et al. Icotinib inhibits the invasion of Tca8113 cells via downregulation of nuclear factor kappaB-mediated matrix metalloproteinase expression. Oncology letters. September 2014;DOI: 10.3892/ol.2014.2311; 8(3):1295–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Kogashiwa Y, Sakurai H, Kimura T, Kohno N. Docetaxel suppresses invasiveness of head and neck cancer cells in vitro. Cancer science. June 2010;DOI: 10.1111/j.1349-7006.2010.01540.x; 101(6):1382–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Lin CW, Chou YE, Chiou HL, et al. Pterostilbene suppresses oral cancer cell invasion by inhibiting MMP-2 expression. Expert opinion on therapeutic targets. August 9 2014;DOI: 10.1517/14728222.2014.947962:1–12. [DOI] [PubMed]
- 291.Shen H, Zeng G, Tang G, et al. Antimetastatic effects of licochalcone A on oral cancer via regulating metastasis-associated proteases. Tumour biology : the journal of the International Society for Oncodevelopmental Biology and Medicine. August 2014;DOI: 10.1007/s13277-014-1985-y; 35(8):7467–7474. [DOI] [PubMed] [Google Scholar]
- 292.Wolf MA, Claudio PP. Benzyl isothiocyanate inhibits HNSCC cell migration and invasion, and sensitizes HNSCC cells to cisplatin. Nutrition and cancer. 2014;DOI: 10.1080/01635581.2014.868912; 66(2):285–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Hwang YS, Park KK, Chung WY. Epigallocatechin-3 gallate inhibits cancer invasion by repressing functional invadopodia formation in oral squamous cell carcinoma. European journal of pharmacology. September 5 2013;DOI: 10.1016/j.ejphar.2013.05.008; 715(1–3):286–295. [DOI] [PubMed] [Google Scholar]
- 294.Chang YC, Chen PN, Chu SC, Lin CY, Kuo WH, Hsieh YS. Black tea polyphenols reverse epithelial-to-mesenchymal transition and suppress cancer invasion and proteases in human oral cancer cells. Journal of agricultural and food chemistry. August 29 2012;DOI: 10.1021/jf302223g; 60(34):8395–8403. [DOI] [PubMed] [Google Scholar]
- 295.Ding Y, Yao H, Yao Y, Fai LY, Zhang Z. Protection of dietary polyphenols against oral cancer. Nutrients. June 2013;DOI: 10.3390/nu5062173; 5(6):2173–2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Yang JS, Lin CW, Hsin CH, Hsieh MJ, Chang YC. Selaginellatamariscina attenuates metastasis via Akt pathways in oral cancer cells. PloS one. 2013;DOI: 10.1371/journal.pone.0068035; 8(6):e68035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Chang WW, Hu FW, Yu CC, et al. Quercetin in elimination of tumor initiating stem-like and mesenchymal transformation property in head and neck cancer. Head & neck. March 2013;DOI: 10.1002/hed.22982; 35(3):413–419. [DOI] [PubMed] [Google Scholar]
- 298.Chien MH, Ying TH, Hsieh YS, et al. Dioscorea nipponica Makino inhibits migration and invasion of human oral cancer HSC-3 cells by transcriptional inhibition of matrix metalloproteinase-2 through modulation of CREB and AP-1 activity. Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association. March 2012;DOI: 10.1016/j.fct.2011.12.016; 50(3–4):558–566. [DOI] [PubMed] [Google Scholar]
- 299.Peng CY, Yang HW, Chu YH, et al. Caffeic Acid phenethyl ester inhibits oral cancer cell metastasis by regulating matrix metalloproteinase-2 and the mitogen-activated protein kinase pathway. Evidence-based complementary and alternative medicine : eCAM. 2012;DOI: 10.1155/2012/732578; 2012:732578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Sun Q, Prasad R, Rosenthal E, Katiyar SK. Grape seed proanthocyanidins inhibit the invasiveness of human HNSCC cells by targeting EGFR and reversing the epithelial-to-mesenchymal transition. PloS one. 2012;DOI: 10.1371/journal.pone.0031093; 7(1):e31093. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 301.Lee TK, Poon RT, Wo JY, et al. Lupeol suppresses cisplatin-induced nuclear factor-kappaB activation in head and neck squamous cell carcinoma and inhibits local invasion and nodal metastasis in an orthotopic nude mouse model. Cancer research. September 15 2007;DOI: 10.1158/0008-5472.CAN-07-0801; 67(18):8800–8809. [DOI] [PubMed] [Google Scholar]
- 302.Nohata N, Hanazawa T, Kikkawa N, et al. Caveolin-1 mediates tumor cell migration and invasion and its regulation by miR-133a in head and neck squamous cell carcinoma. International journal of oncology. January 2011; 38(1):209–217. [PubMed] [Google Scholar]
- 303.Huang WC, Chan SH, Jang TH, et al. miRNA-491–5p and GIT1 serve as modulators and biomarkers for oral squamous cell carcinoma invasion and metastasis. Cancer research. February 1 2014;DOI: 10.1158/0008-5472.CAN-13-1297; 74(3):751–764. [DOI] [PubMed] [Google Scholar]
- 304.Liu CJ, Shen WG, Peng SY, et al. miR-134 induces oncogenicity and metastasis in head and neck carcinoma through targeting WWOX gene. International journal of cancer. Journal international du cancer. February 15 2014;DOI: 10.1002/ijc.28358; 134(4):811–821. [DOI] [PubMed] [Google Scholar]


