Abstract
Undifferentiated pleomorphic sarcoma (UPS) is a high-grade neoplasm that is usually located in the extremities and retroperitoneum. In the past, UPS was considered the most common soft tissue sarcoma in adults; due to improvements in diagnostic techniques, most cases have been reclassified as other lineage-specific tumors. Gnathic bones are rarely affected, and the clinicopathological characteristics of this neoplasm when diagnosed in the jaw remain to be better described. In this report, we present a rare case of mandibular UPS affecting an 88-year-old female who demonstrated a painful swelling on the right side of the mandible that was accompanied by a pathological fracture. Microscopic examination revealed a pleomorphic spindle-cell neoplasm with mitotic figures and necrosis. The patient underwent surgery and adjuvant radiotherapy but experienced metastasis after 12 months of follow-up and died. Diagnosis of UPS is challenging, and oral pathologists must be aware of this entity when dealing with aggressive undifferentiated neoplasms.
Keywords: Soft tissue sarcoma, Pleomorphic sarcoma, Malignant fibrous histiocytoma, Oral cavity, Mandible
I. Introduction
Malignant fibrous histiocytoma (MFH) was first described by Ozzello et al.1 in 1963 and by O’Brien and Stout2 in 1964. It exhibits a fibroblastic/myofibroblastic nature with facultative histiocytic differentiation and was once considered the most common soft tissue sarcoma in adults. However, with the advancement of diagnostic and imaging approaches, many of these previously diagnosed MFHs have been reclassified as a more specific entity based on their lineage of differentiation2-4.
More recently, the World Health Organization classification of soft tissue tumors has modified the term “MFH” to include undifferentiated pleomorphic sarcoma (UPS), which is now the preferred term in the literature and fundamentally represents a diagnosis of exclusion5. Clinically, this malignant tumor preferentially affects deep soft tissues of the extremities (mainly the lower limbs) of older patients during their sixth to seventh decades of life, with a rapidly progressive growth pattern5. Its aggressiveness is also demonstrated by the high frequency of metastases that are identified when the primary tumor is diagnosed4.
UPS that has infiltrated the bone is very uncommon, and involvement of the gnathic bones is even rarer. Only a few case reports currently available in the literature have documented this neoplasm in the oral cavity4-6. Therefore, not only is its microscopic presentation complex, but its rarity makes an oral UPS diagnosis a challenge for diagnosticians. Report of new cases may contribute to a better understanding of its clinical and radiographic features. Therefore, in this manuscript, we aimed to describe an original case of UPS in the mandible of an 88-year-old female patient.
II. Case Report
An 88-year-old female patient was referred to our department chiefly complaining of a mandibular swelling. The patient was a non-smoker and non-drinker, with a positive history of glucose intolerance. During anamnesis, the patient reported that she had a previous diagnosis of a central giant cell lesion affecting the posterior mandible of the right side that was imaged in 2015.(Fig. 1) Five months after the first incisional biopsy, the patient demonstrated persistent strong pain and continuous growth of the lesion. A new radiographic exam revealed a radiolucent image with ill-defined, irregular borders and a pathological fracture on the right side of the mandible.(Fig. 2) The computed tomography scan with tridimensional reconstruction showed that the bone lesion involved half of the mandibular body and three-quarters of the ascending ramus.(Fig. 3) A new biopsy was performed, and the microscopic evaluation revealed the presence of an infiltrative, undifferentiated spindle-cell neoplasm with moderate to severe cellular pleomorphism, atypical mitotic figures, and areas of tissue necrosis with no evidence of osteoid production.(Fig. 4) Immunohistochemistry was carried out and was negative for beta-catenin, h-caldesmon, CD34, epithelial membrane antigen (EMA), desmin, and S100; strongly positive for vimentin; and only focally positive for smooth muscle actin (SMA), cytokeratin (AE1/AE3), and CD68 (Fig. 5), leading us to a final diagnosis of UPS.
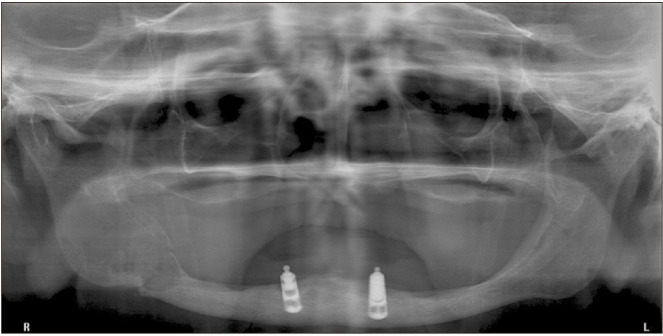
Fig. 1.
Initial radiographic presentation of our patient prior to diagnosis of a central giant cell granuloma. A well-defined, irregular, multilocular radiolucent image is visible on the right side of the posterior region of the mandible.
Fig. 2.
Radiographic appearance of the neoplasm when the second image evaluation was requested. The radiolucent image is larger, with an irregular and ill-defined posterior border. A pathological fracture can also be seen.
Fig. 3.
A tridimensional reconstruction of computed tomography reveals fracture of the mandible and the lesion occupying three-quarters of the ascending ramus.
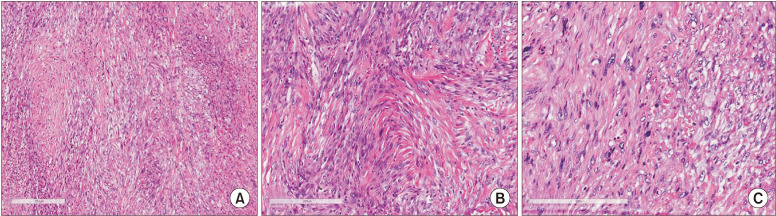
Fig. 4.
Microscopic findings after the second biopsy. A. Under low-power view, the neoplasm showed hypercellularity and a fibrous connective stroma (H&E staining, ×50). B. The tumor was composed of spindle cells with areas resembling a storiform growth pattern (H&E staining, ×100). C. Pleomorphic cells and atypical mitotic figures also were observed (H&E staining, ×200).
Fig. 5.
Immunohistochemistry (DAB stainings, ×200). A. Strong positivity for vimentin. B. Histiocytes demonstrated CD68 reactivity. Focal positive reaction for desmin (C) and α-SMA (D).
The patient underwent right segmental mandibulectomy with condyle disarticulation, right subtotal parotidectomy, and cervical neck dissection. Microscopic analysis of the surgical specimen revealed perineural, muscular, and adipocytic invasions, with positive margins adjacent to the anterior gingival tissue, larynx, floor of the mouth, and retromolar region. The histological aspect of the neoplasm suggested an aggressive spindle-cell neoplasm with multiple multinucleated giant cells. Immunohistochemistry was applied to the surgical specimen and demonstrated diffuse staining for vimentin and CD68 and only focal reactivity for desmin, SMA, and HHF-35; no positivity was found for AE1/AE3, S-100, or CD34 and h-caldesmon, confirming the diagnosis of UPS.
The patient received a cycle of 60-Gy (30 sections of 2 Gy) radiotherapy followed by an additional boost cycle of 6 Gy (3 sections of 2 Gy) for a total of 66 Gy. During anti-neoplastic therapy, the patient exhibited Grade I mucositis, dygeusia, Grade I xerostomy, and Grade II radiodermy. After six months of follow-up, the patient was well, with no evidence of local recurrence and under strict follow-up.(Fig. 6) Unfortunately, 12 months after surgery, she presented with lung metastasis and died.
Fig. 6.
Radiographic image of the patient at six months of follow-up (segmental mandibulectomy with condyle disarticulation).
III. Discussion
UPS was once believed to account for up to 40% of adult soft tissue sarcomas3. However, it is now considered a much less common entity; immunohistochemistry and molecular biology assays indicate that most previously designated MFHs actually represent pleomorphic leiomyosarcomas, rhabdomyosarcomas, liposarcomas, osteosarcomas, malignant nerve sheath tumors, melanomas, lymphomas, and carcinomas. UPS that affects the oral cavity and the gnathic bones is very rare; only a few cases have been described to date: myofibroblastic sarcoma7, low-grade myxofibrosarcoma8, sarcomatoid carcinoma9, osteogenic sarcoma10, Ewing’s sarcoma11, osteosarcoma12, and odontogenic carcinosarcoma13, all involving the mandible. Here, we describe an additional case that will be useful in understanding the clinical and radiographic presentation of the condition.
UPS is a very aggressive neoplasm that predominantly affects the upper and lower extremities and the retroperitoneum; only 10% of cases are diagnosed in the head and neck area and account for less than 0.5% of all malignant neoplasms located in this region3,14. The etiology of UPS is largely unknown, but some cases are thought to originate following radiation therapy3,15. To be considered a post-radiation neoplasm, the tumor must be located within the radiation field and must develop at least three years after radiation therapy in an area that was free of tumors prior to radiation16; there is an estimated lifetime risk of up to 0.3%17. In addition, some authors have also suggested that UPS may develop from its formerly believed benign counterpart, benign fibrous histiocytoma17. The literature shows that radiation-induced sarcoma development may be influenced by factors including radiation dose, patient age at initial exposure, prior exposure to chemotherapeutic agents, and a genetic tendency17.
As demonstrated in the current report, UPS usually develops in older adults in their fifth to seventh decades of life, and males are affected more often than females18. Our review (Table 1)4,5,8,11,18-34identified a mean age of 50.5 years at the diagnosis and a standard deviation of 19.7 years; 21% of patients who undergo radiation treatment eventually develop UPS. Symptoms usually depend on the anatomical site involved, but the neoplasm usually presents as a rapid growth with or without pain16. Our review found that only 30% of patients complained of pain, while 42% sought professional help due to swelling. In the head and neck, the sinonasal tract was the most commonly affected site19, which could potentially lead to dysphagia, pain, and nasal obstruction. In the oral cavity, the mandible was involved in 70% of cases. UPS commonly manifests as diffuse, ill-defined, painful swellings that typically affect the mandible and cause extensive bone destruction that is associated with irregular radiolucent uni- or multilocular images and may also be associated with floating teeth depending on tumor size18,19. The neoplasm may also cause pathological fractures14, as demonstrated in our case.
Table 1.
Clinicopathological features of undifferentiated pleomorphic sarcoma in cases retrieved from the PubMed database4,5,8,11,18-34
| Data | Value (n=33) |
|---|---|
| Sex | |
| Male | 19 (57.6) |
| Female | 14 (42.4) |
| Mean age (yr) | 50.5±19.7 |
| Site | |
| Maxilla | 10 (30.3) |
| Mandible | 23 (69.7) |
| Signs/symptoms | |
| Swelling | 14 (42.4) |
| Pain | 10 (30.3) |
| Ulcer | 4 (12.1) |
| Growth or mass | 14 (42.4) |
| Numbness | 4 (12.1) |
| Cervical lymph node involvement | |
| Yes | 5 (15.2) |
| No | 11 (33.3) |
| Non-contributory | 17 (51.5) |
| Radiation-induced UPS | |
| Yes | 7 (21.2) |
| No | 26 (78.8) |
| Treatment | |
| QT+surgery | 4 (12.1) |
| RT+surgery | 6 (18.2) |
| RQT+surgery | 1 (3.0) |
| Surgery | 21 (63.6) |
| QT | 1 (3.0) |
| Follow-up | |
| Living1 | 14 (42.4) |
| Dead2 | 14 (42.4) |
| Unknown | 5 (15.2) |
(UPS: undifferentiated pleomorphic sarcoma, QT: chemotherapy, RT: radiotherapy, RQT: radiotherapy and chemotherapy)
Mean follow-up length: 1Living, 40.7 months; 2Dead, 12 months.
Values are presented as number (%) or mean±standard deviation.
Histogenesis of UPS remains debatable; in contrast to the original concept, tumors do not seem to have a true histiocytic differentiation and instead are more likely to harbor a fibroblastic/myofibroblastic nature5; therefore, the term “MFH” will likely be completely discontinued in future publications. UPS is a diagnosis of exclusion when no other more specific entity is identified after extensive histological examination and use of ancillary diagnostic techniques. Microscopically, UPS is a heterogeneous malignancy with a variable cellular presentation and a predominantly fibrous stroma. All cases share a marked cytological and nuclear pleomorphism, with atypical mitosis and areas of necrosis, a predominant spindle-cell component, and scattered round histiocyte-like cells. A storiform growth pattern is recognized in most cases. Some cases may reveal extensive inflammatory infiltrate and presence of an evident multinucleated giant cell component18,19. We believe that this microscopic finding along with inadequate sampling of the lesion led to the initial misdiagnosis of a central giant cell lesion in the present case.
Immunohistochemistry is of great importance in achieving a final diagnosis. UPS usually demonstrates strong positivity to vimentin and variable reactivity to the histiocytic marker CD68. However, as illustrated in the present report, focal reactivity to smooth muscle actin (α-SMA), muscle specific actin (HHF-35), and desmin is occasionally observed. However, such findings should not be interpreted as true myogenic differentiation and instead more likely represent a myofibroblastic component, which is further supported by negativity to h-caldesmon16. Focal aberrant expression of cytokeratin may also be observed, as seen in this case. Reactions against S100 and CD34 must also be carried out to exclude melanoma and vascular/perivascular neoplasms, respectively.
The majority of UPS cases we reviewed (73%) included an incisional biopsy, but the clinician selected fine-needle aspiration for diagnosis in 9%. The patients underwent surgery without any evidence of a biopsy (data not shown) in 12% of reviewed case reports. This approach is not the best method of diagnostic management and should be discouraged.
Treatment of the affected patients involves complete surgical removal of the neoplasm and was the definitive treatment in 64% of our reviewed cases. Since regional metastases are only observed in approximately 15% of patients14,18 and were uncommon in the cases we reviewed (Table 1), neck dissections are not advocated in most cases, whereas adjuvant radiotherapy is preferentially recommended in unressectable cases or in those where clear margins cannot be achieved14,18. In the present report, the patient underwent segmental mandibulectomy and total removal of the tumor; however, because of the presence of involved surgical margins, radiotherapy was also administered. Because the initial scheme of 60 Gy (30×2 Gy) was well-tolerated, an additional boost of 6 Gy (3×2 Gy) was additionally recommended. The literature showed that 18% of UPS cases in bone were treated with surgery and adjuvant radiotherapy.
Recurrences are common, and distant metastases are likely to arise in the lung, liver, and bone, as observed in our patient. Some authors report that 25% to 35% of patients with UPS in the head and neck region will develop metastases. Background (primary or recurrent), tumor depth, size, and stage are strongly associated with prognosis; survival rates vary from 30% to 74% after five years of follow-up3,4,16 irrespective of the type of treatment applied for UPS18. Our review demonstrated an average of 40.7 months of follow-up in living patients; after 12 months, 42% of the patients had died.(Table 1) However, aggressive surgical management to achieve clear margins, particularly a minimum of 3-cm tumor-free margins18, is integral to effective treatment of UPS because overall median and five-year survival rates increase with clear surgical margins14. In addition, infiltrative growth behavior, as was seen in our case, is an adverse prognostic factor not only for local control, but also for disease-free and metastasis-free survival14,18.
UPS is an aggressive malignant neoplasm that rarely involves the oral cavity and gnathic bones, and that demands an extensive diagnostic effort to exclude other lineage-specific neoplasms. Therefore, diagnosticians must be aware of this entity when dealing with aggressive tumors that destroy the soft and hard tissues of older patients and also those who have previously undergone radiotherapy.
Footnotes
Author's Contributions
B.M.B. and W.M.S. participated in data collection and wrote the manuscript. C.R.G.C.M.O. and F.P.F. participated in the study design. E.R.F. participated in the study design and coordination and helped to draft the manuscript.
Consent for Publishing Photographs
Written informed consent was obtained from the patients for publication of this article and accompanying images.
Conflict of Interest
No potential conflict of interest relevant to this article was reported.
How to cite this article: Benites BM, Miranda-Silva W, Fonseca FP, de Oliveira CRGCM, Fregnani ER. Undifferentiated pleomorphic sarcoma of the mandible. J Korean Assoc Oral Maxillofac Surg 2020;46:282-287. https://doi.org/10.5125/jkaoms.2020.46.4.282
References
- 1.Ozzello L, Stout AP, Murray MR. Cultural characteristics of malignant histiocytomas and fibrous xanthomas. Cancer. 1963;16:331–44. doi: 10.1002/1097-0142(196303)16:3<331::aid-cncr2820160307>3.0.co;2-f. doi: 10.1002/1097-0142(196303)16:3<331::aid-cncr2820160307>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 2.O'Brien JE, Stout AP. Malignant fibrous xanthomas. Cancer. 1964;17:1445–58. doi: 10.1002/1097-0142(196411)17:11<1445::AID-CNCR2820171112>3.0.CO;2-G. https://doi.org/10.1002/1097-0142(196411) 17:11<1445::aid-cncr2820171112>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 3.Chang RC, Dave SP, Robinson PG. Undifferentiated pleomorphic sarcoma of the parotid gland: a rare pediatric case. Head Neck. 2008;30:970–3. doi: 10.1002/hed.20752. doi: 10.1002/hed.20752. [DOI] [PubMed] [Google Scholar]
- 4.Fleury RN, Damante JH, Soares CT, Sant'Ana E, Mello EJ Jr, Jr, Moreira CR. Malignant fibrous histiocytoma (undifferentiated high-grade pleomorphic sarcoma) occurring in tuberous sclerosis: a case report. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;103:e54–9. doi: 10.1016/j.tripleo.2006.05.014. doi: 10.1016/j.tripleo.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 5.Alfredo E, de Pádua JM, Vicentini EL, Marchesan MA, Comelli Lia RC, da Cruz Perez DE, et al. Oral undifferentiated high-grade pleomorphic sarcoma: report of a case. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;105:e37–40. doi: 10.1016/j.tripleo.2007.07.023. doi: 10.1016/j.tripleo.2007.07.023. [DOI] [PubMed] [Google Scholar]
- 6.Abdul-Karim FW, Ayala AG, Chawla SP, Jing BS, Goepfert H. Malignant fibrous histiocytoma of jaws. A clinicopathologic study of 11 cases. Cancer. 1985;56:1590–6. doi: 10.1002/1097-0142(19851001)56:7<1590::aid-cncr2820560721>3.0.co;2-t. doi: 10.1002/1097-0142(19851001)56:7<1590::aid-cncr2820560721>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 7.Park KR, Jang HW, Won JH, Kim HS, Cha IH, Kim HJ. Myofibroblastic sarcoma of the mandible: a case report. J Korean Assoc Oral Maxillofac Surg. 2012;38:240–4. doi: 10.5125/jkaoms.2012.38.4.240. doi: 10.5125/jkaoms.2012.38.4.240. [DOI] [Google Scholar]
- 8.Park JH, Choi SY, Kwon TG, Kim CS. Low-grade myxofibrosarcoma in the mandible: a case report. J Korean Assoc Oral Maxillofac Surg. 2011;37:67–71. doi: 10.5125/jkaoms.2011.37.1.67. doi: 10.5125/jkaoms.2011.37.1.67. [DOI] [Google Scholar]
- 9.Kwon GY, Choi YJ, Song MS, Yun KI. Sarcomatoid carcinoma of the mandible: report of a case. J Korean Assoc Oral Maxillofac Surg. 2010;36:228–30. doi: 10.5125/jkaoms.2010.36.3.228. doi: 10.5125/jkaoms.2010.36.3.228. [DOI] [Google Scholar]
- 10.Kim HW, Moon HG, Moon C. Surgical treatment of the osteogenic sarcoma in mandible: report of two cases. J Korean Assoc Oral Maxillofac Surg. 1987;13:11–8. [Google Scholar]
- 11.Kim JW, Kim YK. A case report of Ewing's sarcoma of the mandible. J Korean Assoc Oral Maxillofac Surg. 1988;14:22–6. [Google Scholar]
- 12.Kim CS, Kwon TG, Shin HI. Osteosarcoma around the resin implant in the area of the mandible - a case report - J Korean Assoc Oral Maxillofac Surg. 1996;22:222–9. [Google Scholar]
- 13.Kim IK, Pae SP, Cho HY, Cho HW, Seo JH, Lee DH, et al. Odontogenic carcinosarcoma of the mandible: a case report and review. J Korean Assoc Oral Maxillofac Surg. 2015;41:139–44. doi: 10.5125/jkaoms.2015.41.3.139. doi: 10.5125/jkaoms.2015.41.3.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hardison SA, Davis PL 3rd, Browne JD. Malignant fibrous histiocytoma of the head and neck: a case series. Am J Otolaryngol. 2013;34:10–5. doi: 10.1016/j.amjoto.2012.06.010. doi: 10.1016/j.amjoto.2012.06.010. [DOI] [PubMed] [Google Scholar]
- 15.Koyama T, Kobayashi T, Maruyama S, Abé T, Swelam WM, Kodama Y, et al. Radiation-induced undifferentiated high-grade pleomorphic sarcoma (malignant fibrous histiocytoma) of the mandible: report of a case arising in the background of long-standing osteomyelitis with a review of the literature. Pathol Res Pract. 2014;210:1123–9. doi: 10.1016/j.prp.2014.06.028. doi: 10.1016/j.prp.2014.06.028. [DOI] [PubMed] [Google Scholar]
- 16.Thompson LD. Pleomorphic sarcoma of the neck. Ear Nose Throat J. 2015;94:376–7. doi: 10.1177/014556131509400902. doi: 10.1177/014556131509400902. [DOI] [PubMed] [Google Scholar]
- 17.Thiagarajan A, Iyer NG. Radiation-induced sarcomas of the head and neck. World J Clin Oncol. 2014;5:973–81. doi: 10.5306/wjco.v5.i5.973. doi: 10.5306/wjco.v5.i5.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tanaka T, Kobayashi T, Iino M. Transformation of benign fibrous histiocytoma into malignant fibrous histiocytoma in the mandible: case report. J Oral Maxillofac Surg. 2011;69:e285–90. doi: 10.1016/j.joms.2011.02.067. doi: 10.1016/j.joms.2011.02.067. [DOI] [PubMed] [Google Scholar]
- 19.O'Neill JP, Bilsky MH, Kraus D. Head and neck sarcomas: epidemiology, pathology, and management. Neurosurg Clin N Am. 2013;24:67–78. doi: 10.1016/j.nec.2012.08.010. doi: 10.1016/j.nec.2012.08.010. [DOI] [PubMed] [Google Scholar]
- 20.Park SW, Kim HJ, Lee JH, Ko YH. Malignant fibrous histiocytoma of the head and neck: CT and MR imaging findings. AJNR Am J Neuroradiol. 2009;30:71–6. doi: 10.3174/ajnr.A1317. doi: 10.3174/ajnr.A1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li J, Geng ZJ, Lv XF, Zhang XK, Xie CM. Computed tomography and magnetic resonance imaging findings of malignant fibrous histiocytoma of the head and neck. Mol Clin Oncol. 2016;4:888–92. doi: 10.3892/mco.2016.811. doi: 10.3892/mco.2016.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fletcher CDM, Unni KK, Mertens F. Pathology and genetics of tumours of soft tissue and bone. IARC Press; Lyon: 2002. [Google Scholar]
- 23.Weiss SW, Goldblum JR, Enzinger FM. Malignant fibrohistiocytic tumors. In: Weiss SW, Goldblum JR, Enzinger FM, editors. Enzinger and Weiss's soft tissue tumors. 4th ed. Mosby; St. Louis: 2001. pp. 535–69. [Google Scholar]
- 24.Kim CH, Jang JW, Kim MY, Kim YH, Kim HG, Kim JH. Undifferentiated pleomorphic sarcoma in mandible. Maxillofac Plast Reconstr Surg. 2014;36:303–7. doi: 10.14402/jkamprs.2014.36.6.303. doi: 10.14402/jkamprs.2014.36.6.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Senel FC, Bektas D, Caylan R, Onder E, Gunhan O. Malignant fibrous histiocytoma of the mandible. Dentomaxillofac Radiol. 2006;35:125–8. doi: 10.1259/dmfr/24174954. doi: 10.1259/dmfr/24174954. [DOI] [PubMed] [Google Scholar]
- 26.Thomas ME, Koshi R. Electron microscopy in the diagnosis of malignant fibrous histiocytoma of the lung presenting as metastasis to the maxillary gingiva. Int J Oral Maxillofac Surg. 2013;42:99–101. doi: 10.1016/j.ijom.2012.09.019. doi: 10.1016/j.ijom.2012.09.019. [DOI] [PubMed] [Google Scholar]
- 27.Satomi T, Watanabe M, Kaneko T, Matsubayashi J, Nagao T, Chiba H. Radiation-induced malignant fibrous histiocytoma of the maxilla. Odontology. 2011;99:203–8. doi: 10.1007/s10266-011-0001-x. doi: 10.1007/s10266-011-0001-x. [DOI] [PubMed] [Google Scholar]
- 28.Lin SK, How SW, Wang JT, Liu BY, Chiang CP. Oral post-radiation malignant fibrous histiocytoma: a clinicopathological study. J Oral Pathol Med. 1994;23:324–9. doi: 10.1111/j.1600-0714.1994.tb00069.x. doi: 10.1111/j.1600-0714.1994.tb00069.x. [DOI] [PubMed] [Google Scholar]
- 29.Lambade PN, Lambade D, Saha TK, Bande CR, Ramakrishana A. Malignant fibrous histiocytoma: an uncommon sarcoma with pathological fracture of mandible. J Maxillofac Oral Surg. 2015;14(Suppl 1):283–7. doi: 10.1007/s12663-013-0491-x. doi: 10.1007/s12663-013-0491-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barnes L, Kanbour A. Malignant fibrous histiocytoma of the head and neck. A report of 12 cases. Arch Otolaryngol Head Neck Surg. 1988;114:1149–56. doi: 10.1001/archotol.1988.01860220083030. doi: 10.1001/archotol.1988.01860220083030. [DOI] [PubMed] [Google Scholar]
- 31.Kasat VO, Saluja H, Rudagi BM, Kalburge JV, Sachdeva S. Malignant fibrous histiocytoma of maxillary alveolar ridge extending to the hard palate. J Cancer Res Ther. 2014;10:422–4. doi: 10.4103/0973-1482.136678. doi: 10.4103/0973-1482.136678. [DOI] [PubMed] [Google Scholar]
- 32.Suzuki S, Watanabe S, Kato H, Inagaki H, Hattori H, Morita A. A case of cutaneous malignant fibrous histiocytoma with multiple organ metastases. Kaohsiung J Med Sci. 2013;29:111–5. doi: 10.1016/j.kjms.2012.08.019. doi: 10.1016/j.kjms.2012.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Agnihotri R, Bhat KM, Bhat GS. A rare case of malignant fibrous histiocytoma of the gingiva. J Periodontol. 2008;79:955–60. doi: 10.1902/jop.2008.070401. doi: 10.1902/jop.2008.070401. [DOI] [PubMed] [Google Scholar]
- 34.Sato T, Kawabata Y, Morita Y, Noikura T, Mukai H, Kawashima K, et al. Radiographic evaluation of malignant fibrous histiocytoma affecting maxillary alveolar bone: a report of 2 cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;92:116–23. doi: 10.1067/moe.2001.1138. doi: 10.1067/moe.2001.1138. [DOI] [PubMed] [Google Scholar]