Abstract
BACKGROUND:
Stereotactic radiosurgery (SRS) alone is an increasingly accepted treatment for brain metastases, but it requires adherence to frequently scheduled follow-up neuroimaging because of the risk of distant brain metastasis. The effect of disparities in access to follow-up care on outcomes after SRS alone is unknown.
METHODS:
This retrospective study included 153 brain metastasis patients treated consecutively with SRS alone from 2010 through 2016 at an academic medical center and a safety-net hospital (SNH) located in Los Angeles, California. Outcomes included neurologic symptoms, hospitalization, steroid use and dependency, salvage SRS, salvage whole-brain radiotherapy, salvage neurosurgery, and overall survival.
RESULTS:
Ninety-three of the 153 patients were private hospital (PH) patients, and 60 were SNH patients. The median follow-up time was 7.7 months. SNH patients received fewer follow-up neuroimaging studies (1.5 vs 3; P = .008). In a multivariate analysis, the SNH setting was a significant risk factor for salvage neurosurgery (hazard ratio [HR], 13.65; P<.001), neurologic symptoms (HR, 3.74; P = .002), and hospitalization due to brain metastases (HR, 6.25; P<.001). More clinical visits were protective against hospitalizations due to brain metastases (HR, 0.75; P = .002), whereas more neuroimaging studies were protective against death (HR, 0.65; P<.001).
CONCLUSIONS:
SNH patients with brain metastases treated with SRS alone had fewer follow-up neuroimaging studies and were at higher risk for neurologic symptoms, hospitalization for brain metastases, and salvage neurosurgery in comparison with PH patients. Clinicians should consider the practice setting and patient access to follow-up care when they are deciding on the optimal strategy for the treatment of brain metastases.
Keywords: brain metastases, follow-up care, health disparities, neuroimaging, neurologic outcomes, safety-net hospital, stereotactic radiosurgery
INTRODUCTION
The standard of care for the treatment of brain metastases has historically been whole-brain radiotherapy (WBRT) with surgery or stereotactic radiosurgery (SRS) as an adjuvant treatment.1 Recently, SRS alone has become an increasingly accepted treatment option because of the improved neurocognitive preservation demonstrated in 2 randomized controlled trials in comparison with treatment with SRS and WBRT.2,3 SRS delivers a single, high dose of focal radiation to the tumor while sparing adjacent normal brain tissue and is administered in a single session. Multiple randomized controlled trials have shown no improvement in overall survival (OS) with the addition of WBRT to SRS.4,5 The success of SRS alone, however, depends on close clinical observation with neuroimaging because of the increased risk of distant brain metastasis failure associated with the omission of WBRT.3–5
Unfortunately, not all patients have equal access to recommended follow-up clinical care, neuroimaging, and salvage treatment. Disparities in access to cancer care among different racial and socioeconomic groups are well recognized in the medical literature.6–13 The effect of disparities in access to care on patient outcomes is unknown when a strategy of treating brain metastases with SRS alone is used. We compared clinical outcomes between safety-net hospital (SNH) and private hospital (PH) patients treated with SRS alone to test the hypothesis that, because of worse neurologic outcomes, SRS alone with observation may not always be suitable for patients originating from an SNH environment who may have barriers to appropriate follow-up care.
MATERIALS AND METHODS
Study Population
This retrospective cohort study was approved by the institutional review board of the University of Southern California (USC) Keck School of Medicine. Using institutional treatment databases, we included patients who received initial SRS for the treatment ofbrain metastases at the USC Norris Comprehensive Cancer Center (Norris; PH) or the Los Angeles County + USC Medical Center (LAC+USC; SNH) from 2010 to 2016.
Setting and Patient Flow
Both LAC+USC and Norris are USC teaching hospitals, but each hospital has a separate administration. Patients in our study were presented at the same multidisciplinary tumor board, which consisted of neurosurgeons, radiation oncologists, and neuroradiologists, and were deemed to be candidates for SRS on the basis of their clinical history and neuroimaging.
LAC+USC patients were initially evaluated and determined to be candidates for SRS by the LAC+USC team. They were presented at the tumor board and referred to Norris for consultation with the SRS treatment team. The SRS procedure was performed at Keck Hospital of USC. After the SRS procedure, LAC+USC patients returned to LAC+USC for follow-up care.
Norris patients were initially evaluated at Norris and determined to be candidates for SRS by the Norris team. They were presented at the same tumor board, were treated with SRS at Keck Hospital, and then continued their follow-up care at Norris. The recommended routine follow-up interval for clinical and neuroimaging visits after SRS was every 2 to 3 months at Norris and LAC+USC, and this was consistent with National Comprehensive Cancer Center guidelines.14
Radiation Delivery
All patients were treated with single-fraction Gamma Knife radiosurgery with Gamma Knife Perfexion (Elekta AB, Stockholm, Sweden). Patients were immobilized with a stereotactic head frame. The frame was affixed to the cranium of the patient while the patient was under conscious sedation. Magnetic resonance imaging (MRI) of the brain was performed for treatment planning, and radiation therapy was delivered the same day.
Data Source and Approach
Medical records were reviewed to obtain patient demographic information, including age, race, sex, insurance status, and residential zip code. Household incomes were based on aggregate zip code data from the 2014 American Community Survey. All cancer staging was performed with the 7th edition of the American Joint Committee on Cancer guidelines.15 Tumor volumes and maximum diameters were obtained from radiation treatment planning software or were measured manually in the institutional picture archiving and communication system. The number of clinical visits and neuroimaging studies included only those performed as part of routine follow-up. Neurologic symptoms were identified from medical records and were graded with the Common Terminology Criteria for Adverse Events (version 4.03). Only neurologic symptoms attributable to brain metastases were included in the analysis. Severe neurologic symptoms were defined as grade 3 or higher. The date of last follow-up was the last clinical encounter documented in medical records. Survival data were obtained from institutional cancer registries and public online databases.
Statistical Analysis
The baseline patient information, treatment characteristics, number of clinical follow-up visits, number of neuroimaging follow-up visits, steroid use and dependency, and rates of salvage treatments were compared with the Wilcoxon rank-sum test and the Pearson chi-square test. The development of new neurologic symptoms, hospitalizations, salvage surgery, salvage SRS, salvage WBRT, and OS were analyzed as time-dependent variables with the Kaplan-Meier method, with the time calculated from the date of the first radiation treatment to the event and with censoring occurring either at death or on the date of last follow-up. Statistical significance comparisons between the 2 hospitals were calculated with the log-rank test.
Univariate and multivariate analyses were performed with the Cox proportional hazards model. All risk factors, including the institution, age, race, income, education, tumor histology, stage at diagnosis, time from the brain metastasis diagnosis to SRS, clinical visits, neuroimaging studies, Karnofsky performance status (KPS), graded prognostic assessment (GPA), neurologic status at the baseline, number ofbrain metastases, and total tumor volume, were entered into the univariate analysis with each clinical outcome. Risk factors with P values < .10 and other clinically relevant variables were further entered into the multivariate analysis. Afterward, starting with the largest P value, we took risk factors out of the multivariate model one at a time until all of the remaining risk factors had P values < .10 or were clinically relevant. P< .05 was considered significant. All analysis was performed with SAS software (version 9; SAS Institute, Cary, North Carolina) and R software (version 3; R Foundation, Vienna, Austria).
RESULTS
One hundred seventy-six patients, including 110 PH patients and 66 SNH patients, received SRS consecutively for brain metastases from 2010 to 2016. Six PH patients (5%) and 6 SNH patients (9%) were excluded because of prior WBRT. One PH patient (0.9%) was excluded because of treatment with upfront SRS and WBRT, and 10 PH patients (9%) were excluded because of a lack of follow-up after SRS treatment. In total, 153 patients, including 93 PH patients (85%) and 60 SNH patients (91%), were analyzed.
Patient and Treatment Characteristics
The median age was 59 years (interquartile range [IQR], 50–66 years), 78 patients (51%) were female, and 65 patients (42%) were non-Hispanic white (Table 1). SNH patients were significantly younger (median, 56 years for SNH vs 61 years for PH; P = .001), had a lower median household income ($48,754 for SNH vs $72,192 for PH; P< .001), and had a lower median high school graduation rate (69.8% for SNH vs 88.7% for PH; P< .001). Eleven of the SNH patients (18%) were non-Hispanic white, and 32 (53%) were Hispanic, whereas 54 of the PH patients (58%) were non-Hispanic white, and 15 (16%) were Hispanic (P< .001). In addition, more SNH patients had late-stage (III-IV) disease at the diagnosis of cancer (91% for SNH vs 78% for PH; P = .04), and SNH patients had a longer median time from the diagnosis of brain metastases to SRS treatment (43 days for SNH vs 22 days for PH; P < .001). There was no significant difference between the groups in terms of KPS, GPA, baseline neurologic status, tumor histology or mutation status, prior chemotherapy or neurosurgery, number of brain metastases treated, tumor location, total tumor volume, or SRS dose.
TABLE 1.
Baseline Patient and Treatment Characteristics
| Private Hospital (n = 93) | Safety-Net Hospital (n = 60) | All (n = 153) | P | |
|---|---|---|---|---|
| Age, median (IQR), y | 61 (53-69) | 56 (46-63) | 59 (50-66) | .001 |
| Sex, No. (%) | .14 | |||
| Male | 50 (54) | 25 (42) | 75 (49) | |
| Female | 43 (46) | 35 (58) | 78 (51) | |
| Race, No. (%) | <.001 | |||
| Non-Hispanic white | 54 (58) | 11 (18) | 65 (42) | |
| Hispanic (any race) | 15 (16) | 32 (53) | 47 (31) | |
| Non-Hispanic Asian | 17 (18) | 7 (17) | 24 (16) | |
| Non-Hispanic black or other | 7 (8) | 10 (12) | 17 (11) | |
| Insurance status, No. (%) | <.001 | |||
| Private/managed care/Medicare | 87 (94) | 8 (13) | 95 (62) | |
| Medicaid, uninsured, or other | 6 (6) | 52 (87) | 58 (38) | |
| Household income, median (IQR), $ | 72,192 (50,574-92,960) | 48,754 (35,693-60,167) | 59,099 (43,385-80,864) | <.001 |
| KPS, median (IQR) | 80 (75-90) | 80 (80-90) | 80 (80-90) | .23 |
| GPA, median (IQR) | 1.5 (1-2) | 1.75 (1-2.5) | 1.5 (1-2.25) | .67 |
| Neurologically symptomatic at baseline, No. (%) | 35 (38) | 23 (38) | 58 (38) | .93 |
| Tumor histology, No. (%) | .17 | |||
| Lung adenocarcinoma | 22 (24) | 21 (35) | 43 (28) | |
| EGFR mutation | 7 (35) | 4 (25) | 11 (31) | .52 |
| ALK mutation | 4 (20) | 3 (19) | 7 (19) | .93 |
| Breast adenocarcinoma | 11 (12) | 9 (15) | 20 (13) | |
| Melanoma | 16 (17) | 3 (5) | 19 (12) | |
| BRAF mutation | 6 (40) | 2 (67) | 8 (44) | .40 |
| Renal cell carcinoma | 13 (14) | 8 (13) | 21 (14) | |
| Other | 31 (33) | 19 (32) | 50 (33) | |
| Stage at diagnosis, No. (%) | .04 | |||
| I/II | 18 (22) | 5 (9) | 23 (16) | |
| III/IV | 65 (78) | 52 (91) | 117 (84) | |
| Brain metastases diagnosed within 3 mo of primary, No. (%) | 28 (30) | 27 (45) | 55 (36) | .06 |
| Prior surgical resection of brain metastases, No. (%) | 41 (44) | 21 (35) | 62 (41) | .26 |
| Prior systemic therapy, No. (%) | 87 (94) | 53 (88) | 140 (92) | .26 |
| Time from initial consultation to SRS, median (IQR), d | 9 (5-15) | 24 (16.5-37) | 15 (7-25) | <.001 |
| Time from brain metastasis diagnosis to SRS, median (IQR), d | 22 (13.5-48.5) | 43 (33-73.8) | 33 (17-59) | <.001 |
| No. of brain metastases treated, median (IQR) | 2 (1-3) | 2 (1-4) | 2 (1-3) | .64 |
| Total tumor volume, median (IQR), cc | 4.90 (1.33-10.07) | 4.96 (1.61-9.79) | 4.90 (1.46-9.93) | .78 |
| Total treatment volume, median (IQR), cc | 6.61 (1.84-13.76) | 7.05 (2.68-13.82) | 6.7 (2.10-13.73) | .73 |
| SRS dose, median (IQR), Gy | 20 (18-20) | 20 (18-20) | 20 (18-20) | .73 |
| Tumor location, No. (%) | .19 | |||
| Cerebral hemisphere | 183 (76) | 132 (82) | 315 (79) | |
| Cerebellum | 40 (17) | 24 (15) | 64 (16) | |
| Other | 17 (7) | 5 (3) | 22 (5) |
Abbreviations: ALK, anaplastic lymphoma kinase; EGFR, epidermal growth factor receptor; GPA, graded prognostic assessment; IQR, interquartile range; KPS, Karnofsky performance status; SRS, stereotactic radiosurgery.
Clinical and Neuroimaging Follow-Up
The median follow-up time for all patients was 7.70 months. The median follow-up time for SNH patients was 5.93 months (IQR, 2.53-15.09 months), and the median follow-up time for PH patients was 9.15 months (IQR, 3.52-17.72 months; P = .09).
SNH patients, in comparison with PH patients, had significantly lower absolute numbers (median, 1.5 vs 3; P= .008) and monthly rates of neuroimaging studies after SRS (median, 0.228 vs 0.312; P = .007; Table 2). In contrast, SNH patients, in comparison with PH patients, had similar absolute numbers (median, 1.5 vs 2; P= .23) and monthly rates of clinical visits after SRS (median, 0.298 vs 0.288; P = .97).
TABLE 2.
Clinical and Neuroimaging Follow-Up
| Private (n = 93) | Safety Net (n = 60) | P | |
|---|---|---|---|
| Neuroimaging follow-ups, median (IQR) | 3 (1-6) | 1.5 (1-4) | .008 |
| Neuroimaging follow-ups per mo, median (IQR) | 0.312 (0.201-0.418) | 0.228 (0.102-0.304) | .007 |
| Clinical follow-ups, median (IQR) | 2 (1-5) | 1.5 (1-3) | .23 |
| Clinical follow-ups per mo, median (IQR) | 0.288 (0.092-0.401) | 0.298 (0.115-0.416) | .97 |
Abbreviation: IQR, interquartile range.
Incidence of Neurologic Symptoms and Salvage Treatments
SNH patients had significantly higher rates of any neurologic symptoms (33% for SNH vs 19% for PH; P= .05) and severe neurologic symptoms (15% for SNH vs 2% for PH; P = .007) after SRS (Table 3). SNH patients also had higher rates of hospitalization due to brain metastases (25% for SNH vs 7.5% for PH; P < .001) and salvage neurosurgery (17% for SNH vs 6% for PH; P = .04) after SRS. SNH patients did not experience significantly different rates of permanent neurologic symptoms, hospitalization for any reason, steroid requirement or dependency, salvage SRS, or salvage WBRT in comparison with PH patients.
TABLE 3.
Incidence of Neurologic Symptoms and Salvage Treatments
| Private (n = 93), No. (%) | Safety Net (n = 60), No. (%) | P | |
|---|---|---|---|
| Developed any neurologic symptoms | 18 (19) | 20 (33) | .05 |
| Developed severe neurologic symptoms | 2 (2) | 9 (15) | .007 |
| Permanent neurologic symptoms | 10 (11) | 11 (18) | .18 |
| Required steroids | 33 (35) | 22 (37) | .88 |
| Steroid dependencya | 26 (28) | 17 (28) | .96 |
| Hospitalization for any reasonb | 56 (60) | 33 (55) | .41 |
| Hospitalization for brain metastases | 7 (7.5) | 15 (25) | <.001 |
| Salvage neurosurgery | 6 (6) | 10 (17) | .04 |
| Salvage SRS | 31 (33) | 15 (25) | .27 |
| Salvage WBRT | 19 (20) | 15 (25) | .51 |
Abbreviations: SRS, stereotactic radiosurgery; WBRT, whole-brain radiotherapy.
Defined as > 2 weeks.
Other than scheduled chemotherapy or surgery admissions.
Univariate Analysis
The median OS for all patients was 15.4 months. The median OS for SNH patients was 17.5 months, and the median OS for PH patients was 15.1 months (P = .34). SNH patients had a higher risk of developing any neurologic symptoms (hazard ratio [HR], 2.64; 95% confidence interval [CI], 1.35-5.17; P= .003), severe neurologic symptoms (HR, 9.20; 95% CI, 1.98-42.75; P< .001), and permanent neurologic symptoms (HR, 2.33; 95% CI, 1.01-5.52; P= .05). SNH patients were at higher risk for salvage neurosurgery after SRS (HR, 3.29; 95% CI, 1.19-9.08; P = .01). SNH patients did not have a higher risk for any hospitalization (HR, 1.00; 95% CI, 0.65-1.55; P = .99) but did have a higher risk for hospitalization due to brain metastasis progression (HR, 3.64; 95% CI, 1.40-9.44; P = .005). There was no significant difference in the risk of salvage SRS or salvage WBRT. Kaplan-Meier survival curves for OS, salvage neurosurgery, any neurologic symptoms, and hospitalization for brain metastases are shown in Figure 1.
Figure 1.
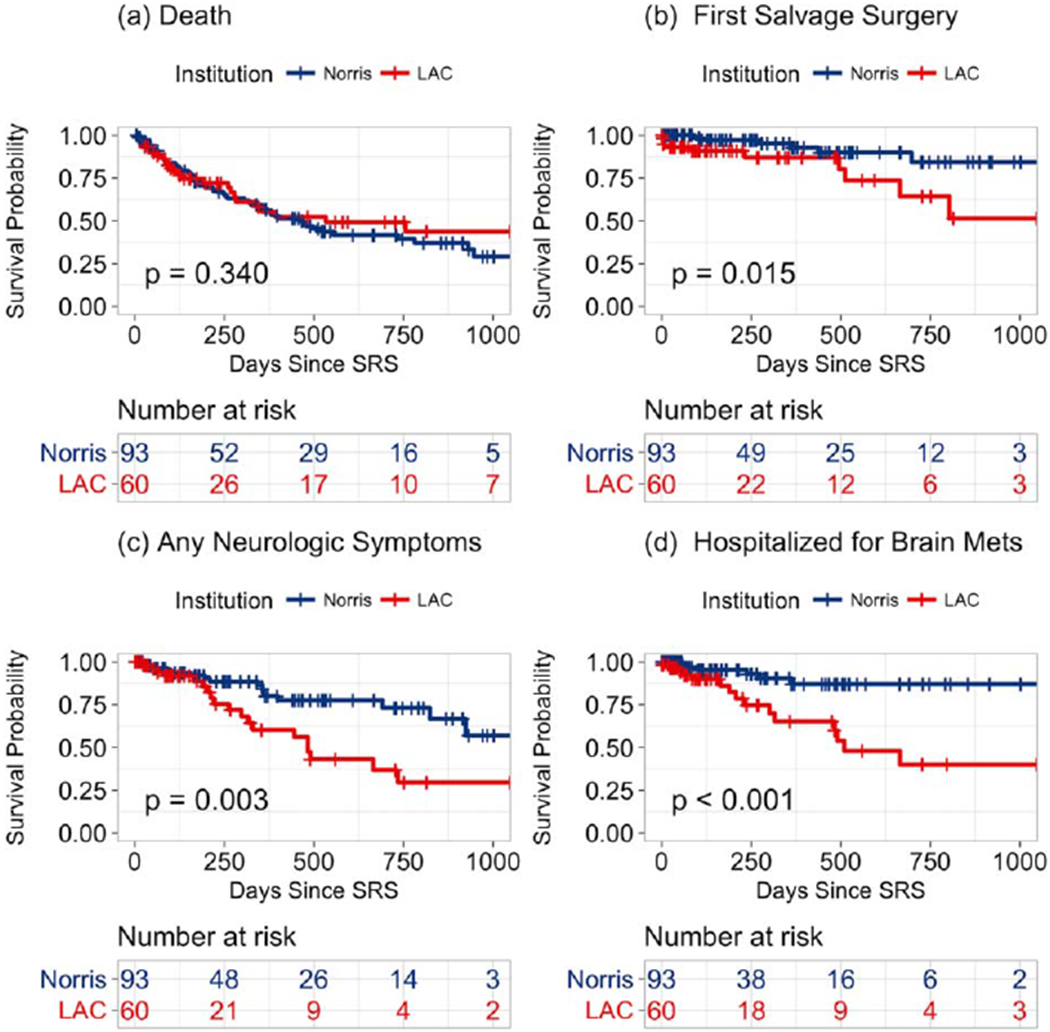
Kaplan-Meier survival curves for (A) overall survival, (B) salvage neurosurgery, (C) any neurologic symptoms, and (D) hospitalization due to brain metastases stratified by the hospital setting. LAC indicates Los Angeles County + USC Medical Center; Norris, Norris Comprehensive Cancer Center; SRS, stereotactic radiosurgery.
Multivariate Analysis
In the multivariate analysis, OS was significantly associated with additional neuroimaging studies (HR, 0.65; 95% CI, 0.58-0.74; P < .001), GPA (HR, 0.66; 95% CI, 0.46-0.94; P = .02), breast histology (HR, 2.20; 95% CI, 1.02-4.73; P = .04), and melanoma histology (HR, 2.91; 95% CI, 1.36-6.22; P = .006; Table 4).
TABLE 4.
Multivariate Models With Significant Risk Factors
| Risk Factor | Unadjusted HR (95% CI) | P | Adjusted HR (95% CI) | P |
|---|---|---|---|---|
| Overall survival | ||||
| Additional neuroimaging follow-up | 0.655 (0.583-0.737) | <.001 | 0.653 (0.578-0.739) | <.001 |
| GPA | 0.657 (0.481 -0.896) | .008 | 0.656 (0.460-0.935) | .02 |
| Additional day from consultation to SRS | 1.003 (0.994-1.011) | .52 | 0.999 (0.989, 1.009) | .83 |
| Tumor histology | ||||
| Lung adenocarcinoma | Reference | Reference | Reference | Reference |
| Breast adenocarcinoma | 1.261 (0.599-2.652) | .54 | 2.199 (1.023-4.726) | .04 |
| Melanoma | 1.526 (0.725-3.211) | .27 | 2.912 (1.363-6.224) | .006 |
| Renal cell carcinoma | 1.256 (0.597-2.644) | .55 | 1.545 (0.730-3.273) | .26 |
| Other | 1.623 (0.887-2.972) | .12 | 1.496 (0.808-2.773) | .20 |
| Salvage neurosurgery | ||||
| Safety-net hospital | 3.293 (1.194-9.082) | .02 | 13.65 (3.311-56.29) | <.001 |
| Neurologic symptoms at baseline | 3.313 (1.218-9.012) | .02 | 11.40 (2.819-46.12) | <.001 |
| Additional brain metastasis | 1.155 (1.002-1.332) | .05 | 1.260 (1.074-1.478) | .005 |
| Additional day from consultation to SRS | 1.001 (0.980,1.022) | .96 | 0.994 (0.955-1.036) | .79 |
| Tumor histology | ||||
| Lung adenocarcinoma | Reference | Reference | Reference | Reference |
| Breast adenocarcinoma | 1.023 (0.092-11.29) | .99 | 0.493 (0.039-6.207) | .58 |
| Melanoma | 7.082 (1.368-36.68) | .02 | 22.73 (3.244-159.29) | .002 |
| Renal cell carcinoma | 3.402 (0.567-20.41) | .18 | 5.763 (0.907-36.64) | .06 |
| Other | 3.537 (0.679-18.41) | .13 | 4.250 (0.790-22.87) | .09 |
| Any neurologic symptoms | ||||
| Safety-net hospital | 2.644 (1.352-5.172) | .004 | 3.739 (1.599-8.743) | .002 |
| Early stage (I/II) at diagnosis | 0.377 (0.183-0.774) | .008 | 0.280 (0.120-0.656) | .003 |
| Additional neuroimaging follow-up | 0.859 (0.759-0.972) | .02 | 0.865 (0.766-0.977) | .02 |
| Additional day from consultation to SRS | 0.997 (0.980-1.014) | .70 | 0.979 (0.957-1.002) | .08 |
| Tumor histology | ||||
| Lung adenocarcinoma | Reference | Reference | Reference | Reference |
| Breast adenocarcinoma | 3.103 (1.120-8.598) | .03 | 3.702 (1.303-10.52) | .01 |
| Melanoma | 2.162 (0.724-6.456) | .17 | 2.851 (0.915-8.877) | .07 |
| Renal cell carcinoma | 1.412 (0.446-4.468) | .56 | 2.301 (0.720-7.360) | .16 |
| Other | 1.922 (0.712-5.190) | .20 | 1.470 (0.532-4.061) | .46 |
| Hospitalization for brain metastases | ||||
| Safety-net hospital | 4.371 (1.693-11.29) | .002 | 6.248 (2.222-17.57) | <.001 |
| Additional clinical follow-up | 0.786 (0.659-0.937) | .007 | 0.749 (0.622-0.902) | .002 |
| Additional brain metastasis | 1.216 (1.003-1.474) | .46 | 1.316 (1.052-1.647) | .02 |
| Additional day from consultation to SRS | 1.000 (0.984-1.017) | .971 | 0.988 (0.958-1.018) | .42 |
| Tumor histology | ||||
| Lung adenocarcinoma | Reference | Reference | Reference | Reference |
| Breast adenocarcinoma | 1.183 (0.283-4.959) | .82 | 2.585 (0.576-11.60) | .22 |
| Melanoma | 2.592 (0.689-9.746) | .16 | 3.360 (0.875-12.90) | .08 |
| Renal cell carcinoma | 2.948 (0.777-11.18) | .11 | 4.584 (1.122-18.72) | .03 |
| Other | 1.570 (0.450-5.471) | .48 | 2.831 (0.727-11.02) | .13 |
Abbreviations: CI, confidence interval; GPA, graded prognostic assessment; HR, hazard ratio; SRS, stereotactic radiosurgery.
Significant risk factors for salvage neurosurgery included an SNH setting (HR, 13.65; 95% CI, 3.31-56.29, P< .001), neurologic symptoms at the baseline (HR, 11.40; 95% CI, 2.82-46.12; P< .001), the number of brain metastases (HR, 1.26; 95% CI, 1.07-1.48; P = .005), and melanoma histology (HR, 22.73; 95% CI, 3.24-159.29; P = .002).
Significant risk factors for the development of any neurologic symptoms included an SNH setting (HR, 3.74; 95% CI, 1.60-8.74; P = .002), an early stage at diagnosis (HR, 0.28; 95% CI, 0.12-0.66; P = .003), follow-up neuroimaging (HR, 0.87; 95% CI, 0.77-0.98; P = .02), and breast histology (HR, 3.70; 95% CI, 1.30-10.52; P = .01).
Significant risk factors for hospitalization due to brain metastasis progression included an SNH setting (HR, 6.25; 95% CI, 2.22-17.57; P< .001), follow-up clinical visits (HR, 0.75; 95% CI, 0.62-0.90; P= .002), the number of brain metastases (HR, 1.32; 95% CI, 1.05-1.65; P = .02), and renal histology (HR, 4.58; 95% CI, 1.12-18.72; P = .03).
DISCUSSION
The goal of our study was to examine the effects of the hospital setting and the quality offollow-up on neurologic outcomes for brain metastasis patients treated with SRS alone. Although it is currently accepted that patients undergoing SRS alone require close clinical monitoring with neuroimaging because of the increased distant brain metastasis failure rate with the omission of WBRT, we are not aware of any studies that have directly correlated follow-up clinical visits and neuroimaging studies with clinical outcomes. Furthermore, we did not find any studies that examined health care disparities in the brain metastasis population treated with SRS and how their treatment outcomes might depend on the clinical setting and patient demographics such as race, household income, and insurance status. Given the unique affiliation between the SNH and the PH as teaching hospitals with collaboration for SRS treatment, we were especially well positioned to perform such a study.
We found that after SRS, SNH patients had a higher incidence and risk of any neurologic symptoms, severe neurologic symptoms, hospitalizations for brain metastases, and salvage neurosurgeries. Despite this, SNH and PH patients had similar OS. OS was high in both groups in comparison with historic survival after a diagnosis of brain metastases, and this may be attributed to patient selection for SRS and improved systemic therapy. 16 The observation that SNH patients did not have worse median OS despite higher rates of neurologic symptoms and hospitalizations for brain metastases could be explained by excellent neurosurgical care, which allowed the successful salvage of patients with brain metastasis progression, because SNH patients also had higher rates of salvage neurosurgery. In addition, there may have been undetected differences in systemic disease burdens between the cohorts, or our study may have been underpowered to detect differences in survival.
Although the 2 patient populations differed in terms of multiple baseline factors, including a younger age, a more advanced stage at the diagnosis of cancer, and a longer time from the diagnosis of brain metastases to SRS treatment for SNH patients, they had similar baseline KPS values, GPA values, histologies, numbers of brain metastases, and total tumor volumes. Studies have shown that the strongest predictors of outcomes after the treatment of brain metastases with SRS include age, KPS, histology, number of brain metastases, and total tumor volume, all of which, besides age, were similar in the 2 groups.17–21 Furthermore, the SNH patient population was significantly younger than the PH patient population, and this typically confers better outcomes.
In the multivariate analysis, the SNH setting remained associated with an increased risk for salvage neurosurgery, any neurologic symptoms, and hospitalization for brain metastases even after we had controlled for multiple other risk factors, including the tumor histology and the time from the initial consultation to SRS treatment. In an attempt to assess the quality of follow-up for patients after SRS, we recorded the number of routine follow-up clinical visits and neuroimaging studies that patients had. In comparison with PH patients, SNH patients received similar numbers offollow-up clinical visits but fewer neuroimaging studies.
In the multivariate analysis, more follow-up clinical visits were correlated with fewer hospitalizations for brain metastases, whereas more follow-up neuroimaging studies were associated with better OS and less risk for the development of any neurologic symptoms. These findings indicate that the poor outcomes observed in the SNH patient group were at least partly attributable to fewer neuroimaging studies after SRS. We confirm and reemphasize the need for close clinical and neuroimaging surveillance after a treatment strategy of SRS alone for brain metastases. The comparatively small effect magnitude of clinical and neuroimaging follow-up visits on these outcomes in comparison with an SNH setting could potentially be due to challenges in quantifying the quality of follow-up, such as differentiating symptom-triggered visits from routine visits, accounting for differences in clinical follow-up visits due to varying systemic therapy regimens, and determining whether patients received care at institutions outside our health care network.22 Nonetheless, our results suggest that there may be other unaccounted-for risk factors associated with an SNH practice setting, such as more patient comorbidities, less access to and compliance with systemic therapies, fewer hospital resources, and a lower quality of medical care.23–26
There are numerous possible explanations for the disparity in the number of neuroimaging follow-up visits at the 2 hospitals, including differences in age, race, income, education, language, social supports, distance from the treatment center, access to transportation, ability to take time off from work, and severity of disease.23 The fact that SNH patients still received similar clinical follow-up suggests that the underlying reasons are either specific to neuroimaging or affect compliance with neuroimaging studies more than clinical visits. An institution-specific barrier that we identified is the number of MRI scanners available. LAC+USC, which has 650 hospital beds, has three 1.5-T MRI scanners, whereas Keck Hospital, which has 471 hospital beds, has two 1.5-T scanners and three 3-T MRI scanners (5 total) available for patient use. There is a general consensus among providers at our institutions that there are longer scheduling wait times for neuroimaging appointments at LAC+USC. Currently, LAC+USC has a longer than 4-month backlog, which is defined as the time between the date on which the examination is ordered and the date of the neuroimaging appointment, whereas Keck Hospital has no backlog or waiting queue for scheduling neuroimaging appointments.
The finding that an increasing number of brain metastases was associated with an increased risk for salvage neurosurgery and hospitalization for brain metastases is consistent with other analyses that found the number of brain metastases to be a significant prognostic factor after treatment with SRS.17–20 These studies focused on the effect of the number of brain metastases on OS, but we now report that it may have prognostic significance for other neurologic outcomes such as salvage neurosurgery and hospitalization as well. Because SNH and PH patients had similar numbers of brain metastases, this factor alone did not explain the difference in neurologic outcomes.
Limitations
The main limitations of our study are its retrospective nature, relatively small sample size, and heterogeneous cohorts. Patients at the PH and patients at the SNH had significantly different baseline characteristics for which we attempted to account in the multivariate analysis. There were challenges in quantifying the quality of follow-up that may have lessened the true magnitude of the effect on outcomes. Neurologic symptoms were determined retrospectively, and thus this study was less reliable than a prospective study with real-time data on the neurologic status. Despite this, it is unlikely that the observed differences in neurologic outcomes between the hospitals can be accounted for merely by retrospective bias.
Our study was conducted jointly at Norris, an academic medical center, and LAC+USC, one of the largest SNHs in the United States, both located in Los Angeles, California, and thus the results may or may not be generalizable to other practice settings where indigent patients are managed. Further study in this area in other indigent care settings is needed to confirm our findings.
In conclusion, patients with brain metastases followed in an SNH setting after treatment with SRS alone experienced higher rates of neurologic symptoms, severe neurologic symptoms, hospitalizations for brain metastases, and salvage neurosurgeries in comparison with PH patients. During follow-up, SNH patients received fewer neuroimaging studies. In the multivariate analysis, an early stage at diagnosis, more neuroimaging studies, and more clinical visits were protective against neurologic outcomes, whereas an SNH setting and a higher number of brain metastases were risk factors for poor neurologic outcomes.
The treatment strategy of SRS alone with observation for brain metastases may be challenging to perform in the SNH setting because of fewer follow-up neuroimaging studies and other unidentified barriers associated with practice in an SNH setting. Patients and clinicians should consider patient access to follow-up care when deciding on the optimal strategy for the treatment ofbrain metastases. Further studies are needed to investigate potential barriers to receiving appropriate brain metastasis follow-up care after SRS alone and potential interventions for improving compliance rates and neurologic outcomes in this setting.
Acknowledgments
FUNDING SUPPORT
The research support of the Ginsburg, Kozak, and Phillip families is gratefully acknowledged.
Footnotes
CONFLICT OF INTEREST DISCLOSURES
Eric L. Chang received a speaker’s honorarium from Brainlab.
REFERENCES
- 1.Patchell RA. The management of brain metastases. Cancer Treat Rev. 2003;29:533–540. [DOI] [PubMed] [Google Scholar]
- 2.Brown PD, Jaeckle K, Ballman KV, et al. Effect of radiosurgery alone vs radiosurgery with whole brain radiation therapy on cognitive function in patients with 1 to 3 brain metastases: a randomized clinical trial. JAMA. 2016;316:401–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chang EL, Wefel JS, Hess KR, et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol. 2009;10:1037–1044. [DOI] [PubMed] [Google Scholar]
- 4.Aoyama H, Shirato H, Tago M, et al. Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: a randomized controlled trial. JAMA. 2006;295:2483–2491. [DOI] [PubMed] [Google Scholar]
- 5.Kocher M, Soffietti R, Abacioglu U, et al. Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: results of the EORTC 22952–26001 study. J Clin Oncol. 2011;29:134–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Amini A, Rusthoven CG, Waxweiler TV, et al. Association of health insurance with outcomes in adults ages 18 to 64 years with melanoma in the United States. J Am Acad Dermatol. 2016;74:309–316. [DOI] [PubMed] [Google Scholar]
- 7.Martinez SR, Tseng WH, Canter RJ, Chen AM, Chen SL, Bold RJ. Do radiation use disparities influence survival in patients with advanced breast cancer?: breast cancer radiation disparities. Cancer. 2012;118:196–204. [DOI] [PubMed] [Google Scholar]
- 8.Martinez SR, Beal SH, Chen SL, et al. Disparities in the use of radiation therapy in patients with local-regionally advanced breast cancer. Int J Radiat Oncol. 2010;78:787–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang EH, Yu JB, Abouassally R, et al. Disparities in treatment of patients with high-risk prostate cancer: results from a population-based cohort. Urology. 2016;95:88–94. [DOI] [PubMed] [Google Scholar]
- 10.Akinyemiju T, Sakhuja S, Vin-Raviv N. Racial and socio-economic disparities in breast cancer hospitalization outcomes by insurance status. Cancer Epidemiol. 2016;43:63–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rose TL, Deal AM, Krishnan B, et al. Racial disparities in survival among patients with advanced renal cell carcinoma in the targeted therapy era: racial disparities in advanced RCC. Cancer. 2016;122: 2988–2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koroukian SM, Bakaki PM, Raghavan D. Survival disparities by Medicaid status: an analysis of 8 cancers. Cancer. 2012;118:4271–4279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holmes JA, Carpenter WR, Wu Y, et al. Impact of distance to a urologist on early diagnosis of prostate cancer among black and white patients. J Urol. 2012;187:883–888. [DOI] [PubMed] [Google Scholar]
- 14.Nabors LB, Ammirati M, Bierman PJ, et al. Central nervous system cancers. J Natl Compr Canc Netw. 2013;11:1114–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471–1474. [DOI] [PubMed] [Google Scholar]
- 16.Lippitz B, Lindquist C, Paddick I, Peterson D, O’Neill K, Beaney R. Stereotactic radiosurgery in the treatment of brain metastases: the current evidence. Cancer Treat Rev. 2014;40:48–59. [DOI] [PubMed] [Google Scholar]
- 17.Bian SX, Routman D, Liu J, et al. Prognostic factors for melanoma brain metastases treated with stereotactic radiosurgery. J Neurosurg. 2016;125(suppl 1):31–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kano H, Iyer A, Kondziolka D, Niranjan A, Flickinger JC, Lunsford LD. Outcome predictors of gamma knife radiosurgery for renal cell carcinoma metastases. Neurosurgery. 2011;69:1232–1239. [DOI] [PubMed] [Google Scholar]
- 19.Liew DN, Kano H, Kondziolka D, et al. Outcome predictors of Gamma Knife surgery for melanoma brain metastases. Clinical article. J Neurosurg. 2011;114:769–779. [DOI] [PubMed] [Google Scholar]
- 20.Ferrel E, Roehrig A, Kaya E, et al. Retrospective study of metastatic melanoma and renal cell carcinoma to the brain with multivariate analysis of prognostic pre-treatment clinical factors. Int J Mol Sci. 2016;17:400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gonda DD, Kim TE, Goetsch SJ, et al. Prognostic factors for stereotactic radiosurgery-treated patients with cerebral metastasis: implications on randomised control trial design and inter-institutional collaboration. Eur J Cancer. 2014;50:1148–1158. [DOI] [PubMed] [Google Scholar]
- 22.Wu X, Lin H. Patient adherence to follow-up in clinical research: a systematic review of measurements, associated factors and intervention strategies. J Clin Res Ophthalmol. 2015;2:058–064. [Google Scholar]
- 23.Jin J, Sklar GE, Min Sen Oh V, Chuen Li S. Factors affecting therapeutic compliance: a review from the patient’s perspective. Ther Clin Risk Manag. 2008;4:269–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murphy CC, Tiro JA, Jean GW, Balasubramian BA, Alvarez CA. High initiation of adjuvant hormonal therapy among uninsured stages I-III breast cancer patients treated in a safety-net healthcare system. J Womens Health (Larchmt). 2017;26:655–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Balasubramanian BA, Garcia MP, Corley DA, et al. Racial/ethnic differences in obesity and comorbidities between safety-net- and non safety-net integrated health systems. Medicine (Baltimore). 2017;96:e6326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Werner RM, Goldman LE, Dudley RA. Comparison of change in quality of care between safety-net and non-safety-net hospitals. JAMA. 2008;299:2180–2187. [DOI] [PubMed] [Google Scholar]


