Abstract
Introduction
There is evidence that the combination of ipilimumab and stereotactic radiosurgery (SRS) for brain metastases improves outcomes. We investigated clinical outcomes, radiation toxicity, and impact of ipilimumab timing in patients treated with SRS for melanoma brain metastases.
Methods
We retrospectively identified 91 patients treated with SRS at our institution for melanoma brain metastases from 2006 to 2015. Concurrent ipilimumab administration was defined as within ±4 weeks of SRS procedure. Acute and late toxicities were graded with CTCAE v4.03. Overall survival (OS), local failure, distant brain failure, and failure-free survival were analyzed with the Kaplan–Meier method. OS was analyzed with Cox regression.
Results
Twenty-three patients received ipilimumab concurrent with SRS, 28 patients non-concurrently, and 40 patients did not receive ipilimumab. The median age was 62 years and 91% had KPS ≥ 80. The median follow-up time was 7.4 months. Patients who received ipilimumab had a median OS of 15.1 months compared to 7.8 months in patients who did not (p = 0.02). In multivariate analysis, ipilimumab (p = 0.02) and diagnosis-specific graded prognostic assessment (p = 0.02) were associated with OS. There were no differences in intracranial control by ipilimumab administration or timing. The incidence of radiation necrosis was 5%, with most events occurring in patients who received ipilimumab.
Conclusions
Patients who received ipilimumab had improved OS even after adjusting for prognostic factors. Ipilimumab did not appear to increase risk for acute toxicity. The majority of radiation necrosis events, however, occurred in patients who received ipilimumab. Our results support the continued use of SRS and ipilimumab as clinically appropriate.
Keywords: Melanoma brain metastases, Ipilimumab immunotherapy, Stereotactic radiosurgery, Clinical outcomes, Radiation toxicity
Introduction
There are 76,000 cases of melanoma diagnosed and 9000 deaths due to melanoma each year in the United States. The incidence of melanoma has tripled over the past 30 years and it is currently the second most common invasive malignancy in people under 39 years old [1]. Brain metastases are common in melanoma and carry a particularly poor prognosis with a historical median survival of 2–5 months [2–4].
The advent of targeted therapy agents, however, has shown promise in the treatment of patients with metastatic melanoma. Ipilimumab, a human monoclonal antibody, blocks cytotoxic T-lymphocyte antigen-4 (CTLA-4) and has been demonstrated to improve overall survival (OS) in two large randomized trials [5, 6]. Although ipilimumab is not able to cross an intact blood–brain barrier (BBB), there is evidence that ipilimumab is either able to penetrate tumors at leaky BBB sites or activates cytotoxic T lymphocytes which in turn infiltrate the tumor environment and induce anti-tumor activity [7–10]. In addition, some postulate that radiotherapy can induce an abscopal effect that enhances the immunologic effectiveness of ipilimumab [11–14]. Several retrospective studies have now found an OS and/or intracranial control benefit of ipilimumab when used in combination with stereotactic radiosurgery (SRS) for treatment of melanoma brain metastases [15–19].
Despite this, the impact of combination treatment and the timing of administration of ipilimumab relative to SRS on toxicity, cancer control outcomes, and survival remains unclear. In the present study, we aim to describe OS, intracranial tumor control, and radiation toxicity in patients treated with combination ipilimumab and SRS compared to patients treated with SRS alone. We explore the effect of ipilimumab timing relative to SRS and identify associated prognostic factors, making this one of the most complete discourses on the clinical impact of this combination of treatments to date.
Materials and methods
This retrospective cohort study was approved by the University of Southern California (USC) Keck School of Medicine Institutional Review Board. We identified 107 consecutive patients who were treated with SRS at our institution for melanoma brain metastases between 2006 and 2015. Patients who did not have clinical follow-up were excluded. Ipilimumab, if administered, was at a dose of either 3 or 10 mg/ kg every 3 weeks. Concurrent ipilimumab administration was defined as within ± 4 weeks of SRS procedure. All patients received SRS using Gamma Knife® Perfexion™ (Elekta AB, Stockholm, Sweden). Radiation doses were based on factors including tumor size, shape, and location as described in RTOG 9005 with variation allowed at the discretion of the treating clinician [20].
Cancer staging was performed according to the American Joint Committee on Cancer (AJCC) 7th edition guidelines [21]. The diagnosis-specific graded prognostic assessment (DS-GPA) score for melanoma was calculated based on the study by Sperduto et al. [22]. Acute and late radiation toxicities were defined as events that occurred ≤ 90 or > 90 days after SRS, respectively, and were graded with the Common Terminology Criteria for Adverse Events (CTCAE), version 4.03.
Baseline patient and treatment characteristics were compared with the Kruskal–Wallis test and Pearson Chi square test. OS, local failure (LF), distant brain failure (DBF), and failure-free survival (FFS) were calculated from the date of first SRS treatment and analyzed with the Kaplan–Meier method and log-rank test. LF was analyzed per patient as the date of first in-field tumor progression. DBF was analyzed per patient as the date of first new distant brain metastasis. FFS was defined as the first occurrence of LF, DBF, or death. Univariate and multivariate analysis were performed using the Cox proportional hazards model. Clinically relevant risk factors were entered into univariate analysis and significant variables were further entered into multivariate analysis. Acute toxicities were analyzed per SRS procedure and late toxicities were analyzed per patient with comparison using Fisher’s exact test. Significance was defined as p ≤ 0.05. Statistical calculations were performed in JMP Pro (version 13; SAS Institute, Cary, NC).
Results
Patient and treatment characteristics
A total of 91 patients were included in this analysis, 51 (56%) of whom received ipilimumab and 40 (44%) of whom did not. Twenty-three patients received ipilimumab concurrent with SRS and 28 received ipilimumab non-concurrently. Among all patients, the median age was 62 years (range 27–85 years), 29 (32%) were female, and 82 (91%) had KPS ≥ 80. The median follow-up time was 7.4 months. Of patients who received ipilimumab, 36 (77%) were treated at a dose of 3 mg/kg and 11 (23%) were treated at 10 mg/ kg. The median cycles of ipilimumab administered was 4 (range 1–6). During initial SRS, 256 brain metastases with a median tumor volume of 0.27 cm3 (range 0.01–30.33 cm3) were treated to a median marginal dose of 20 Gy (range 12–22 Gy). The number of surgical resection cavities treated at initial SRS was 29 out of 256 (11%). A total of 155 SRS treatments were given, with 46 (50%) patients receiving 1 SRS treatment, 37 (41%) patients receiving 2 SRS treatments, and 8 (9%) receiving 3 or more SRS treatments.
Overall, patient and treatment characteristics were similar between patients who did not receive ipilimumab, received ipilimumab concurrently, and received ipilimumab non-concurrently (Table 1). However, patients who received non-concurrent ipilimumab received prior chemotherapy at a higher rate (39 vs. 13%) and more often received ipilimumab at a dose of 10 mg/kg (35 vs. 10%) compared to concurrent ipilimumab patients. In addition, patients who did not receive ipilimumab were treated with SRS at an earlier median year (2009) compared to those who received concurrent ipilimumab (2011) and non-concurrent ipilimumab (2010).
Table 1.
Patient and treatment characteristics
| Characteristic | No ipilimumab (n = 40) | Concurrent ipilimumab (n = 23) | Non-concurrent ipilimumab (n = 28) | p Value |
|---|---|---|---|---|
| Age, median (range) | 62.5 (35–84) | 62 (29–85) | 59 (27–76) | 0.38 |
| Sex | 0.59 | |||
| Male | 29 (73%) | 16 (70%) | 17 (61%) | |
| Female | 11 (27%) | 7 (30%) | 11 (39%) | |
| KPS, median (range) | 90 (60–100) | 90 (70–100) | 90 (60–100) | 0.29 |
| DS-GPA, median (range) | 2 (0–4) | 3 (1–4) | 3 (1–4) | 0.47 |
| Stage at diagnosis of cancer | 0.15 | |||
| I-II | 5 (25%) | 5 (31%) | 10 (56%) | |
| III-IV | 15 (75%) | 11 (69%) | 8 (44%) | |
| Brain metastases diagnosed within 3 months of primary | 0.72 | |||
| Yes | 5 (13%) | 3 (13%) | 2 (7%) | |
| No | 35 (87%) | 20 (87%) | 26 (93%) | |
| Time from primary cancer to brain metastasis, in years, median (range) | 3.38 (0–22.74) | 1.6 (0–20.10) | 7.14 (0.04–29.10) | 0.007 |
| Extracranial metastases | 0.15 | |||
| Yes | 27 (68%) | 20 (87%) | 23 (82%) | |
| No | 13 (32%) | 3 (13%) | 5 (18%) | |
| Neurologically symptomatic at baseline | 0.71 | |||
| Yes | 7 (18%) | 4 (17%) | 7 (25%) | |
| No | 33 (82%) | 19 (83%) | 21 (75%) | |
| Prior WBRT | 0.69 | |||
| Yes | 3 (8%) | 1 (4%) | 3 (11%) | |
| No | 36 (92%) | 22 (96%) | 25 (89%) | |
| Prior chemotherapy | 0.04 | |||
| Yes | 16 (41%) | 3 (13%) | 11 (39%) | |
| No | 23 (59%) | 20 (87%) | 17 (61%) | |
| Prior targeted therapy | 0.15 | |||
| Yes | 15 (38%) | 9 (39%) | 17 (61%) | |
| No | 24 (62%) | 14 (61%) | 11 (39%) | |
| Prior neurosurgery | 0.60 | |||
| Yes | 17 (44%) | 8 (35%) | 9 (32%) | |
| No | 22 (56%) | 15 (65%) | 19 (68%) | |
| BRAF therapy | 0.65 | |||
| Yes | 4 (10%) | 3 (13%) | 5 (18%) | |
| No | 36 (90%) | 20 (87%) | 23 (82%) | |
| Pembrolizumab therapy | 0.03 | |||
| Yes | 1 (3%) | 5 (22%) | 6 (21%) | |
| No | 39 (97%) | 18 (78%) | 22 (79%) | |
| Ipilimumab dose | 0.04 | |||
| 3 mg/kg | – | 19 (90%) | 17 (65%) | |
| 10 mg/kg | – | 2 (10%) | 9 (35%) | |
| Ipilimumab cycles, median (range) | – | 4 (2–5) | 4 (1–6) | 0.81 |
| Year treated with SRS, median (range) | 2009 (2006–2011) | 2011 (2008–2014) | 2010 (2007–2015) | < 0.001 |
| 2006–2010 | 34 (85%) | 6 (26%) | 16 (57%) | |
| 2011–2015 | 6 (15%) | 17 (74%) | 12 (43%) | |
| Time from brain metastasis diagnosis to SRS, in days, median (range) | 21 (0–285) | 25 (6–154) | 22 (3–1663) | 0.63 |
| Number of brain metastases treated, mean (standard deviation) | 2.45 (1.84) | 3.61 (3.60) | 2.68 (2.28) | 0.20 |
| Number of brain metastases treated, median (range) | 2 (1–8) | 3 (1–16) | 2 (1–9) | 0.49 |
| Number of surgical resection cavities treated (% of total) | 11 (11%) | 11 (13%) | 7 (9%) | 0.74 |
| Tumor volume, cm3, median (range) | 0.42 (0.01–21.80) | 0.20 (0.01–30.33) | 0.20 (0.01–10.60) | 0.10 |
DS-GPA diagnosis-specific graded prognostic assessment, Gy gray, SRS stereotactic radiosurgery, WBRT whole-brain radiotherapy
Overall survival
The median OS among all patients was 10.6 months. Patients who received ipilimumab had a median OS of 15.1 months compared to 7.8 months in patients who did not receive ipilimumab (p = 0.02). The median survival of patients treated with SRS between 2006 and 2010 was 11.7 months compared to 10.0 months for patients treated from 2011 to 2015 (p = 0.53). Patients who received non-concurrent ipilimumab had the most favorable OS (median 18.7 months), followed by concurrent ipilimumab (median 11.8 months) and no ipilimumab (median 7.8 months). At 1 year, OS was 63, 50, and 28%, respectively, in patients who received non-concurrent ipilimumab, concurrent ipilimumab, and no ipilimumab (p = 0.02) (Fig. 1a). In multivariate analysis, non-concurrent ipilimumab administration (HR, 0.51; 95% CI 0.28–0.92; p = 0.02) and DS-GPA (HR, 0.69; 95% CI 0.50–0.96; p = 0.02) were significantly associated with OS (Table 2). Any ipilimumab was borderline significant for improved OS (p = 0.06). Time from primary cancer to brain metastasis of ≤ 5 years and number of brain metastases treated were significant in univariate analysis but not multivariate anlaysis.
Fig. 1.
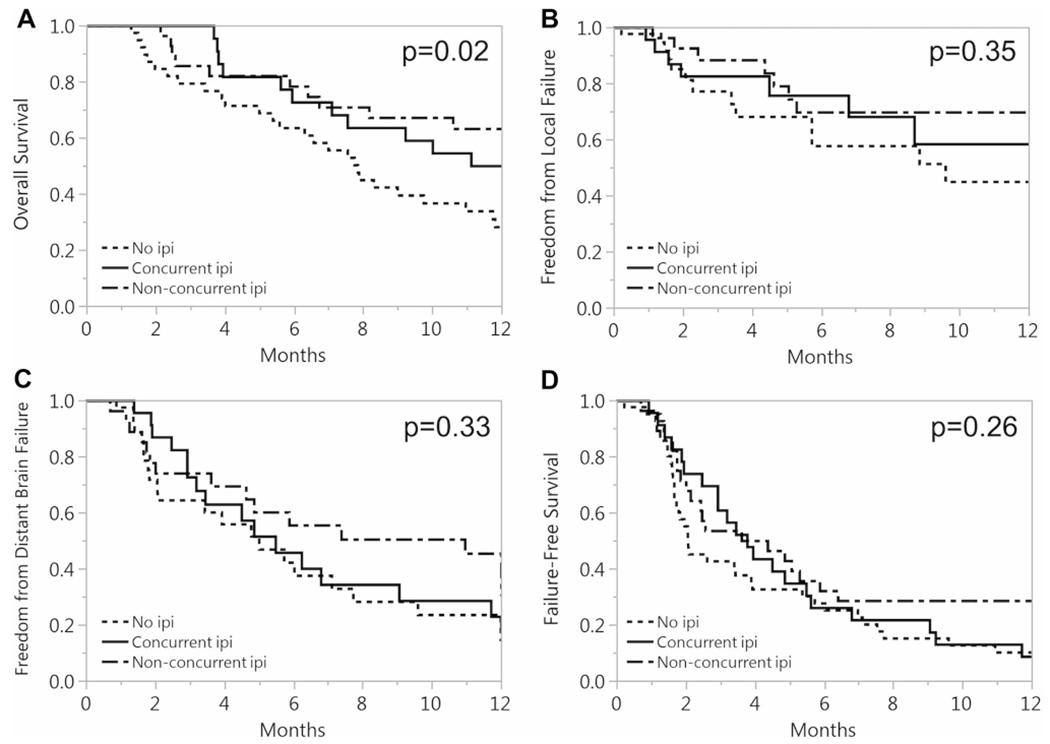
Kaplan–Meier 1-year survival curves for a overall survival, b freedom from local failure, c freedom from distant brain failure, and d failure-free survival for no ipilimumab, concurrent ipilimumab, and non-concurrent ipilimumab
Table 2.
Univariate and multivariate analysis of overall survival
| Variable | Univariate |
multivariate |
||
|---|---|---|---|---|
| HR (95% CI) | p Value | HR (95% CI) | p Value | |
| Age (per year) | 1.00 (0.98–1.02) | 0.98 | – | – |
| Sex | ||||
| Male | 1.35 (0.83–2.29) | 0.23 | – | – |
| Female | Ref. | Ref. | – | – |
| Group | Global p = 0.10 | Global p = 0.06 | ||
| No ipilimumab | Ref. | Ref. | Ref. | Ref. |
| Concurrent ipilimumab | 0.77 (0.43–1.35) | 0.37 | 0.60 (0.32–1.11) | 0.11 |
| Non-concurrent ipilimumab | 0.55 (0.30–0.95) | 0.03 | 0.51 (0.28–0.92) | 0.02 |
| Ipilimumab dose | ||||
| 3 mg/kg | Ref. | Ref. | – | – |
| 10 mg/kg | 0.72 (0.30–1.55) | 0.41 | – | – |
| Ipilimumab cycles (per cycle) | 1.24 (0.91–1.73) | 0.17 | – | – |
| Pembrolizumab therapy | ||||
| Yes | 0.63 (0.26–1.28) | 0.21 | – | – |
| No | Ref. | Ref. | – | – |
| KPS | ||||
| ≥80 | 0.72 (0.45–1.17) | 0.06 | – | – |
| <80 | Ref. | Ref. | – | – |
| DS-GPA (per unit increase) | 0.60 (0.46–0.78) | < 0.001 | 0.69 (0.50–0.96) | 0.02 |
| Stage at diagnosis | ||||
| I–II | Ref. | Ref. | – | – |
| III–IV | 0.89 (0.48–1.71) | 0.50 | – | – |
| Time from primary cancer to brain metastasis (years) | ||||
| >5 | 0.57 (0.35–0.92) | 0.02 | 0.70 (0.40–1.20) | 0.20 |
| ≤5 | Ref. | Ref. | Ref. | Ref. |
| Extracranial metastases | ||||
| Yes | 1.36 (0.78–2.50) | 0.29 | – | – |
| No | Ref. | Ref. | – | – |
| Year treated with SRS | ||||
| 2006–2010 | Ref. | Ref. | – | – |
| 2011–2015 | 1.41 (0.87–2.28) | 0.16 | – | – |
| Number of brain metastases treated | ||||
| 1 | Ref. | Ref. | Ref. | Ref. |
| 2–4 | 2.35 (1.35–4.17) | 0.003 | 1.64 (0.86–3.16) | 0.13 |
| 5+ | 2.46 (1.27–4.69) | 0.009 | 1.28 (0.54–3.02) | 0.57 |
| Prior WBRT | ||||
| Yes | 1.37 (0.57–2.81) | 0.45 | – | – |
| No | Ref. | Ref. | – | – |
| Prior chemotherapy | ||||
| Yes | 0.78 (0.46–1.28) | 0.33 | – | – |
| No | Ref. | Ref. | – | – |
| Prior targeted therapy | ||||
| Yes | 0.83 (0.52–1.33) | 0.44 | – | – |
| No | Ref. | Ref. | – | – |
| Prior neurosurgery | ||||
| Yes | 1.00 (0.61–1.61) | 0.99 | – | – |
| No | Ref. | Ref. | – | – |
CI confidence interval, DS-GPA diagnosis-specific graded prognostic assessment, SRS stereotactic radiosurgery, WBRT whole-brain radiotherapy
Cancer control outcomes
The 1-year freedom from any LF was 45, 58, and 70%, respectively, in patients receiving no ipilimumab, concurrent ipilimumab, and non-concurrent ipilimumab (p = 0.35) (Fig. 1b). The 1-year freedom from DBF was 23, 23, and 45%, respectively, in patients receiving no ipilimumab, concurrent ipilimumab, and non-concurrent ipilimumab (p = 0.33) (Fig. 1c). The 1-year FFS was 10, 9, and 29%, respectively, in patients receiving no ipilimumab, concurrent ipilimumab, and non-concurrent ipilimumab (p = 0.26) (Fig. 1d). Furthermore, there were no significant differences in pairwise testing between any of the treatment groups for LF, DBF, or FFS.
Acute and late toxicities
Acute toxicities were analyzed per SRS procedure performed (Table 3). Out of a total of 155 courses of SRS, 59 were given in patients who did not receive ipilimumab, 23 were given concurrently with ipilimumab, and 73 were given non-concurrently with ipilimumab. Overall, 25 (16%) SRS treatments resulted in an acute toxicity. The incidence of acute toxicity was 14, 26, and 15%, respectively, following no ipilimumab, concurrent ipilimumab, and non-concurrent ipilimumab SRS procedures (p = 0.36). There was no significant difference in the distribution of acute toxicity grades among the treatment groups (p = 0.51). There were four total grade 3–4 events and no grade 5 events. Grade 3–4 events included two cases of cerebral edema and two cases of cerebral hemorrhage in four unique patients. One of these patients was deceased prior to 90 days; however, none of the other three patients went on to develop late toxicities.
Table 3.
Acute toxicity events for each SRS procedure
| Treatment group | Maximum grade of acute toxicitya |
Acute toxicityb |
Total | ||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | Yes | No | ||
| No ipilimumab | 3 | 3 | 1 | 1 | 8 (14%) | 51 (86%) | 59 |
| Concurrent ipilimumab | 4 | 1 | 1 | 0 | 6 (26%) | 17 (74%) | 23 |
| Non-concurrent ipilimumab | 6 | 4 | 0 | 1 | 11 (15%) | 62 (85%) | 73 |
| Total | 13 | 8 | 2 | 2 | 25 (16%) | 130 (84%) | 155 |
p = 0.51 with Fisher’s exact test
p = 0.36 with Fisher’s exact test
Late toxicities were analyzed per patient (Table 4). Out of a total of 91 patients, 12 (13%) experienced a late toxicity. The incidence of late toxicity was 13, 17, and 11%, respectively, in patients receiving no ipilimumab, concurrent ipilimumab, and non-concurrent ipilimumab (p = 0.79). There was no significant difference in the distribution of late toxicity grades among the treatment groups (p = 0.29). There were five total grade 3–4 events, all of which were pathologically-confirmed cases of RN, and no grade 5 events. However, four out of five cases of RN were in patients who received ipilimumab prior to the event. The relative frequency of RN events among no ipilimumab, concurrent ipilimumab, and non-concurrent ipilimumab was 3, 9, and 7%, respectively. Of the RN events, median time from SRS to event was 6.6 months (range 3.5–42 months). The single non-ipilimumab event was associated with the longest time to event of 42 months, compared to the longest time to event of 11.4 months among ipilimumab-associated events.
Table 4.
Late toxicity events for each patient
| Treatment group | Max grade of late toxicitya |
Radiation necrosis |
Late toxicityb |
Total | |||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | Yes | No | Yes | No | ||
| No ipilimumab | 0 | 4 | 1 | 0 | 1 (3%) | 39 (97%) | 5 (13%) | 35 (87%) | 40 |
| Concurrent ipilimumab | 0 | 2 | 1 | 1 | 2 (9%) | 21 (91%) | 4 (17%) | 19 (83%) | 23 |
| Non-concurrent ipilimumab | 1 | 0 | 2 | 0 | 2 (7%) | 26 (93%) | 3 (11%) | 25 (89%) | 28 |
| Total | 1 | 6 | 4 | 1 | 5 (5%) | 86 (95%) | 12 (13%) | 79 (87%) | 91 |
p = 0.29 with Fisher’s exact test
p = 0.79 with Fisher’s exact test
Discussion
The combination of ipilimumab and SRS has shown promise for improving cancer outcomes in patients with melanoma brain metastases in multiple retrospective studies [15–19]. However, owing to the small number of patients receiving this combination of treatments who have been reported on to date, additional data is needed to elucidate the effect of treatment on clinical outcomes and radiation toxicity, as well as the impact that timing of ipilimumab administration relative to SRS has on these measures [23]. In the current study, to our knowledge, we describe results from our experience with the largest single-institution cohort of patients with melanoma brain metastases treated with SRS and ipilimumab to date (n = 51).
With a median follow-up of 7.4 months, we found that patients who received ipilimumab had a median OS of 15.1 vs. 7.8 months for control patients who did not receive ipilimumab. The OS of control patients observed in our cohort correlates closely with historical data presented by Sperduto et al., in which patients with melanoma brain metastases who received SRS had a median OS of 7.26 months [22]. The increased OS observed in patients receiving ipilimumab supports prior studies with similar findings [15–19]. Although patients who did not receive ipilimumab were treated with SRS in earlier years, we did not find a significant difference in survival based on SRS treatment year. Prior to 2011, ipilimumab was available primarily through clinical trials, for which brain metastases were frequently an exclusion criteria. As KPS and DS-GPA did not differ significantly between treatment groups, it is most likely that patients in the control group did not receive ipilimumab due to restricted access and/or different practice patterns at the time. Furthermore, the observed improved survival in our study persisted after controlling for DS-GPA score and number of brain metastases in multivariate analysis, suggesting that the survival differences were truly due to treatment effect as opposed to pre-treatment selection bias.
We found that patients who had ipilimumab delivered non-concurrently with SRS had the most favorable survival outcomes. On the other hand, there were significant baseline differences between the non-concurrent and concurrent ipilimumab groups. Patients who received ipilimumab non-concurrently more frequently received prior chemotherapy (39 vs. 13%) and a higher dose of ipilimumab (35 vs. 10% received 10 mg/kg). In addition, we hypothesize that patients who received ipilimumab non-concurrently may have had less aggressive disease, as non-concurrent ipilimumab patients had the longest time from diagnosis of primary cancer to brain metastasis development.
Although these factors may partially explain the observed differences in survival, we were not able to completely explain the differences even when controlling for these factors during analysis. An additional possibility is that administering ipilimumab non-concurrently actually is associated with improved survival. Although the mechanism of interaction between ipilimumab and SRS continues to be elucidated, a study published by Twyman-Saint Victor et al. found that anti-CTLA4 antibodies such as ipilimumab promote expansion of T cells whereas radiotherapy may help shape the T-cell receptor repertoire of these clonal expansions [24]. Based on such a model, it is conceivable that ipilimumab requires time to prime the immune system for a greater response to subsequent radiotherapy. Despite this, the optimal timing of ipilimumab with radiotherapy remains to be determined [25].
In our study, there were no significant differences in terms of LF, DBF, or FFS among the treatment groups, although we observed a trend to reduced LF with ipilimumab, particularly in the non-concurrent ipilimumab group. Recent studies have come to mixed results regarding intracranial control with combination SRS and ipilimumab, with some studies not finding any difference in terms of LF or DBF [19, 26, 27], one study finding improved DBF but similar LF [18], and other studies finding improvements in LF based on the timing of ipilimumab [28–30]. Due to the heterogeneity of findings and methodologies in these studies, there is no clear consensus on the impact of administration or timing of ipilimumab on intracranial control. Our observation that tumor control was similar among treatment groups yet OS favored the ipilimumab groups suggests that the improved survival outcomes with ipilimumab were likely more related to improvements in systemic disease control than to intracranial disease control. This finding is consistent with previous literature in which systemic disease, rather than intracranial disease, was typically the limiting survival factor in brain metastasis patients [31].
The relative risk of toxicity in patients treated with combination ipilimumab and SRS has not been well-defined in part because only a few existing studies with small sample sizes have compared combination treatment with a SRS-only control group (n = 62 total among all studies), and none of these studies provided detailed toxicity reporting [16, 26, 27, 32]. Recently, the results of a phase I study that enrolled 11 patients treated with SRS and ipilimumab were published, in which only 1 patient experienced grade ≥ 3 neurotoxicity, occurring prior to ipilimumab administration [33]. In the present study, we did not find statistically significant differences in the incidence of acute radiation toxicities following SRS procedures by ipilimumab or timing of ipilimumab. In general, grade ≥ 3 acute toxicities were rare, occurring after only 3% of SRS procedures overall.
There were no statistically significant differences in the incidence of late toxicities. Late grade ≥ 3 radiation toxicities occurred in 5% of patients overall. All late toxicities were pathologically-confirmed RN, and 4 out of 5 of these events occurred in patients who received ipilimumab previously. This raises concern that ipilimumab immunotherapy may increase the risk for symptomatic RN—a result that other studies have previously reported [28, 34, 35]. Furthermore, the relatively short time to onset of RN observed among patients who received ipilimumab in the present study compared to historical cohorts suggests that ipilimumab may be associated with faster time to onset of RN as well [36]. However, the low incidence of toxicities and absence of fatal toxicity events in the ipilimumab groups, combined with the documented OS benefit, supports the continued use of combination SRS and ipilimumab for melanoma brain metastases as clinically appropriate. Our institutional experience with ipilimumab suggests that it is safe to administer ipilimumab concurrently with SRS, not requiring a washout period prior to SRS or a wait period after SRS, although the optimal timing of administration remains to be defined. Larger prospective studies are needed to further investigate the safety, efficacy, and mechanisms of combination SRS and ipilimumab therapy.
Limitations
The current study was performed as a retrospective review and thus there was likely some selection bias that occurred when patients elected to undergo various treatments. The relatively small sample size raises the possibility that the study was underpowered to detect differences in outcomes including intracranial control and toxicities within subgroups. Although we attempted to control for prognostic factors in multivariate analysis, there may have been baseline differences between groups that remained unaccounted for. Standardized symptom and/or quality of life assessments were not administered prospectively, making highly accurate toxicity detection and assessment difficult. We did not report on non-radiation related toxicities and were not able to definitively differentiate between RN and tumor progression except when surgical pathology was available.
Conclusions
In our experience with the largest single-institution cohort of patients with brain metastases treated with SRS and ipilimumab to date, patients who received ipilimumab had an improved OS of 15.1 months compared to 7.8 months for patients who did not receive ipilimumab. Other prognostic factors for OS included DS-GPA score. Non-concurrent ipilimumab had the most favorable survival outcomes; however, this observation may in part be due to selection bias. Ipilimumab did not appear to significantly increase risk for acute toxicity. The majority of RN events, however, occurred in patients who received ipilimumab, with a trend towards faster time to onset of RN. Ipilimumab appears safe to administer concurrently with SRS, not requiring a washout period prior to SRS or a wait period after SRS. Our results support the continued use of SRS and ipilimumab for treatment of melanoma brain metastases as clinically appropriate.
Acknowledgements
We wish to gratefully acknowledge the research support of the Kozak, Ginsburg, and Phillip families for the research stipend of Kevin Diao. Our sponsors were not involved in the research project.
Footnotes
Conflict of interest Eric L. Chang received speaker’s honorarium from Brainlab.
References
- 1.Siegel R, DeSantis C, Virgo K et al. (2012) Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin 62:220–241. 10.3322/caac.21149 [DOI] [PubMed] [Google Scholar]
- 2.Fonkem E, Uhlmann EJ, Floyd SR et al. (2012) Melanoma brain metastasis: overview of current management and emerging targeted therapies. Expert Rev Neurother 12:1207–1215. 10.1586/ern.12.111 [DOI] [PubMed] [Google Scholar]
- 3.Fife KM, Colman MH, Stevens GN et al. (2004) Determinants of outcome in melanoma patients with cerebral metastases. J Clin Oncol 22:1293–1300. 10.1200/JCO.2004.08.140 [DOI] [PubMed] [Google Scholar]
- 4.Staudt M, Lasithiotakis K, Leiter U et al. (2010) Determinants of survival in patients with brain metastases from cutaneous melanoma. Br J Cancer 102:1213–1218. 10.1038/sj.bjc.6605622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hodi FS, O’Day SJ, McDermott DF et al. (2010) Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 363:711–723. 10.1056/NEJMoa1003466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Robert C, Thomas L, Bondarenko I et al. (2011) Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med 364:2517–2526. 10.1056/NEJMoa1104621 [DOI] [PubMed] [Google Scholar]
- 7.Di Giacomo AM, Margolin K (2015) Immune checkpoint blockade in patients with melanoma metastatic to the brain. Semin Oncol 42:459–465. 10.1053/j.seminoncol.2015.02.006 [DOI] [PubMed] [Google Scholar]
- 8.Wilson EH, Weninger W, Hunter CA (2010) Trafficking of immune cells in the central nervous system. J Clin Invest 120:1368–1379. 10.1172/JCI41911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Engelhardt B, Coisne C (2011) Fluids and barriers of the CNS establish immune privilege by confining immune surveillance to a two-walled castle moat surrounding the CNS castle. Fluids Barriers CNS 8:4 10.1186/2045-8118-8-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cohen JV, Kluger HM (2016) Systemic Immunotherapy for the treatment of brain metastases. Front Oncol. 10.3389/fonc.2016.00049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Margolin K, Ernstoff MS, Hamid O et al. (2012) Ipilimumab in patients with melanoma and brain metastases: an open-label, phase 2 trial. Lancet Oncol 13:459–465. 10.1016/S1470-2045(12)70090-6 [DOI] [PubMed] [Google Scholar]
- 12.Golden EB, Demaria S, Schiff PB et al. (2013) An abscopal response to radiation and ipilimumab in a patient with metastatic non-small cell lung cancer. Cancer Immunol Res 1:365–372. 10.1158/2326-6066.CIR-13-0115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Postow MA, Callahan MK, Barker CA et al. (2012) Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med 366:925–931. 10.1056/NEJMoa1112824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stamell EF, Wolchok JD, Gnjatic S et al. (2013) The abscopal effect associated with a systemic anti-melanoma immune response. Int J Radiat Oncol Biol Phys 85:293–295. 10.1016/j.ijrobp.2012.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tazi K, Hathaway A, Chiuzan C, Shirai K (2015) Survival of melanoma patients with brain metastases treated with ipilimumab and stereotactic radiosurgery. Cancer Med 4:1–6. 10.1002/cam4.315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Silk AW, Bassetti MF, West BT et al. (2013) Ipilimumab and radiation therapy for melanoma brain metastases. Cancer Med 2:899–906. 10.1002/cam4.140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Knisely JP, Yu JB, Flanigan J et al. (2012) Radiosurgery for melanoma brain metastases in the ipilimumab era and the possibility of longer survival. J Neurosurg 117:227–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kiess AP, Wolchok JD, Barker CA et al. (2015) Stereotactic radiosurgery for melanoma brain metastases in patients receiving ipilimumab: safety profile and efficacy of combined treatment. Int J Radiat Oncol Biol Phys 92:368–375. 10.1016/j.ijrobp.2015.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shoukat S, Marcus D, Rizzo M et al. (2014) Outcome with stereotactic radiosurgery (SRS) and ipilimumab (Ipi) for malignant melanoma brain metastases (mets). J Clin Oncol 32:9076 [Google Scholar]
- 20.Shaw E, Scott C, Souhami L et al. (2000) Single dose radiosurgical treatment of recurrent previously irradiated primary brain tumors and brain metastases: final report of RTOG protocol 90-05. Int J Radiat Oncol Biol Phys 47:291–298 [DOI] [PubMed] [Google Scholar]
- 21.Edge SB, Compton CC (2010) The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol 17:1471–1474. 10.1245/s10434-010-0985-4 [DOI] [PubMed] [Google Scholar]
- 22.Sperduto PW, Chao ST, Sneed PK et al. (2010) Diagnosis-specific prognostic factors, indexes, and treatment outcomes for patients with newly diagnosed brain metastases: a multi-institutional analysis of 4,259 patients. Int J Radiat Oncol Biol Phys 77:655–661. 10.1016/j.ijrobp.2009.08.025 [DOI] [PubMed] [Google Scholar]
- 23.Salama AKS, Postow MA, Salama JK (2016) Irradiation and immunotherapy: from concept to the clinic. Cancer 122:1659–1671. 10.1002/cncr.29889 [DOI] [PubMed] [Google Scholar]
- 24.Twyman-Saint Victor C, Rech AJ, Maity A et al. (2015) Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature 520:373–377. 10.1038/nature14292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Frey B, Rubner Y, Kulzer L et al. (2014) Antitumor immune responses induced by ionizing irradiation and further immune stimulation. Cancer Immunol Immunother 63:29–36. 10.1007/s00262-013-1474-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mathew M, Tam M, Ott PA et al. (2013) Ipilimumab in melanoma with limited brain metastases treated with stereotactic radiosurgery. Melanoma Res 23:191–195. 10.1097/CMR.0b013e32835f3d90 [DOI] [PubMed] [Google Scholar]
- 27.Patel KR, Shoukat S, Oliver DE et al. (2017) Ipilimumab and stereotactic radiosurgery versus stereotactic radiosurgery alone for newly diagnosed melanoma brain metastases. Am J Clin Oncol 40:444–450 [DOI] [PubMed] [Google Scholar]
- 28.Diao K, Bian SX, Routman DM et al. (2018) Combination ipilimumab and radiosurgery for brain metastases: tumor, edema, and adverse radiation effects. J Neurosurg. 10.3171/2017.7.JNS171286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.An Y, Jiang W, Kim BYS et al. (2017) Stereotactic radiosurgery of early melanoma brain metastases after initiation of anti-CTLA-4 treatment is associated with improved intracranial control. Radiother Oncol 125:80–88. 10.1016/j.radonc.2017.08.009 [DOI] [PubMed] [Google Scholar]
- 30.Cohen-Inbar O, Shih H-H, Xu Z et al. (2017) The effect of timing of stereotactic radiosurgery treatment of melanoma brain metastases treated with ipilimumab. J Neurosurg 127:1007–1014. 10.3171/2016.9.JNS161585 [DOI] [PubMed] [Google Scholar]
- 31.Cairncross JG, Kim J-H, Posner JB (1980) Radation therapy for brain metastases. Ann Neurol 7:529–541. 10.1002/ana.410070606 [DOI] [PubMed] [Google Scholar]
- 32.Nguyen SM, Castrellon A, Vaidis O, Johnson AE (2017) Stereotactic radiosurgery and ipilimumab versus stereotactic radiosurgery alone in melanoma brain metastases. Cureus 9:e1511 10.7759/cureus.1511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Williams NL, Wuthrick EJ, Kim H et al. (2017) Phase I study of ipilimumab combined with whole brain radiation therapy or radiosurgery for melanoma patients with brain metastases. Int J Radiat Oncol. 10.1016/j.ijrobp.2017.05.028 [DOI] [PubMed] [Google Scholar]
- 34.Colaco RJ, Martin P, Kluger HM et al. (2016) Does immunotherapy increase the rate of radiation necrosis after radiosurgical treatment of brain metastases? J Neurosurg 125:17–23. 10.3171/2015.6.JNS142763 [DOI] [PubMed] [Google Scholar]
- 35.Du Four S, Wilgenhof S, Duerinck J et al. (2012) Radiation necrosis of the brain in melanoma patients successfully treated with ipilimumab, three case studies. Eur J Cancer 48:3045–3051. 10.1016/j.ejca.2012.05.016 [DOI] [PubMed] [Google Scholar]
- 36.Siddiqui ZA, Johnson MD, Baschnagel AM et al. (2016) Predictors of radiation necrosis in long-term survivors of stereotactic radiosurgery for brain metastases. Int J Radiat Oncol Biol Phys 96:E104. 10.1016/j.ijrobp.2016.06.855 [DOI] [PMC free article] [PubMed] [Google Scholar]


