Abstract
Multikinase inhibitors are effective treatments for thyroid cancers, acting primarily as antiangiogenic agents. This year, advances have been made in selective targeting of RET and BRAF in patients with medullary and anaplastic thyroid cancers, respectively. However, Hürthle cell carcinomas have a unique genomic landscape with no dominant truncal drivers, precluding simplistic approaches to therapeutic targeting.
The genomic landscapes of the main types of primary thyroid cancer have largely been defined. Cancers derived from thyroid follicular cells are enriched for BRAF and RAS mutations, as well as fusions involving receptor tyrosine kinases, primarily RET. Medullary thyroid carcinomas (MTCs), which are tumours derived from calcitonin-secreting C cells, comprise approximately 5% of all thyroid cancers and usually have somatic or germline RET point mutations1. Truncal driver mutations can confer vulnerabilities that could be targeted with specific therapies, as genetic or pharmacological inhibition of their activity suppresses cell growth. Despite this vulnerability, treatment of radioiodine-refractory metastatic thyroid cancers has relied on the multikinase inhibitors (MKIs) lenvatinib and sorafenib, the efficacy of which is due to their antiangiogenic activity rather than effects on the mutant oncoproteins. The high frequency of RET mutations in MTC led to the repurposing and FDA approval of vandetanib and cabozantinib, which inhibit rearranged-during transfection (RET) kinase activity in vitro. However, their narrow therapeutic window due to their broad spectrum of kinase inhibition probably prevents reaching doses that suppress RET kinase profoundly in vivo. Currently, no selective inhibitors of either BRAF or RET are approved for treatment of advanced thyroid cancer.
Therapy of anaplastic thyroid carcinoma (ATC) remains challenging. Surgery, when feasible, followed by chemoradiotherapy is the current standard of care1. No effective therapies are available for patients with distant metastases (stage IVc), for whom prognosis is poor. Approximately 45% of ATCs have BRAFV600E mutations1. A basket study of vemurafenib showed significant responses in patients with BRAF-mutant ATC1. However, thyroid cancers exhibit primary resistance to RAF kinase inhibition through relief of negative feedback1, and hence a combination of RAF and MEK kinase inhibitors is a logical choice to attain potent MAPK pathway inhibition (the MAPK signalling pathway is upregulated in tumours with BRAF mutations). An open-label phase II study of 16 patients with BRAFV600E-mutant ATC treated with dabrafenib and trametinib showed a remarkable overall response rate (ORR) of 69% in heavily pretreated patients2. By contrast, the ORR of BRAFV600E-driven, differentiated thyroid cancers (DTCs) to the same combination was only 33%3. The greater ORR in ATC than DTC was unexpected, as ATCs have a greater mutation burden and a more aggressive biological behaviour. DTC and ATC differ in their immune microenvironments, and it is intriguing to consider whether this feature might have a role in these differential responses. Interestingly, dabrafenib and trametinib treatment enabled neoadjuvant surgery for BRAF-mutant ATC in a case series of six patients who were initially deemed to have unresectable tumours4. The adverse effects of dabrafenib and trametinib were consistent with those of previously reported studies.
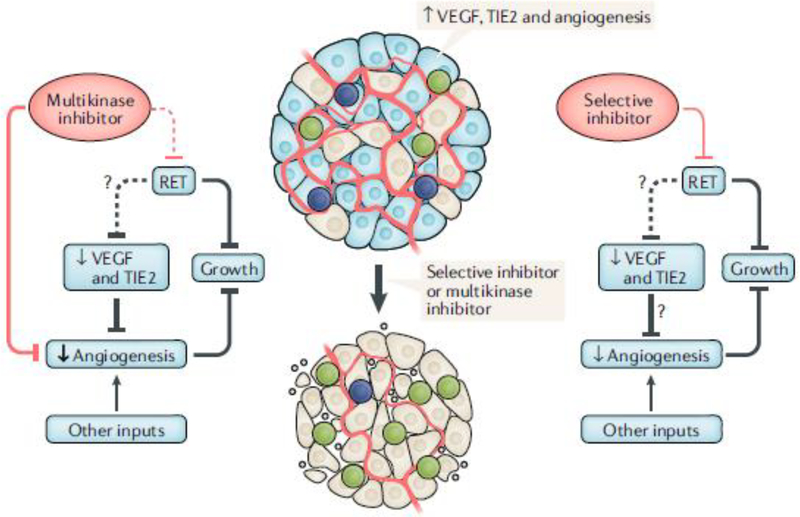
The MKIs vandetanib and cabozantinib improve progression-free survival of patients with unresectable and/or metastatic MTC compared with placebo but have frequent adverse events, often leading to dose interruption or reduction1. These effects are mostly due to their activity on kinases other than RET, raising the question of whether targeting RET with greater selectivity would be beneficial and have fewer toxicities (FIG. 1). The preclinical and early clinical studies of two selective RET kinase inhibitors — BLU-667 and LOXO-292 — were recently reported5,6. BLU-667 inhibits the protein product of RETM918T, the most common mutation in MTC, with greater potency than vandetanib and cabozantinib. It also inhibits the protein products of RETV804L/m gatekeeper mutations; the proteins encoded by these mutations confer resistance to the MKIs by sterically preventing their binding to the oncoprotein. BLU-667 is an order of magnitude less potent than the MKIs on VEGFR2 kinase activity in vitro. In contrast with cabozantinib, the drug did not alter plasma levels of soluble vascular endothelial growth factor receptor 2 (VEGFR2) or VEGFA, which are in vivo biomarkers of VEGFR2 inhibition.
Fig. 1 |. Highly selective versus promiscuous kinase driver inhibitors.
Tumour growth in RET-mutant thyroid cancer is driven by constitutive RET activation and enhanced angiogenesis. The multikinase inhibitors vandetanib and cabozantinib inhibit VEGFR2 and decrease angiogenesis but might not consistently inhibit RET kinase in vivo because of their narrow therapeutic window. The selective RET inhibitors LOXO-292 and BLU-667 do not target vascular endothelial growth factor (VEGF) receptor 2 (VEGFR2), MET or EGFR but probably exert their antitumour effects through blocking the primary oncogenic driver of the disease and seem to have a better safety profile. RET, rearranged-during transfection; TIE2, tyrosine kinase with immunoglobulin-like and EGF-like domains 1.
Subbiah and colleagues also described the activity of BLU-667 in two patients with RET-mutant, metastatic MTC who were enrolled in the dose escalation ARROW trial. One patient presented with a locally invasive primary tumour and distant metastases, and the other had metastatic disease that progressed on vandetanib. Both achieved a partial response with a maximal size reduction of target lesions of 47%5. The preliminary results of the ARROW trial of BLU-667 were presented at the 2019 ASCO meeting7. Sixty patients with RET-mutant MTC and five with RET-fusion PTC were included. Among these, 49 were eligible for analysis. The ORR was 47% (2 complete responses and 21 partial responses). Treatment-related toxicity was low grade and reversible and included decreased white blood cell count and increased transaminases, creatinine and phosphate; in addition, fewer than 15% of patients experienced hypertension.
LOXO-292 is a highly selective RET kinase inhibitor with nanomolar potency against the canonical RET MTC drivers, RET gatekeeper mutations and RET fusions6. It has a 20-fold to 1,700-fold greater growth inhibitory activity on RET-mutant cancer cell lines than in 83 cell lines that were RET wild type and markedly decreased potency on engineered cell lines expressing VEGFR2, FGFR1, hERG or Aurora kinase compared with those expressing RET. One patient with MTC that had a RETM918T mutation was treated consecutively with six MKIs over a 6-year period, ultimately showing disease progression, at which point cell-free DNA revealed a RETV804M mutation not detected in the primary tumour. LOXO-292 treatment resulted in a 54% tumour mass reduction after 7 months6. Ongoing results of the LIBRETTO-001 trial of LOXO-292 for RET-mutant MTC and RET-fusion thyroid cancer were reported at ESMO in September 2019 (REF8). A primary analysis set of 55 patients with RET-mutant MTC previously treated with vandetanib or cabozantinib had an ORR of 56%. Among these patients were three who had RETV804M/L gatekeeper mutations. Within the primary analysis set, a cohort of 26 patients with RET-fusion thyroid cancers had an ORR of 62%. Adverse events in ≥15% of patients included xerostomia, elevated levels of transaminases, diarrhoea and hypertension, which were mostly grade 1 and 2.
Despite their efficacy, current MKI treatments of RET-mutant MTC probably do not adequately inhibit RET kinase activity at doses that are safe and tolerable, as selective RET inhibitors show responses in patients who progressed on these treatments5,6. This finding strengthens the premise that selective targeting of truncal oncogenic drivers remains a rational approach for the treatment of many cancers.
Not all types of thyroid cancer offer easily tractable therapeutic vulnerabilities. Hürthle cell carcinomas (HCCs) account for 5% of DTC. HCCs are characterized by an abundance of dysfunctional mitochondria. Two recent studies defined their genomic landscape, demonstrating that they represent a distinct entity9,10. Mutations in the RAS–RAF–MAPK and PI3K–AKT–mTOR pathways were present in 55% of tumours. Alterations in genes encoding proteins involved in DNA-damage and DNA-repair pathways were also present, as well as chromatin modifier gene alterations and TERT promoter mutations. There was a striking global loss of heterozygosity (LOH) across most of the genome, resulting from one copy of most chromosomes being lost. Some haploid tumours probably undergo whole chromosome duplication, resulting in uniparental disomy. The haploidization of the genome would favour the inactivation of tumour suppressor genes. Chromosome 7 was never lost and in many tumours underwent amplification, which was associated with tumour recurrence. Both studies reported frequent mutations of complex I mitochondrial DNA genes. These were commonly homoplasmic (present in most mitochondria), and when found in the primary tumour they were also present in metastases, consistent with a role in tumour evolution. In conclusion, HCC have a distinct chromosome state with widespread LOH and recurrent mitochondrial and somatic mutations. Aberrant bioenergetics probably has a central role in HCC pathogenesis. How this finding might be exploited for therapeutic advantage is currently unclear.
Although these advances in the treatment of BRAF-driven and RET-driven thyroid cancers with selective kinase inhibitors are highly encouraging, they should be followed in due course by studies to determine effects on overall survival compared with the current standards of care, as well as by detailed investigation of mechanisms of acquired resistance to these drugs that will almost certainly occur.
Key advances.
The combination of BRAF and MEK inhibition with dabrafenib and trametinib induces durable responses in patients with BRAFV600E-mutant anaplastic thyroid cancer, providing a road map for advancing the treatment of the most aggressive form of thyroid cancer2,4.
Two new selective RET inhibitors — BLU-667 and LOXO-292 — have potent antitumour effects in RET-mutant medullary thyroid carcinoma and RET-fusion papillary thyroid carcinomas, with a favourable safety profile5,6.
Hürthle cell carcinomas are a distinct entity characterized by mitochondrial dysfunction and widespread loss of heterozygosity9,10.
Acknowledgements
The authors are supported in part by NIH RO1-CA50706, NIH RO1-CA72597, NIH P50-CA72012 and P30-CA008748.
Competing interests
J.A.F. has received consultancy fees from Loxo Oncology and a grant from Eisai. V.T. declares no competing interests.
References
- 1.Fagin JA & Wells SA Jr. Biologic and clinical perspectives on thyroid cancer. N. Engl. J. Med 375, 2307 (2016). [DOI] [PubMed] [Google Scholar]
- 2.Subbiah V et al. Dabrafenib and trametinib treatment in patients with locally advanced or metastatic BRAF V600-mutant anaplastic thyroid cancer. J. Clin. Oncol 36, 7–13 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shah MH et al. Results of randomized phase II trial of dabrafenib versus dabrafenib plus trametinib in BRAF-mutated papillary thyroid carcinoma. J. Clin. Oncol 35 (Suppl. 15), 6022 (2017). [Google Scholar]
- 4.Wang JR et al. Complete surgical resection following neoadjuvant dabrafenib plus trametinib in BRAF(V600E)-mutated anaplastic thyroid carcinoma. Thyroid 29, 1036–1043 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Subbiah V et al. Precision targeted therapy with BLU-667 for RET-driven cancers. Cancer Discov 8, 836–849 (2018). [DOI] [PubMed] [Google Scholar]
- 6.Subbiah V et al. Selective RET kinase inhibition for patients with RET-altered cancers. Ann. Oncol 29, 1869–1876 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taylor MH et al. Activity and tolerability of BLU-667, a highly potent and selective RET inhibitor, in patients with advanced RET-altered thyroid cancers [abstract]. J. Clin. Oncol 37 (Suppl. 15), 6018 (2019). [Google Scholar]
- 8.Wirth LJ et al. Registrational results of LOXO-292 in patients with RET-altered thyroid cancers [abstract]. Ann. Oncol 30 (Suppl. 5), v851–v934 (2019). [Google Scholar]
- 9.Ganly I et al. Integrated genomic analysis of Hürthle cell cancer reveals oncogenic drivers, recurrent mitochondrial mutations, and unique chromosomal landscapes. Cancer Cell 34, 256–270 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gopal RK et al. Widespread chromosomal losses and mitochondrial DNA alterations as genetic drivers in Hurthle cell carcinoma. Cancer Cell 34, 242–255 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]