To the Editor: Non-alcoholic fatty liver disease (NAFLD) is the most common chronic liver disease worldwide. It is associated with visceral obesity and is considered to be the hepatic component of metabolic syndrome, as insulin resistance plays a major role in this process. Despite its known association, the pathogenesis remains uncertain. Recent works from both our groups and other groups have demonstrated that zinc-α2-glycoprotein (ZAG) or alpha-2-glycoprotein 1, zinc-binding, a novel adipokine, is closely linked to obesity and obesity-related metabolic disease. Because of this, it has been proposed as a candidate factor in the regulation of lipid metabolism and insulin sensitivity.[1] Despite its link, the clinical relevance of ZAG in NAFLD remains largely unknown. We conducted a case-control study to measured serum ZAG levels as well as its association with various metabolic parameters in NAFLD patients.
Three hundred and eight Chinese individuals, including 168 patients with NAFLD (aged 25–64 years; male/female, 89/79) and 140 healthy controls (aged 22–66 years, male/female 79/61), were recruited after their routine physical examination. All participants completed a uniform questionnaire that included demographics, medical history, recent medication history, and lifestyle factors (smoking and alcohol). NAFLD was diagnosed based on ultrasonic imaging. The severity (three grades) of NAFLD was defined according to the degree of steatosis.[2] The controls, on the other hand, were defined as those who did not have metabolic syndrome and hepatic steatosis.
Measurement parameters included height, weight, waist circumference, and blood pressure. These were measured using a standardized protocol. An analyzer of bioelectrical impedance was used to measure the percentage of body fat (Fat %). Lipid profile, glucose, and insulin were also measured as previously described.[3] Serum ZAG concentrations were determined by commercially available human ZAG enzyme-linked immunosorbent assay kits (Biovendor, Modrice, Czech Republic) according to the manufacturer’ instructions. The study was approved by the Human Research Ethics Committee of the hospital, following the principles of the Declaration of Helsinki. Written informed consent was obtained from all subjects. SPSS version 17.0 (Chicago, IL, USA) statistical software was applied to analyze the data.
Here, we showed that serum levels of ZAG in patients with NAFLD were significantly decreased compared to those in the controls (41.21 ± 8.21 vs. 51.32 ± 9.87 mg/L, P < 0.001; Figure 1A). More interestingly, we found that the decreasing trend of serum ZAG level was associated with the increasing ultrasonographic severity of hepatic steatosis (P for linear trend < 0.001; Figure 1B). Nevertheless, the reason for the decrease of serum ZAG level is unknown but it is consistent with previous studies. Evidence has demonstrated that ZAG expression was decreased in palmitic acid-treated hepatocytes and liver tissue of NAFLD patients and obese mice. This demonstrates that ZAG over-expression could protect against NAFLD by ameliorating hepatic steatosis, insulin resistance, and inflammation.[1] Hence, we speculate that ZAG may serve as a protective factor associated with the pathogenesis of NAFLD. However, the current cross-sectional study still has difficulty deducing the causal relationship between serum ZAG levels and NAFLD. A follow-up study will be necessary. It is worth mentioning that our result is inconsistent with observations reported by Yilmaz et al.[4] They found that serum ZAG concentrations did not differ in patients with NAFLD compared to healthy controls. This discrepancy may be due to the differences in ethnicities, adiposity, or the severity of NAFLD. Furthermore, it is possible that adiposity or metabolic disorders cause the resistance of ZAG actions such as insulin or leptin resistance. This in part leads to the increased secretion and release of ZAG.
Figure 1.
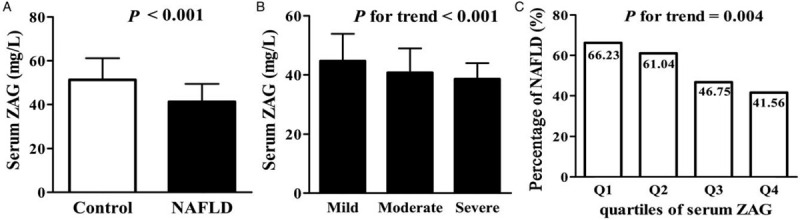
Serum zinc-α2-glycoprotein levels in the study population. (A) Serum zinc-α2-glycoprotein concentrations in control subjects (n = 140) and NAFLD patients (n = 168). (B) Serum zinc-α2-glycoprotein levels in different NAFLD severity. (C) Percentage of NAFLD according to quartiles of serum zinc-α2-glycoprotein. Values are given as mean ± standard deviation. NAFLD: Non-alcoholic fatty liver disease; ZAG: Zinc-α2-glycoprotein.
Next, we investigated the relationship between serum ZAG levels, anthropometric parameters, and biochemical indexes in all subjects. The analysis demonstrated a significant negative association of serum ZAG with blood pressure, lipid profile (increased total cholesterol, triglyceride, and decreased high-density lipoprotein cholesterol), glucose metabolic parameters (fasting blood glucose and fasting insulin), insulin resistance indices (homeostasis model assessment of insulin resistance, HOMA-IR), and parameters of obesity including body mass index, waist circumference, and Fat % (P < 0.05). To determine which parameters were independently associated with serum ZAG, multiple linear regression analysis was performed. The analysis involved the parameters mentioned above including triglyceride, waist circumference, and HOMA-IR. These parameters were found to be independently associated with serum ZAG levels (P < 0.01). Based on the observations mentioned above, it could be speculated that decreasing ZAG levels might contribute to NAFLD through obesity and insulin resistance-dependent pathways. However, we do not rule out the involvement of other unknown factors.
Furthermore, serum ZAG concentrations were strongly related to NAFLD even after adjustment for age, gender, body mass index, lipid profile, and glucose metabolic parameters in an additive multivariate logistic regression model (OR = 0.824, 95% confidence interval = 0.722–0.941, P = 0.009). Therefore, we analyzed the relationship between serum ZAG levels and the prevalence of NAFLD. We found that decreasing levels of ZAG showed a significant linear trend and were independently associated with NAFLD, especially when concentrations were analyzed by the row mean scores differ and the Cochran-Armitage trend test. All subjects were further categorized into four groups based on serum ZAG levels. There was a borderline difference of NAFLD percentage among the four groups (P = 0.004; Figure 1C). Compared to the higher serum ZAG level group (quartile 4), the risk of NAFLD was increased by 24.67% in lower serum ZAG level group (quartile 1).
Overall, our study demonstrated that serum ZAG levels have negative association with the prevalence of NAFLD and obesity-related metabolic parameters. ZAG might be a novel protective factor in the development of NAFLD. The underlying mechanisms may partly attribute to the interactions of both obesity and insulin resistance.
Funding
This work was supported by grants from the National Natural Science Foundation of China (No. 81870595) and Major Special Projects of Hunan Provincial Health and Family Planning Commission (No. A2017011).
Conflicts of interest
None.
Footnotes
How to cite this article: Qi XY, Li JY, Wang YD, Zeng YW, Liao ZZ, Ran L, Yang J, Wen GB, Liu JH, Xiao XH, Xiao XH. Association of serum zinc-α2-glycoprotein with non-alcoholic fatty liver disease. Chin Med J 2020;133:1882–1883. doi: 10.1097/CM9.0000000000000873
References
- 1.Xiao XH, Wang YD, Qi XY, Wang YY, Li JY, Li JY, et al. Zinc alpha2 glycoprotein protects against obesity-induced hepatic steatosis. Int J Obes (Lond) 2018; 42:1418–1430. doi: 10.1038/s41366-018-0151-9. [DOI] [PubMed] [Google Scholar]
- 2.Sheng X, Che H, Ji Q, Yang F, Lv J, Wang Y, et al. The Relationship Between Liver Enzymes and Insulin Resistance in Type 2 Diabetes Patients with Nonalcoholic Fatty Liver Disease. Horm Metab Res 2018; 50:397–402. doi: 10.1055/a-0603-7899. [DOI] [PubMed] [Google Scholar]
- 3.Li JY, Wang YD, Qi XY, Ran L, Hong T, Yang J, et al. Serum CCN3 levels are increased in type 2 diabetes mellitus and associated with obesity, insulin resistance and inflammation. Clin Chim Acta 2019; 494:52–57. doi: 10.1016/j.cca.2019.03.006. [DOI] [PubMed] [Google Scholar]
- 4.Yilmaz Y, Yonal O, Eren F, Kurt R, Celikel CA, Ozdogan O, et al. Serum zinc-(2-glycoprotein concentrations in patients with non-alcoholic fatty liver disease. Clin Chem Lab Med 2011; 49:93–97. doi: 10.1515/CCLM.2011.022. [DOI] [PubMed] [Google Scholar]


