Abstract
The sodium iodide symporter (NIS) is required for iodide uptake, which facilitates thyroid hormone biosynthesis. NIS has been exploited for over 75 years in ablative radioiodine (RAI) treatment of thyroid cancer, where its ability to transport radioisotopes depends on its localization to the plasma membrane. The advent of NIS-based in vivo imaging and theranostic strategies in other malignancies and disease modalities has recently increased the clinical importance of NIS. However, NIS trafficking remains ill-defined. Here, we used tandem mass spectrometry followed by coimmunoprecipitation and proximity ligation assays to identify and validate two key nodes—ADP-ribosylation factor 4 (ARF4) and valosin-containing protein (VCP)—controlling NIS trafficking. Using cell-surface biotinylation assays and highly inclined and laminated optical sheet microscopy, we demonstrated that ARF4 enhanced NIS vesicular trafficking from the Golgi to the plasma membrane, whereas VCP—a principal component of endoplasmic reticulum (ER)–associated degradation—governed NIS proteolysis. Gene expression analysis indicated VCP expression was particularly induced in aggressive thyroid cancers and in patients who had poorer outcomes following RAI treatment. Two repurposed FDA-approved VCP inhibitors abrogated VCP-mediated repression of NIS function, resulting in significantly increased NIS at the cell-surface and markedly increased RAI uptake in mouse and human thyroid models. Collectively, these discoveries delineate NIS trafficking and highlight the new possibility of systemically enhancing RAI therapy in patients using FDA- approved drugs.
Significance:
These findings show that ARF4 and VCP are involved in NIS trafficking to the plasma membrane and highlight the possible therapeutic role of VCP inhibitors in enhancing radioiodine effectiveness in radioiodine-refractory thyroid cancer.
Introduction
Since the 1940s, radioiodine (RAI) treatment has been the central post-surgical therapy for patients with differentiated thyroid cancer (DTC) and ablative treatment with RAI is recommended in moderate and high-risk tumors (1). However, at least a quarter of patients with DTC do not uptake sufficient RAI for effective ablation (2, 3), which remains an urgent problem in metastatic disease. There are two cohorts of thyroid cancer patients: those who respond to RAI and have an excellent prognosis (radiosensitive tumors), and those who do not respond (radioresistant tumors) and whose outcome is dire (1). Despite efforts to improve outcomes, no substantial changes have been made to the way RAI is administered therapeutically. Troublingly, DTC is now the most rapidly increasing cancer in the UK and the USA, with 300,000 new cases reported worldwide per annum, and more than 40,000 deaths annually (4).
The sodium iodide symporter (NIS) is the sole human transporter responsible for iodide uptake (5), exploitation of which represents the first—and most specifically targeted—internal radiotherapy in existence. High-energy β-emitting 131I is utilized to destroy remaining thyroid cells post-surgery, and target metastases. More recently, the interest in NIS has been enhanced due to its use as a novel reporter gene in preclinical and translational imaging systems and in theranostic strategies in nonthyroidal tumors (6, 7). Breast tumors, for instance, can uptake RAI (8), with functional NIS expression confirmed in up to ~80% of breast cancers (9). However, NIS is rarely localized to the plasma membrane (PM) in breast cancers, limiting its clinical utility (9, 10).
Decreased levels of NIS expression and/or diminished targeting of NIS to the PM represent the principal mechanisms behind radioiodine-refractory thyroid cancer (RAIR-TC; ref. 11). Numerous studies have addressed the common pathways of NIS regulation in vitro and in vivo (12–14), such as key transcriptional and epigenetic alterations, which silence thyroid-specific genes including NIS (or SLC5A5; refs. 15, 16). Clinical approaches to improve treatment of thyroid cancer have involved the use of retinoids (17), PPARγ agonists (18), MAPK pathway/BRAF inhibitors (19, 20), multitargeted kinase inhibitors (21), and histone deacetylase (HDAC) inhibitors (22). Multiple biologically targeted drugs have been evaluated in phase I, II, and III trials, with several agents including sorafenib (21), lenvatinib (23), and dabrafenib (24) showing promising responses and/or disease stabilization. However, issues of toxicity and drug resistance remain.
To actively transport iodide for thyroid hormone biosynthesis and radioisotopes, NIS must be present at the basolateral PM. However, little is known about the mechanisms that govern NIS trafficking. Thyroid-stimulating hormone (TSH) induces iodide uptake through upregulation of NIS expression and modulation of its subcellular localization (25). Yet, many thyroid cancers demonstrate reduced NIS activity through diminished PM retention (26, 27). BRAF-mutant tumors (60%–70% of thyroid cancers) are more likely to be resistant to RAI, partly due to decreased NIS expression (12), but also impaired PM targeting (13, 28), through mechanisms that remain ill-defined. Currently, PTTG1-binding factor (PBF) is the only protein shown to bind NIS and modulate its subcellular localization (29).
Here, we performed mass spectrometry (MS/MS) to identify previously undefined proteins that interact with NIS and regulate its trafficking or retention at the PM. We now report two proteins—ADP-ribosylation factor 4 (ARF4) and valosin-containing protein (VCP)—which specifically bind NIS and directly regulate its function in both breast and thyroid cancer models. Critically, the ability to inhibit VCP activity via FDA-approved drugs indicates a therapeutic possibility of systemically enhancing RAI uptake in patients. This provides new hope that NIS activity may be stimulated in RAIR-TC patients, but also supports the prospect of exploiting NIS trafficking and function to facilitate radioisotope uptake for in vivo imaging and therapy in nonthyroidal tumors.
Materials and Methods
Cell culture and lentiviral cell line generation
Breast (MDA-MB-231) and thyroid (8505C, BCPAP, SW1736, TPC-1, and CAL62) cancer cell lines were maintained in RPMI-1640 (Life Technologies), whereas HeLa cervical cancer cells were maintained in DMEM (Sigma-Aldrich). Media were supplemented with 10% fetal bovine serum (FBS), penicillin (105 U/L), and streptomycin (100 mg/L), and cell lines were maintained at 37°C and 5% CO2 in a humidified environment. Cell lines were obtained from ECACC (HeLa and MDA-MB-231) and DSMZ (8505C and BCPAP), whereas SW1736 and TPC-1 cell lines were kindly provided by Dr. Rebecca Schweppe (University of Colorado, Denver, CO). Cells were cultured at low passage, authenticated by short tandem repeat analysis (NorthGene), and tested for Mycoplasma contamination (EZ-PCR kit; Geneflow).
Stable NIS-expressing MDA-MB-231 and TPC-1 cell lines were generated by lentiviral transduction, as per the manufacturer’s instructions. In brief, ready-to-transduce lentiviral particles containing a precision lentiORF construct (pLOC) housing cDNA coding for red fluorescent protein (RFP; OHS5833) or the full-length human NIS cDNA without a stop codon within the open reading frame (ORF; OHS5900–224632369) were purchased from Dharmacon. For lentiviral transduction, the manufacturer’s protocol was followed. In brief, 1 day after plating, MDA-MB-231 and TPC-1 cells were infected with the lentiviral vector containing NIS diluted in antibiotic-free and serum-free RPMI containing 8 μg/mL or 14 μg/mL polybrene, respectively. Cells transduced with the RFP-containing lentiviral vector served as the control. After 24 hours, medium was replaced with RPMI containing 10% FBS. After a further 48 hours, cells were maintained in RPMI containing 10% FBS, penicillin (105 U/L), and streptomycin (100 mg/L) as well as 15 or 5 μg/mL blasticidin S for the MDA-MB-231 and TPC-1 cell lines, respectively. Twenty-four hours later, infection efficiency was assessed by fluorescence microscopy. Upon cell expansion and selection of single-cell colonies, NIS expression was assessed by quantitative PCR (qPCR) and Western blotting, with NIS function confirmed via the radioiodine uptake assay.
Mass spectrometry
NIS interactors were isolated by coimmunoprecipitation (co-IP; anti-NIS antibody) and separated by SDS-PAGE. Proteins were reduced, alkylated, and trypsinized. Resulting peptides were separated on an acetonitrile gradient on the UltiMate 3000 HPLC (Thermo Fisher Scientific). Eluted peptides passed through the AmaZon ETD ion trap and tandem mass spectrometer (Bruker). Mass spectra were processed using the Bruker DataAnalysis software and analyzed using the Mascot search engine (Matrix Science). Data sets were filtered based on peptide number, DAVID functional classification (30, 31), and literature review (Supplementary Table S1; refs. 32–36).
Nucleic acids and transfection
Plasmids containing human NIS cDNA with an HA- or MYC-tag have been described (37) and GFP-tagged NIS was kindly provided by Dr. Takahiko Kogai (Dokkyo Medical University, Mibu, Japan). NIS mutants 475ALAS478 and 574AAAK577 were generated using the Quik-Change Site-directed Mutagenesis Kit (Agilent Technologies). Untagged-ARF4 plasmid was purchased from Origene (#SC119092), whereas wild-type (WT) and mutant (QQ) VCP (rat) plasmid were kindly provided by Dr. Yihong Ye (National Institutes of Health, Bethesda, MD; refs. 11, 12). Human VCP cDNA was generated by site-directed mutagenesis. Further details on nucleic acids and siRNA are provided (Supplementary Tables S2 and S3). Plasmid DNA and siRNA transfections were performed with TransIT-LT1 (Mirus Bio) and Lipofectamine RNAiMAX (Thermo Fisher Scientific) following standard protocols in accordance with the manufacturer’s guidelines.
VCP inhibitors and dynasore
All drugs were resuspended in dimethyl sulfoxide (DMSO), diluted in RPMI, and then added directly to cells at the appropriate final concentration. Cells were treated with VCP inhibitors: Eeyarestatin-1 (ES-1; Cayman Chemicals), NMS-873 (SelleckChem), astemizole (Sigma-Aldrich), clotrimazole (Sigma-Aldrich), and ebastine (Sigma-Aldrich) or the dynamin inhibitor dynasore (Sigma-Aldrich).
Western blotting, cell-surface biotinylation, co-IP assays, and RAI uptake
Western blotting, coimmunoprecipitation, cell-surface biotinylation assays and RAI (125I) uptake assays were performed as described (29, 38) following dynasore treatment (2 hours), VCP inhibitor treatment (24 hours), DNA plasmid transfection (48 hours), or siRNA transfection (72 hours) as indicated.
Blots were probed with specific antibodies (Supplementary Table S4) and NIS expression quantified by densitometry in ImageJ relative to β-actin or Na+/K+ ATPase, as indicated.
Highly inclined and laminated optical sheet microscopy and proximity ligation assay
Live-cell highly inclined and laminated optical sheet (HILO) microscopy was performed on an Olympus IX81 inverted fluorescence microscope. Images were acquired every 2 seconds for up to 5 minutes and then integrated at 5 frames/second using the Olympus xCellence build 3554 software (Supplementary Movies S1 to S3). Mean velocity and distance traveled of NIS-GFP was quantified using the TrackMate plugin (ImageJ). The Duolink in situ proximity ligation assay (PLA) was performed according to the manufacturer’s instructions (Sigma-Aldrich).
Gene expression data analyses
Normalized gene expression data and clinical information (Supplementary Tables S5–S7) for papillary thyroid cancer (PTC) and breast cancer were downloaded from The Cancer Genome Atlas (TCGA) via cBioPortal (39, 40) and FireBrowse (41). In total, RNA-seq data for 501 papillary thyroid cancer (THCA) and 1,093 breast cancer (BRCA) TCGA samples were analyzed. Patient-derived poorly differentiated thyroid cancer (PDTC) and anaplastic thyroid cancer (ATC) samples were selected from the Memorial Sloan Kettering Cancer Center pathology department files from 1986 to 2015 according to the classification outlined in (42). In total, mRNA expression data from fresh-frozen tissue were obtained from 17 PDTC and 20 ATC samples via MSK-IMPACT targeted sequencing. All 37 tumor samples were also expression profiled via the Affymetrix U133 plus 2.0 array and Agilent SurePrint G3 CGH 1 × 1M arrayCGH platform to validate copy-number calls.
Statistical analyses
Data were analyzed using GraphPad Prism and Microsoft Excel (Supplementary Materials and Methods).
Results
Identification and manipulation of NIS interactors
We performed MS/MS in MDA-MB-231 cells with lentivirally expressed NIS (NIS+) to identify interactors in whole-cell and PM extracts. NIS and its protein interactors were subjected to in-gel tryptic digest and resulting peptides were fingerprinted using the AmaZon ETD ion trap and tandem mass spectrometer prior to shortlisting (Fig. 1A and B; Supplementary Fig. S1A and S1B). Five proteins with established roles related to subcellular trafficking, endocytosis, PM targeting, and/or endosomal transport were selected (Supplementary Fig. S1A and S1B; Supplementary Table S1) and underwent an initial endoribonuclease-prepared siRNA (esiRNA) screen to investigate their impact upon NIS function (Fig. 1C and D). An important finding was that ARF4 depletion repressed iodine-125 (RAI; 125I) uptake in MDA-MB-231 NIS+ cells, whereas VCP ablation resulted in a significant induction of RAI uptake (Fig. 1D). As a positive control, esiRNA knockdown of PBF, the only protein known to specifically modulate NIS subcellular localization and function (29), significantly increased 125I uptake (Fig. 1C and D). In thyroidal TPC-1 NIS+ cells, treatment with ARF4, VCP, or PBF esiRNA also significantly altered RAI uptake (Supplementary Fig. S2A).
Figure 1.
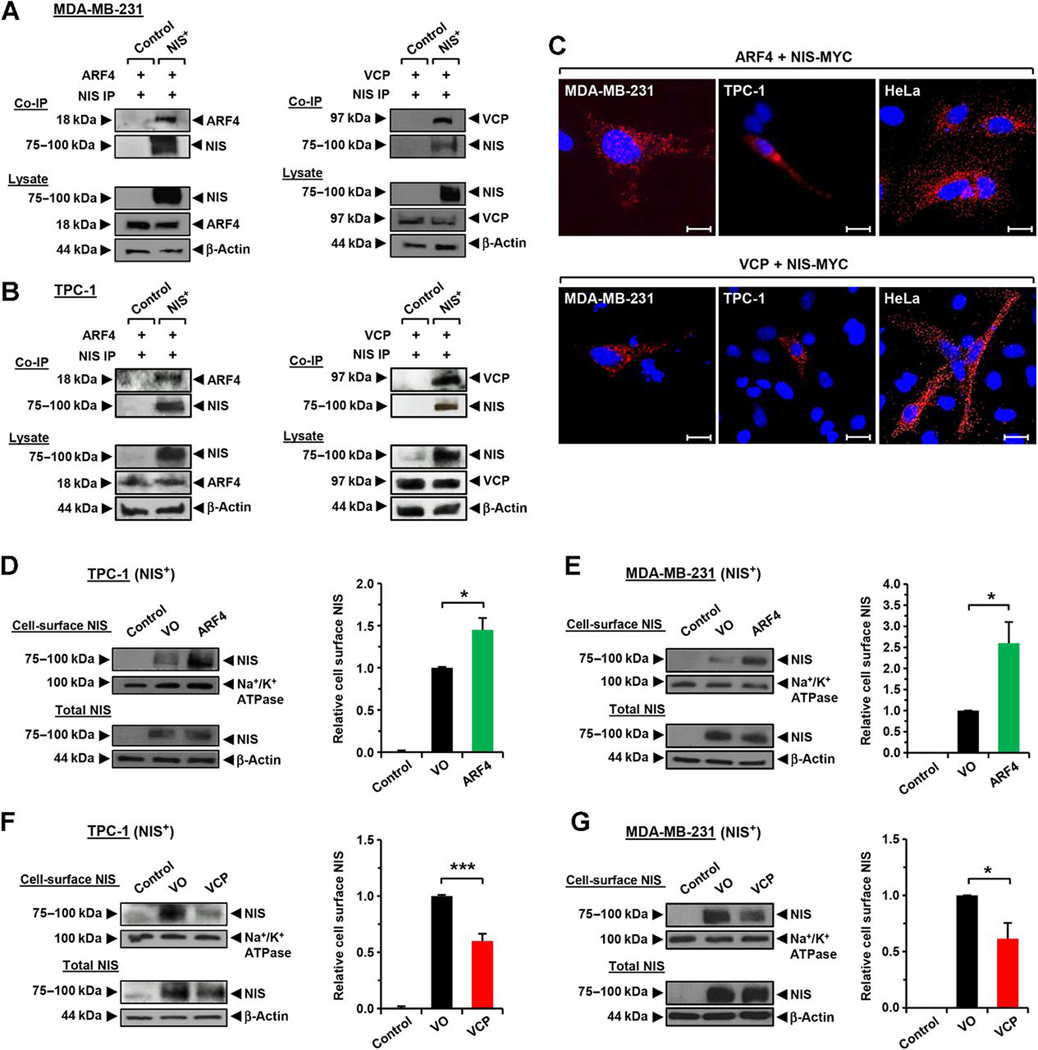
Identification of ARF4 and VCP as regulators of NIS activity. A, Western blot analysis of whole-cell lysate and PM fraction in MDA-MB-231 (NIS+) cells used in MS/MS. B, Top hits for putative NIS interactors identified by MS/MS (peptides ≥ 6). C and D, Western blot analysis and RAI uptake of MDA-MB-231 (NIS+) cells transfected with esiRNA specific for indicated NIS interactors. NT, nontransfected cells. E and F, Western blot analysis and RAI uptake in MDA-MB-231 (NIS+) cells, TPC-1 (NIS+) cells, and human primary thyrocytes transfected with ARF4 siRNA (E) or ARF4 (F). G and H, Same as E and F, but cells transfected with VCP siRNA (G) or VCP (H). NS, not significant; *, P < 0.05; **, P < 0.01; ***, P < 0.001.
ARF4 and VCP were thus considered novel putative functional partners of NIS and further investigated. Subsequent ARF4 ablation in TPC-1 and MDA-MB-231 NIS+ cells, as well as in human primary thyroid cells, confirmed a ~60% to 80% decrease in 125I uptake (Fig. 1E) with different siRNA sequences (Supplementary Table S2). In contrast, transient ARF4 overexpression resulted in significantly increased 125I uptake in all three cellular settings (Fig. 1F; Supplementary Fig. S2B), suggesting its impact on NIS function is bidirectional. A ~50% to 80% increase in 125I uptake was further validated in VCP-siRNA–depleted TPC-1 and MDA-MB-231 NIS+ cells, with human primary thyrocytes demonstrating a ~35% increase (Fig. 1G; Supplementary Table S2). VCP induction by transient transfection reversed these effects, resulting in markedly repressed 125I uptake in all three cell models (Fig. 1H; Supplementary Fig. S2C).
Thus, we identify two novel modulators of NIS function that alter RAI uptake both in thyroid and breast cells lentivirally transduced with NIS, and in human primary thyrocytes with endogenous NIS expression.
ARF4 and VCP bind NIS in vitro and modulate its expression
Having identified that manipulation of ARF4 and VCP expression altered RAI uptake, we sought to challenge our MS/MS data that indicated specific binding between each protein and NIS. Co-IP confirmed that NIS specifically interacts with ARF4 and VCP in both MDA-MB-231 and TPC-1 NIS+ cells (Fig. 2A and B). Control co-IPs further demonstrated specific interaction between NIS with ARF4 and VCP (Supplementary Fig. S3A–S3D). Additionally, PLA demonstrated specific binding between ARF4 and NIS in three different cell types, which appeared to occur generally throughout the cytoplasm with some binding within the endoplasmic reticulum (ER)/Golgi also suggested (Fig. 2C; Supplementary Fig. S3E and S3F). Similarly, PLA confirmed the VCP–NIS interaction, which occurred more generally within the cytoplasm of MDA-MB-231, TPC-1, and HeLa cells (Fig. 2C; Supplementary Fig. S3E and S3F).
Figure 2.
ARF4 and VCP bind NIS in vitro and modulate PM NIS. A, Co-IP assays in MDA-MB-231 (NIS+) cells showing specific interaction between NIS and ARF4 (left) or VCP (right). B, Same as A, but in TPC-1 (NIS+) cells. C, PLA demonstrating specific interaction between NIS-MYC and ARF4 (top) or VCP (bottom) in MDA-MB-231, TPC-1, and HeLa cells. Red fluorescent spots, specific interactions. Blue, DAPI nuclear staining. Magnification, ×100. Scale bars, 10 μm. D and E, Western blot analysis of NIS protein levels at the PM relative to Na+/K+ ATPase following the CSBA in TPC-1 (NIS+) cells (D) and MDA-MB-231 (NIS+) cells (E) after ARF4 transfection. F and G, Same as D and E, but after VCP transfection. *, P < 0.05; ***, P < 0.001.
We next performed cell-surface biotinylation assays to quantify whether ARF4 and VCP modulate the amount of NIS present at the PM. Exogenous expression of ARF4 in NIS+ cells demonstrated an approximate doubling of NIS protein in PM preparations compared with vector only (VO) controls (Fig. 2D and E). In contrast, VCP overexpression resulted in significantly diminished NIS protein PM abundance (Fig. 2F and G). Together, these data suggest that while ARF4 potentiates NIS presence at the PM, VCP inhibits its expression at its key site of symporter activity.
ARF4 modulates NIS trafficking at the PM
Given that ARF4 and VCP overexpression was associated with altered NIS PM localization, we next investigated their trafficking using HILO microscopy—a technique used to visualize and quantify molecular dynamics in cells. We identified ARF4-dsRED and NIS-GFP trafficking in coincident vesicles at the PM in HeLa cells (Fig. 3A and B; Supplementary Fig. S4A and S4B; Supplementary Movie S1). By contrast, no such cotrafficking was apparent for VCP-dsRED and NIS-GFP, suggesting the site of functional interaction between VCP and NIS was distant to the PM (Supplementary Fig. S4C; Supplementary Movie S2). Of particular significance, and in contrast to VCP, the presence of ARF4 led to an overall induction in both the mean velocity and distance traveled by NIS-GFP-positive vesicles (P < 0.001; Fig. 3C and D; Supplementary Fig. S5A–S5C).
Figure 3.
Involvement of ARF4 in trafficking NIS at the PM. A and B, HILO microscopy images demonstrating trafficking of ARF4-dsRED (red), NIS-GFP (green), and colocalization (yellow) to the PM in HeLa cells. Video capture times (hr:min:sec) are indicated. PM regions in framed areas are magnified in bottom right, which highlight the movement of ARF4 and NIS (white and orange arrowheads; see bottom). Scale bars, 10 μm. C, Representative images of NIS-GFP movement patterns tracked using ImageJ software. Scale bars, 10 μm. D, Box-whisker plot of velocity (μm/sec) and distance traveled (μm) of NIS-GFP in HeLa cells transfected with ARF4 (n = 475) or VO (n = 339). E, RAI uptake in TPC-1 (NIS+) and MDA-MB-231 (NIS+) cells transfected with ARF4 and treated with dynasore for 1 hour prior to addition of 125I. F, Western blot analysis of NIS expression levels in TPC-1 (NIS+) cells as described in E. G, RAI uptake in TPC-1 cells transfected with ARF4, as well as WT NIS, 574AAAK577-mutant NIS, or 475ALAS478-mutant NIS. H, Representative co-IP assay for ARF4 with WT or mutant NIS. NS, not significant; *, P < 0.05; ***, P < 0.001.
In control experiments, we confirmed that NIS is endosomally trafficked in association with clathrin (Supplementary Fig. S5D; Supplementary Movie S3) and found a significant increase in the overall distance traveled by NIS-GFP in cells overexpressing clathrin (Supplementary Fig. S5A). We then appraised the impact of inhibiting clathrin-mediated endocytosis on NIS function using 100 μmol/L dynasore, a cell-permeable inhibitor of dynamin, which is required for the budding and formation of clathrin-coated vesicles. Treatment of NIS+ cells overexpressing ARF4 with dynasore resulted in significantly greater RAI uptake (Fig. 3E) without any effect on total NIS protein levels (Fig. 3F; Supplementary Fig. S5E), suggesting ARF4 is not primarily enhancing NIS recycling to the PM.
ARF4 binds NIS via a C-terminal VXPX motif
ARF4 binds to the terminal VXPX motif of Rhodopsin (43). We identified two putative VXPX motifs within the extracellular loop of NIS at positions 475–478 (sequence VLPS) and the C-terminus at positions 574–577 (sequence VAPK). Abrogation of both motifs (mutations 475ALAS478 and 574AAAK577) resulted in NIS proteins that retained endogenous functionality, but the 574AAAK577 NIS mutant could no longer be augmented in terms of RAI uptake by ARF4 overexpression in TPC-1 thyroid cells (Fig. 3G). Co-IP assays demonstrated that 475ALAS478-mutant NIS still avidly bound ARF4, whereas 574AAAK577-mutant NIS lost interaction (Fig. 3H). Hence, NIS has a C-terminal VXPX ARF4 recognition sequence, which is required for ARF4 potentiation of function.
Overall, our results indicate that ARF4 and VCP appear to have different modes of action; ARF4 is implicated in the trafficking of NIS to the PM, whereas VCP binds NIS and modulates NIS function elsewhere in the cell. In support of this, significantly altered total NIS protein levels were apparent in NIS+ cells after siRNA ablation or exogenous expression of VCP but not following modulation of ARF4 expression (Supplementary Fig. S6A and S6B).
Selective VCP inhibitors promote RAI uptake
VCP functions chiefly as a chaperone in disassembling protein complexes and facilitating the extraction and/or proteasomal degradation of proteins from the ER, but is also implicated in a wider range of other cellular actions (44). Several specific VCP inhibitors already exist that target different facets of VCP structure/activity (45). Critically, a significant induction of RAI uptake (Fig. 4A) was evident in NIS+ cells treated with the allosteric VCP inhibitor ES-1, which inhibits ER-cytosol dislocation and subsequent degradation of substrates (46). Similarly, the VCP inhibitor NMS-873, which is a potent and specific allosteric inhibitor of VCP (47), yielded a significant 3- to 4-fold increase in RAI uptake in NIS+ cells (Fig. 4B).
Figure 4.
Inhibition of VCP enhances NIS function. A, RAI uptake of MDA-MB-231 (NIS+) and TPC-1 (NIS+) cells treated with ES-1 for 24 hours. B, Same as A, but cells treated with NMS-873. C, Western blot analysis following ES-1 or NMS-873 treatment in TPC-1 (NIS+) cells. D, PLA showing specific interaction (red fluorescent spots) between NIS-MYC and VCP in TPC-1 cells treated with ES-1. Blue, DAPI nuclear stain. Magnification, ×100. Scale bars, 10 μm. E, Co-IP assays demonstrating interaction of VCP and NIS in TPC-1 (NIS+) cells treated with ES-1 or NMS-873. F and G, RAI uptake and relative NIS protein levels in parental TPC-1 cells treated with ES-1, NMS-873, or DMSO. H, Time course of RAI uptake (top) and relative NIS protein levels (bottom) in TPC-1 (NIS+) cells treated with 2.5 μmol/L ES-1. I, Same as H, but cells treated with 5 μmol/L NMS-873. J, RAI uptake and Western blot analysis in TPC-1 (NIS+) cells transfected with VCP or Scr siRNA, then treated with ES-1. K, RAI uptake and Western blot analysis in MDA-MB-231 (NIS+) cells, TPC-1 (NIS+) cells, and human primary thyrocytes transfected with WT VCP or QQ VCP mutant. NS, not significant; *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Importantly, there were no significant reductions in cell viability at the optimal doses of VCP inhibitors used (Supplementary Fig. S6C), nor any changes in VCP protein expression (Fig. 4C). PLA and co-IP assays also revealed that NIS and VCP retained the ability to bind in the presence of VCP inhibitors (Fig. 4D and E). Notably, we also observed a significant induction in RAI uptake after 24 hours of ES-1 and NMS-873 inhibitor treatment on native thyroidal cells (i.e., without lentiviral NIS) with different mutation profiles, including TPC-1 (Fig. 4F and G), CAL-62, 8505C, BCPAP, and SW1736 cells (Supplementary Fig. S6D–S6G). This important finding raises the possibility that VCP inhibitors can be used to induce RAI uptake in thyroid cells that have inherently repressed NIS function.
Dissecting the VCP mechanism of action
We next examined the dynamics of therapeutically targeting VCP and found that ES-1 was associated with increased detectable NIS protein after 12 hours, accompanied by a concomitant increase in RAI uptake by 24 hours (Fig. 4H). Similar data were apparent for NMS-873, although RAI uptake and NIS expression were detected at 6 hours posttreatment (Fig. 4I). To explore the dependency of ES-1 on the presence of VCP to modulate RAI uptake, we characterized VCP-ablated TPC-1 NIS+ cells, which demonstrated no induction of RAI uptake after ES-1 (Fig. 4J) or NMS-873 treatment (Supplementary Fig. S7A), thus confirming that inhibitors ES-1 and NMS-873 require VCP in order to exert their effects on NIS function.
VCP’s canonical function lies in facilitating the extraction of mis-folded proteins from the ER, a “segregase” process that generally requires ATPase activity. We next used an ATPase-deficient dominant-negative VCP mutant (QQ VCP) to investigate whether VCP required ATPase activity to modulate NIS function. The QQ VCP mutant behaved identically to WT VCP in repressing RAI uptake when overexpressed in multiple cell models (Fig. 4K) and retained the ability to decrease NIS localization at the PM in cell-surface biotinylation assays (Supplementary Fig. S7B). Additionally, ATP-competitive VCP inhibitors N2,N4-dibenzylquinazoline-2,4-diamine (DBeQ; ref. 48) and sorafenib, which inhibits VCP activity at the PM by blocking its phosphorylation, failed to increase RAI uptake in NIS+ cells (Supplementary Fig. S7C and S7D). Collectively, these results suggest that VCP functions in an ATPase-independent manner to affect NIS function, a facet that has been reported in VCP’s ability to unfold proteins for subsequent proteasomal degradation (49).
Recently, three new drugs—astemizole, clotrimazole, and ebastine—have been identified as repurposed small molecules that specifically and allosterically inhibit VCP activity (50). Clotrimazole and ebastine are well tolerated in vivo and FDA approved (50). As ES-1 and NMS-873 may have limited clinical utility (51), we investigated whether astemizole, clotrimazole, and ebastine also enhance RAI uptake. All three drugs significantly induced RAI uptake (Fig. 5A) without affecting cell viability (Supplementary Fig. S6C). There was no induction of RAI uptake after treatment of VCP-ablated TPC-1 NIS+ cells, thus confirming these VCP inhibitors require VCP in order to exert their effect on NIS function (Fig. 5B and C). Additionally, VCP inhibitors increased NIS expression, particularly at the PM (Fig. 5D and E) but did not alter VCP protein levels (Fig. 5F).
Figure 5.
VCP inhibitors increase PM NIS expression and function. A, TPC-1 (NIS+) cells treated with VCP inhibitors (ebastine, clotrimazole, and astemizole). B and C, Western blot (B) and RAI uptake (C) analyses of VCP expression following VCP-siRNA depletion and treatment with 0.5 μmol/L ebastine (top), 0.25 μmol/L clotrimazole (middle), 0.25 μmol/L astemizole (bottom) in TPC-1 (NIS+) cells. D and E, Cell-surface biotinylation assay analysis of NIS protein levels at the PM relative to Na+/K+ ATPase in the TPC-1 (NIS+) cell line after treatment with 0.5 μmol/L ebastine, 0.25 μmol/L clotrimazole, or 0.25 μmol/L astemizole. F, Western blot analysis of VCP expression in TPC-1 (NIS+) cells following treatment with 0.5 μmol/L ebastine, 0.25 μmol/L clotrimazole, or 0.25 μmol/L astemizole. G, Schematic indicating protocol for obtaining mouse thyrocytes. H and I, RAI uptake (H) and Western blot analysis (I) in mouse thyrocytes treated with 0.5 μmol/L ebastine (n = 8) or 0.25 μmol/L clotrimazole (n = 8). J, RAI uptake in human thyrocytes treated as described with ebastine or clotrimazole at varying of doses of VCP inhibitors due to previous evidence of variability in VCP inhibitor sensitivity (50). *, P < 0.05; **, P < 0.01.
We next progressed to our model of mouse thyroid function. Primary thyrocytes were isolated from C57BL/6 mice and treated with ebastine or clotrimazole; both drugs significantly enhanced RAI uptake and NIS protein expression (Fig. 5G to I). Finally, we tested our drugs in human primary thyroid cultures, which were confirmed TSH responsive. Here, both ebastine and clotrimazole significantly increased RAI uptake (Fig. 5J).
VCP and ARF4 expression correlates with poorer survival and response to RAI
Having identified VCP and ARF4 as novel regulators of NIS function, we appraised their expression profiles and clinical relevance in thyroid cancer using TCGA papillary thyroid cancer (THCA) data set. Of significance, ARF4 was underexpressed in PTC compared with normal tissue (Fig. 6A), whereas VCP was significantly induced in PTC, PDTC, and ATC (Fig. 6A and B). Genetic drivers in PTC have distinct signaling consequences and have been categorized into BRAF- like and RAS-like PTCs according to distinct gene signatures (52). Interestingly, VCP mRNA expression did not differ between BRAF- like and RAS-like PTC, whereas ARF4 mRNA expression was lower in BRAF-like than RAS-like tumors across the THCA series (Fig. 6C).
Figure 6.
VCP and ARF4 expression is associated with poorer survival and response to RAI. A, ARF4 (left) and VCP (right) expression in normal thyroid and PTC in the THCA TCGA data set. B, VCP expression in normal thyroid (n = 12), PDTC (n = 17), and ATC (n = 20). C, ARF4 (left) and VCP (right) expression in PTC with BRAF-like (n = 272) or RAS-like genetic signatures (n = 119). D, ARF4 expression in PTC with indicated genetic alterations. E, Frequency (%) of indicated genetic alterations in PTC with low (Q1Q2) versus high ARF4 (Q3Q4) expression. F and G, Same as D and E, but for VCP expression. H–J, DFS for THCA with high (Q3Q4; >12.71) versus low (Q1Q2; <12.71) VCP expression for the entire PTC cohort (H), RAI-treated patients (I) and non–RAI-treated patients (J). K, Hazard ratios ±95% CI for patients stratified on median VCP and ARF4 tumoral expression in THCA with the indicated treatment and genetic signature or alteration. L and M, DFS for THCA with high (Q3Q4; >11.84) versus low (Q1Q2; <11.84) ARF4 expression for RAI-treated PTC patients (L) and non–RAI-treated PTC patients (M). NS, not significant; *, P < 0.05; **, P < 0.01; ***, P < 0.001.
In agreement with these findings, there was greater reduction of ARF4 expression in BRAF-mutant PTC versus the non–BRAF-mutant tumors (Fig. 6D). The frequency of BRAF alterations was also higher in PTC with low ARF4 expression compared with PTC with high ARF4 (Fig. 6E). By comparison, VCP expression was most significantly elevated in PTC with BRAF-mutant, RET fusion, and PAX8 fusion genes (Fig. 6F). In contrast to ARF4, there was a similar frequency of BRAF alterations in PTC with either high or low VCP expression (Fig. 6G).
We next evaluated whether VCP and ARF4 expression was associated with patient outcome, treatment (RAI vs. non-RAI) or disease classification. Overall, log-rank analysis using the entire cohort (n = 413) did not detect any difference in survival between PTC patients with high tumoral VCP compared with those with low VCP (PL = NS), although a significant difference was evident at early (PB = 0.016) and intermediate time points (PT = 0.022; Fig. 6H). Similar to THCA, higher VCP expression was present in breast tumors versus normal tissue in the BRCA cohort (Supplementary Fig. S8A), which again failed to correlate with a significant reduction in survival (PL = NS; Supplementary Fig. S8B).
A key finding, however, was that the subgroup of RAI-treated PTC patients with high tumoral VCP expression (n = 195) had significantly reduced disease-free survival (DFS) than those with low VCP (PL = 0.016; Fig. 6I; Supplementary Fig. S8C). By comparison, there was no significant difference in DFS of patients who did not receive RAI treatment when stratified on median tumoral VCP (Fig. 6J). Cox regression analysis further highlighted that higher tumoral VCP in RAI-treated PTC patients was associated with an increased risk of recurrence (hazard ratio 3.57; Fig. 6K). Interestingly, the risk of recurrence was even greater for RAI-treated patient subgroups associated with BRAF-like (HR 4.63) or BRAF alterations (HR 4.97; Fig. 6K; Supplementary Fig. S8D–S8G). RAI-treated patients with lower tumoral ARF4 expression receiving a dose ≥100 mCi also had reduced survival (Fig. 6L and M; Supplementary Fig. S8H and S8I) and a higher risk of recurrence (HR 2.93; Fig. 6K). In contrast, there was no increase in the risk of recurrence of non–RAI-treated PTC patients with higher tumoral VCP or lower ARF4 expression (Fig. 6K). Overall, disease classification indicated that RAI-treated patients had significantly more advanced thyroid disease than non–RAI-treated patients (Supplementary Fig. S9A; Supplementary Table S5). Importantly, there were no significant differences in clinical staging attributes for RAI or non-RAI treatment groups stratified for VCP or ARF4 tumoral expression (Supplementary Fig. S9B and S9C; Supplementary Tables S6 and S7).
Collectively, we show that ARF4 and VCP are significantly dysregulated in PTC, with expression profiles that fit the repression of RAI uptake generally apparent in thyroid cancers. We propose that dysregulated ARF4 and VCP results in reduced trafficking of NIS to the PM via repressed ARF4 function and increased VCP activity in targeting NIS for degradation. We further identify that VCP and ARF4 are associated with poorer survival characteristics in RAI-treated patients and represent promising new drug targets in patients who are RAI refractory. A model of the proposed functional interaction of NIS with VCP and ARF4 is outlined in Fig. 7.
Figure 7.
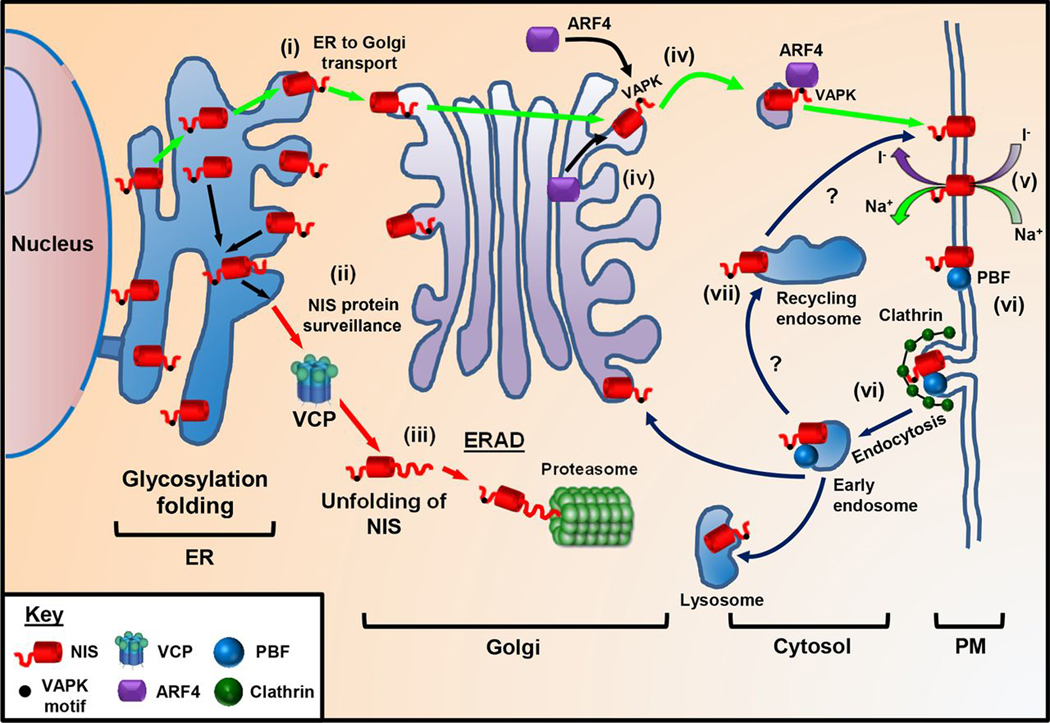
Putative model of NIS trafficking. NIS maintains a delicate balance between protein synthesis, folding, assembly, trafficking, and degradation. (i) We propose NIS is glycosylated in the ER and upon correct folding transported to the Golgi. (ii) Protein surveillance pathways exist that target NIS for ERAD. As VCP does not require ATPase activity to inhibit NIS function, it is likely VCP acts to unfold NIS (iii) prior to proteasomal degradation. (iv) ARF4 recognizes the VAPK motif in the NIS C-terminus and promotes vesicular trafficking to the PM, where NIS is active (v). (vi) PBF has a YARF endocytosis motif and acts to bind and internalize NIS away from the PM in a clathrin-dependent process. (vii) Although inhibition of recycling by dynasore suggests that ARF4 shuttles NIS to the PM, other proteins must promote recycling of NIS to the PM as with most PM transporters.
Discussion
Extensive studies have sought to enhance NIS expression and function in patients with RAIR-TC and hence resensitize tumors to RAI therapy. This is essential because patients with RAIR-TC, particularly those with metastatic disease, have a life expectancy of 3 to 5 years and represent a group for whom there is a clear unmet medical need (2, 3). Most investigations so far have focused on “redifferentiation agents,” which stimulate the expression of thyroid-specific genes including NIS. Constitutive activation of the MAPK pathway in thyroid cancer results in dysregulated NIS expression and function, decreased RAI uptake, and a poor patient prognosis. A substantial number of studies have therefore focused on selective inhibitors of the MAPK pathway to restore RAI avidity (20, 53).
Importantly, new drug strategies, such as combining BRAF or MEK inhibitors with pan-PI3K inhibitors, are showing preclinical promise by rescuing NIS gene expression in thyroid cancer (19). However, we propose that augmenting NIS trafficking to the PM is fundamental to boosting the efficacy of radioisotope treatment, given that we are now close to having the necessary tools to restore NIS expression. With the advent of NIS-based in vivo imaging and therapeutic approaches in nonthyroidal malignancies and other disease modalities, the exploitation and enhancement of NIS function via FDA-approved VCP inhibitors have widespread clinical potential. For example, NIS expression can now be induced via administration of engineered viruses or mesenchymal stem cells in tumoral and nontumoral settings, including cardiac disease and tissue regeneration (6, 54, 55).
Here, we have identified five allosteric inhibitors of VCP that all enhanced RAI uptake in vitro by a minimum of 2-fold. Specific ARF4 agonists do not currently exist, and hence we pursued VCP inhibition as our central therapeutic strategy. To circumvent potential issues of toxicity, we evaluated alternative drugs to enhance RAI uptake, of which ebastine and clotrimazole are FDA approved and well tolerated in vivo (50). Given that RAI uptake was enhanced within 24 hours of ebastine or clotrimazole treatment, our findings necessitate the need for clinical trials to address whether patients receiving these drugs at the time of RAI therapy uptake more 131I. One important finding in support of this is that native, relatively dedifferentiated thyroid cells that have negligible endogenous RAI uptake in our hands showed measurable NIS function following VCP inhibition.
Previously PBF, which, unlike NIS, has a functional endocytosis motif (38), was the only protein shown to bind NIS and diminish its function due to altered subcellular localization via endocytosis (29). Now, our MS/MS, co-IP, and PLA assays have identified and validated ARF4 and VCP as novel NIS interactors. ARF4 binds NIS via a C-terminal 574VXPX577 motif to traffic NIS toward the PM and enhance NIS function. One surprising facet observed via new imaging technologies was the rapid movement of vesicles expressing ARF4 and NIS, which hint at a more dynamic process of NIS trafficking than previously suggested. ARF4 has a range of subcellular roles, from recruiting adaptor proteins for packaging proteins into vesicles destined for the PM, to recycling proteins from the PM through endosomes (56). Our data suggest ARF4 is involved in the vesicular transport of NIS to the PM, given that inhibition of endocytosis had no impact on the ARF4-mediated increase in RAI uptake.
An important clinical observation was that high tumoral VCP expression, which acts predominately in the ER, and hence early in the progression of NIS to the PM, was strongly correlated with DFS, both overall, but particularly in patients who received RAI treatment. Similarly, PTC patients with low tumoral ARF4 expression who received higher doses of RAI (≥100 mCi) also had a significantly worse DFS. In accordance with ATA guidelines (1), RAI-treated patients in TCGA had more advanced disease than non–RAI-treated patients. However, there was no significant difference in clinical staging attributes for RAI or non-RAI treatment groups stratified for VCP or ARF4 tumoral expression. Importantly, these findings suggest that altered expression of VCP or ARF4 do not contribute to the advancement of thyroid disease. Instead, we propose that high VCP or low ARF4 expression significantly influence outcomes following RAI treatment through modulating the function of NIS. Our hypothesis is that high tumoral VCP expression in patients with PTC results in increased NIS degradation, permitting less NIS to be trafficked to the PM by ARF4, which we show is repressed in PTC. Hence the function of NIS, which is already at very low expression levels or predominantly localized to intracellular compartments, in PTC, is further attenuated, resulting in worse DFS. In agreement with this, VCP or ARF4 expression did not significantly influence disease recurrence in patients who had not received RAI treatment.
Several gaps in our knowledge remain. As a protein expressed mainly in basolateral membranes, cell polarization is important to NIS function. No human polarized cell models are physiologically relevant to thyroid cell biology, however. Despite this, our studies in nonpolarized cell systems demonstrate a universal mechanism of NIS trafficking in transformed thyroid and breast cells, as well as primary human and mouse thyrocytes. Further, because our dedifferentiated TPC-1 thyroid cells, as well as our MDA-MB-231 breast cells, are unlikely to express physiologically relevant levels of the TSH receptor, we did not investigate the potential effects of the canonical regulator of NIS-TSH-on NIS trafficking (57). Our hypothesis is that TSH is more important to the expression of NIS than its subcellular trafficking. This reflects the surprising timescales of trafficking we observed in our HILO microscopy, whereby TSH is known to influence NIS function over the course of hours and days (57), in contrast to the vesicular movement of NIS close to the PM, which was unexpectedly dynamic. We thus propose that NIS function at the PM is considerably more rapid than currently envisaged.
Collectively, we now identify two novel NIS interactors, VCP and ARF4, with critical roles in modulating NIS function as well as correlating markedly with clinical outcome in PTC. Notably, VCP is specifically druggable with FDA-approved inhibitors, resulting in enhanced RAI uptake in breast and thyroid cancer models. Strategies that manipulate the function/expression of VCP or ARF4 will thus offer a promising new therapeutic strategy for RAIR-TC in addition to augmenting NIS function for novel in vivo imaging and therapeutic strategies across a broad disease spectrum.
Supplementary Material
Acknowledgments
This work was supported by the Medical Research Council (MR/P000509/1 to C.J. McCabe, V.E. Smith, and K. Boelaert) and Wellcome Trust (RG_05–052 to A. Fletcher). C.J. McCabe also received funding from Cancer Research UK, Get A-Head Charitable Trust, and a PhD studentship from the Medical Research Council/University of Birmingham. We acknowledge the contribution to this study by the Human Biomaterials Resource Centre (University of Birmingham), A. Di Maio and D. Calebiro for expertise in HILO, and D. Nasteska and D. Hodson for C57BL/6 mice.
Footnotes
Note: Supplementary data for this article are available at Cancer Research Online (http://cancerres.aacrjournals.org/).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American thyroid association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid 2016;26:1–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schlumberger M, Brose M, Elisei R, Leboulleux S, Luster M, Pitoia F, et al. Definition and management of radioactive iodine-refractory differentiated thyroid cancer. Lancet Diabetes Endocrinol 2014;2:356–8. [DOI] [PubMed] [Google Scholar]
- 3.Spitzweg C, Bible KC, Hofbauer LC, Morris JC. Advanced radioiodine-refractory differentiated thyroid cancer: the sodium iodide symporter and other emerging therapeutic targets. Lancet Diabetes Endocrinol 2014;10:830–42. [DOI] [PubMed] [Google Scholar]
- 4.La Vecchia C, Malvezzi M, Bosetti C, Garavello W, Bertuccio P, Levi F, et al. Thyroid cancer mortality and incidence: a global overview. Int J Cancer 2015; 136:2187–95. [DOI] [PubMed] [Google Scholar]
- 5.Dai G, Levy O, Carrasco N. Cloning and characterization of the thyroid iodide transporter. Nature 1996;379:458–60. [DOI] [PubMed] [Google Scholar]
- 6.Ravera S, Reyna-Neyra A, Ferrandino G, Amzel LM, Carrasco N. The sodium/iodide symporter (NIS): molecular physiology and preclinical and clinical applications. Annu Rev Physiol 2017;79:261–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Urnauer S, Schmohl KA, Tutter M, Schug C, Schwenk N, Morys S, et al. Dual-targeted NIS polyplexes—a theranostic strategy toward tumors with heterogeneous receptor expression. Gene Ther 2019;26:93–108. [DOI] [PubMed] [Google Scholar]
- 8.Eskin BA, Parker JA, Bassett JG, George DL. Human breast uptake of radioactive iodine. Obstet Gynecol 1974;44:398–402. [PubMed] [Google Scholar]
- 9.Tazebay UH, Wapnir IL, Levy O, Dohan O, Zuckier LS, Zhao QH, et al. The mammary gland iodide transporter is expressed during lactation and in breast cancer. Nat Med 2000;6:871–8. [DOI] [PubMed] [Google Scholar]
- 10.Moon DH, Lee SJ, Park KY, Park KK, Ahn SH, Pai MS, et al. Correlation between 99mTc-pertechnetate uptakes and expressions of human sodium iodide symporter gene in breast tumor tissues. Nucl Med Biol 2001;28: 829–34. [DOI] [PubMed] [Google Scholar]
- 11.Spitzweg C, Harrington KJ, Pinke LA, Vile RG, Morris JC. Clinical review 132: The sodium iodide symporter and its potential role in cancer therapy. J Clin Endocrinol Metab 2001;86:3327–35. [DOI] [PubMed] [Google Scholar]
- 12.Riesco-Eizaguirre G, Gutierrez-Martinez P, Garcia-Cabezas MA, Nistal M, Santisteban P. The oncogene BRAF V600E is associated with a high risk of recurrence and less differentiated papillary thyroid carcinoma due to the impairment of Na+/I- targeting to the membrane. Endocr Relat Cancer 2006;13:257–69. [DOI] [PubMed] [Google Scholar]
- 13.Riesco-Eizaguirre G, Rodriguez I, De la Vieja A, Costamagna E, Carrasco N, Nistal M, et al. The BRAFV600E oncogene induces transforming growth factor beta secretion leading to sodium iodide symporter repression and increased malignancy in thyroid cancer. Cancer Res 2009;69:8317–25. [DOI] [PubMed] [Google Scholar]
- 14.Kogai T, Sajid-Crockett S, Newmarch LS, Liu YY, Brent GA. Phosphoinositide-3-kinase inhibition induces sodium/iodide symporter expression in rat thyroid cells and human papillary thyroid cancer cells. J Endocrinol 2008; 199:243–52. [DOI] [PubMed] [Google Scholar]
- 15.Mancikova V, Buj R, Castelblanco E, Inglada-Perez L, Diez A, de Cubas AA, et al. DNA methylation profiling of well-differentiated thyroid cancer uncovers markers of recurrence free survival. Int J Cancer 2014;135:598–610. [DOI] [PubMed] [Google Scholar]
- 16.Kitazono M, Robey R, Zhan Z, Sarlis NJ, Skarulis MC, Aikou T, et al. Low concentrations of the histone deacetylase inhibitor, depsipeptide (FR901228), increase expression of the Na(+)/I(−) symporter and iodine accumulation in poorly differentiated thyroid carcinoma cells. J Clin Endocrinol Metab 2001;86: 3430–5. [DOI] [PubMed] [Google Scholar]
- 17.Kogai T, Kanamoto Y, Che LH, Taki K, Moatamed F, Schultz JJ, et al. Systemic retinoic acid treatment induces sodium/iodide symporter expression and radioiodide uptake in mouse breast cancer models. Cancer Res 2004; 64:415–22. [DOI] [PubMed] [Google Scholar]
- 18.Kebebew E, Peng M, Reiff E, Treseler P, Woeber KA, Clark OH, et al. A phase II trial of rosiglitazone in patients with thyroglobulin-positive and radioiodine-negative differentiated thyroid cancer. Surgery 2006;140:960–6. [DOI] [PubMed] [Google Scholar]
- 19.Nagarajah J, Le M, Knauf JA, Ferrandino G, Montero-Conde C, Pillarsetty N, et al. Sustained ERK inhibition maximizes responses of BrafV600E thyroid cancers to radioiodine. J Clin Invest 2016;126:4119–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dunn LA, Sherman EJ, Baxi SS, Tchekmedyian V, Grewal RK, Larson SM, et al. Vemurafenib redifferentiation of BRAF mutant, RAI-refractory thyroid cancers. J Clin Endocrinol Metab 2019;104:1417–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoftijzer H, Heemstra KA, Morreau H, Stokkel MP, Corssmit EP, Gelderblom H, et al. Beneficial effects of sorafenib on tumor progression, but not on radioiodine uptake, in patients with differentiated thyroid carcinoma. Eur J Endocrinol 2009; 161:923–31. [DOI] [PubMed] [Google Scholar]
- 22.Piekarz RL, Frye R, Turner M, Wright JJ, Allen SL, Kirschbaum MH, et al. Phase II multi-institutional trial of the histone deacetylase inhibitor romidepsin as monotherapy for patients with cutaneous T-cell lymphoma. J Clin Oncol 2009; 27:5410–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schlumberger M, Tahara M, Wirth LJ, Robinson B, Brose MS, Elisei R, et al. Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. N Engl J Med 2015;372:621–30. [DOI] [PubMed] [Google Scholar]
- 24.Rothenberg SM, McFadden DG, Palmer EL, Daniels GH, Wirth LJ. Redifferentiation of iodine-refractory BRAF V600E-mutant metastatic papillary thyroid cancer with dabrafenib. Clin Cancer Res 2015;21:1028–35. [DOI] [PubMed] [Google Scholar]
- 25.Kogai T, Curcio F, Hyman S, Cornford EM, Brent GA, Hershman JM. Induction of follicle formation in long-term cultured normal human thyroid cells treated with thyrotropin stimulates iodide uptake but not sodium/iodide symporter messenger RNA and protein expression. J Endocrinol 2000;167: 125–35. [DOI] [PubMed] [Google Scholar]
- 26.Castro MR, Bergert ER, Beito TG, Roche PC, Ziesmer SC, Jhiang SM, et al. Monoclonal antibodies against the human sodium iodide symporter: utility for immunocytochemistry of thyroid cancer. J Endocrinol 1999;163:495–504. [DOI] [PubMed] [Google Scholar]
- 27.Dohan O, Baloch Z, Banrevi Z, Livolsi V, Carrasco N. Rapid communication: predominant intracellular overexpression of the Na(+)/I(−) symporter (NIS) in a large sampling of thyroid cancer cases. J Clin Endocrinol Metab 2001;86: 2697–700. [DOI] [PubMed] [Google Scholar]
- 28.Riesco-Eizaguirre G, Santisteban P. A perspective view of sodium iodide symporter research and its clinical implications. Eur J Endocrinol 2006;155: 495–512. [DOI] [PubMed] [Google Scholar]
- 29.Smith VE, Read ML, Turnell AS, Watkins RJ, Watkinson JC, Lewy GD, et al. A novel mechanism of sodium iodide symporter repression in differentiated thyroid cancer. J Cell Sci 2009;122:3393–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang DW, Sherman BT, Tan Q, Collins JR, Alvord WG, Roayaei J, et al. The DAVID gene functional classification tool: a novel biological module-centric algorithm to functionally analyze large gene lists. Genome Biol 2007;8:R183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang DW, Sherman BT, Tan Q, Kir J, Liu D, Bryant D, et al. DAVID Bioinformatics Resources: expanded annotation database and novel algorithms to better extract biology from large gene lists. Nucleic Acids Res 2007; 35:W169–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cotomacci G, Sarkis JJ, Furstenau CR, Barreto-Chaves ML. Thyroid hormones are involved in 5ʹ -nucleotidase modulation in soluble fraction of cardiac tissue. Life Sci 2012;91:137–42. [DOI] [PubMed] [Google Scholar]
- 33.Li S, Esterberg R, Lachance V, Ren D, Radde-Gallwitz K, Chi F, et al. Rack1 is required for Vangl2 membrane localization and planar cell polarity signaling while attenuating canonical Wnt activity. Proc Natl Acad Sci U S A 2011;108: 2264–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Myklebust LM, Akslen LA, Varhaug JE, Lillehaug JR. Receptor for activated protein C kinase 1 (RACK1) is overexpressed in papillary thyroid carcinoma. Thyroid 2011;21:1217–25. [DOI] [PubMed] [Google Scholar]
- 35.Tamajusuku AS, Carrillo-Sepulveda MA, Braganhol E, Wink MR, Sarkis JJ, Barreto-Chaves ML, et al. Activity and expression of ecto-5ʹ -nucleotidase/CD73 are increased by thyroid hormones in vascular smooth muscle cells. Mol Cell Biochem 2006;289:65–72. [DOI] [PubMed] [Google Scholar]
- 36.Yang J, Liao X, Yu J, Zhou P. Role of CD73 in disease: promising prognostic indicator and therapeutic target. Curr Med Chem 2018;25:2260–71. [DOI] [PubMed] [Google Scholar]
- 37.Ye Y, Meyer HH, Rapoport TA. The AAA ATPase Cdc48/p97 and its partners transport proteins from the ER into the cytosol. Nature 2001;414:652–6. [DOI] [PubMed] [Google Scholar]
- 38.Smith VE, Sharma N, Read ML, Ryan G, Martin A, Boelaert K, et al. Manipulation of PBF/PTTG1IP phosphorylation status; a new therapeutic strategy for improving radioiodine uptake in thyroid and other tumours. J Clin Endocrinol Metab 2013;98:2876–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov 2012;2:401–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO, et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal 2013;6:pl1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Broad Institute TCGA Genome Data Analysis Center. Analysis-ready standardized TCGA data from Broad GDAC Firehose 2016_01_28 run. Cambridge (MA): Broad Institute of MIT and Harvard; 2016. [Google Scholar]
- 42.Landa I, Ibrahimpasic T, Boucai L, Sinha R, Knauf JA, Shah RH, et al. Genomic and transcriptomic hallmarks of poorly differentiated and anaplastic thyroid cancers. J Clin Invest 2016;126:1052–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Deretic D, Williams AH, Ransom N, Morel V, Hargrave PA, Arendt A. Rhodopsin C terminus, the site of mutations causing retinal disease, regulates trafficking by binding to ADP-ribosylation factor 4 (ARF4). Proc Natl Acad Sci U S A 2005;102:3301–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bastola P, Neums L, Schoenen FJ, Chien J. VCP inhibitors induce endoplasmic reticulum stress, cause cell cycle arrest, trigger caspase-mediated cell death and synergistically kill ovarian cancer cells in combination with salubrinal. Mol Oncol 2016;10:1559–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chapman E, Maksim N, de la Cruz F, La Clair JJ. Inhibitors of the AAA+ chaperone p97. Molecules 2015;20:3027–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang Q, Li L, Ye Y. Inhibition of p97-dependent protein degradation by Eeyarestatin I. J Biol Chem 2008;283:7445–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Magnaghi P, D’Alessio R, Valsasina B, Avanzi N, Rizzi S, Asa D, et al. Covalent and allosteric inhibitors of the ATPase VCP/p97 induce cancer cell death. Nat Chem Biol 2013;9:548–56. [DOI] [PubMed] [Google Scholar]
- 48.Chou TF, Brown SJ, Minond D, Nordin BE, Li K, Jones AC, et al. Reversible inhibitor of p97, DBeQ, impairs both ubiquitin-dependent and autophagic protein clearance pathways. Proc Natl Acad Sci U S A 2011; 108:4834–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Song C, Wang Q, Song C, Rogers TJ. Valosin-containing protein (VCP/p97) is capable of unfolding polyubiquitinated proteins through its ATPase domains. Biochem Biophys Res Commun 2015;463:453–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Segura-Cabrera A, Tripathi R, Zhang X, Gui L, Chou TF, Komurov K. A structure- and chemical genomics-based approach for repositioning of drugs against VCP/p97 ATPase. Sci Rep 2017;7:44912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bastola P, Oien DB, Cooley M, Chien J. Emerging cancer therapeutic targets in protein homeostasis. AAPS J 2018;20:94. [DOI] [PubMed] [Google Scholar]
- 52.Cancer Genome Atlas Research N. Integrated genomic characterization of papillary thyroid carcinoma. Cell 2014;159:676–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang H, Chen D. Synergistic inhibition of MEK/ERK and BRAF V600E with PD98059 and PLX4032 induces sodium/iodide symporter (NIS) expression and radioiodine uptake in BRAF mutated papillary thyroid cancer cells. Thyroid Res 2018;11:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hickey RD, Mao SA, Amiot B, Suksanpaisan L, Miller A, Nace R, et al. Noninvasive 3-dimensional imaging of liver regeneration in a mouse model of hereditary tyrosinemia type 1 using the sodium iodide symporter gene. Liver Transpl 2015;21:442–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Knoop K, Schwenk N, Schmohl K, Muller A, Zach C, Cyran C, et al. Mesenchymal stem cell-mediated, tumor stroma-targeted radioiodine therapy of metastatic colon cancer using the sodium iodide symporter as theranostic gene. J Nucl Med 2015;56:600–6. [DOI] [PubMed] [Google Scholar]
- 56.Wang J, Fresquez T, Kandachar V, Deretic D. The Arf GEF GBF1 and Arf4 synergize with the sensory receptor cargo, rhodopsin, to regulate ciliary membrane trafficking. J Cell Sci 2017;130:3975–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Riedel C, Dohan O, De la Vieja A, Ginter CS, Carrasco N. Journey of the iodide transporter NIS: from its molecular identification to its clinical role in cancer. Trends Biochem Sci 2001;26:490–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.