Abstract
Context
Research interest in monoamine oxidase (MAO) as a promising drug target for neurodegenerative diseases has a long history. However, efforts to develop MAO inhibitors (MAOIs) from marine sources have been limited, despite the increasing number of interesting marine natural products.
Objective
To review the potential of marine natural products as MAOIs source, including their activities and selectivity on MAO.
Methods
Public databases such as SciFinder, MarinLit and PubMed were systematically searched from 1991 until Dec 2019. MAO and MAOI were the key terms searched combined with marine natural products and marine.
Results
Six classes of marine natural products with good selectivity between the two MAO subtypes were organized with their selectivity and sources.
Conclusions
This is the first review to investigate the potential of marine natural products as MAOIs source. Despite the small number of known MAOIs from marine sources, marine natural products are potential leads for the further development of MAOI drugs with novel chemical frames and good selectivity.
Keywords: MAOIs, aplysinopsins, piloquinones, anithiactins, bromopyrroles, caulerpins, astaxanthin
Introduction
Monoamine oxidases (MAOs, E.C. 1.4.3.4) are members of the flavin-containing amine oxidoreductases which catalyze the oxidative deamination (Al-Nuaimi et al. 2012). In humans, these ubiquitous mitochondrial enzymes have been categorized into two subtypes, MAO-A and MAO-B. Both subtypes are found in neurons and astroglia, and are distributed unevenly outside the central nervous system (Shih and Chen 2004). Each isoform has preferred substrates, stemming from structural differences in the substrate binding site (Gaweska and Fitzpatrick 2011). MAO-A is principally responsible for degrading serotonin, norepinephrine and epinephrine, whereas MAO-B prefers dopamine and β-phenylethylamine as substrates (Westlund et al. 1985; Bolasco et al. 2010). The central role of MAO-A in the degradation of the neurotransmitter serotonin and biogenic amines may be linked with the pathogenesis of mental disorders such as depression and anxiety (Shih et al. 1999). The substrate preference of MAO-B for dopamine results in the specificity of monoamine oxidase inhibitors (MAOIs) in clinical applications, and inhibitors of MAO-B have been studied in relation to Parkinson’s disease (Dezsi and Vecsei 2017).
Research interest in MAO as a drug target goes back to the 1950s, and MAOIs are a good example of drug-repositioning, as they were originally anti-tuberculosis drugs which were later used to manage patients’ tempers (Crne 1956). Understanding of this empirical approach was modernised by recent advances in our understanding of the 3D structure of human MAO-A and -B (Hubálek et al. 2005; Son et al. 2008). Elucidation of the full structure of the enzymes and their substrates has provided critical information, such as on the size of the binding cavity, size of the entrance cavity, hydrophobicity of the cavities, and amino acids that are responsible for binding.
There are several classes of MAOI drugs on the market based on reversibility and selectivity (Shulman et al. 2013; Entzeroth and Ratty 2017). Reversibility means the way of the inhibitor binding into the enzyme whether covalently or non-covalently. Selectivity is a preference for one of the MAO subtypes. Non-selective and irreversible MAOI such as isocarboxazid, phenelzine and tranylcypromine were approved to treat depression in the US (Shulman et al. 2013). Selective MAO-A inhibitors with reversibility such as brofaromin and moclobemide were employed to treat social anxiety disorder (Entzeroth and Ratty 2017). However, the results were contradictory that these were not approved in the US. Irreversible MAO-B inhibitors, selegiline and rasagiline, were used to treat the symptoms of Parkinson's disease (Dezsi and Vecsei 2017). At present, the development of reversible, specific and safe MAOIs is required.
Previously, the search for novel MAOIs has mainly focussed on herbal sources (Erdogan Orhan 2016). Microbial sources were also extensively studied, such as in the case of pimprinine, trans-cinnamic acid amid and phenethylamine (Takeuchi et al. 1973). However, there are only four papers reporting on marine MAOIs which have been published since 1973 (Lee et al. 1999; Lee et al. 2015; Lee, Choi, et al. 2017; Lee, Kim, et al. 2017), and these are mostly focussed on Streptomycetes as a source.
Additionally, the interest in marine natural products in relation to this subject has been limited. Considering the increasing number of marine natural products, only a handful of MAOIs have been reported so far. In this review, we describe the various MAOIs of marine origin (Table 1). For the purpose of this review, synthetic analogs were indicated with the letter attached to the mother natural product number.
Table 1.
List of marine natural products used as monoamine oxidase (MAO) inhibitors.
| Compounds | Source | Potency (compound number, SIa) | Reference |
|---|---|---|---|
| Aplysinopsins | Sponge (Aplysinopsis sp.) synthetic analogs | MAO-A IC50 = 0.0056 µM (1a, 80.2) MAO-A IC50 = 0.035 µM (1c, 290.3) |
(Baird-Lambert et al. 1982; Aoki et al. 2001; Segraves and Crews, 2005) |
| Piloquinones | Bacteria (Streptomyces sp.) | MAO-B IC50 = 1.21 µM (2, 0.19) MAO-B IC50 = 14.5 µM (3, NAb) |
(Lee, Choi, et al. 2017) |
| Anithiactins | Bacteria (Streptomyces sp.) | MAO-A IC50 = 13.0 µM (4, 14.1) | (Lee et al. 2015) |
| Bromopyrroles | Sponge synthetic analogs | MAO-A IC50 = 2.4 µM (7a, 15.9) MAO-B IC50 = 2.1 µM (7b, 0.08) |
(Rane, Sahu, et al. 2014; Rane, Napahde, et al. 2014) |
| Caulerpins | Algae synthetic analogs | In silico prediction | (Lorenzo et al. 2015) |
| Astaxanthin | Algae Shrimps | MAO-A inhibitory activity at high dose (9, NBb) |
(Safarova et al. 2016; Jiang et al. 2017) |
aSI stands for ‘selectivity index’, defined by the ratio of IC50 (MAO-B)/IC50 (MAO-A).
b NA/NB stands for ‘no inhibition on MAO-A/MAO-B’.
Discussion
Aplysinopsins
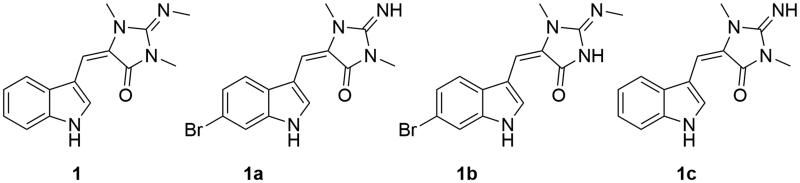
Aplysinopsins have been isolated from various classes of marine organisms such as molluscs, corals, sea anemones and particularly from marine sponges. Aplysinopsin was reported for the first time in 1977 from Australian sponges belonging to the genus Thorecta, which has been re-assigned as Aplysinopsis (Kazlauskas et al. 1977). Aplysinopsins are tryptophan-derived indole-bearing natural products with a modified functional group within. Over two dozen aplysinopsins have been reported from marine organisms, with a wide range of bioactivities including antimicrobial, antimalarial and antitumor activity (Bialonska and Zjawiony 2009). Among them, methylaplysinopsin (1, Figure 1) has shown potent in vivo antidepressant activity, and was later shown to have reversible inhibitory activity against MAO (Baird-Lambert et al. 1982). Detailed pharmacokinetic studies followed this study, which showed the drug-like property of 1. Successive studies developed synthetic analogs as MAOIs with improved IC50 values down to 5 nM against MAO-A (Aoki et al. 2001; Segraves and Crews 2005). These studies, covering 50 synthetic analogs, improved not only the activities but also selectivity in discriminating between the two isoforms of MAO. Some of the most potent aplysinopsin synthetic analogs are shown in Figure 1. It is interesting that the addition of a bromine atom in the indole ring system results in a dramatic increase in the activity.
Figure 1.
MAO inhibiting aplysinopsins.
Piloquinones
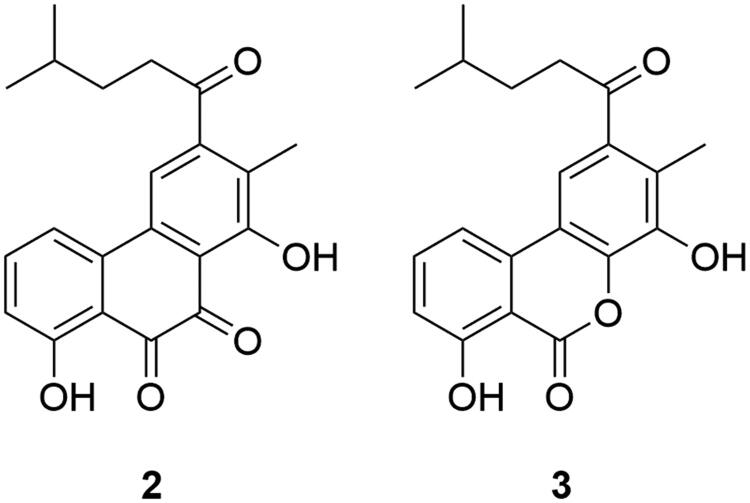
Piloquinone (2, Figure 2) was reported in 1963 from the mycelium of Streptomyces pilosus (Connor et al. 1963). However, the inhibitory activities of 2 on recombinant human MAO were not studied until five decades later, with a derivative (3) from Streptomyces sp. CNQ-027 (Lee, Choi, et al. 2017). Interestingly, both piloquinones showed MAO-B selectivity over MAO-A, with a good selective index value of 0.19 (2, MAO-B IC50 1.21 μM), or no MAO-A inhibitory activity up to 80 μM (3, MAO-B IC50 14.5 μM). This finding was in conflict with a previous study which showed MAO-A selectivity with a dibenzopyrone frame fungal secondary metabolite (Lee, Kim, et al. 2017). The attached pentyl chain may be responsible for the selective conversion of piloquinones. Compound 3 showed potent inhibitory activity comparable with current Parkinson’s disease drugs, based on a MAO-B inhibitory mechanism. It is noteworthy that 3 is the most potent MAO-B inhibitor derived from microbial sources.
Figure 2.
MAO inhibiting piloquinones.
Anithiactins
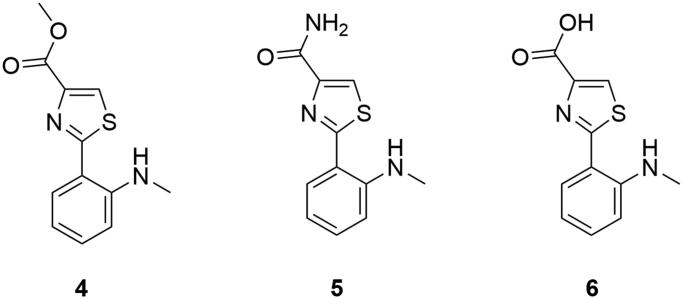
Anithiactins (4–6, Figure 3) were the first reported aniline bearing 2-phenylthiazole natural products in the class. Initially, moderate acetylcholine esterase (AChE) inhibitory activity of anithiactins was reported, with IC50 values of 63, 53, and 58 μM, respectively, and without cytotoxicity up to 100 μM (Kim et al. 2014). Anithiactin A (4) showed moderate inhibitory activity for MAO-A with a selectivity index of 14.1 over MAO-B (Lee et al. 2015). The absence of a methyl ester functional group attached to the thiazole ring resulted in dramatic activity differences among anithiactins (Figure 3). The substitution of the methyl ester with an amine resulted in the complete loss of MAO-B activity of the anithiactin B (5), whereas the presence of a carboxylic acid group resulted in IC50 value of over 170 μM for anithiactin C (6) against MAOs. Anithiactin A (4, MAO-A IC50 13.0 μM) showed a reversible MAO-A inhibitory activity with selectivity over MAO-B, and showed moderate AChE inhibitory activity.
Figure 3.
MAO inhibiting anithiactins.
Bromopyrroles
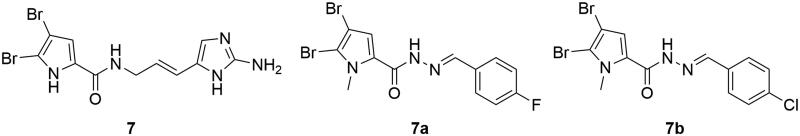
Bromopyrroles are marine alkaloids exclusive to sponge with a wide range of bio-activities, such as antibacterial, antifungal, antimalarial, anticoagulant, antiprotozoal, antiviral, antihistamininc, anticancer and anti-inflammatory activities (Rane, Sahu, et al. 2014). Among them, synthetic analogs based on oroidin (7, Figure 4) have been prepared to improve the affinity and selectivity for MAOs (Rane, Napahde, et al. 2014). In vivo mouse experiments have been performed to demonstrate the drug-like properties of these analogs with promising pharmacological activities. The 4,5-dibromopyrrole carboxamide frame of oroidin (7) was retained in these analogs. It is interesting that the substitution of a fluorine atom in compound 7a to a chlorine in 7b dramatically changes the MAO selectivity, with the selectivity index altered from 0.06 (7a, MAO-A IC50 2.4 μM) to 12.80 (7b, MAO-B IC50 2.1 μM). The presence of an N-methyl group at the pyrrole ring was one of the favoured features for in vivo mouse antidepressant activity.
Figure 4.
MAO inhibiting bromopyrroles.
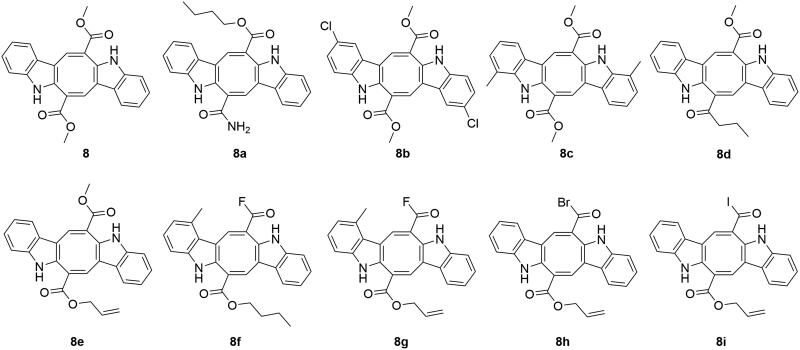
Caulerpins
Caulerpin (8, Figure 5) has shown calcium channel inhibitory activity (Cavalcante-Silva et al. 2013), with antinociceptive and anti-inflammatory activity (De Souza et al. 2009). Through computer-aided drug design, nine caulerpin analogs have been suggested as potential MAO-B inhibitors from among 108 entities (Lorenzo et al. 2015). In this study, three-dimensional structures were built on the Volsurf descriptors and drug-like scores were calculated using DRAGON software. These virtual screened analogs showed more similarity to Moldock energy than caulerpin (8), with improved drug-like scores. However, experimental validation is needed for validation of the in silico results.
Figure 5.
MAO inhibiting caulerpins.
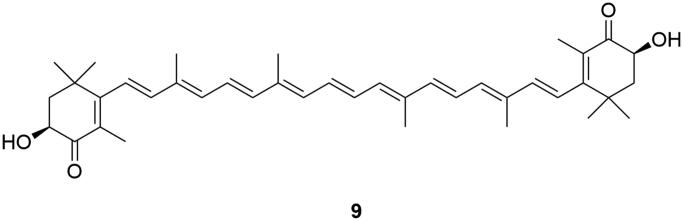
Astaxanthin
Astaxanthin (9, Figure 6) is a well-known xanthophyll terpenoid that has a wide range of food industry applications as a colouring agent. This lipophilic pigment is responsible for the red colour of marine organisms such as shrimp and algae. The in vivo antidepressant effects of 9 have been studied in the mice, along with its MAO inhibitory effects (Jiang et al. 2017). Furthermore, the molecular binding of 9 with MAO-A and -B enzyme has been studied in silico and compared with the known MAOIs isatin, and lazabemide (Safarova et al. 2016). It is interesting that compound 9 showed a protective effect against cognitive dysfunction in the mouse model (Feng et al. 2018). However, evidence of 9 as an MAOI is limited and requires additional proof.
Figure 6.
MAO inhibiting astaxanthin (9).
Conclusions
In this review, several marine natural products with MAO inhibitory activities were discussed, which possess chemical framework such as indole-imidazole, dibromo-pyrrole, naphthoquinone, phenylthiazole, bisindole and xanthophyll. It is interesting that most of these MAOIs have a nitrogen-containing heterocyclic ring system or quinone moiety. This tendency is consistent with previously reported MAOIs from other sources (Bolasco et al. 2010; Erdogan Orhan 2016). Producers of these marine MAOIs include sponges, algae and marine Streptomyces (Table 1). The most potent MAO-A activity was observed with the aplysinopsin analog (1a). Interestingly, most of these marine origin inhibitors show good selectivity between the two MAO subtypes. Aplysinopsin analog (1c) has a three hundred times higher affinity to MAO-A than B, whereas the bromopyrrole analog (7b) favours MAO-B eight times more than MAO-A. Improved activities with functional group modification were also notable in aplysinopsins and bromopyrroles. It is clear that the current examples of MAOIs from marine natural products are not well understood, and require further research. Some of the examples have only early assay data or virtual experiments available, so discussion of their potential as good drug leads is contingent on further studies.
In conclusion, there are a small number of marine natural products that show MAO inhibitory activities. These inhibitors, with novel chemical structures, are potential leads for the further development of MAOI drugs from marine sources.
Funding Statement
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Science and ICT under Grant No. NRF-2017R1D1A1B03028172 (to S.-J.N.), 2018R1A5A2025286 (to K.-M.L.).
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Al-Nuaimi SK, MacKenzie EM, Baker GB.. 2012. Monoamine oxidase inhibitors and neuroprotection: a review. Am J Ther. 19(6):436–448. [DOI] [PubMed] [Google Scholar]
- Aoki S, Ye Y, Higuchi K, Takashima A, Tanaka Y, Kitagawa I, Kobayashi M.. 2001. Novel neuronal nitric oxide synthase (nNOS) selective inhibitor, aplysinopsin-type indole alkaloid, from marine sponge Hyrtios erecta. Chem Pharm Bull. 49(10):1372–1374. [DOI] [PubMed] [Google Scholar]
- Baird-Lambert J, Davis PA, Taylor KM.. 1982. Methylaplysinopsin: a natural product of marine origin with effects on serotonergic neurotransmission. Clin Exp Pharmacol Physiol. 9(2):203–212. [DOI] [PubMed] [Google Scholar]
- Bialonska D, Zjawiony JK.. 2009. Aplysinopsins-marine indole alkaloids: chemistry, bioactivity and ecological significance. Mar Drugs. 7(2):166–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolasco A, Carradori S, Fioravanti R.. 2010. Focusing on new monoamine oxidase inhibitors. Expert Opin Ther Pat. 20(7):909–939. [DOI] [PubMed] [Google Scholar]
- Cavalcante-Silva LHA, De Carvalho Correia AC, Barbosa-Filho JM, Da Silva BA, De Oliveira Santos BV, De Lira DP, Sousa JCF, De Miranda GEC, Cavalcante FA, Alexandre-Moreira MS.. 2013. Spasmolytic effect of caulerpine involves blockade of Ca2+ influx on guinea pig ileum. Mar Drugs. 11(5):1553–1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor JB, Polonsky J, Cohen P, Lederer E.. 1963. Piloquinone: a new phenanthrene-O-quinone isolated from the mycelium of Streptomyces pilosus. Nature. 199:285–286. [DOI] [PubMed] [Google Scholar]
- Crne GE. 1956. The psychiatric side-effects of iproniazid. Am J Psychiatry. 112:494–497. [DOI] [PubMed] [Google Scholar]
- De Souza ÉT, De Lira DP, De Queiroz AC, Da Silva DJC, De Aquino AB, Campessato Mella EA, Lorenzo VP, De Miranda GEC, De Araújo-Júnior JX, De Oliveira Chaves MC, et al. 2009. The antinociceptive and anti-inflammatory activities of caulerpin, a bisindole alkaloid isolated from seaweeds of the genus Caulerpa. Mar Drugs. 7(4):689–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dezsi L, Vecsei L.. 2017. Monoamine oxidase B inhibitors in Parkinson's disease. CNS Neurol Disord Drug Targets. 16(4):425–439. [DOI] [PubMed] [Google Scholar]
- Entzeroth M, Ratty AK.. 2017. Monoamine oxidase inhibitors – revisiting a therapeutic principle. OJD. 06(02):31–68. [Google Scholar]
- Erdogan Orhan I. 2016. Potential of natural products of herbal origin as monoamine oxidase inhibitors. Curr Pharm Des. 22(3):268–276. [DOI] [PubMed] [Google Scholar]
- Feng Y, Chu A, Luo Q, Wu M, Shi X, Chen Y.. 2018. The protective effect of astaxanthin on cognitive function via inhibition of oxidative stress and inflammation in the brains of chronic T2DM rats. Front Pharmacol. 9:748–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaweska H, Fitzpatrick PF.. 2011. Structures and mechanism of the monoamine oxidase family. Biomol Concepts. 2(5):365–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubálek F, Binda C, Khalil A, Li M, Mattevi A, Castagnoli N, Edmondson DE.. 2005. Demonstration of isoleucine 199 as a structural determinant for the selective inhibition of human monoamine oxidase B by specific reversible inhibitors. J Biol Chem. 280(16):15761–15766. [DOI] [PubMed] [Google Scholar]
- Jiang X, Zhu K, Xu Q, Wang G, Zhang J, Cao R, Ye J, Yu X.. 2017. The antidepressant-like effect of trans-astaxanthin involves the serotonergic system. Oncotarget. 8(15):25552–25563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazlauskas R, Murphy PT, Quinn RJ, Wells RJ.. 1977. Aplysinopsin, a new tryptophan derivative from a sponge. Tetrahedron Lett. 18(1):61–64. [Google Scholar]
- Kim H, Yang I, Patil RS, Kang S, Lee J, Choi H, Kim M-S, Nam S-J, Kang H.. 2014. Anithiactins A-C, modified 2-phenylthiazoles from a mudflat-derived Streptomyces sp. J Nat Prod. 77(12):2716–2719. [DOI] [PubMed] [Google Scholar]
- Lee HW, Choi H, Nam S-J, Fenical W, Kim H.. 2017. Potent inhibition of monoamine oxidase B by a piloquinone from marine-derived Streptomyces sp. CNQ-027. J Microbiol Biotechnol. 27(4):785–790. [DOI] [PubMed] [Google Scholar]
- Lee HW, Jung WK, Kim HJ, Jeong YS, Nam SJ, Kang H, Kim H.. 2015. Inhibition of monoamine oxidase by anithiactins from Streptomyces sp. J Microbiol Biotechnol. 25(9):1425–1428. [DOI] [PubMed] [Google Scholar]
- Lee HW, Kim YJ, Nam SJ, Kim H.. 2017. Potent selective inhibition of monoamine oxidase a by alternariol monomethyl ether isolated from Alternaria brassicae. J Microbiol Biotechnol. 27(2):316–320. [DOI] [PubMed] [Google Scholar]
- Lee I-K, Yun B-S, Oh S, Kim Y-H, Lee M-K, Yoo I-D.. 1999. 5-Methylmellein and nectriapyrone, two new monoamine oxidase inhibitors. Med Sci Res. 27:463–465. [Google Scholar]
- Lorenzo VP, Filho JMB, Scotti L, Scotti MT.. 2015. Combined structure- and ligand-based virtual screening to evaluate caulerpin analogs with potential inhibitory activity against monoamine oxidase B. Braz J. Pharmacogn. 25(6):690–697. [Google Scholar]
- Rane R, Sahu N, Shah C, Karpoormath R.. 2014. Marine bromopyrrole alkaloids: Synthesis and diverse medicinal applications. Curr Top Med Chem. 14(2):253–273. [DOI] [PubMed] [Google Scholar]
- Rane RA, Napahde S, Bangalore P. u, Sahu NU, Shah N, Kulkarni YA, Barve K, Lokare L, Karpoormath R.. 2014. Synthesis and evaluation of novel marine bromopyrrole alkaloid-based derivatives as potential antidepressant agents. Chem Biol Drug Des. 84(5):593–602. [DOI] [PubMed] [Google Scholar]
- Safarova G, Safarov N, Gasanov R.. 2016. Molecular docking of astaxanthin to monoamine oxidase. Adv Biol Earth Sci. 1:45–50. [Google Scholar]
- Segraves NL, Crews P.. 2005. Investigation of brominated tryptophan alkaloids from two thorectidae sponges: Thorectandra and Smenospongia. J Nat Prod. 68(10):1484–1488. [DOI] [PubMed] [Google Scholar]
- Shih JC, Chen K.. 2004. Regulation of MAO-A and MAO-B gene expression. Curr Med Chem. 11(15):1995–2005. [DOI] [PubMed] [Google Scholar]
- Shih JC, Chen K, Ridd MJ.. 1999. Monoamine oxidase: from genes to behavior. Annu Rev Neurosci. 22:197–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman KI, Herrmann N, Walker SE.. 2013. Current place of monoamine oxidase inhibitors in the treatment of depression. CNS Drugs. 27(10):789–797. [DOI] [PubMed] [Google Scholar]
- Son S-Y, Ma J, Kondou Y, Yoshimura M, Yamashita E, Tsukihara T.. 2008. Human monoamine oxidase A: structure and control of opening the entry for substrates/inhibitors. Proc Natl Acad Sci USA. 105:5739–5744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi T, Ogawa K, Iinuma H, Suda H, Ukita K, Nagatsu T, Kato M, Umezawa H.. 1973. Monoamine oxidase inhibitors isolated from fermented broths. J Antibiot. 26(3):162–167. [DOI] [PubMed] [Google Scholar]
- Westlund KN, Denney RM, Kochersperger LM, Rose RM, Abell CW.. 1985. Distinct monoamine oxidase A and B populations in primate brain. Science. 230(4722):181–183. [DOI] [PubMed] [Google Scholar]