Abstract
Cancer immunotherapy suppresses and destroys tumors by re-activating and sustaining the tumor-immune process, and thus improving the immune response of the body to the tumor. Immunotherapeutic strategies are showing promising results in pre-clinical and clinical trials, however, tumor microenvironment (TME) is extremely immunosuppressive. Thus, their translation from labs to clinics still faces issues. Recently, nanomaterial-based strategies have been developed to modulate the TME for robust immunotherapeutic responses. The combination of nanotechnology with immunotherapy potentiates the effectiveness of immunotherapy by increasing delivery and retention, and by reducing immunomodulation toxicity. This review aims to highlight the barriers offered by TME for hindering the efficiency of immunotherapy for cancer treatment. Next, we highlight various nano-carriers based strategies for modulating those barriers for achieving better therapeutic efficacy of cancer immunotherapy with higher safety. This review will add to the body of scientific knowledge and will be a good reference material for academia and industries.
Keywords: Tumor microenvironment, immunotherapy, nanotechnology
1. Introduction
Cancer has become one of the world’s most significant health problems. Global population projections have projected rising incidences of cancer over the upcoming years, with 420 million new cancer cases anticipated per year by 2025 (Zaheer et al., 2019). It is traditionally treated with medicines and radiations used to treat anticancer. These therapies, however, are associated with certain disadvantages such as high recurrence possibilities and limited therapeutic efficacy. The clinical intensity of radiation or chemotherapeutic medications at the target sites is accomplished by significant penetration of the majority of the body, contributing to unacceptable side effects (Oshita et al., 1992; Glen and Dubrova, 2012; Huang et al., 2017). Clinicians have treated cancer with assurance in recent years through the use of immunotherapeutic moieties. This strategy also has many benefits including its efficacy against metastasized cancer and low risk of recurrence (Liu and Guo, 2018).
Unlike conventional therapies, immunotherapy targets the immune system to cause systemic therapeutic efficacy. Clinical studies with immune checkpoint inhibitors have demonstrated enormous lasting responses (Farkona et al., 2016; Shi et al., 2018). Specifically, it can inhibit tumor metastasis and relapse by improving the immune system, amplifying the immune response, and triggering immune memory while reducing off-target adverse effects (Shi et al., 2018).
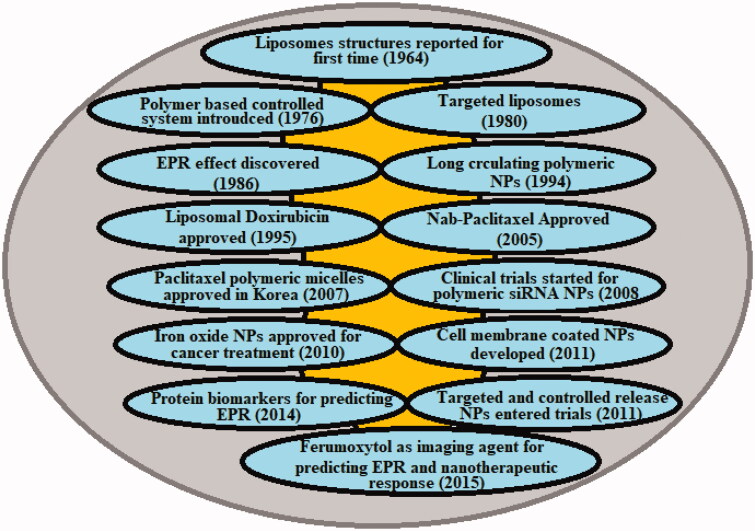
The Medical Standing Committee of the European Science Foundation states that ‘Nanomedicine is the science and technology of diagnosing, treating, and preventing disease and traumatic injury, of relieving pain, and of preserving and improving human health, using molecular tools, and molecular knowledge of the human body.’ (Webster, 2006) The past years have fueled the formulation and module of a myriad of nanomaterials including, Nanoparticles (NPs) made from noble metals, carbon, heavy metals, etc., in many forms, such as spherical or non-sphere NPs, nanofilms, nanotubes, and nanowires (Chen et al., 2013). Such nanomaterials have special properties that could be investigated for use in theranostics. Carbon nanotubes, for example, are known and reputable, with high durability; iron oxide NPs are superparamagnetic; while gold NPs have distinctive spectral (optical) characteristics (Awasthi et al., 2018). To enhance the therapeutic advantages of nanomedicine, numerous approaches have been developed, particularly active nanomedicine targeting, tumor-responsive nanomedicine, and optimization of nanomedicine’s physiochemical parameters similar to a scale, and charge (Pérez-Herrero and Fernández-Medarde, 2015; Awasthi et al., 2018). With time there are various breakthroughs in the field of nanomedicine for cancer management (Figure 1). Nevertheless, these approaches rely on the advanced production of nanomedicine alone, which cannot resolve the above-mentioned tumor microenvironmental distribution obstacles (Garg et al., 2018). Correspondingly, Tumor Microenvironment (TME) alteration was considered as an effective tool for improving the delivery of cancer nanomedicine (Zhang et al., 2017a).
Figure 1.
Historical timeline of major developments in the field of cancer nanomedicine.
This review aims to highlight the barriers offered by TME for the efficiency of nanomedicine. Next, we highlight the various strategies to modulate those barriers through NPs and in combination therapy with NPs.
2. Challenges offered by TME to nanomedicine
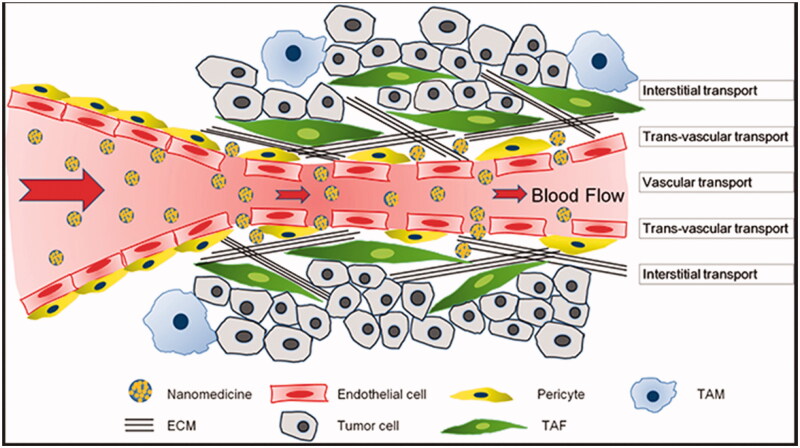
Given the increasing understanding of tumor growth and advancement, it is practically difficult to ascertain the sequence of actions from the primary phase of tumor production and the unregulated proliferation of cells to a mature high-grade tumor (Feitelson et al., 2015). Occasionally one would be inclined to equate an occurrence induced by the proliferation of tumor cells with an event taking place inside the TME, however, this association is far from the fact because it is the changing interplay of all TME elements that will eventually be responsible for the regulation and development of tumors (Hanahan and Weinberg, 2011; Netea-Maier et al., 2018). TME offers several challenges for the transport of therapeutic material to the site of action and thus hinder the therapeutic efficiency (Figure 2). For simplification, here we will focus individually on each element of the TME. Nevertheless, it is important to note that with a particular tumor or tumor type, any of these events happening with each component may be caused differently, thereby influencing all the other TME components differently, leading to specific outcomes.
Figure 2.
The transport barriers for tumor nanomedicine delivery imposed by a complicated tumor microenvironment (Reproduced from Zhang et al., 2017a).
The explosive growth of tumor cells leads to such a restriction of the supply of oxygen and nutrients through neighboring blood vessels could not withstand (Hanahan and Weinberg, 2011). The oxygen shortage faced by rising tumor cells induces the cellular reaction to hypoxia, mainly through factors caused by hypoxia (HIF) (Vaupel and Multhoff, 2018). The transcriptional factor HIF family is made up of HIF2, HIF3, and HIF1, proteins that trigger genes involved in the biosynthesis of glucose, angiogenesis, cell proliferation, and migration, and immune response (Graham and Presnell, 2017; Schito et al., 2017). Alongside high energy requirements, the HIF reply in tumor cells induces a metabolic change from oxidative phosphorylation to aerobic glycolysis called the Warburg effect (Gwangwa et al., 2018). Though oxygen presence, this metabolic switch results in increase secretion of lactate into the extravascular environment and corresponding acidification of TME (Lu et al., 2019). The increased proliferation and glycolytic metabolism of tumor cells contribute to a rise in the development of reactive oxygen species (ROS), which in effect attack cellular components, including certain DNA, fostering genomic instability, which affects the morphology of cells, and also stimulates antioxidant capacity (Gwangwa et al., 2018). Such incidents, along with the up-regulation of efflux pumps for the secretion of lactic and carbonic acid, provide a benefit for tumor cells to live and succeed in extreme conditions (Roma-Rodrigues et al., 2019). Intriguingly, the HIF proteins that act as tumor suppressor genes instead of oncogenic promoters in malignant cells (Nakazawa et al., 2016). Nonetheless, HIF-mediated paracrine contact among tumor cells and populations, such as immune system cells, and extracellular matrix modulation and stromal cell metastases, facilitates the growth of tumors and allows HIF proteins oncogenic at TME level (Sormendi and Wielockx, 2018).
The production of vascular endothelial growth factor A (VEGFA) by TME components promotes the growth of adjacent vessels by attaching in endothelial cells to VEGF receptors (VEGFR). The increasing incidence of angiogenic signals at the TME lead in the development of vessels with damaged or undefined basal cells, resulting in the leakage of the vasculature with a disorderly structure unequally applied around the tumor, with cancer areas enriched by vessels and improperly provided cancer areas (Dirkx et al., 2006; Klein, 2018). This restricts the nutrient and oxygen supply to the TME, promoting hypoxia, and difficult the chemotherapeutic agents’ distribution throughout the tumor (Dirkx et al., 2003; 2006). The unstable composition of the blood vessels contributes to the abnormal production of cytokines implicated in inflammatory and coagulation functions at TME (Tei et al., 2002). However, it less structured rusty vasculature enables nanomedicines to selectively attack the source of the tumor. VEGF-D and VEGF-C secreted by cancer cells, stromal cells and immune cells promote the development of lymphatic vessels at TME, termed tumor-associated lymphangiogenesis (Partanen et al., 2000). Thus, lymphatic endothelial cells (LECs) develop single-layer lymph capillaries of the reduced basal lamina, which connect lymph vessels with a basal lamina and valves to avoid regressive discharge (Weitman et al., 2013). Lymphatic vessel development at the TME is associated with a bad prognosis since it promotes metastatic proliferation in distal organs (Albrecht and Christofori, 2011). But on the other hand, LECs play a major role in the regulation of the immune system at TME which contributes to anti-tumor immunity (Weitman et al., 2013; Farnsworth et al., 2014). The faulty lymph drainage once again supports the aggregation of nanomedicines at both the locus by the EPR.
Concerning immune system cells, the TME differs greatly during the production of tumors and across the different kinds of tumors (Netea-Maier et al., 2018). Owing to the constant shifts and modifications that arise at the TME, multiple activation factors (e.g. chemokines and cytokines) are naturally produced, culminating in the identification of cells both from adaptive and innate immune systems (Chen and Mellman, 2013). Notably, TME’s composition of molecular signals influences the therapeutic result by facilitating tumor escape through immunosurveillance or cancer restraint (Gun et al., 2019). Since in the TME, monocytes may distinguish into two major groups of macrophages based on the chemical makeup of the tumor site, M1-type macrophages are produced in the existence of interferon-gamma (IFN-π), and M2-type macrophages just before subjected to various interleukins (IL, e.g. IL-4 or IL-10), translating growth factor-beta (TGF-β), stimulative granulocyte-macrophage colony (Martinez and Gordon, 2014; Mulder et al., 2017). This polarization of macrophages is important for tumor diagnosis and treatment as M1-type is linked with a strong prognosis, whereas tumor-associated macrophages (TAMs) typically has M2 phenotype and lead to metastasis tumor formation, and angiogenesis and invasion (Lim et al., 2017; Schülke, 2018). Inflammation is typically seen in TME, initially stimulated by tumor cells (intrinsic pathway) and maintained and/or exacerbated by other elements of TME (Schülke, 2018). A pro-inflammatory condition normally comes with a bad prognosis (Inácio Pinto et al., 2015). The TAMs-mediated IL-1 cytokine production leads to systemic inflammation and promotes a pro-inflammatory microenvironment (Landskron et al., 2014). The lymphoid descendant cells often have a contrasting function in the growth of tumors. Thus, B cells and regulatory T cells build innate cytotoxic lymphocytes, immunosuppressive microenvironment, and NKT and natural killer cells (NK) cells lead to the immunostimulant TME (Balato et al., 2009; Vivier et al., 2012; Krijgsman et al., 2018). The improved expression of GM-CSF and VEGF induces the formation of myeloid-derived suppressive cells (MDSCs) at the bone marrow, which is deployed to the TME while cells stay undifferentiated (Vetsika et al., 2019; Horikawa et al., 2020). MDSCs are generally associated with bad prognosis as they include angiogenesis and inhibition CD8 + cytotoxic T cells and NK cells (Horikawa et al., 2020).
In epithelial cancers, increasing tumor cells and parts of TME cells are endorsed in an ECM with disorder characterized and biomechanical characteristics comparison with healthy tissues (Brauchle et al., 2018). The reduced oxygenation and inflammatory environment cause changes in ECM proteins that lead to desmoplasia, accompanied by high rigidity (Poltavets et al., 2018) Collagen types I, III and IV, fibronectin, laminin, hyaluronic acid (HA) and osteonectin are the key contributors of ECM to desmoplasia (Mouw et al., 2014).
Stromal cells are often essential to the growth and prognosis of tumors. Because of the inflammatory environment, mesenchymal stromal cells (MSCs) are recruited into the tumor and can facilitate or impede tumor progression as per the chemical composition at the TME (Zhou et al., 2013; Plava et al., 2019). The activation and consequent release of TGF-β in the TME cause the transformation of fibroblasts into fibroblasts consistent with cancer (CAFs) (Sloin et al., 2018). In addition to tumor cells, CAFs are the most prevalent type of cell at the TME and play a significant role in increased TME desmoplasia (Kilari et al., 2018). Enhanced desmoplasia and Hypoxia, interactions between the different TME players facilitate the epithelial-to-mesenchymal transformation (EMT) of tumor cells contributing to the development of stem cells of cancer (SCC) (Martin et al., 2016). EMT leads to disturbance of intracellular adhesion and lack of cell polarity, granting CSC migratory capacity to reach neighboring blood or lymph vessels at TME and move to some other anatomical position when they through undergo mesenchymal-to-epithelial transformation (MET) and potentiate metastatic groove development (Roma-Rodrigues et al., 2019). Matrix metalloproteinases (MMPs) play an essential part in EMT and are responsible for the formation of tumor cells from ECM that promote CSC development (Nisticò et al., 2012; Tsai and Yang, 2013).
In the early stages of tumorigenesis, tumor growth is determined by the genomic makeup of tumor cells. As the tumor grows, TME and tumor advancement are dictated by cell signaling between cancer and surrounding tissue, adding value to intra and intertumor heterogeneous nature (Han et al., 2013; Nielsen and Schmid, 2017). Exosomes are crucial for the interaction between cells. Exosomes are endosomal vesicles with a diameter of 30–100 nm, consisting of a lipid bilayer comprising membrane proteins, trapping soluble proteins, signaling molecules including chemokines, growth factors, and cytokines, and nucleic acids including miRNA and mRNA (Corrado et al., 2013). Notably, the composition of exosomes relies on the precursor cells, which also represents the cell’s physiological response (Simons and Raposo, 2009). Upon release into the extracellular world, secondary cells lateral to the primary cell can embrace exosomes, or migrate across the vascular or lymphatic network to another anatomical place where local cells will internalize them. Ever since internalized, exosomes can modify the participant cell’s phenotype, which could adapt to incoming signals (Mashouri et al., 2019). Tumor cells generated exosomes (TCDEs) play a significant role in the development of tumors, and the regulation of the immune system, leading to the natural tumor movement of neighboring cells and planning the metastatic niche at a newly anatomical position (Mashouri et al., 2019). TME advancement, as mentioned, is remarkably analogous among different cancers, displaying many other similar characteristics in structure and organization. TME characteristics are often highly tissue/organ based, though. Hematological tumors for instance may display the decreased angiogenesis in the bone marrow at the TME area (Han et al., 2016; Zheng et al., 2016).
Given the increasing understanding of the TME’s function in tumorigenesis, tumor development and organism metastasis, report focus to create models, replicate environments, and test experimental drugs. Moreover, many of these showings and evaluations are performed utilizing conventional cell lines which only represent the actual tumor in the persons, leaving the entire heterogeneity of the intra- and inter-tumors benefit of the entire. Among the most pivotal role in the development of biological diagnostics of effects and treatment interventions for tumor has promptly developed suitable results to replace variable and expensive in vivo models (Jean-Quartier et al., 2018).
3. Strategies to modulate TME through NPs
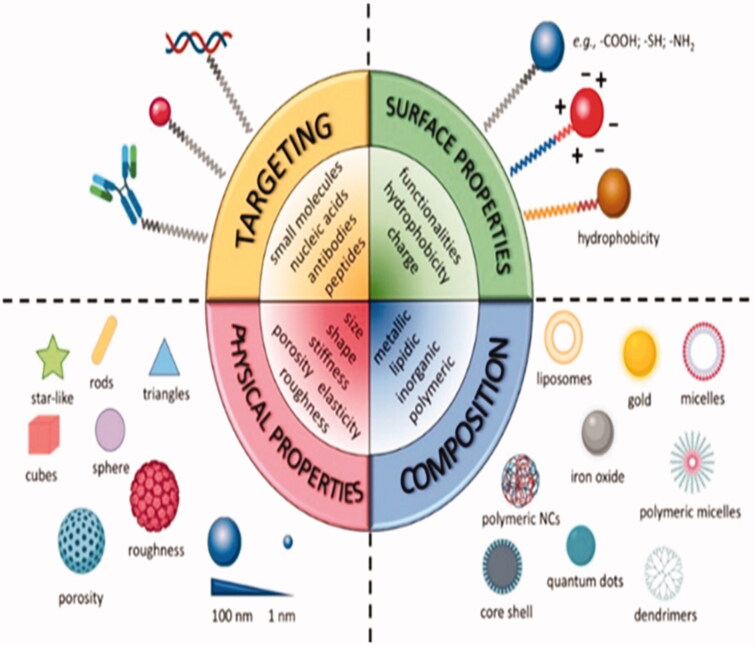
NPs targeting systems have proved to be suitable carriers for effective co-delivery of a range of cargoes, including medications, medicinal peptides, and organic compounds. NPs exhibit various tunable properties that can modulate the TME and improve the therapy (Figure 3). The TME’s particular hallmarks, including such weakly acidic pH extracellular matrix, redox potential, hypoxia, etc., were exploited to develop highly targeted delivery systems (Haider et al., 2020). By triggering CTLs, the localized provision of acceptable antagonists and inhibitors can serve as an incentive. Many authors have recently investigated the tremendous potential of engineered NPs to modulate the TME by breaking down potential obstacles (Blanco et al., 2015; Gao, 2016b; Yang and Gao, 2017; Zhou et al., 2020). Some of them are elaborated below.
Figure 3.
Tunable physical and chemical properties of nanocarriers (NCs) (Reproduced from Salvioni et al., 2019).
3.1. Reverting immune suppression
Tumors use Immune Control strategies to escape. Many cancer immunotherapies have the principal purpose of maintaining successful immune surveillance. Among many of the numerous processes that control immune escape, tumor microenvironment-associated soluble factors, and/or surface-bound molecules are largely responsible for tumor-specific cell defective behavior (Papaioannou et al., 2016). Such complex immunosuppressive networks inhibit multilevel tumor rejection while reducing immunotherapy performance. Strategies to distribution based on NP allowed immune suppression to be reversed by hindering the IDO pathway. GuangjunNie’s group intended a peptide-assembled nanostructure (DEAP-DPPA-1) usually contains hydrophobic and hydrophilic domains where PLGLAG, a peptide substratum of abundantly expressed proteinases, e.g. matrix metalloproteinase-2 (MMP-2) and functional 3-dimethyl aminopropyl isothiocyanate (DEAP) were combined to make a hydrophobic domain, whereas the hydrophilic domain consisted of a peptide antatic domain (Tang et al., 2013). Within physiological conditions the administered NLG919, a well-known IDO inhibitor, co-assembled into micelle-like NPs. The undistributed hydrophobic nucleus of NPs in the acidic tumor niche allowed MMP-2 to hydrolyze and cleave the peptide substratum and start releasing modified DPPA-1 and NLG919 accurately (Saeed et al., 2019). The controlled release of updated DPPA-1 and NLG919 meant that immunosuppressive channels, such as PD-L1 and IDO, were inhibited, simultaneously and eventually rescued. After treatment, the levels of IFN-ÿ and IL-2, and the amount of NK cells were increased (Guerrouahen et al., 2019).
3.2. Improving permeability through inflammatory mediators
Inflammatory mediators including TNFα, prostaglandin analogs, VEGF, and nitric oxide (NO) donors, which are capable of improving vascular permeability, were reported to improve the concentration of nanomedicine in tumors up to 2-6 times more than the control group (Abdulkhaleq et al., 2018). In addition to increased vascular permeability, vasodilatation, and optimizing blood flow by the use of inflammatory mediators have led to improving the production of nanomedicine for cancers (Nehoff et al., 2014). Nevertheless, a sequence of actions of the above listed inflammatory mediators may also contribute to increased IFP against the delivery of nanomedicine. The accretion of nanomedicine in cancers is therefore highly based on such variables. Because inflammation may potentially facilitate the growth of cancer cells, local application (or direct distribution to the tumor cells of inflammatory mediators) must be incorporated (Dawulieti et al., 2020).
3.3. Vessel normalization strategy to improve the delivery of nanomedicine
Various approaches are adopted to manipulate the blood and tumor vessels to modulate the TME and facilitate drug delivery. Some of them are discussed below.
3.3.1. Normalization of tumor vasculature
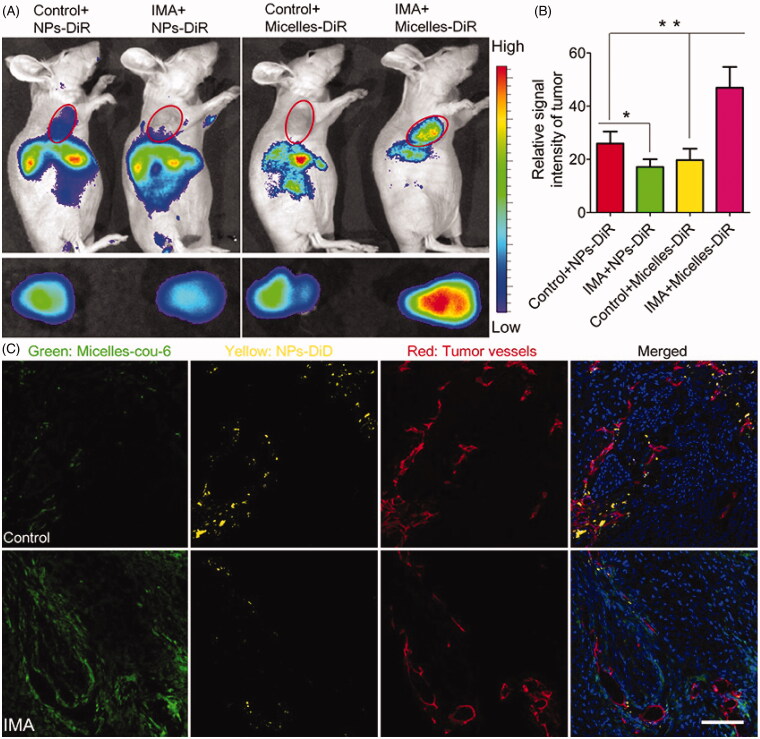
The freshly developed tumor vessels are still tortuous and leaky, enabling both for extravasation of nanomedicine while at the same time the IFP, which inhibits sufficient and homogeneous nanomedicine blood circulation and systemic flow (Chen et al., 2017). Standardization of the vessels has emerged as an important solution to optimizing nanomedicine distribution for tumor therapy. Regularization of the vessels turns the pathological phenotype of the tumor vessels into a phenotype that strongly matches that of normal completely functioning vessels by restoring the basal layer and growing pericyte distribution and eventually reducing vessel leakage (Zhang et al., 2019; Wang et al., 2020). Increasing the tumor vessel architecture could greatly reduce fluid extravasation and lower IFP, and instead restore tumor blood flow, thus working to improve nanomedicine vascular transport. Several proangiogenic molecules, such as VEGF, fibroblast growth factor (FGF), and PDGF, are over-expressed in tumors and engaged in angiogenesis, which induces disorderly structural formation in such freshly developed tumor vessels (Zhao and Adjei, 2015; Zhang et al., 2016). Consequently, techniques were developed to suppress these proangiogenic signaling molecules and restore tumor vessels. In the diagnosis of metastatic colorectal cancer, for example, VEGF inhibitors Bevacizumab, the FDA-approved antiangiogenic monoclonal antibody (mAb), capable of restoring irregular tumor vessel configuration to a more natural phenotype, is added (Kong et al., 2017). It has culminated in the creation of therapies to inhibit these proangiogenic signaling molecules and rebuild tumor vessels. In the treatment of metastatic colorectal cancer, for example, VEGF inhibitors Bevacizumab, an antiangiogenic monoclonal antibody (mAb) approved by the FDA, which can return abnormal tumor structure to a more normal phenotype (Saxton and Sabatini, 2017), Notch 1 signaling (Lee et al., 2015), and D2 receptors-angiopoietin 1 signaling (Chauvet et al., 2017). Often interested in vessel standardization, to boost the production of nanomedicine. By previous work, imatinib mesylate (IMA) has also been shown to normalize tumor vessels of A549 tumors by preventing the signaling mechanism of the platelet-derived growth factor (Zhang et al., 2016). Interestingly, treatment with IMA could significantly reduce the aggregation of NPs (NPs) by about 110 nm but increased the accumulation of micelles by about 23 nm. In comparison, IMA therapy reduced the distribution of NPs within tumors but enhanced that of micelles with a more homogeneous pattern (Figure 4) (Gao, 2016a; Zhang et al., 2016). Eventually, the anti-cancer effectiveness analysis found that pretreatment with IMA could substantially increase the therapeutic impact of paclitaxel-laden micelles. As tumor vessel normalization narrowed endothelial space, tumor cells could be stopped from splitting into tumor vessels, and tumor metastasis could be decreased to some degree.
Figure 4.
The effects of IMA treatment on tumor NP delivery. (A) In vivo fluorescence imagery of A549 xenograft-bearing mice (upper row) treated with IMA or water as a buffer, ex vivo fluorescence imagery of their respective xenografts (lower row), and (B) Relative tumor tissue signal intensity 24 hours after DiR-labeled NPs or micelles are injected. *p < .05, compared with Control + NP group. **p < .01 compared with the IMA + Micelles group. (C) In vivo dissemination of micelles and NPs from A549 tumor xenograft-bearing mouse models treated with IMA or water 24 h after i.v. in tumor slices Injection of a combination of DiD-labeled NPs and micelles labeled with coumarin-6. During 3 weeks the oral dose of IMA was 50 mg/kg/d. All the coumarin-6 and DiD concentrations were 0.05 mg/kg. 100 μm was indicated in the slot (Reproduced from Zhang et al., 2016).
Four concerns must be taken into consideration to use vessel normalization strategy to improve the delivery of nanomedicine for tumor treatment. First, the technique will only boost the distribution of low molecular weight drugs or comparatively smaller nanomedicines varying from 20 to 40 nm but reduces the transmission of large nanomedicines by about 100 nm because it eliminates cancer vessel endothelial gaps (Mattheolabakis and Mikelis, 2019). Second, the regularization is reversible and the nanomedicine that occurs will be introduced in the normalization cycle (Chauhan et al., 2012). Third, it is strongly advised that a judicious dosage of vascular normalizer avoids unnecessary pruning of tumor arteries, which may impede vascular capacity and therefore the transmission of simultaneous therapy (Cheng and Saltzman, 2012). Fourthly, provided that vasculatures are still extremely cramped in strongly desmoplastic tumors and refractory to vasculature normalizers, this technique should either be used during tumors that are relatively porous and not extremely desmoplastic or at least paired with the other techniques that can restart crushed vessels (Jiang et al., 2015).
3.3.2. Tumor vessel dilation
Vasoconstrictive endothelin-1 (ET1) and its ETA receptor, by which ET-1 mediates vasoconstriction, are also present in cancer cells to preserve the tumor vessel contractile signal. The frequency of expression of ET1 and ETA in tumor vessels was 13 and 5 times greater than that of regular vessels balanced in scale, accordingly (Kowalczyk et al., 2015). BQ123, a selective antagonist against ETA, can prevent ET1-ETA signaling, stimulate dilation of the tumor vessels, and trigger tumor-specific blood flow growth. The BQ123-induced boost in the blood flow increased the distribution of free drugs to tumors following a rise in IFP (Zamora et al., 1993). Furthermore, it has been shown that BQ123 could promote the levels of photothermal nanomedicine by about 100 nm for successful photothermal treatment of tumors (Zamora et al., 1993). A few inflammation variables, including such bradykinin, which can dilate vessels, also could boost tumor perfusion directly. In our previous research, captopril, a commonly used hypotensor in clinics, has been shown to dilate tumor blood vessels by growing the bradykinin expression and also growing the permeability of tumor vessels to increase the distribution of nanomedicine for cancer treatment (Zhang et al., 2017a).
An IDO pathway is designated as a crucial immunosuppression regulator. Engineered NPs allowed immune suppression to be reversed by blocking the IDO pathways (Mbongue et al., 2015). The findings indicate that the NP dependent immunostimulatory formulations have a strong antitumor effect (Feng et al., 2019). Feng et al. (2018) demonstrated that The TME can be mediated by constructing a dual-activatable (acidic and reduced) binary cooperative drug NP(BCPN) in which amphiphilic oxaliplatin (OXA) and NLG919 have been assembled for enhanced immunotherapy treatment. BCPN assured increased absorption and penetration attributable to charging reversing characteristics of deshielded polyethylene glycol (PEG) in acidic TME, whereas OXA prodrug and NLG919 were enabled in the TME to minimize. BCPN can cause ICD successfully and can reverse the immunosuppressive process due to the involvement of OXA and NLG919, collectively. The apparent rise in apoptosis of tumor cells and reduce in tumorigenesis were ascribed to the activation of DCs, CTLs, and pro-inflammatory cytokines (IFN-ÿ). NLG919 has also been found to be beneficial in suppressing intratumoral Treg cell infiltration
3.4. Improving transvascular delivery of therapeutics to TME
For strongly desmoplastic tumors, the pericyte penetration levels on endothelium were around 70%, far higher than porous and permeable tumors, greatly restricting nanomedicine’s transvascular movement through tumor interstitium (Aguilera and Brekken, 2014). Strategies were then formulated utilizing a low dose of a TGF-β receptor, LY364947 to decrease the pericyte distribution of endothelium and to increase the size differences between endothelium to improve the therapeutic benefits of gemcitabine-charged liposomes for pancreatic cancer and Doxil for dispersing gastric tumor (Miao et al., 2015).
Platelets are widely believed to lead a great deal to hemostasis. Besides its position in the creation of thrombosis, platelets are also deeply engaged in tumor progression and metastasis. Additionally, it may also help tumor vascular homeostasis and preserve tumor vessel integrity (Gay and Felding-Habermann, 2011). The research found that the removal of platelets caused bleeding at the tumor site and decreased tumor vasculature leakiness. Platelet elimination in thrombocytopenic mice also improved the effectiveness of breast cancer therapies (Wang et al., 2018b). To avoid potential bleeding in normal organs caused by low platelet counts, a recent study by Li et al. (2017) designed a tumor microenvironment-responsive NP worthy of distributing antiplatelet antibody R300 to specifically reduce platelets in cancer cells, thereby increasing endothelial dysfunction and enhancing the distribution of nanomedicine to tumors. Platelet reduction was a viable candidate for increased transvascular nanomedicine delivery to tumors.
3.5. Targeting lymph nodes targeting strategy to modulate TME
Although all of the NP structures either spread in the bloodstream or are meant to concentrate in the tumor, attacking the lymph nodes is another critical field where NPs may have a major effect. Irvine et al. have reported many papers on NPs in the polymer that travel to lymph nodes (Liu et al., 2014). To provide higher therapeutic benefits on cancer vaccines, vaccine adjuvants should accrue in lymph nodes, in which naïve T and B cells are fully prepared. CpG is a DNA sequence that binds TLR9 and may be a strong immunostimulant, but free CpG does not concentrate on lymph nodes. Irvine et al. conjugated CpG to a lipophilic albumin-binding domain and demonstrated that such peptide vaccines travel to lymph nodes through albumin hitchhiking, based on the nanoparticle. One week after injection, the concentration of albumin-binding CpG-liposomes was 6 times higher than those of soluble CpG in lymph nodes but this mechanism also contributed to a persistent regression of tumors in murine melanoma models (Liu et al., 2014; Mehta et al., 2015). Injecting vaccines into lymph nodes improves the efficacy, however timely clearance of vaccines remains a concern. To overcome this problem, Irvine et al. merged nanoparticle-based vaccinations with intralymph node injections. The combination of intralymph node vaccination strategies with a PLGA micro- or nanoparticular-conjugated TLR3 agonist improved lymph node aggregation, enhanced T-cell cytokine development and resulted in more sustained DC stimulation in immunized mice (Andorko et al., 2014; Mehta et al., 2015).
Through comparison, in tumor-bearing mice, Swartz et al. inserted lymph node-targeting nanoparticle-conjugated TAA and adjuvant intradermally. Those NPs collected efficiently in the lymph nodes given the particular distribution pathway. Besides that, once bonded to NPs and inserted into the TAA-primed tumor-draining lymph node the vaccine had stronger therapeutic effects. Following vaccine administration, the immunosuppressive condition of the tumor-draining lymph nodes was restored toward a more immunogenic setting (Maisel et al., 2017). Swarz et al. have used pyridyl disulfide NPs aimed at tumor-draining lymph nodes to distribute hydrophobic DC inducing agents like TLR9 agonist and TLR4 agonist paclitaxel. They demonstrated higher DC ripening, additional production of IL-12, and slower tumor growth utilizing this delivery method (Stewart and Keselowsky, 2017). Similar findings suggest that functionalized NPs are capable of transmitting adjuvant cancer vaccines to lymph nodes and growing immune responses, using a range of distribution routes. Using NPs can boost the adjuvant’s circulation time due to the complex’s larger size, and functionalized particles could even specifically attack crucial areas like the lymph nodes.
3.6. Physical stimulus
Radiation may enhance the distribution of nanomedicine intended for tumors (Xin et al., 2017). Several potential pathways are as follows: firstly, through triggering the hypoxia-inducible factor 1 (HIF1), radiation may regulate cell the amount of the vascular endothelial growth factor (VEGF) (Moeller et al., 2004) or via numerous mitogen-activated protein kinase reliant paths to boost tumor vessel permeability. Evidence indicated that perhaps the permeation of magnetic resonance contrast agent with molecular weight over 200 kDa by the cancerous cells was improved by 32.8% after irradiation (10 Gy) (Reitan et al., 2010). Furthermore, radiation will easily destroy the cells of the susceptible tumor. The decreased cell density helped to alleviate tension burden from tumor cells, reopen closed arteries and thereby improve the blood supply of tumors (Delarue et al., 2014). The impact of radiation on tumors is dynamic and depends on the amount of dosage, duration, and tumor (Dolega et al., 2017). Milosevic’s recent review provides further evidence of the same
Koning et al. (2010) pioneered Enhanced vascular permeability with the usage of moderate hyperthermia (HT) for nanomedicine extravasation of tumor tissues. Research has also shown that mild HT may also help increase tumor perfusion and decrease IFP, potentially by vascular fenestration and vascular endothelial disruption, thus allowing for deep nanomedicine infiltration into the cancers instead of perivascular aggregation (Stylianopoulos, 2017). There has been, nevertheless, no clear evidence to prove the tumor vessel pore size change after HT treatment. The extent and severity of extravasation in tumor interstitium were known to differ widely between cancer cell forms, which depends on the morphology of the endothelial lining and the intrinsic properties of the underlying tumor microenvironment, such as interstitial matrix composition. Highly desmoplastic tumors, other than cancers of a certain vascular component, also could react well with mild HT therapy (Golombek et al., 2018). Temperature is a key component in the heat source, in which data showed that 41–43 °C was suitable that a very maximum temperature could harm the tumor vessel’s endothelial lining and stimulate response to coagulation. The creation of thrombins could choke the vessels and negotiation the delivery of nanomedicine. Conversely, inadequate temperature could have a limited impact on the endothelium of the tumor vessel to raise the endothelial gap (Cicha, 2015; Karagkiozaki et al., 2016).
Ultrasound was used to boost the transmission of nanomedicine to tumors through both mechanical and HT impact (Tharkar et al., 2019). For structural results, several studies have shown that gas-filled bubbles may be used to transiently create pores in blood vessels or cell membranes (sonoporation) through which nanomedicines of various types may easily extravasate tumor vessels or penetrate tumor cells, thereby enhancing nanomedicine distribution (Han et al., 2017). Besides, ultrasound also generates energy at a time-dependent, acoustic intensity. Frazier previously using magnetic resonance imaging-guided, high-intensity oriented ultrasound (HIFU) to achieve a nearly uniform heating pattern of 43 °C in a xenograft tumor model and increased the aggregation of Evans blue dye in warmed tumors to nearly 2-fold better than those in unheated cancers (Hijnen et al., 2014)
3.7. Companion diagnostic
The companion diagnosis, which corresponds to a patient stratification dependent on tumor properties, is a compelling approach to boost the effectiveness of nanomedicine. There are presently numerous approaches under review, focused on the usage of biomarker signatures and image evidence (Hare et al., 2017). The very first sought to assess TME-associated circulatory proteins highly linked to the EPR effect. For example, the proportion of MMP9 to the metalloproteinase tissue inhibitor 1, the collagen content in the capillary walls, and other angiogenesis markers has also been shown to forecast the EPR agent (Salvioni et al., 2019). But on the other side, radio-labeled and ferumoxytol-charged NCs are being adopted to supervise their bioavailability using noninvasive techniques (e.g. Tomography and magnetic resonance imaging measured single-particle emission or electron absorption, respectively). The finished step is to obtain students with the best probability of responding positively to a particular clinical intervention (Greish et al., 2018). These methods, though, should be further tested by reliable correlative tests, establishing a consistent range of parameters and requirements that can forecast the clinical result. Table 1 summarizes TME modulation strategies for improving tumor nanomedicine delivery.
Table 1.
Summary of TME modulation strategies for improving tumor nanomedicine delivery.
| Modulation approach | Working mechanism | Agents | Tumor model | Reference |
|---|---|---|---|---|
| Improving interstitial transport | Reprogramming or depletion of TAF | Quercetin NP downregulating the expression of Wnt16 | Bladder tumor | Hu et al. (2017) |
| Losartan | Human pancreatic, skin and breast tumors | Diop-Frimpong et al. (2011); Chauhan et al. (2013) | ||
| VDR ligand | Pancreatic cancer | Sherman et al. (2014) | ||
| ATAR | Pancreatic cancer | Chronopoulos et al. (2016) | ||
| ECM degradation | PEGPH20 (PEGylated hyaluronidase) | Pancreatic cancer | Hingorani et al. (2016) | |
| Matrix metalloproteinases-1 and − 8 | Sarcoma | Mok et al. (2007) | ||
| rtPA | Melanoma and Lung cancer | Kirtane et al. (2017) | ||
| ECM reduction through inhibiting TAF activity | Cyclopamine | Pancreatic cancer | Jiang et al. (2017) | |
| IPI-926 | Pancreatic cancer | Olive et al. (2009) | ||
| Advancing tumor perfusion | Tumor vessel dilation | BQ123 | Colorectal carcinoma | Wang et al. (2017) |
| Captopril | Glioma | Zhang et al. (2017b) | ||
| Tumor vessel normalization | Chloroquine (Notch 1 signaling inhibition) | Melanoma | Maes et al. (2014) | |
| Imatinib mesylate | Lung cancer | Zhang et al. (2016) | ||
| DC101 | Colon adenocarcinoma, small cell lung carcinoma, glioblastoma multiforme, Mammary carcinoma, | Tong et al. (2004) | ||
| Rapamycin | Melanoma | Guo et al. (2014) | ||
| Dopamine | Colon and prostate tumor | Chakroborty et al. (2011) | ||
| Improving nanomedicine extravasation | Platelet depletion | R300 (Antiplatelet antibody) | Breast cancer | Li et al. (2017) |
| Inflammatory mediators for enhancing vessel permeability | VEGF | Colon and Glioma carcinoma | Monsky et al. (1999) | |
| TNF- alpha | Melanoma and lymphoma | Curnis et al. (2002) | ||
| Prostaglandin | Hepatocellular carcinoma | Tanaka et al. (2003) | ||
| Pericyte depletion by inhibiting TGF signal pathway | TGF- type I receptor (TR-I) inhibitor | Gastric cancer, Pancreatic cancer. | Kano et al. (2007) | |
| A small-molecule TGF-β inhibitor, LY364947 | Pancreatic cancer | Meng et al. (2013) | ||
| ID11 (anti-TGF-β mAb) | Breast cancer | Liu et al. (2012) |
4. Nps in combination therapies for modulating the tumor microenvironment
After a mixture of traditional treatments, such as surgery, radiotherapy, and chemotherapy, cancer relapses contribute to clinical failure. The implementation of new approaches to fight cancer is crucial. Specific approaches will be based on increasing immune reaction, which will stimulate immune memory to resolve the cancer relapse (Subhash et al., 2015). NPs can be used in a joint venture with different therapeutic approaches to modulate the TME. Some of them are discussed below.
4.1. Nps with immunotherapies to modulate TME
ICD-inducing chemotherapeutic entity, such as doxorubicin, oxaliplatin, and cisplatin, was delivered with NP-based formulations, resulting in complementary immunotherapy reactions when coupled with IDO inhibitor (indoximod) or immune control blockade (Wang et al., 2016). Differential diagnoses, like chemo-PDT therapy, are not successful toward metastasis; nevertheless, the mixture of chemo-PDT therapy with checkpoint blockade therapy could not only prevent tumor growth and also display promising results toward cancer growth owing to the reverse of T – cells exhaustion (Agostinis et al., 2011). Chlorine e6 and doxorubicin-loaded hollow manganese dioxide nano platform (H-MnO2-PEG/C&D) will alleviate tumor suppression, although checkpoint blocking (PD-L1 blocking) promotes higher TNF-α secretion and improved immune response of CD4 + and CD8 + T cells that chemo-PDT therapy (Yang et al., 2017). For enhancing the ability of immunotherapy, Zhou et al. designed the nanoplatform by combining the OXA prodrug and PEGylated Photosensitizer (PS) that induced ICD and cancer cell phagocytosis by blocking CD47. The application of ICD activation and CD47 blockade strengthened T-cell – mediated reaction, DC maturation tolerance to antitumor, resulting in clinical outcomes, tumor metastasis, and tumor relapse prevention (Gao et al., 2019). Lymphatic metastasis inhibition was due to combined therapy in the B16‐F10 model (Potez et al., 2018). An apparent rise in recruitment of DCs, tumor-infiltrating CTL (CD8+ and CD4+), and as well as a substantial decrease in Treg cells is reported when paired with PDT immunotherapy. As a result, more than 90% of tumor growth was due to iron NP-based relaxed immunosuppression and penetration of T cells (Chen et al., 2016).
TLR agonists and control-point blockade strategies are combined with photothermal and radiotherapy to achieve effective therapeutic efficacy and immunological memory (Chen et al., 2016). The integration of the NP-based sonodynamic treatment framework of immunotherapy blockade and immune adjuvant avoids tumor metastasis and induces an antitumor and immune response by inducing robust immune responses, including increased maturation of DCs, CD4 + and CD8 + lymphocyte infiltration, CD45 + leucocytes, and cytokine secretion (Saeed et al., 2019). The integration of NP-based immunotherapy with other treatment regimens will also unlock the capacity for cancer therapies. Even so, a fuller knowledge of the factors involved in the immune systems and the NPs-mediated toxic effects must be given immense attention (Saeed et al., 2019). Furthermore, NPs are also reported to modulate the TME and improve the efficiency of CAR-T therapy. It can ease the manufacturing process of CAR-T cell production and modulate to complex solid TME to enhance the efficiency of therapy (Nawaz et al., 2020).
Perhaps the more productive method to anticancer treatment is to target a combination of the TME’s vascular, ECM, and immune cells and the actual tumor cells as well. Liu et al. conducted a study wherein liposomes have been used to encompass anti-VEGF agents and adorned via an antagonist CXCR4 to attack all angiogenic and immune responses in a model of hepatocellular carcinoma (Martin et al., 2020). CXCR4 is abundantly expressed both in cancer and immune cells inside the TME and acted as both the targeting ligand and the immune response modulation process (Xu et al., 2015). These CXCR4-targeting liposomes were first compared with sorafenib, a currently licensed anti-VEGF small-molecule medication, and combined therapy was shown to be more successful than both therapies alone. Those who then substituted sorafenib to anti-VEGF siRNA, load current combination with the targeting liposomes delegated authority vessel density and inhibited tumor growth, and prevented TAM from infiltration into the cancer cell (McCallion et al., 2019). NP-based approaches may be implemented to leverage the ICD-inducing properties of traditional therapies to enhance cancer immunotherapy’s therapeutic ability. Therapeutic agents focused on NP, including phototherapy, photodynamic therapy, radiation therapy, and immunotherapy chemotherapy enhance clinical efficiency by allowing the concurrent distribution of different therapeutic substances (Lim et al., 2019; Wu et al., 2020).
4.2. Nps in combination with the drug for TME modulation
In addition, drug combination can attack both the TME and the tumors cells oneself. A further cohort reported recently a multivolume nanocarrier capable of delivering multiple anticancer agents and assembling them within the TME into ‘drug delivery depots.’ Whose pH-sensitive carrier produced HA, that traffic toward tumor cells overexpressing both the CD44 HA receptor and hyaluronidase (HAase). As near to the tumor site, HAase cleaves HA, which causes the crosslinking of certain nanocarrier elements, creating depots that are slowly destroyed by the TME acidity. Once packed with TNF-related apoptosis-inducing ligand and anti-angiogenic drug cilengitide, such carriers resided at the tumor site, collated in depots, continued cargo activation, decreased tumor vascularization, and slowed significantly tumor growth without adverse side effects. Furthermore, those who recommended that this platform for NPs can also be used to carry a wide range of cargo like small-molecule chemotherapeutic agents and immune modulators (Hu et al., 2016). Finally, Jiao and coworkers used drug combination in tumor theranostics and often has medicinal advantages for a diagnostic agent. They paired gold NPs with a chimeric tumor binding antibody, anti-GD2, adjusted to improve NK cell activity by interacting with the Fc receptor. The NPs successfully trafficked to cancer cells expressing GD2 and increased computed tomographic contrast, so even small tumors had been visible on diagnostic scans. Antibodies Fc regions bound to the Fc receptors on NK cell surfaces and induced an immune response to cancer cells. Intriguingly, once conjugated with the NPs, the antibodies had a larger impact on NK stimulation than it was when using individually, likely owing to the arrangement of several antibodies linked from each NP (Jiao et al., 2016). Thereby, drug combination can significantly impact numerous facets of TME, target tumor cells and TME, or provide both diagnostic and therapeutic impacts.
4.3. Np based combined chemotherapies to modulate TME
Damage-associated molecular patterns (DAMPs) are revealed to the surface by dying/stressed cells, released or secreted. DAMPs, including surface-exposed CRT, passively released HMGB1, secreted ATP, and heat-shock proteins that may act as either hazardous signals or immune system adjuvants to induce ICD in cancer cells (Krysko et al., 2013; Land, 2015). When tumor cells die, DAMPs are emitted that can serve as a warning or combinatorial code to activate various inflammatory cells. ICD adjuvants, like chemotherapeutic agents (anthracyclines and oxaliplatin) and photodynamic therapy (PDT), may activate immunologic apoptosis where dendritic cells swallow their bodies and present T-cell tumor-specific antigens to cause an immune reaction to antitumor (Hou et al., 2013).
By inciting the threat signaling pathways, ICD may be caused by the generation of ROS and endoplasmic reticulum tension (ER). The pressure-specific pathways include the release of main DAMPs, including CRT and ATP (Farooqi et al., 2015; Cubillos-Ruiz et al., 2017). Thus, PDT may cause ICD in cancer cells by inducing ER stress-dependent on ROS. Cells treated with anthracycline will also cause ICD and provoke an immune reaction to the antitumor without any adjuvant. The tumor cells which experience ICD may cause CRT to be translocated on their cell membrane, which eventually results in tumor-specific cytotoxic T lymphocyte-mediated immune responses (Zhou et al., 2019). While PDT and chemotherapy that activate an anti-tumor immune response, T cell fatigue significantly impairs the mediated immune response by PD-1/PD-L1 up-regulation. T cell exhaustion may be altered by obstructing the pathways to the immune control point (for example PD-1) (Asadzadeh et al., 2020). Immunotherapy with a blockade at the immune control point could be used to measure the intensity and usability of tumor-specific T cells to intensify antitumor effectiveness (Wang et al., 2018c).
Wang et al. (2013) designed combining several features including ultra-pH-sensitive diblock (PDPA), and siRNA and pheophorbide A photosensitizer (PPa), may improve therapeutic ability. Hydrolyzed amphiphilic polycation, e.g. 1,2-epoxytetradecane alkylated oligoethylenimine (OEI-C14), having an intrinsic binding affinity with siRNA, thereby promoting proton sponge to ensure the endosomal escape of siRNA. PDPA-OEI-C14-PPa (POP) micelleplex was activated precisely in acidic pH (6.2) while maintaining an intact epithelial microenvironment. The pdna-PD-L1-conjugated micelleplexes (POP-PD-L1) efficiently triggered blockade of PD-L1 and therefore rescued the tumor cells from immunosuppression through silencing the expression of PD-L1. The immune reaction to antitumor was intensified by photodynamic therapy (PDT), where it effectively eradicated the tumor and remote metastasis in the B16-F10 melanoma model through encouraging cytokine production (TNF-α and IFN-ÿ) and tumor invasion lymphocyte frequency (CD8+ and CD4+). Within a week of combination therapy, its most apoptotic cancer death was observed than individual modalities and was thus ultimately caused by the existence of activated immune cells.
Doxorubicin-charged lipoprotein-mimicking nanodiscs (sHDL-DOX) may impose antitumor effectiveness by activating tumor cells ICD (Kuai et al., 2018). Composite chemoimmunotherapy may instruct cancer cells to blockage of the immune checkpoint and potentiate the cell-mediated response to antitumor T. Through delivering chemotherapeutic agents via nanodisks, the removal of therapeutic action (MC38 and CT26 colon carcinoma) in 80–88% of animals was achieved. Survivors are shielded from tumor relapse because of the mediated antitumor memory (Wang et al., 2018a).
Conclusion
TME has also been involved in growing and metastasizing cancer. With-tumor awareness, tumors are shown to develop in increasingly heterogeneous but diverse microenvironments composed of ECM elements, immune cells, vasculature, TAMs, and CAFs. Recent advances indicate that TME modification as well as its unusual structure is an effective technique for curbing tumor growth, invasion, and metastasis. New strategies for addressing the increasing cancer challenge have grown with the introduction of nanotechnology in the drug discovery field. Even so, the sophistication of the TME has already shown an important so far provocative role in the regulation of deeper nano-chemotherapeutic tumor absorption and consequently its biochemical mechanisms. Strategies have been proposed to tackle this challenge utilizing nanotechnology to resolve the resistance mechanism caused by the tumor coverage.
Funding Statement
This work was supported by Zhejiang Provincial Science and Technology Projects [grants no. GF20H020041 to HS, LGD19H160001 to JKT], Shaoxing Municipal Science and Technology Projects [grant no. 2018C30016 to FXL], and National Natural Science Foundation of China [Grants No. 81772537 to JKT; 81374014 to JKT].
Disclosure statement
All the authors declare that they have no conflict of interest to declare.
References
- Abdulkhaleq LA, Assi MA, Abdullah R, et al. (2018). The crucial roles of inflammatory mediators in inflammation: a review. Vet World 11:627–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agostinis P, Berg K, Cengel KA, et al. (2011). Photodynamic therapy of cancer: an update. CA Cancer J Clin 61:250–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguilera KY, Brekken RA. (2014). Recruitment and retention: factors that affect pericyte migration. Cell Mol Life Sci 71:299–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrecht I, Christofori G. (2011). Molecular mechanisms of lymphangiogenesis in development and cancer. Int J Dev Biol 55:483–94. [DOI] [PubMed] [Google Scholar]
- Andorko JI, Tostanoski LH, Solano E, et al. (2014). Intra-lymph node injection of biodegradable polymer particles. JoVE e50984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asadzadeh Z, Safarzadeh E, Safaei S, et al. (2020). Current approaches for combination therapy of cancer: the role of immunogenic cell death. Cancers 12:1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awasthi R, Roseblade A, Hansbro PM, et al. (2018). Nanoparticles in cancer treatment: opportunities and obstacles. Curr Drug Targets 19:1696–709. [DOI] [PubMed] [Google Scholar]
- Balato A, Unutmaz D, Gaspari AA. (2009). Natural killer T cells: an unconventional T-cell subset with diverse effector and regulatory functions. J Invest Dermatol 129:1628–42. [DOI] [PubMed] [Google Scholar]
- Blanco E, Shen H, Ferrari M. (2015). Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat Biotechnol 33:941–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brauchle E, Kasper J, Daum R, et al. (2018). Biomechanical and biomolecular characterization of extracellular matrix structures in human colon carcinomas. Matrix Biol 68–69:180–93. [DOI] [PubMed] [Google Scholar]
- Chakroborty D, Sarkar C, Yu H, et al. (2011). Dopamine stabilizes tumor blood vessels by up-regulating angiopoietin 1 expression in pericytes and Kruppel-like factor-2 expression in tumor endothelial cells. Proc Natl Acad Sci USA 108:20730–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauhan VP, Martin JD, Liu H, et al. (2013). Angiotensin inhibition enhances drug delivery and potentiates chemotherapy by decompressing tumour blood vessels. Nat Commun 4:2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauhan VP, Stylianopoulos T, Martin JD, et al. (2012). Normalization of tumour blood vessels improves the delivery of nanomedicines in a size-dependent manner. Nat Nanotechnol 7:383–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauvet N, Romanò N, Lafont C, et al. (2017). Complementary actions of dopamine D2 receptor agonist and anti-vegf therapy on tumoral vessel normalization in a transgenic mouse model. Int J Cancer 140:2150–61. [DOI] [PubMed] [Google Scholar]
- Chen DS, Mellman I. (2013). Oncology meets immunology: the cancer-immunity cycle. Immunity 39:1–10. [DOI] [PubMed] [Google Scholar]
- Chen H, Zhang W, Zhu G, et al. (2017). Rethinking cancer nanotheranostics. Nat Rev Mater 2:17024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Zhen Z, Todd T, et al. (2013). Nanoparticles for improving cancer diagnosis. Mater Sci Eng R Rep 74:35–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Xu L, Liang C, et al. (2016). Photothermal therapy with immune-adjuvant nanoparticles together with checkpoint blockade for effective cancer immunotherapy. Nat Commun 7:13193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng CJ, Saltzman WM. (2012). Nanomedicine: downsizing tumour therapeutics. Nat Nanotechnol 7:346–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chronopoulos A, Robinson B, Sarper M, et al. (2016). ATRA mechanically reprograms pancreatic stellate cells to suppress matrix remodelling and inhibit cancer cell invasion. Nat Commun 7:12630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicha I. (2015). Thrombosis: novel nanomedical concepts of diagnosis and treatment. World J Cardiol 7:434–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrado C, Raimondo S, Chiesi A, et al. (2013). Exosomes as intercellular signaling organelles involved in health and disease: basic science and clinical applications. Int J Mol Sci 14:5338–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cubillos-Ruiz JR, Bettigole SE, Glimcher LH. (2017). Tumorigenic and immunosuppressive effects of endoplasmic reticulum stress in cancer. Cell 168:692–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curnis F, Sacchi A, Corti A. (2002). Improving chemotherapeutic drug penetration in tumors by vascular targeting and barrier alteration. J Clin Invest 110:475–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawulieti J, Sun M, Zhao Y, et al. (2020). Treatment of severe sepsis with nanoparticulate cell-free DNA scavengers. Sci Adv 6:eaay7148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delarue M, Montel F, Vignjevic D, et al. (2014). Compressive stress inhibits proliferation in tumor spheroids through a volume limitation. Biophys J 107:1821–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diop-Frimpong B, Chauhan VP, Krane S, et al. (2011). Losartan inhibits collagen I synthesis and improves the distribution and efficacy of nanotherapeutics in tumors. Proc Natl Acad Sci USA 108:2909–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirkx AEM, oude Egbrink MGA, Castermans K, et al. (2006). Anti-angiogenesis therapy can overcome endothelial cell anergy and promote leukocyte-endothelium interactions and infiltration in tumors. Faseb J 20:621–30. [DOI] [PubMed] [Google Scholar]
- Dirkx AEM, Oude Egbrink MGA, Kuijpers MJE, et al. (2003). Tumor angiogenesis modulates leukocyte-vessel wall interactions in vivo by reducing endothelial adhesion molecule expression. Cancer Res 63:2322–9. [PubMed] [Google Scholar]
- Dolega ME, Delarue M, Ingremeau F, et al. (2017). Cell-like pressure sensors reveal increase of mechanical stress towards the core of multicellular spheroids under compression. Nat Commun 8:14056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farkona S, Diamandis EP, Blasutig IM. (2016). Cancer immunotherapy: the beginning of the end of cancer? BMC Med 14:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farnsworth RH, Lackmann M, Achen MG, et al. (2014). Vascular remodeling in cancer. Oncogene 33:3496–505. [DOI] [PubMed] [Google Scholar]
- Farooqi AA, Li KT, Fayyaz S, et al. (2015). Anticancer drugs for the modulation of endoplasmic reticulum stress and oxidative stress. Tumour Biol 36:5743–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feitelson MA, Arzumanyan A, Kulathinal RJ, et al. (2015). Sustained proliferation in cancer: mechanisms and novel therapeutic targets. Semin Cancer Biol 35(Suppl):S25–S54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng B, Zhou F, Hou B, et al. (2018). Binary cooperative prodrug nanoparticles improve immunotherapy by synergistically modulating immune tumor microenvironment. Adv Mater Weinheim 30:e1803001. [DOI] [PubMed] [Google Scholar]
- Feng X, Xu W, Li Z, et al. (2019). Immunomodulatory nanosystems. Adv Sci (Weinh) 6:1900101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao A, Hu XL, Saeed M, et al. (2019). Overview of recent advances in liposomal nanoparticle-based cancer immunotherapy. Acta Pharmacol Sin 40:1129–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao H. (2016. a). Progress and perspectives on targeting nanoparticles for brain drug delivery. Acta Pharm Sin B 6:268–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao H. (2016. b). Shaping tumor microenvironment for improving nanoparticle delivery. Curr Drug Metab 17:731–6. [DOI] [PubMed] [Google Scholar]
- Garg NK, Tandel N, Jadon RS, et al. (2018). Lipid-polymer hybrid nanocarrier-mediated cancer therapeutics: current status and future directions. Drug Discov Today 23:1610–21. [DOI] [PubMed] [Google Scholar]
- Gay LJ, Felding-Habermann B. (2011). Contribution of platelets to tumour metastasis. Nat Rev Cancer 11:123–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glen CD, Dubrova YE. (2012). Exposure to anticancer drugs can result in transgenerational genomic instability in mice. Proc Natl Acad Sci USA 109:2984–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golombek SK, May JN, Theek B, et al. (2018). Tumor targeting via EPR: strategies to enhance patient responses. Adv Drug Deliv Rev 130:17–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham AM, Presnell JS. (2017). Hypoxia inducible factor (HIF) transcription factor family expansion, diversification, divergence and selection in eukaryotes. PLoS One 12:e0179545–e0179545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greish K, Mathur A, Bakhiet M, et al. (2018). Nanomedicine: is it lost in translation? Ther Deliv 9:269–85. [DOI] [PubMed] [Google Scholar]
- Guerrouahen BS, Maccalli C, Cugno C, et al. (2019). Reverting immune suppression to enhance cancer immunotherapy. Front Oncol 9:1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gun SY, Lee SWL, Sieow JL, et al. (2019). Targeting immune cells for cancer therapy. Redox Biol 25:101174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo S, Lin CM, Xu Z, et al. (2014). Co-delivery of cisplatin and rapamycin for enhanced anticancer therapy through synergistic effects and microenvironment modulation. ACS Nano 8:4996–5009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwangwa MV, Joubert AM, Visagie MH. (2018). Crosstalk between the Warburg effect, redox regulation and autophagy induction in tumourigenesis. Cell Mol Biol Lett 23:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haider T, Sandha KK, Soni V, et al. (2020). Recent advances in tumor microenvironment associated therapeutic strategies and evaluation models. Mater Sci Eng C 116:111229. [DOI] [PubMed] [Google Scholar]
- Han H, Lee H, Kim K, et al. (2017). Effect of high intensity focused ultrasound (HIFU) in conjunction with a nanomedicines-microbubble complex for enhanced drug delivery. J Control Release 266:75–86. [DOI] [PubMed] [Google Scholar]
- Han N, Yang YY, Wang S, et al. (2013). Polymer-based cancer nanotheranostics: retrospectives of multi-functionalities and pharmacokinetics. Curr Drug Metab 14:661–74. [DOI] [PubMed] [Google Scholar]
- Han Y, Wang X, Wang B, et al. (2016). The progress of angiogenic factors in the development of leukemias. Intractable Rare Dis Res 5:6–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. (2011). Hallmarks of cancer: the next generation. Cell 144:646–74. [DOI] [PubMed] [Google Scholar]
- Hare JI, Lammers T, Ashford MB, et al. (2017). Challenges and strategies in anti-cancer nanomedicine development: an industry perspective. Adv Drug Deliv Rev 108:25–38. [DOI] [PubMed] [Google Scholar]
- Hijnen N, Langereis S, Grüll H. (2014). Magnetic resonance guided high-intensity focused ultrasound for image-guided temperature-induced drug delivery. Adv Drug Deliv Rev 72:65–81. [DOI] [PubMed] [Google Scholar]
- Hingorani SR, Harris WP, Beck JT, et al. (2016). Phase Ib study of PEGylated recombinant human hyaluronidase and gemcitabine in patients with advanced pancreatic cancer. Clin Cancer Res 22:2848–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horikawa N, Abiko K, Matsumura N, et al. (2020). Anti-VEGF therapy resistance in ovarian cancer is caused by GM-CSF-induced myeloid-derived suppressor cell recruitment. Br J Cancer 122:778–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou W, Zhang Q, Yan Z, et al. (2013). Strange attractors: DAMPs and autophagy link tumor cell death and immunity. Cell Death Dis 4:e966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu K, Miao L, Goodwin TJ, et al. (2017). Quercetin remodels the tumor microenvironment to improve the permeation, retention, and antitumor effects of nanoparticles. ACS Nano 11:4916–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Q, Sun W, Lu Y, et al. (2016). Tumor microenvironment-mediated construction and deconstruction of extracellular drug-delivery depots. Nano Lett 16:1118–26. [DOI] [PubMed] [Google Scholar]
- Huang CY, Ju DT, Chang CF, et al. (2017). A review on the effects of current chemotherapy drugs and natural agents in treating non-small cell lung cancer. Biomedicine (Taipei) 7:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inácio Pinto N, Carnier J, Oyama LM, et al. (2015). Cancer as a proinflammatory environment: metastasis and cachexia. Mediators Inflamm 2015:791060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jean-Quartier C, Jeanquartier F, Jurisica I, et al. (2018). In silico cancer research towards 3R. BMC Cancer 18:408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang T, Zhang B, Shen S, et al. (2017). Tumor microenvironment modulation by cyclopamine improved photothermal therapy of biomimetic gold nanorods for pancreatic ductal adenocarcinomas. ACS Appl Mater Interfaces 9:31497–508. [DOI] [PubMed] [Google Scholar]
- Jiang W, Huang Y, An Y, et al. (2015). Remodeling tumor vasculature to enhance delivery of intermediate-sized nanoparticles. ACS Nano 9:8689–96. [DOI] [PubMed] [Google Scholar]
- Jiao P, Otto M, Geng Q, et al. (2016). Enhancing both CT imaging and natural killer cell-mediated cancer cell killing by a GD2-targeting nanoconstruct. J Mater Chem B 4:513–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kano MR, Bae Y, Iwata C, et al. (2007). Improvement of cancer-targeting therapy, using nanocarriers for intractable solid tumors by inhibition of TGF-beta signaling. Proc Natl Acad Sci USA 104:3460–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karagkiozaki V, Pappa F, Arvaniti D, et al. (2016). The melding of nanomedicine in thrombosis imaging and treatment: a review. Future Sci OA 2:FSO113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilari S, Yang B, Sharma A, et al. (2018). Increased transforming growth factor beta (TGF-β) and pSMAD3 signaling in a murine model for contrast induced kidney injury. Sci Rep 8:6630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirtane AR, Sadhukha T, Kim H, et al. (2017). Fibrinolytic enzyme cotherapy improves tumor perfusion and therapeutic efficacy of anticancer nanomedicine. Cancer Res 77:1465–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein D. (2018). The tumor vascular endothelium as decision maker in cancer therapy. Front Oncol 8:367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong DH, et al. (2017). A review of anti-angiogenic targets for monoclonal antibody cancer therapy. Int J Mol Sci 18:1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koning GA, Eggermont AMM, Lindner LH, et al. (2010). Hyperthermia and thermosensitive liposomes for improved delivery of chemotherapeutic drugs to solid tumors. Pharm Res 27:1750–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalczyk A, Kleniewska P, Kolodziejczyk M, et al. (2015). The role of endothelin-1 and endothelin receptor antagonists in inflammatory response and sepsis. Arch Immunol Ther Exp (Warsz) 63:41–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krijgsman D, Hokland M, Kuppen PJK. (2018). The role of natural killer T cells in cancer – a phenotypical and functional approach. Front Immunol 9:367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krysko O, Løve Aaes T, Bachert C, et al. (2013). Many faces of DAMPs in cancer therapy. Cell Death Dis 4:e631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuai R, Yuan W, Son S, et al. (2018). Elimination of established tumors with nanodisc-based combination chemoimmunotherapy. Sci Adv 4:eaao1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Land WG. (2015). The role of damage-associated molecular patterns (DAMPs) in human diseases: part II: DAMPs as diagnostics, prognostics and therapeutics in clinical medicine. Sultan Qaboos Univ Med J 15:e157–70. [PMC free article] [PubMed] [Google Scholar]
- Landskron G, De la Fuente M, Thuwajit P, et al. (2014). Chronic inflammation and cytokines in the tumor microenvironment. J Immunol Res 2014:149185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HJ, Kim MY, Park HS. (2015). Phosphorylation-dependent regulation of Notch1 signaling: the fulcrum of Notch1 signaling. BMB Rep 48:431–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Zhang Y, Wang J, et al. (2017). Nanoparticle-mediated local depletion of tumour-associated platelets disrupts vascular barriers and augments drug accumulation in tumours. Nat Biomed Eng 1:667–79. [DOI] [PubMed] [Google Scholar]
- Lim JE, Chung E, Son A neuropeptide Y. (2017). A neuropeptide, Substance-P, directly induces tissue-repairing M2 like macrophages by activating the PI3K/Akt/mTOR pathway even in the presence of IFNγ. Sci Rep 7:9417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim S, Park J, Shim MK, et al. (2019). Recent advances and challenges of repurposing nanoparticle-based drug delivery systems to enhance cancer immunotherapy. Theranostics 9:7906–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Moynihan KD, Zheng Y, et al. (2014). Structure-based programming of lymph-node targeting in molecular vaccines. Nature 507:519–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Liao S, Diop-Frimpong B, et al. (2012). TGF-β blockade improves the distribution and efficacy of therapeutics in breast carcinoma by normalizing the tumor stroma. Proc Natl Acad Sci USA 109:16618–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Guo F. (2018). Recent updates on cancer immunotherapy. Precis Clin Med 1:65–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu LL, Smith MT, Yu KKQ, et al. (2019). IFN-γ-independent immune markers of Mycobacterium tuberculosis exposure. Nat Med 25:977–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes H, Kuchnio A, Peric A, et al. (2014). Tumor vessel normalization by chloroquine independent of autophagy. Cancer Cell 26:190–206. [DOI] [PubMed] [Google Scholar]
- Maisel K, Sasso MS, Potin L, et al. (2017). Exploiting lymphatic vessels for immunomodulation: rationale, opportunities, and challenges. Adv Drug Deliv Rev 114:43–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin JD, Cabral H, Stylianopoulos T, et al. (2020). Improving cancer immunotherapy using nanomedicines: progress, opportunities and challenges. Nat Rev Clin Oncol 17:251–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin JD, Fukumura D, Duda DG, et al. (2016). Reengineering the tumor microenvironment to alleviate hypoxia and overcome cancer heterogeneity. Cold Spring Harb Perspect Med 6:a027094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez FO, Gordon S. (2014). The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep 6:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashouri L, Yousefi H, Aref AR, et al. (2019). Exosomes: composition, biogenesis, and mechanisms in cancer metastasis and drug resistance. Mol Cancer 18:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattheolabakis G, Mikelis CM. (2019). Nanoparticle delivery and tumor vascular normalization: the chicken or the egg? Front Oncol 9:1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mbongue JC, Nicholas DA, Torrez TW, et al. (2015). The role of indoleamine 2, 3-dioxygenase in immune suppression and autoimmunity. Vaccines (Basel) 3:703–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCallion C, Peters AD, Booth A, et al. (2019). Dual-action CXCR4-targeting liposomes in leukemia: function blocking and drug delivery. Blood Adv 3:2069–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta NK, Moynihan KD, Irvine DJ. (2015). Engineering new approaches to cancer vaccines. Cancer Immunol Res 3:836–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng H, Zhao Y, Dong J, et al. (2013). Two-wave nanotherapy to target the stroma and optimize gemcitabine delivery to a human pancreatic cancer model in mice. ACS Nano 7:10048–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao L, Lin CM, Huang L. (2015). Stromal barriers and strategies for the delivery of nanomedicine to desmoplastic tumors. J Control Release 219:192–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller BJ, Cao Y, Li CY, et al. (2004). Radiation activates HIF-1 to regulate vascular radiosensitivity in tumors: role of reoxygenation, free radicals, and stress granules. Cancer Cell 5:429–41. [DOI] [PubMed] [Google Scholar]
- Mok W, Boucher Y, Jain RK. (2007). Matrix metalloproteinases-1 and -8 improve the distribution and efficacy of an oncolytic virus. Cancer Res 67:10664–8. [DOI] [PubMed] [Google Scholar]
- Monsky WL, Fukumura D, Gohongi T, et al. (1999). Augmentation of transvascular transport of macromolecules and nanoparticles in tumors using vascular endothelial growth factor. Cancer Res 59:4129–35. [PubMed] [Google Scholar]
- Mouw JK, Ou G, Weaver VM. (2014). Extracellular matrix assembly: a multiscale deconstruction. Nat Rev Mol Cell Biol 15:771–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulder R, Banete A, Seaver K, et al. (2017). M(IL-4) tissue macrophages support efficient interferon-gamma production in antigen-specific CD8+ T cells with reduced proliferative capacity. Front Immunol 8:1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazawa MS, Eisinger-Mathason TSK, Sadri N, et al. (2016). Epigenetic re-expression of HIF-2α suppresses soft tissue sarcoma growth. Nat Commun 7:10539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawaz W, Xu S, Li Y, et al. (2020). Nanotechnology and immunoengineering: how nanotechnology can boost CAR-T therapy. Acta Biomater 109:21–36. [DOI] [PubMed] [Google Scholar]
- Nehoff H, Parayath NN, Domanovitch L, et al. (2014). Nanomedicine for drug targeting: strategies beyond the enhanced permeability and retention effect. Int J Nanomedicine 9:2539–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netea-Maier RT, Smit JWA, Netea MG. (2018). Metabolic changes in tumor cells and tumor-associated macrophages: a mutual relationship. Cancer Lett 413:102–9. [DOI] [PubMed] [Google Scholar]
- Nielsen SR, Schmid MC. (2017). Macrophages as key drivers of cancer progression and metastasis. Mediators Inflamm 2017:9624760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisticò P, Bissell MJ, Radisky DC. (2012). Epithelial-mesenchymal transition: general principles and pathological relevance with special emphasis on the role of matrix metalloproteinases. Cold Spring Harbor Perspect Biol 4:a011908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olive KP, Jacobetz MA, Davidson CJ, et al. (2009). Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science 324:1457–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshita F, Fujiwara Y, Saijo N. (1992). Radiation sensitivities in various anticancer-drug-resistant human lung cancer cell lines and mechanism of radiation cross-resistance in a cisplatin-resistant cell line. J Cancer Res Clin Oncol 119:28–34. [DOI] [PubMed] [Google Scholar]
- Papaioannou NE, Beniata OV, Vitsos P, et al. (2016). Harnessing the immune system to improve cancer therapy. Ann Transl Med 4:261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partanen TA, Arola J, Saaristo A, et al. (2000). VEGF-C and VEGF-D expression in neuroendocrine cells and their receptor, VEGFR-3, in fenestrated blood vessels in human tissues. Faseb J 14:2087–96. [DOI] [PubMed] [Google Scholar]
- Pérez-Herrero E, Fernández-Medarde A. (2015). Advanced targeted therapies in cancer: drug nanocarriers, the future of chemotherapy. Eur J Pharm Biopharm 93:52–79. [DOI] [PubMed] [Google Scholar]
- Plava J, Cihova M, Burikova M, et al. (2019). Recent advances in understanding tumor stroma-mediated chemoresistance in breast cancer. Mol Cancer 18:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poltavets V, Kochetkova M, Pitson SM, et al. (2018). The role of the extracellular matrix and its molecular and cellular regulators in cancer cell plasticity. Front Oncol 8:431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potez M, Trappetti V, Bouchet A, et al. (2018). Characterization of a B16-F10 melanoma model locally implanted into the ear pinnae of C57BL/6 mice. PloS One 13:e0206693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitan NK, Thuen M, Goa PE, et al. (2010). Characterization of tumor microvascular structure and permeability: comparison between magnetic resonance imaging and intravital confocal imaging. J Biomed Opt 15:036004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roma-Rodrigues C, Pombo I, Raposo L, et al. (2019). Nanotheranostics targeting the tumor microenvironment. Front Bioeng Biotechnol 7:197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeed M, Gao J, Shi Y, et al. (2019). Engineering nanoparticles to reprogram the tumor immune microenvironment for improved cancer immunotherapy. Theranostics 9:7981–8000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvioni L, Rizzuto MA, Bertolini JA, et al. (2019). Thirty years of cancer nanomedicine: success, frustration, and hope. Cancers 11:1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxton RA, Sabatini DM. (2017). mTOR signaling in growth, metabolism, and disease. Cell 168:960–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schito L, Rey S, Konopleva M. (2017). Integration of hypoxic HIF-α signaling in blood cancers. Oncogene 36:5331–40. [DOI] [PubMed] [Google Scholar]
- Schülke S. (2018). Induction of interleukin-10 producing dendritic cells as a tool to suppress allergen-specific T helper 2 responses. Front Immunol. 9: 455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman MH, Yu RT, Engle DD, et al. (2014). Vitamin D receptor-mediated stromal reprogramming suppresses pancreatitis and enhances pancreatic cancer therapy. Cell 159:80–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi T, et al. (2018). Cancer immunotherapy: a focus on the regulation of immune checkpoints. Int J Mol Sci 19:1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons M, Raposo G. (2009). Exosomes-vesicular carriers for intercellular communication. Curr Opin Cell Biol 21:575–81. [DOI] [PubMed] [Google Scholar]
- Sloin HE, Ruggiero G, Rubinstein A, et al. (2018). Interactions between the circadian clock and TGF-β signaling pathway in zebrafish. PLoS One 13:e0199777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sormendi S, Wielockx B. (2018). Hypoxia pathway proteins as central mediators of metabolism in the tumor cells and their microenvironment. Front Immunol 9:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart JM, Keselowsky BG. (2017). Combinatorial drug delivery approaches for immunomodulation. Adv Drug Deliv Rev 114:161–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stylianopoulos T. (2017). The solid mechanics of cancer and strategies for improved therapy. J Biomech Eng 139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subhash VV, Yeo MS, Tan WL, et al. (2015). Strategies and advancements in harnessing the immune system for gastric cancer immunotherapy. J Immunol Res 2015:308574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka S, Akaike T, Wu J, et al. (2003). Modulation of tumor-selective vascular blood flow and extravasation by the stable prostaglandin 12 analogue beraprost sodium. J Drug Target 11:45–52. [DOI] [PubMed] [Google Scholar]
- Tang C, Qiu F, Zhao X. (2013). Molecular design and applications of self-assembling surfactant-like peptides. J Nanomater 2013:1–9. [Google Scholar]
- Tei K, Kawakami-Kimura N, Taguchi O, et al. (2002). Roles of cell adhesion molecules in tumor angiogenesis induced by cotransplantation of cancer and endothelial cells to nude rats. Cancer Res 62:6289–96. [PubMed] [Google Scholar]
- Tharkar P, Varanasi R, Wong WSF, et al. (2019). Nano-enhanced drug delivery and therapeutic ultrasound for cancer treatment and beyond. Front Bioeng Biotechnol 7:324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong RT, Boucher Y, Kozin SV, et al. (2004). Vascular normalization by vascular endothelial growth factor receptor 2 blockade induces a pressure gradient across the vasculature and improves drug penetration in tumors. Cancer Res 64:3731–6. [DOI] [PubMed] [Google Scholar]
- Tsai JH, Yang J. (2013). Epithelial-mesenchymal plasticity in carcinoma metastasis. Genes Dev 27:2192–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaupel P, Multhoff G. (2018). Hypoxia-/HIF-1α-driven factors of the tumor microenvironment impeding antitumor immune responses and promoting malignant progression. Adv Exp Med Biol 1072:171–5. [DOI] [PubMed] [Google Scholar]
- Vetsika EK, Koukos A, Kotsakis A. (2019). Myeloid-derived suppressor cells: major figures that shape the immunosuppressive and angiogenic network in cancer. Cells 8:1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivier E, Ugolini S, Blaise D, et al. (2012). Targeting natural killer cells and natural killer T cells in cancer. Nat Rev Immunol 12:239–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Cheng L, Liu Z. (2013). Upconversion nanoparticles for photodynamic therapy and other cancer therapeutics. Theranostics 3:317–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Jiang W, Zhu F, et al. (2018. a). Modulation of the tumor microenvironment by intratumoral administration of IMO-2125, a novel TLR9 agonist, for cancer immunotherapy. Int J Oncol 53:1193–203. [DOI] [PubMed] [Google Scholar]
- Wang P, Jiang F, Chen B, et al. (2020). Bioinspired red blood cell membrane-encapsulated biomimetic nanoconstructs for synergistic and efficacious chemo-photothermal therapy. Colloids Surf B Biointerfaces 189:110842. [DOI] [PubMed] [Google Scholar]
- Wang S, Campos J, Gallotta M, et al. (2016). Intratumoral injection of a CpG oligonucleotide reverts resistance to PD-1 blockade by expanding multifunctional CD8+ T cells. Proc Natl Acad Sci U S A 113:E7240–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Li Z, Xu R. (2018. b). Human cancer and platelet interaction, a potential therapeutic target. Int J Mol Sci 19: 1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Li H, Liu X, et al. (2017). Enhanced photothermal therapy of biomimetic polypyrrole nanoparticles through improving blood flow perfusion. Biomaterials 143:130–41. [DOI] [PubMed] [Google Scholar]
- Wang YJ, Fletcher R, Yu J, et al. (2018. c). Immunogenic effects of chemotherapy-induced tumor cell death. Genes Dis 5:194–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster TJ. (2006). Nanomedicine: what’s in a definition? Int J Nanomedicine 1:115–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weitman E, Cuzzone D, Mehrara BJ. (2013). Tissue engineering and regeneration of lymphatic structures. Future Oncol 9:1365–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G, Deng Z, Jin Z, et al. (2020). Identification of prognostic immune-related genes in pancreatic adenocarcinoma and establishment of a prognostic nomogram: a bioinformatic study. Biomed Res Int 2020:1346045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin Y, Yin M, Zhao L, et al. (2017). Recent progress on nanoparticle-based drug delivery systems for cancer therapy. Cancer Biol Med 14:228–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C, et al. (2015). CXCR4 in breast cancer: oncogenic role and therapeutic targeting. Drug Des Devel Ther 9:4953–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang G, Xu L, Chao Y, et al. (2017). Hollow MnO2 as a tumor-microenvironment-responsive biodegradable nano-platform for combination therapy favoring antitumor immune responses. Nat Commun 8:902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S, Gao H. (2017). Nanoparticles for modulating tumor microenvironment to improve drug delivery and tumor therapy. Pharmacol Res 126:97–108. [DOI] [PubMed] [Google Scholar]
- Zaheer S, Shah N, Maqbool SA, et al. (2019). Estimates of past and future time trends in age-specific breast cancer incidence among women in Karachi, Pakistan: 2004-2025. BMC Public Health 19:1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamora MA, Dempsey EC, Walchak SJ, et al. (1993). BQ123, an ETA receptor antagonist, inhibits endothelin-1-mediated proliferation of human pulmonary artery smooth muscle cells. Am J Respir Cell Mol Biol 9:429–33. [DOI] [PubMed] [Google Scholar]
- Zhang B, Hu Y, Pang Z. (2017. a). Modulating the tumor microenvironment to enhance tumor nanomedicine delivery. Front Pharmacol 8:952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Jiang T, Tuo Y, et al. (2017. b). Captopril improves tumor nanomedicine delivery by increasing tumor blood perfusion and enlarging endothelial gaps in tumor blood vessels. Cancer Lett 410:12–19. [DOI] [PubMed] [Google Scholar]
- Zhang B, Shi W, Jiang T, et al. (2016). Optimization of the tumor microenvironment and nanomedicine properties simultaneously to improve tumor therapy. Oncotarget 7:62607–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YR, Lin R, Li HJ, et al. (2019). Strategies to improve tumor penetration of nanomedicines through nanoparticle design. Wiley Interdiscip Rev Nanomed Nanobiotechnol 11:e1519. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Adjei AA. (2015). Targeting angiogenesis in cancer therapy: moving beyond vascular endothelial growth factor. Oncologist 20:660–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Sun Y, Yu X, et al. (2016). Angiogenesis in liquid tumors: an in vitro assay for leukemic-cell-induced bone marrow angiogenesis. Adv Healthc Mater 5:1014–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Wang G, Chen Y, et al. (2019). Immunogenic cell death in cancer therapy: present and emerging inducers. J Cell Mol Med 23:4854–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, et al. (2020). Overcoming the biological barriers in the tumor microenvironment for improving drug delivery and efficacy. J Mater Chem B. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Zhang P, Ren J, et al. (2013). Synergistic effects of ultrasound-targeted microbubble destruction and TAT peptide on gene transfection: an experimental study in vitro and in vivo. J Control Release 170:437–44. [DOI] [PubMed] [Google Scholar]