Abstract
Analysis of the literature data reveals that while inhibition of cancer-related carbonic anhydrase IX and XII isoforms continues to be an important enrichment factor for designing anticancer agent development libraries, exclusive reliance on the in vitro inhibition of these two recombinant isozymes in nominating candidate compounds for evaluation of their effects on cancer cells may lead not only to identifying numerous compounds devoid of the desired cellular efficacy but also to overlooking many promising candidates which may not display the best potency in biochemical inhibition assay. However, SLC-0111, now in phase Ib/II clinical trials, was developed based on the excellent agreement between the in vitro, in vivo and more recently, in-patient data.
Keywords: Carbonic anhydrase inhibitors, cancer-related IX and XII isoforms, anticancer agents, screening funnel, enrichment factor
Introduction
Tumour growth and proliferation are strongly associated with hypoxic stress due to poor vascularisation and oxygen deprivation of neoplastic tissues1. Adaptive metabolic changes observed in cancer cells include elevated production of acidic metabolites which leads to tumour acidosis2–4. Human carbonic anhydrase (hCA) isoforms IX and XII are crucial effectors that regulate extracellular pH thus mediating cancer cell proliferation, invasion, and metastasis5,6. These membrane-bound proteins are zinc metalloenzymes that catalyse the reversible hydration of CO2 to bicarbonate anion and proton on cell surface (Equation (1))7. Many studies confirmed hCA IX/XII upregulation in hypoxic tumours8,9. In particular, hCA IX which has limited expression in normal tissues, is a marker of aggressive and drug-resistant cancer cell phenotypes indicating poor prognosis for patients10. Arguably, hCA XII is widely distributed in the human body and considered a marker for less malignant tumours11. Targeting the catalytic activity of the cell surface carbonic anhydrases has been and continues to be considered a promising therapeutic approach in the antineoplastic field, either for suppressing tumour growth or overcoming drug resistance of cancer cells12,13.
| (1) |
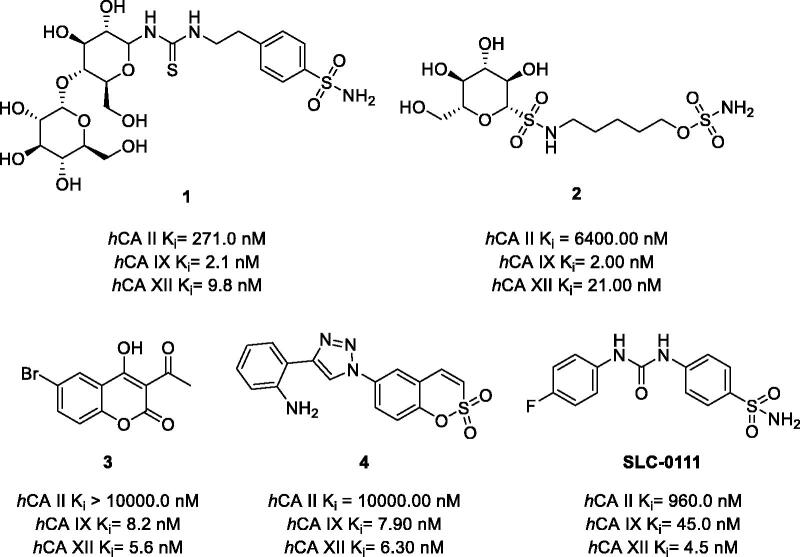
Much progress has been made in the last decade towards anticancer agents based on small-molecule inhibitors of hCA IX and/or XII14,15. These efforts typically involved screening of compound libraries in search for potent and selective blockers of these isozymes, followed by detailed in vitro and in vivo characterisation of the most promising candidates. Many new compounds have been thus identified capable of inhibiting recombinant hCA IX and/or XII in the low nanomolar to subnanomolar range with remarkable selectivity over other carbonic anhydrase isoforms. These chemotypes often contained a primary sulphonamide or sulfamate (e.g. 1 and 2) group or were based on a coumarin and sulfocoumarin core (such as 3 and 4, respectively)16–19. Of these classes, ureido-substituted benzene sulphonamides (USBs) made the most progress with SLC-0111 recently entering phase Ib/II clinical trials (Figure 1)20,21.
Figure 1.
Potent and selective hCA IX/XII inhibitors from among sulphonamides (1), sulfamates (2), coumarins (3), and sulfocumarins (4) and SLC-0111 with its CA inhibition profile.
Despite significant advances, the discovery of hCA-targeted anticancer agents is not a straightforward endeavour, with many aspects remain unclear. In fact, our own efforts as well as those reported in the literature often yield structures, which display frustratingly modest antiproliferative effect, although possessing profound inhibition of isolated hCA IX/XII. Such inconsistencies occurring between the ability of some hCA inhibitors to block the recombinant enzyme catalytic function and their effects on cancer cells constitute a challenge that has been much less addressed, in comparison to those related to the SAR and selectivity studies22–24. Thus, although the potential of hCA IX and XII as anticancer targets is supported by much evidence, including the well-documented efficacy of hCA-inhibitory antibodies in vitro and in vivo, the success rate of small-molecule inhibitors upon the transition to cell culture setting is still below the desirable level18,25–29. Taking this into account, attempts to re-evaluate the conventional drug discovery workflow that has existed in this field are of importance. Indeed, while factors affecting the activity in cells are poorly understood, the drug-discovery funnel beginning with recombinant protein-based profiling should be critically evaluated. Therefore, we undertook a literature survey and analysis regarding possible disconnects for hCA inhibitors, leading to a dramatic change of the compound’s activity upon the transition from the recombinant isolated protein to the cell-based models.
Discussion
Disappointingly, a large fraction of publications on the subject reported compounds’ cytotoxicity data obtained in normoxic conditions thus making the involvement of hCA IX and XII debateable30–45. Although such testing can be relevant in some cases, we consider these conditions irrelevant as long as antiproliferative activity of hCA inhibitors is concerned. This is primarily due to limited expression of the target isoforms, if any, reported for many cancer cell lines under these conditions8,46. The studies which employ normoxic MCF-10A cells lacking hCAIX/XII on their surface are illustrative47. Furthermore, some investigations involved cell lines displaying robust hCA IX activity in the cytosol, such as MDA-MB-231 and MCF-748. The said non-default localisation of hCA IX could lead to overestimation of the protein expression and activity on the cell membrane, thus leading to the problematic interpretation of the data obtained16. Thus, the choice of experimental conditions often renders the results ambiguous, making them rather difficult to analyse.
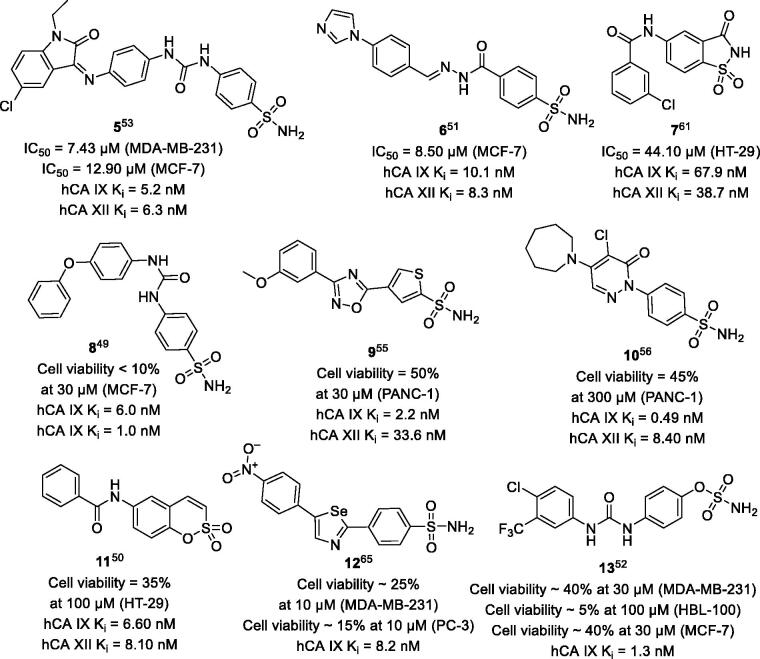
To our delight, however, there is a large number of mechanistically relevant experiments reported in the literature which address the ability of hCA IX/XII inhibitors to block the growth of hypoxic cancer cells overexpressing the target isozymes49–67. Moreover, a certain cohort of studies revealed compounds possessing both favourable hCA inhibitory profile and significant anticancer activity, as exemplified by structures 5–13 (Figure 2)49–56,58–61,64–67. In fact, quite a number of single-digit nanomolar hCA IX/XII inhibitors significantly suppressed cancer cell growth. Additional data underscoring the specificity of this antiproliferative action in cancer over normoxic and non-cancerous cells highlight the potential of these findings for practical applications. Meanwhile, the drug-like character of some frontrunners makes them intriguing starting points for medicinal chemistry optimisation. Once selected based on their hCA inhibitory profiles, these compounds might be useful indeed in designing antineoplastic drugs either of combinative or single-agent use49–53,55,56,61,65.
Figure 2.
Potent hCA IX/XII inhibitors displaying good translation of their activity into antiproliferative activity against cancer cells under hypoxia.
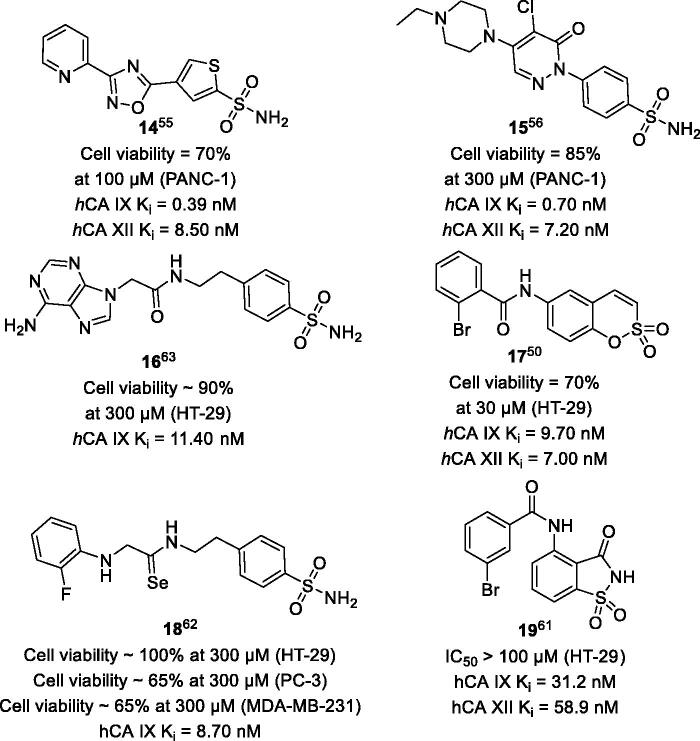
In contrast to the aforementioned examples of good correlation between hCA IX/XII inhibition and cytotoxicity against cancer cells in hypoxic conditions, the reverse is true for a strikingly large number of examples50,55–57,60–63,67. In these cases, many potent hCA IX/XII inhibitors, such as 14–19, did not possess a substantial activity against cancer cells. Furthermore, these examples often share structural similarity with the hit compounds originating from the same screening series (cf. examples in Figure 2). Despite the obvious resemblance, no effect observed under identical conditions, again, indicates our limited understating of the factors influencing hCA inhibitors activity in the intact cells (Figure 3)50,55,56,61–63. According to the existing discovery workflow in this field, if these compounds are selected for the detailed evaluation based solely on their enzyme inhibitory profile, this could have diverted the investigator’s attention from potentially efficacious anticancer agents.
Figure 3.
Potent hCA IX/XII exhibiting poor antiproliferative activity under hypoxic conditions.
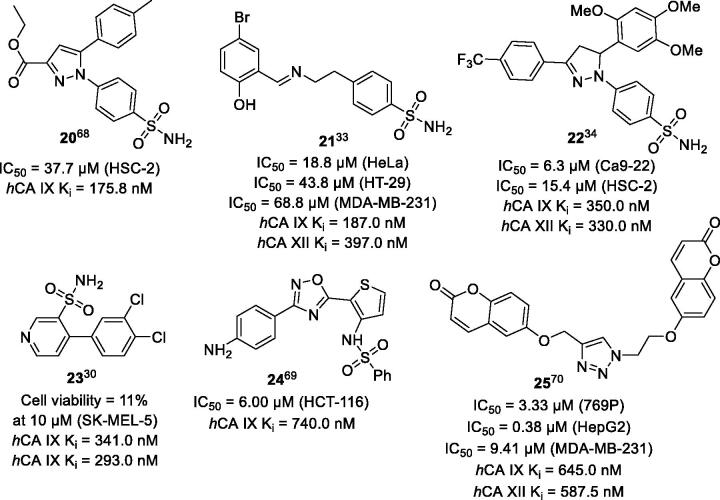
A large portion of compounds may be overlooked in the anticancer agent development projects because of their moderate inhibitory properties towards hCA IX/XII cancer-related isozyme duo. This fairly populated category of compounds possessing moderate to low inhibitory properties towards recombinant hCA IX/XII features many compounds that are based on privileged chemotypes (e.g. 20–25). Evidently, in most studies, these relatively inactive molecules would have not been progressed based solely on their inhibitory profile. At the same time, several reports reveal that and unexpectedly good cytotoxicity towards cancer cells can be demonstrated by such compounds (Figure 4)30,33,34,68–70.
Figure 4.
Sulphonamides and coumarins demonstrating potent antiproliferative properties despite being weak inhibitors of the recombinant hCA IX/XII.
These occasional findings supported the questioning of the direct predictive power of hCA IX/XII inhibitory profile for a compound’s anticancer activity. Understanding the significance of the candidate molecule prioritisation problem in question is essential while ignoring it may lead to many promising compounds being overlooked in want of more potent hCA IX/XII inhibitors. Aiming to draw a more informative picture, we have graphically represented the relationship between the anticancer effect (in this case, irrespective of the specific experimental conditions employed in various studies) and the hCA IX/XII inhibitory activity of the compounds30–45,49–58,71–74. In order to summarise the somewhat heterogeneous literature data, we have visualised the reported antiproliferative agents in the plots with the Y-axis displaying Ki’s against hCA IX (Figures S1 and S2) and hCA XII (Figures S3 and S4). Several groups of compounds were distinguished and marked by a different colour with regard to their effect on cancer cells: (1) <50% cell survival at 10 µM (or IC50=0.1–10 µM), green; (2) <50% cell survival at 50 µM (or IC50=11–50 µM), blue; (3) IC50 51–100 µM, tangerine; (4) IC50=101–150 µM, cyan; (5) IC50=151–200 µM, magenta; (6) IC50 >200 µM, red; (7) <50% cell growth inhibition at 10 or 50 µM, yellow. As is evident from Figures S1–S4, potent cancer cell growth suppressors can possess vastly different levels of inhibitory activity against hCA IX/XII. However, the low nanomolar region of Ki values appears to be most populated with compound possessing strong effect on cancer cells (Figures S1 and S3). Meanwhile, a fairly large portion of compounds did not possess noticeable antiproliferative activity while displaying various levels of Ki values towards recombinant hCAs (Figures S2 and S4). The prevailing occurrence of these compounds in the nanomolar range of hCA IX/XII inhibitory activity is likely the result of the currently applied drug discovery funnel where the best hCA IX/XII inhibitors are primarily selected for subsequent evaluation for their effects on cancer cells.
The supremacy of in vitro potency for drug discovery decision making has been much debated with respect to different fields of medicinal chemistry75. In complex biological environment, multiple variables such as ligand residence time or metabolic stability76,77 can determine the observed efficacy. However, the said parameters are not routinely looked at when screening for potential hCA IX/XII inhibitors. While tens to hundreds nanomolar levels of on-target activity prevail among clinically used drugs77, such activity levels are easily achievable for primary sulphonamides hCA IX and\or XII isoforms, as can be deduced from Figures S1–S4. This gives us an idea of a different approach to the discovery of anticancer agents based on hCA IX/XII inhibition. Considering, that multiple factors appear to be at play when cytotoxicity towards cancer cells is concerned, front-loading hCA IX/XII inhibition as a candidate selection criterion should be taken with caution. In vitro screening is indispensable for identifying chemotypes with a clear tendency to inhibit the target isozymes. Once identified and expanded into larger, SAR-informative follow-on libraries, these candidate chemotypes should perhaps be screened primarily against cancer cells so as not to overlook promising candidates which may not necessarily be endowed with the best hCA IX/XII inhibitory potency.
Conclusions
Literature data indicate that inhibition of the recombinant protein possesses a somewhat limited predictive power for the compounds’ ability to block cancer cell growth. Not questioning the role of the cell surface hCA isoforms in tumour growth and survival, this conclusion, however, highlights the imperfection of the existing approach to the design and discovery of antineoplastic drugs in this field. In fact, by selecting compounds for in vitro and in vivo characterisation based solely on their inhibitory profile, one could discard many promising hit-structures. Resources expended for optimising molecules’ potency and selectivity towards the target recombinant protein may also be wasted as they do not necessarily ensure antiproliferative activity. On the other hand, a wealth of positive examples in the literature of a strong correlation between hCA IX/XII inhibition and cytotoxicity towards cancer cells indicates that demonstrated tendency of new chemotypes to inhibit these isoforms can serve as a robust enrichment factor for designing screening libraries that target cancer cells. Moreover, detailed SAR studies of different carbonic anhydrase inhibitors with regard to hCA IX and/or XII are of importance due to the significance of developing new ligands of these targets for tumour imaging, drug delivery and other purposes78.
Supplementary Material
Funding Statement
This research was supported by the Russian Federation Government Megagrant 14.W03.031.0025. Tatiana Sharonova is grateful to the Russian Foundation for Basic Research for the graduate student research grant (project 19-33-90017).
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- 1.Bailey KM, Wojtkowiak JW, Hashim AI, Gillies RJ.. Targeting the metabolic microenvironment of tumors. Adv Pharmacol 2012;65:63–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chiche J, Brahimi-Horn MC, Pouysségur J.. Tumour hypoxia induces a metabolic shift causing acidosis: a common feature in cancer. J Cell Mol Med 2010;14:771–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Webb BA, Chimenti M, Jacobson MP, Barber DL.. Dysregulated pH: a perfect storm for cancer progression. Nat Rev Cancer 2011;11:671–7. [DOI] [PubMed] [Google Scholar]
- 4.McDonald PC, Chafe SC, Dedhar S.. Overcoming hypoxia-mediated tumor progression: combinatorial approaches targeting pH regulation, angiogenesis and immune dysfunction. Front Cell Dev Biol 2016;4:16–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singh S, Lomelino CL, Mboge MY, et al. . Cancer drug development of carbonic anhydrase inhibitors beyond the active site. Molecules 2018;23:1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pastorek J, Pastorekova S.. Hypoxia-induced carbonic anhydrase IX as a target for cancer therapy: from biology to clinical use. Semin Cancer Biol 2015;31:52–64. [DOI] [PubMed] [Google Scholar]
- 7.Supuran CT. Structure and function of carbonic anhydrases. Biochem J 2016;473:2023–32. [DOI] [PubMed] [Google Scholar]
- 8.Švastová E, Hulíková A, Rafajová M, et al. . Hypoxia activates the capacity of tumor-associated carbonic anhydrase IX to acidify extracellular pH. FEBS Lett 2004;577:439–45. [DOI] [PubMed] [Google Scholar]
- 9.Waheed A, Sly WS.. Carbonic anhydrase XII functions in health and disease. Gene 2017;623:33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Becker HM, Klier M, Deitmer JW. Carbonic anhydrases and their Interplay with acid/base-coupled membrane transporters. In: Frost S, McKenna R, eds. Carbonic anhydrase: mechanism, regulation, links to disease, and industrial applications. Subcellular Biochemistry. Vol 75. Dordrecht: Springer; 2014. [DOI] [PubMed] [Google Scholar]
- 11.Watson PH, Chia SK, Wykoff CC, et al. . Carbonic anhydrase XII is a marker of good prognosis in invasive breast carcinoma. Br J Cancer 2003;88:1065–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Neri D, Supuran CT.. Interfering with pH regulation in tumours as a therapeutic strategy. Nat Rev Drug Discov 2011;10:767–77. [DOI] [PubMed] [Google Scholar]
- 13.Güttler A, Theuerkorn K, Riemann A, et al. . Cellular and radiobiological effects of carbonic anhydrase IX in human breast cancer cells. Oncol Rep 2019;41:2585–94. [DOI] [PubMed] [Google Scholar]
- 14.Koch G. Medicinal chemistry. Chimia 2017;71:643. [PubMed] [Google Scholar]
- 15.McDonald PC, Chia S, Bedard PL, et al. . A phase 1 study of SLC-0111, a novel inhibitor of carbonic anhydrase IX, in patients with advanced solid tumors. Am J Clin Oncol 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Williams KJ, Gieling RG.. Preclinical evaluation of ureidosulfamate carbonic anhydrase IX/XII inhibitors in the treatment of cancers. Int J Mol Sci 2019;20:6080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gieling RG, Babur M, Mamnani L, et al. . Antimetastatic effect of sulfamate carbonic anhydrase IX inhibitors in breast carcinoma xenografts. J Med Chem 2012;55:5591–600. [DOI] [PubMed] [Google Scholar]
- 18.Touisni N, Maresca A, McDonald PC, et al. . Glycosyl coumarin carbonic anhydrase IX and XII inhibitors strongly attenuate the growth of primary breast tumors. J Med Chem 2011;54:8271–7. [DOI] [PubMed] [Google Scholar]
- 19.Krasavin M, Žalubovskis R, Grandāne A, et al. . Sulfocoumarins as dual inhibitors of human carbonic anhydrase isoforms IX/XII and of human thioredoxin reductase. J Enzyme Inhib Med Chem 2020;35:506–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mboge MY, Mahon BP, Lamas N, et al. . Structure activity study of carbonic anhydrase IX: selective inhibition with ureido-substituted benzenesulfonamides. Eur J Med Chem 2017;132:184–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mboge MY, Chen Z, Wolff A, et al. . Selective inhibition of carbonic anhydrase IX over carbonic anhydrase XII in breast cancer cells using benzene sulfonamides: disconnect between activity and growth inhibition. PLOS One 2018;13:e0207417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.A study of SLC-0111 and gemcitabine for metastatic pancreatic ductal cancer in subjects positive for CAIX (SLC-0111-17-01) ; 2018. Available from: https://clinicaltrials.gov/ct2/show/NCT03450018.
- 23.Han T, Goralski M, Gaskill N, et al. . Anticancer sulfonamides target splicing by inducing RBM39 degradation via recruitment to DCAF15. Science 2017;356:eaal3755. [DOI] [PubMed] [Google Scholar]
- 24.Uehara T, Minoshima Y, Sagane K, et al. . Selective degradation of splicing factor CAPERα by anticancer sulfonamides. Nat Chem Biol 2017;13:675–80. [DOI] [PubMed] [Google Scholar]
- 25.Podolski-Renić A, Dinić J, Stanković T, et al. . Sulfocoumarins, specific carbonic anhydrase IX and XII inhibitors, interact with cancer multidrug resistant phenotype through pH regulation and reverse P-glycoprotein mediated resistance. Eur J Pharm Sci 2019;138:105012. [DOI] [PubMed] [Google Scholar]
- 26.Murri-Plesko MT, Hulikova A, Oosterwijk E, et al. . Antibody inhibiting enzymatic activity of tumour-associated carbonic anhydrase isoform IX. Eur J Pharmacol 2011;657:173–83. [DOI] [PubMed] [Google Scholar]
- 27.Battke C, Kremmer E, Mysliwietz J, et al. . Generation and characterization of the first inhibitory antibody targeting tumour-associated carbonic anhydrase XII. Cancer Immunol Immunother 2011;60:649–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pastorekova S, Gillies RJ.. The role of carbonic anhydrase IX in cancer development: links to hypoxia, acidosis, and beyond. Cancer Metastasis Rev 2019;38:65–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Supuran CT. Carbonic anhydrase inhibition and the management of hypoxic tumors. Metabolites 2017;7:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sławiński J, Szafrański K, Vullo D, Supuran CT.. Carbonic anhydrase inhibitors. Synthesis of heterocyclic 4-substituted pyridine-3-sulfonamide derivatives and their inhibition of the human cytosolic isozymes I and II and transmembrane tumor-associated isozymes IX and XII. Eur J Med Chem 2013;69:701–10. [DOI] [PubMed] [Google Scholar]
- 31.Żołnowska B, Sławiński J, Pogorzelska A, et al. . Carbonic anhydrase inhibitors. Synthesis, and molecular structure of novel series N-substituted N′-(2-arylmethylthio-4-chloro-5-methylbenzenesulfonyl)guanidines and their inhibition of human cytosolic isozymes I and II and the transmembrane tumor-associated isozymes IX and XII. Eur J Med Chem 2014;71:135–47. [DOI] [PubMed] [Google Scholar]
- 32.Ibrahim HS, Allam HA, Mahmoud WR, et al. . Dual-tail arylsulfone-based benzenesulfonamides differently match the hydrophobic and hydrophilic halves of human carbonic anhydrases active sites: selective inhibitors for the tumor-associated HCA IX isoform. Eur J Med Chem 2018;152:1–9. [DOI] [PubMed] [Google Scholar]
- 33.Koyuncu I, Gonel A, Kocyigit A, et al. . Selective inhibition of carbonic anhydrase-IX by sulphonamide derivatives induces pH and reactive oxygen species-mediated apoptosis in cervical cancer HeLa cells. J Enzyme Inhib Med Chem 2018;33:1137–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gul HI, Yamali C, Sakagami H, et al. . New anticancer drug candidates sulfonamides as selective HCA IX or HCA XII inhibitors. Bioorg Chem 2018;77:411–9. [DOI] [PubMed] [Google Scholar]
- 35.Peerzada MN, Khan P, Ahmad K, et al. . Synthesis, characterization and biological evaluation of tertiary sulfonamide derivatives of pyridyl-indole based heteroaryl chalcone as potential carbonic anhydrase IX inhibitors and anticancer agents. Eur J Med Chem 2018;155:13–23. [DOI] [PubMed] [Google Scholar]
- 36.Koyuncu I, Gonel A, Durgun M, et al. . Assessment of the antiproliferative and apoptotic roles of sulfonamide carbonic anhydrase IX inhibitors in HeLa cancer cell line. J Enzyme Inhib Med Chem 2019;34:75–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vanchanagiri K, Emmerich D, Bruschke M, et al. . Synthesis and biological investigation of new carbonic anhydrase IX (CAIX) inhibitors. Chem Biol Interact 2018;284:12–23. [DOI] [PubMed] [Google Scholar]
- 38.Ghorab MM, Alsaid MS, Ceruso M, et al. . Carbonic anhydrase inhibitors: synthesis, molecular docking, cytotoxic and inhibition of the human carbonic anhydrase isoforms I, II, IX, XII with novel benzenesulfonamides incorporating pyrrole, pyrrolopyrimidine and fused pyrrolopyrimidine moieties. Bioorg Med Chem 2014;22:3684–95. [DOI] [PubMed] [Google Scholar]
- 39.Mojzych M, Bielawska A, Bielawski K, et al. . Pyrazolo[4,3-e][1,2,4]triazine sulfonamides as carbonic anhydrase inhibitors with antitumor activity. Bioorg Med Chem 2014;22:2643–7. [DOI] [PubMed] [Google Scholar]
- 40.Wang ZC, Duan YT, Qiu HY, et al. . Novel metronidazole-sulfonamide derivatives as potent and selective carbonic anhydrase inhibitors: design, synthesis and biology analysis. RSC Adv 2014;4:33029–38. [Google Scholar]
- 41.Yilmaz Ö, Özbaş Turan S, Akbuʇa J, et al. . Synthesis of pro-apoptotic indapamide derivatives as anticancer agents. J Enzyme Inhib Med Chem 2015;30:967–80. [DOI] [PubMed] [Google Scholar]
- 42.Mojzych M, Ceruso M, Bielawska A, et al. . New pyrazolo[4,3-e][1,2,4]triazine sulfonamides as carbonic anhydrase inhibitors. Bioorg Med Chem 2015;23:3674–80. [DOI] [PubMed] [Google Scholar]
- 43.Abdel Gawad NM, Amin NH, Elsaadi MT, Mohamed FM.. Synthesis of 4-(thiazol-2-ylamino)-benzenesulfonamides with carbonic anhydrase I, II and IX inhibitory activity and cytotoxic effects against breast cancer cell lines. Bioorg Med Chem 2016;24:3043–51. [DOI] [PubMed] [Google Scholar]
- 44.Eldehna WM, Abo-ashour MF, Nocentini A, et al. . Novel 4/3-((4-Oxo-5-(2-oxoindolin-3-ylidene)thiazolidin-2-ylidene)amino) benzenesulfonamides: synthesis, carbonic anhydrase inhibitory activity, anticancer activity and molecular modelling studies. Eur J Med Chem 2017;139:250–62. [DOI] [PubMed] [Google Scholar]
- 45.Eldehna WM, Nocentini A, Al-Rashood ST, et al. . Tumor-associated carbonic anhydrase isoform IX and XII inhibitory properties of certain isatin-bearing sulfonamides endowed with in vitro antitumor activity towards colon cancer. Bioorg Chem 2018;81:425–32. [DOI] [PubMed] [Google Scholar]
- 46.Cecchi A, Hulikova A, Pastorek J, et al. . Carbonic anhydrase inhibitors. Design of fluorescent sulfonamides as probes of tumor-associated carbonic anhydrase IX that inhibit isozyme IX-mediated acidification of hypoxic tumors. J Med Chem 2005;48:4834–41. [DOI] [PubMed] [Google Scholar]
- 47.Li Y, Wang H, Oosterwijk E, et al. . Expression and activity of carbonic anhydrase IX is associated with metabolic dysfunction in MDA-MB-231 breast cancer cells. Cancer Invest 2009;27:613–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Klier M, Jamali S, Ames S, et al. . Catalytic activity of human carbonic anhydrase isoform IX is displayed both extra- and intracellularly. FEBS J 2016;283:191–200. [DOI] [PubMed] [Google Scholar]
- 49.Winum JY, Carta F, Ward C, et al. . Ureido-substituted sulfamates show potent carbonic anhydrase IX inhibitory and antiproliferative activities against breast cancer cell lines. Bioorg Med Chem Lett 2012;22:4681–5. [DOI] [PubMed] [Google Scholar]
- 50.Grandane A, Tanc M, Di Cesare Mannelli L, et al. . 6-Substituted sulfocoumarins are selective carbonic anhydrase IX and XII inhibitors with significant cytotoxicity against colorectal cancer cells. J Med Chem 2015;58:3975–83. [DOI] [PubMed] [Google Scholar]
- 51.Allam HA, Fahim SH, Abo-Ashour MF, et al. . Application of hydrazino and hydrazido linkers to connect benzenesulfonamides with hydrophilic/phobic tails for targeting the middle region of human carbonic anhydrases active site: selective inhibitors of HCA IX. Eur J Med Chem 2019;179:547–56. [DOI] [PubMed] [Google Scholar]
- 52.Bozdag M, Ferraroni M, Ward C, et al. . Carbonic anhydrase inhibitors based on sorafenib scaffold: design, synthesis, crystallographic investigation and effects on primary breast cancer cells. Eur J Med Chem 2019;182:111600. [DOI] [PubMed] [Google Scholar]
- 53.Eldehna WM, Abo-ashour MF, Nocentini A, et al. Eur J Med Chem 2018;162:147–160. [DOI] [PubMed] [Google Scholar]
- 54.Tanini D, Ricci L, Capperucci A, et al. . Synthesis of novel tellurides bearing benzensulfonamide moiety as carbonic anhydrase inhibitors with antitumor activity. Eur J Med Chem 2019;181:111586. [DOI] [PubMed] [Google Scholar]
- 55.Krasavin M, Shetnev A, Sharonova T, et al. . Continued exploration of 1,2,4-oxadiazole periphery for carbonic anhydrase-targeting primary arene sulfonamides: discovery of subnanomolar inhibitors of membrane-bound HCA IX isoform that selectively kill cancer cells in hypoxic environment. Eur J Med Chem 2019;164:92–105. [DOI] [PubMed] [Google Scholar]
- 56.Krasavin M, Shetnev A, Baykov S, et al. . Pyridazinone-substituted benzenesulfonamides display potent inhibition of membrane-bound human carbonic anhydrase IX and promising antiproliferative activity against cancer cell lines. Eur J Med Chem 2019;168:301–14. [DOI] [PubMed] [Google Scholar]
- 57.Abo-Ashour MF, Eldehna WM, Nocentini A, et al. . 3-Hydrazinoisatin-based benzenesulfonamides as novel carbonic anhydrase inhibitors endowed with anticancer activity: synthesis, in vitro biological evaluation and in silico insights. Eur J Med Chem 2019;184:111768. [DOI] [PubMed] [Google Scholar]
- 58.Said MA, Eldehna WM, Nocentini A, et al. . Sulfonamide-based ring-fused analogues for CAN508 as novel carbonic anhydrase inhibitors endowed with antitumor activity: design, synthesis, and in vitro biological evaluation. Eur J Med Chem 2020;189:112019. [DOI] [PubMed] [Google Scholar]
- 59.Khalil OM, Kamal AM, Bua S, et al. . Pyrrolo and pyrrolopyrimidine sulfonamides act as cytotoxic agents in hypoxia via inhibition of transmembrane carbonic anhydrases. Eur J Med Chem 2020;188:112021. [DOI] [PubMed] [Google Scholar]
- 60.Akocak S, Alam MR, Shabana AM, et al. . PEGylated bis-sulfonamide carbonic anhydrase inhibitors can efficiently control the growth of several carbonic anhydrase IX-expressing carcinomas. J Med Chem 2016;59:5077–88. [DOI] [PubMed] [Google Scholar]
- 61.Coviello V, Marchi B, Sartini S, et al. . 1,2-Benzisothiazole derivatives bearing 4-, 5-, or 6-alkyl/arylcarboxamide moieties inhibit carbonic anhydrase isoform IX (CAIX) and cell proliferation under hypoxic conditions. J Med Chem 2016;59:6547–52. [DOI] [PubMed] [Google Scholar]
- 62.Angeli A, Tanini D, Peat TS, et al. . Discovery of new selenoureido analogues of 4-(4-fluorophenylureido)benzenesulfonamide as carbonic anhydrase inhibitors. ACS Med Chem Lett 2017;8:963–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nocentini A, Bua S, Lomelino CL, et al. . Discovery of new sulfonamide carbonic anhydrase IX inhibitors incorporating nitrogenous bases. ACS Med Chem Lett 2017;8:1314–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kazokaitė J, Niemans R, Dudutienė V, et al. . Novel fluorinated carbonic anhydrase IX inhibitors reduce hypoxia-induced acidification and clonogenic survival of cancer cells. Oncotarget 2018;9:26800–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Angeli A, Trallori E, Ferraroni M, et al. . Discovery of new 2, 5-disubstituted 1,3-selenazoles as selective human carbonic anhydrase IX inhibitors with potent anti-tumor activity. Eur J Med Chem 2018;157:1214–22. [DOI] [PubMed] [Google Scholar]
- 66.Queen A, Khan P, Idrees D, et al. . Biological evaluation of P-toluene sulphonylhydrazone as carbonic anhydrase IX inhibitors: an approach to fight hypoxia-induced tumors. Int J Biol Macromol 2018;106:840–50. [DOI] [PubMed] [Google Scholar]
- 67.Nocentini A, Trallori E, Singh S, et al. . 4-Hydroxy-3-nitro-5-ureido-benzenesulfonamides selectively target the tumor-associated carbonic anhydrase isoforms IX and XII showing hypoxia-enhanced antiproliferative profiles. J Med Chem 2018;61:10860–74. [DOI] [PubMed] [Google Scholar]
- 68.Yamali C, Gul HI, Ece A, et al. . Synthesis, biological evaluation and in silico modelling studies of 1,3,5-trisubstituted pyrazoles carrying benzenesulfonamide as potential anticancer agents and selective cancer-associated HCA IX isoenzyme inhibitors. Bioorg Chem 2019;92:103222. [DOI] [PubMed] [Google Scholar]
- 69.Shamsi F, Hasan P, Queen A, et al. . Synthesis and SAR studies of novel 1,2,4-oxadiazole-sulfonamide based compounds as potential anticancer agents for colorectal cancer therapy. Bioorg Chem 2020;98:103754. [DOI] [PubMed] [Google Scholar]
- 70.Kurt BZ, Dag A, Doğan B, et al. . Synthesis, biological activity and multiscale molecular modeling studies of bis-coumarins as selective carbonic anhydrase IX and XII inhibitors with effective cytotoxicity against hepatocellular carcinoma. Bioorg Chem 2019;87:838–50. [DOI] [PubMed] [Google Scholar]
- 71.Zengin Kurt B, Sonmez F, Ozturk D, et al. . Synthesis of coumarin-sulfonamide derivatives and determination of their cytotoxicity, carbonic anhydrase inhibitory and molecular docking studies. Eur J Med Chem 2019;183:111702. [DOI] [PubMed] [Google Scholar]
- 72.Eldehna WM, Abdelrahman MA, Nocentini A, et al. . Bioorganic chemistry synthesis, biological evaluation and in silico studies with 4-benzylidene-2-phenyl-5(4H)-imidazolone-based benzenesulfonamides as novel selective carbonic anhydrase IX inhibitors endowed with anticancer activity. Bioorg Chem 2019;90:103102. [DOI] [PubMed] [Google Scholar]
- 73.Koyuncu I, Tülüce Y, Slahaddin Qadir H, et al. . Evaluation of the anticancer potential of a sulphonamide carbonic anhydrase IX inhibitor on cervical cancer cells. J Enzyme Inhib Med Chem 2019;34:703–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Petreni A, Bonardi A, Lomelino C, et al. . Inclusion of a 5-fluorouracil moiety in nitrogenous bases derivatives as human carbonic anhydrase IX and XII inhibitors produced a targeted action against MDA-MB-231 and T47D breast cancer cells. Eur J Med Chem 2020;190:112112. [DOI] [PubMed] [Google Scholar]
- 75.Hann MM. Molecular obesity, potency and other addictions in drug discovery. Med Chem Commun 2011;2:349–55. [Google Scholar]
- 76.Copeland RA, Pompliano DL, Meek TD.. Drug-target residence time and its implications for lead optimization. Nat Rev Drug Discov 2006;5:730–9. [DOI] [PubMed] [Google Scholar]
- 77.Gleeson MP, Hersey A, Montanari D, Overington J.. Probing the links between in vitro potency, ADMET and physicochemical parameters. Nat Rev Drug Discov 2011;10:197–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Supuran CT. Carbonic anhydrase inhibitors and their potential in a range of therapeutic areas. Expert Opin Ther Pat 2018;28:709–12. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.