Abstract
Objective
Pain after coronary artery by-pass (CAB) surgery is severe. Analgesic administration by mouth is unreliable until after gastrointestinal function has recovered. We evaluated the bioavailability of oxycodone co-administered with naloxone by mouth in patients after CAB surgery using either a conventional extracorporeal circulation (CECC) or off-pump surgery (OPCAB).
Methods
Twenty-four patients, 50–73 years, 12 with CECC and 12 with OPCAB, were administered a 10/5 mg oxycodone-naloxone controlled-release tablet by mouth on the preoperative day and for the first seven postoperative days (PODs) thereafter. Blood samples were collected up to 24 h after the preoperative administration, and then randomly either on POD1 and POD3 or on POD2 and POD4. The oxycodone concentration in plasma was analyzed using liquid chromatography-mass spectrometry.
Results
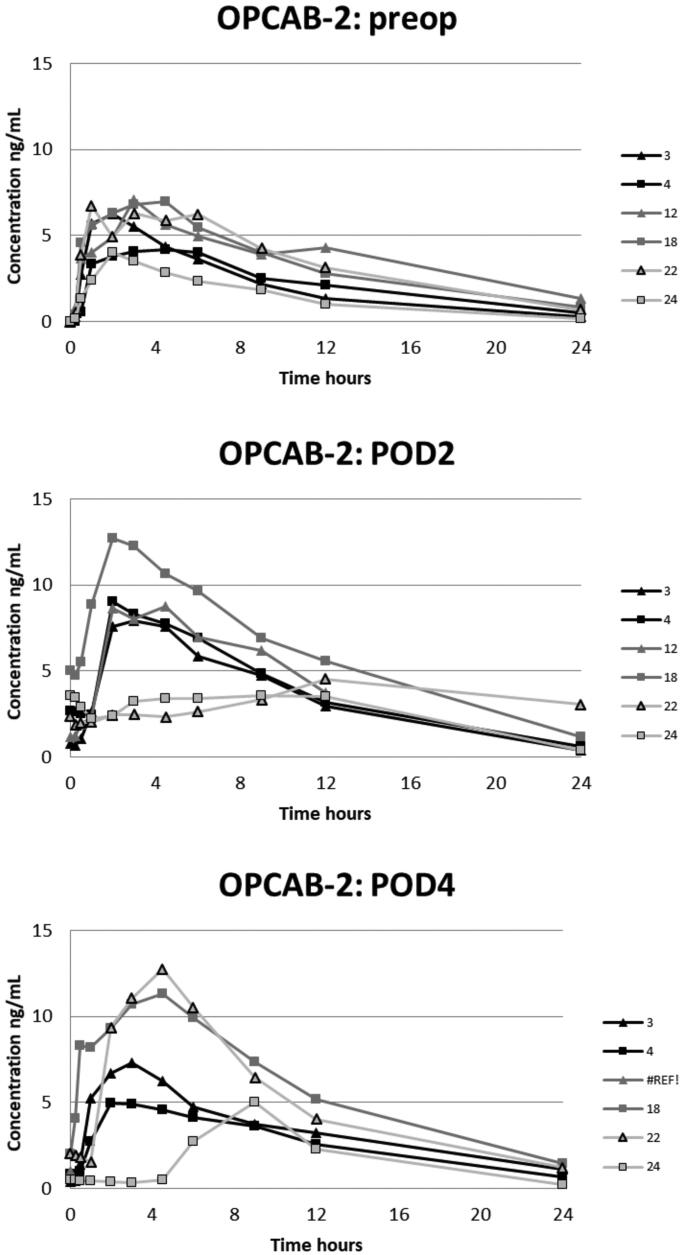
On POD1 oxycodone absorption was markedly delayed in five of six patients after CECC and in all six patients after OPCAB surgery; median of tmax after CECC 630 [range 270–1420] minutes and after OPCAB 1020 [720–1410] minutes, compared to median of 120–315 min preoperatively and on POD2-POD4. The carry-over corrected AUC0–24 values on the PODs did not differ from the preoperative values, but were higher on POD3 compared with POD1 in both CECC and OPCAB groups. The rate and extent of oxycodone absorption equaled preoperative values on POD2 and onwards in patients with CAB surgery.
Conclusions
Bioavailability of oxycodone by mouth was similar after CAB surgery via CECC or having OPCAB. Data indicate that POD2 is an appropriate time to start oxycodone administration by mouth after CAB surgery.
Keywords: Oxycodone, pharmacokinetics, area under the curve, postoperative, coronary artery bypass, off-pump
Introduction
Early postoperative pain after coronary artery bypass (CAB) surgery is often severe. In the majority of patients, moderate or severe pain persists for the few first days after surgery. In our earlier study, at four days after surgery, half of CAB surgery patients still had severe pain at rest, two-thirds during movement and four out of five patients during coughing. Most of the patients experienced more postoperative pain than they anticipated.1
The need for efficient pain management is essential in CAB surgery patients because severe postoperative pain increases the risks for cardiovascular and thromboembolic complications, pneumonia, delays postoperative rehabilitation, and decreases function and health-related quality of life.2 One of the major concerns is that severe acute postoperative pain is associated with an increased risk for persistent pain 12 months after surgery. Data indicate that the longer a patient has severe postoperative pain, the higher the risk for chronic postsurgical pain.1,3
Early postoperative analgesia after CAB surgery is based on opioid analgesics. Acetaminophen (paracetamol) is often included as a part of a multimodal approach,4,5 but non-steroidal anti-inflammatory drugs are contraindicated in the early phase of recovery.6 In the early phase after CAB surgery opioid analgesics are commonly administered parenterally, in co-operative patients via an intravenous (IV) patient-controlled analgesia (PCA) pump, as the absorption of compounds given by mouth is unpredictable.7,8 However, opioid administration by mouth is preferred as soon as gastrointestinal function is restored, because the costs of IV PCA medication are rather high, and the PCA pump and IV lines interfere with patient mobility.9
Oxycodone is a highly effective opioid analgesics, and its use has surpassed that of morphine by several-fold during the last decade in several countries.10 Some recent trials have shown that oxycodone administration by mouth is feasible also in CAB patients.11 Moreover, novel controlled-release tablet formulations may allow a twice daily dosing. In healthy young adults a controlled-release oxycodone tablets the fast absorption component comprised 35% of the dose with an absorption half-life of 0.3 h and the slower absorption component comprised 65% of the dose with an absorption half-life of 4.8 h.12 In vitro release rate data correlate well with the rate of absorption data of oxycodone from controlled-release tablets in young adults.13 This kind of tablet formulation could be feasible also in CAB patients, but to the best of our knowledge there are no pharmacokinetic (PK) data that show when gastrointestinal function is recovered after CAB to allow opioid administration by mouth.
Postoperative ileus is common after major surgery. Many surgery- and anesthesia-related factors contribute to delay gastrointestinal transit, and in addition of anesthetics and perioperative opioids, surgical stress and associated inflammatory reaction may contribute.14 Off-pump CAB surgery (OPCAB) is assumed to be associated with less inflammatory reaction by avoiding the use of a conventional extracorporeal circulation (CECC), maintaining pulsatile blood flow and generally having lesser need for fluid resuscitation during the operation.15 In this study our hypothesis was that the surgical trauma to the body is less after OPCAB surgery than after CAB surgery with CECC, and as a result, the gastrointestinal function is less disturbed and the absorption of oxycodone by mouth from a controlled-release tablet formulation is restored earlier after OPCAB surgery compared to CAB surgery with CECC. To test this hypothesis, we conducted the present PK study where the primary outcome measure was the absorption of oxycodone co-administered with naloxone by mouth on the preoperative and first four postoperative days (PODs) in patients scheduled for CAB with CECC or OPCAB surgery.
Material and methods
Patients
The study population consisted of 24 patients, aged between 50 and 73 years, who were scheduled for elective CAB surgery at Kuopio University Hospital, Kuopio, Finland between November 2015 and December 2016.
The patients were provided oral and written information about the trial protocol, and they all provided written consent by the cardiac surgeon (AV). The study protocol was approved by the Research Ethics Committee of the Northern Savo Hospital District, Kuopio, Finland (January 24, 2012; Ref. 119//2011), the Finnish Medicines Agency was notified (Ref. 63//2012), and it was registered in the European Clinical Trials Database (Eudra CT: 2011-004894-96) prior to patient enrollment. The study was conducted in accordance with the Declaration of Helsinki and had institutional approval.
All patients between 18 and 75 years of age were included if they had no contraindication to oxycodone or naloxone. Patients who had used oxycodone during the previous week prior to surgery were excluded, as were patients with a previous surgery of the upper gastrointestinal tract, disease or any other condition that could interfere with gastric absorption, respiratory depression with hypoxia and/or hypercapnia, chronic obstructive pulmonary disease, moderate or severe hepatic or renal impairment, or history of opioid abuse. Patients taking concomitant cytochrome P450 3A4 (CYP3A4) inhibitors (such as ketoconazole), CYP3A inducers (such as rifampin), CYP2D6 inhibitors (such as paroxetine), or MAO-inhibitors were also excluded.
Oxycodone administration
Patients arrived at the hospital on the day before surgery. For the preoperative administration, the patients fasted for at least 3 h before the oxycodone-naloxone administration. Each patient was given a controlled-release (CR) oxycodone-naloxone tablet 10/5 mg (Targiniq, Mundipharma, Vantaa, Finland) by mouth with a glass of water (150 mL) between 8 and 10 a.m. After this, they were asked to remain in an upright position for at least 30 min, either sitting on a chair or walking around the ward. Fasting was continued after the test drug administration, and at noon the patients were served a light meal.
After surgery, the patients were given a CR oxycodone-naloxone tablet 10/5 mg at 7 a.m. for the first seven PODs after an overnight fast and two hours before breakfast was served.
Blood samples
Patients in both the CECC and OPCAB groups were randomized into two arms. In one study arm, postoperative blood samples were collected on POD1 and POD3, and in the other arm, samples were collected on POD2 and POD4. Thus, blood samples were collected for the PK analysis from each subject on three days: on the preoperative day and on two PODs. The randomization was computer generated (www.randomization.com) by the principal investigator (Figure 1).
Figure 1.
Flow chart. CECC = conventional extracorporeal circulation; OPCAB = Off-pump coronary artery bypass surgery; PREOP = preoperative day; POD = postoperative day.
Blood samples (3 mL) were obtained with an indwelling catheter inserted into an antecubital vein at baseline (before drug administration), and at 0.25, 0.5, 1, 2, 3, 4.5, 6, 9, 12 and 24 h after the oxycodone-naloxone administration. The baseline sample was obtained before the 7 a.m. administration, and the 24 h sample was obtained before the next 7 a.m. oxycodone-naloxone administration. Blood was collected into EDTA tubes, and plasma was obtained within 60 min of collection by centrifugation at 2100 g for 10 min at +20 °C. The separated plasma was stored at −76 °C until analysis. Arterial blood pressure, heart rate and rhythm, peripheral capillary oxygen saturation (SpO2), respiratory rate, end-tidal CO2 (ETCO2) and adverse effects were recorded after each blood sample. Pain was also evaluated during the blood collection visits, at rest, with coughing and during a deep breath, using an 11-point numeric rating scale (NRS, 0 = no pain, 10 = most pain). Morphine consumption for rescue analgesia via the IV PCA pump was recorded in 12-h intervals from 7 a.m. to 7 p.m.
Outcome measures
The primary outcome measure was the area under the oxycodone curve from time zero to 24 h calculated with the carry-over subtraction (corrected AUC0–24; see Pharmacokinetic parameters) on the PODs compared to that on the preoperative day. The secondary outcome measures were the observed AUC0–24 and peak concentration (Cmax), time to peak concentration (tmax) and terminal half-life (t½). For the clinical outcome measure we used pain scores, the consumption of IV PCA morphine for rescue analgesia and adverse events (AE).
Anesthetic management and extracorporeal circulation
Before surgery, the patients received premedication by mouth: diazepam 0.25 mg/kg up to 20 mg. Nitrides, beta-blockers, statins, cortisone and medication for chronic pulmonary diseases were given from their drug list. A standardized anesthesia protocol was used for each patient. Anesthesia was induced with intravenous midazolam, sufentanil, propofol and pancuronium. Anesthesia was maintained with propofol infusion, and sufentanil and pancuronium boluses i.v. Sevoflurane was added if the patient was hypertensive. Customized perfusion sets were used in the CECC group as previously described.8
Postoperative pain
Each patient was provided with multimodal postoperative pain management. In addition to seven daily single dose oxycodone-naloxone CR 10/5 mg tablets administered at 7 a.m., the patients received acetaminophen 1 g IV or by mouth three times per day. Regarding rescue analgesia, for the first five PODs, the patients had an IV PCA pump with morphine, single dose 2 mg, lock-out time 10 min, maximum dose 20 mg/4 h. For patients with meaningful pain, indicated by a pain score >3/10 at rest or >5/10 during coughing or a deep breath, nurses were allowed to administer 5 mg IV morphine. Following the study period, the patients were prescribed acetaminophen/codeine 500/30 mg tablets to be used up to 8 tablets/24 h as required.
Plasma oxycodone and metabolites concentrations
Oxycodone and metabolites concentrations were analyzed with an ultra-performance liquid chromatography mass spectrometry system in three patches at Admescope Ltd., Oulu, Finland as previously described in detail.16 The linear calibration ranges (ng/mL) were fitted as follows: oxycodone 0.02–500, oxymorphone 0.05–200, noroxymorphone 0.1–500, and noroxycodone 0.2–200. Accuracies were between 84 and 125% at the lowest limit of quantification (LLoQ) and 83–112% above the LLoQ. Precisions were 0.6–17% over the entire range of calibration. All concentrations of oxycodone and its metabolites are reported as free bases.
Pharmacokinetic parameters
The AUC0–24, Cmax, tmax, t1/2, and terminal elimination rate constant (Kel) were determined using noncompartmental analysis with Phoenix WinNonlin version 6.3 software (Certara, Princeton, NJ, USA). The observed AUC0–24 was calculated using the linear-up and log-down trapezoidal rule, and the carry-over concentration from the previous dose at time zero (C0) was used as such without any subtraction. The Kel and t1/2 were determined only when there was a clear log-linear terminal phase.
The carry-over corrected AUC0–24 was calculated assuming an exponential decline in the carry-over concentration:
where C24(calc) is the calculated carry-over concentration at the last sampling time (24 h). In this correction Kel was taken from the same dosing event of the individual patient when available, and otherwise from the previous studied dosing event of the same patient.
Pharmacokinetic population modeling
A 2-compartment PK model with a lag-time, first-order absorption and first-order elimination was used to describe oxycodone disposition. The model was parameterized in terms of CL (L/h/70 kg), V (L/70 kg), intercompartmental CL (Q, L/h/70 kg), absorption half-life (tabs, h) and absorption lag-time (tlag, h). The absorption lag-time was used to quantify the delay in oxycodone-naloxone controlled-release tablet absorption after cardiac surgery. Data were pooled with those available in patients given the intravenous formulation after laparotomy17 and urological procedures18,19 to make an estimate of the relative oral bioavailability (F). Parameter estimates were scaled using theory based allometry to a 70 kg total body weight individual.
where Fsize is a variable describing the fractional difference from a standard adult weighing 70 kg e.g. CL. EXP describes the allometric exponent; ¾ for functional processes such as CL, 1 for volumes and ¼ for parameters such as half-life which are dependent on the ratio of V over CL. Absorption was assumed independent of size.
Absorption on POD1 was slow and this was quantified by adding an additional factor to account for this delay (Fabs)
Lag time (tlag) was also prolonged after cardiac surgery but returned to preoperative values over the 5-day study period. This was quantified using an additional factor (Flag) and a recovery half-time (LagT)
Population parameter estimates were obtained using mixed effects models with ADVAN4 TRANS4 (NONMEM 7.4, ICON Development Solutions, Ellicott City, MD, USA) with first-order conditional estimation and a convergence criterion set to 3 significant digits. Population parameter variability (PPV) was described using an exponential model for the random effect variables (η); these variables were assumed to have a mean of zero and variance denoted by ω2.
where P is the parameter (e.g. CL) for the ith individual, PTV is the typical value for that parameter and η is the random effects variable. Between occasion variability for CL, V and F was added to the model because oral oxycodone was administered daily for seven PODs. Population parameter variability comprises both between subject variability (BSV) and between occasion variability (BOV); BOV was estimated for CL, V1 and F.
Residual unidentified variability (RUV) was accounted for using a combined residual error model (consisting of additive and proportional error terms) for oxycodone PK. BSV in the residual error model was estimated for each observation. Estimates of the proportional (θRUV_CV) and additive (θRUV_SD) residual error parameters were obtained. The population parameter variability of the RUV (ηPPV_RUV) was also estimated:
where OBSij is the observation (oxycodone plasma concentration) in the ith individual at the jth time. Individual predictions of concentration were calculated using the following equation with the random effects () fixed to 1.
Model selection
The decrease in objective function value (OBJ; [−2log likelihood]) was used as a guide during the model building process, with a lower OBJ within nested models indicating a superior model. Model selection was also based on inspection of prediction corrected visual predictive check (PC-VPC) plots20 and confidence intervals surrounding parameter estimates obtained by bootstrapping. Bootstrap methods provide a means to evaluate parameter uncertainty. Parameter medians and their associated 95% confidence intervals were obtained after 100 bootstrap replications.
Statistical analysis
No formal sample size calculation was performed but 24 patients were assumed to provide sufficient data on PK after CAB surgery. Statistical analysis was performed with SigmaPlot version 13.0 software (Systat Software, Inc., San Jose, CA, USA). Between-group differences in the preoperative day AUC0–24, Cmax, and t1/2 values were analyzed with the Kruskall–Wallis test, and between-day differences in the AUC0–24 and Cmax values within each group were analyzed with the Friedman test. Tukey’s test was used in all pair-wise comparisons. Differences were regarded as statistically significant if the p-value was less than .05. Data are expressed as the number of cases and median with minimum and maximum values where appropriate.
Results
Patient characteristics are presented in Table 1. The groups were similar regarding sex, age and body mass index. There were no blood samples for one subject in the OPCAB group on POD4. Most subjects had carry-over concentration from the previous dose. The median oxycodone C0 on POD1 was 0.07 [0.0–0.15] ng/mL, POD2 1.8 [0.0–5.0] ng/mL, POD3 1.6 [0.41–5.1] ng/mL and POD4 1.0 [0.39–3.4] ng/mL, respectively.
Table 1.
Patient characteristics.
| Parameter | Conventional extracorporeal circulation groups |
Off-pump groups |
||
|---|---|---|---|---|
| Samples on POD1 and POD3 | Samples on POD2 and POD4 | Samples on POD1 and POD3 | Samples on POD2 and POD4h | |
| n = 6 | n = 6 | n = 6 | n = 6 | |
| Sex: male/female | 6/– | 5/1 | 6/– | 5/1 |
| Age, years | 63 [57–73] | 61 [50–73] | 66 [59–72] | 66 [56–70] |
| Height, m | 1.73 [1.63–1.83] | 1.72 [1.71–1.82] | 1.75 [1.70–1.84] | 1.78 [1.61–1.82] |
| Weight, kg | 86 [60–102] | 93 [85–99] | 81 [65–98] | 81 [65–104] |
| BMI, kg/m2 | 27.6 [22.4–34.9] | 30.4 [29.1–32.4] | 25.8 [21.2–33.7] | 25.2 [22.2–35.0] |
Data are presented as the number of cases or median [minimum – maximum].
Abbreviations: POD, postoperative day; BMI, body mass index.
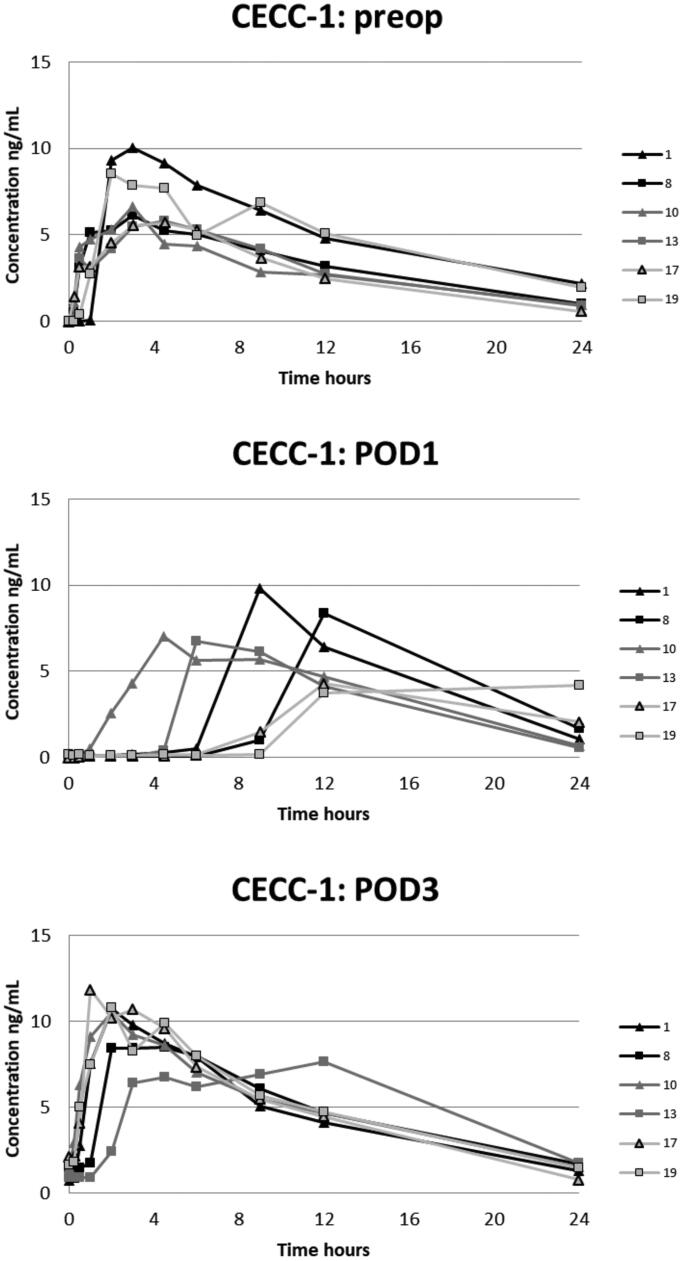
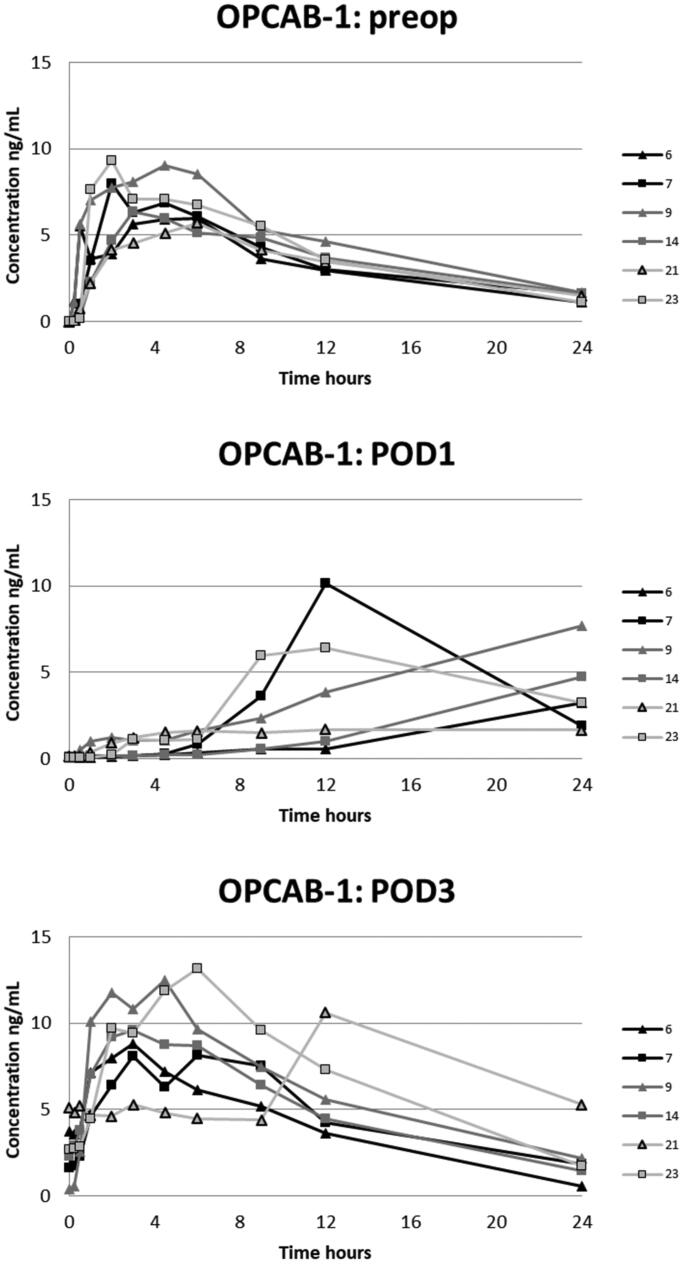
The plasma oxycodone concentration curves for each patient are presented in Figures 2–5. On POD1 oxycodone absorption was markedly delayed in five of six patients after CECC surgery and in all six patients after OPCAB surgery. On POD1 the median of tmax after CECC was 630 [range, 270–1420] minutes and after OPCAB 1020 [720–1410] minutes, respectively. These values were substantially longer than the preoperative values, tmax before CECC was 180 [30–270] minutes and before OPCAC 180 [60–360] minutes (Table 2).
Figure 2.
Plasma concentrations of oxycodone in the CECC-group with blood samples in the preoperative, and 1st and 3rd postoperative days.
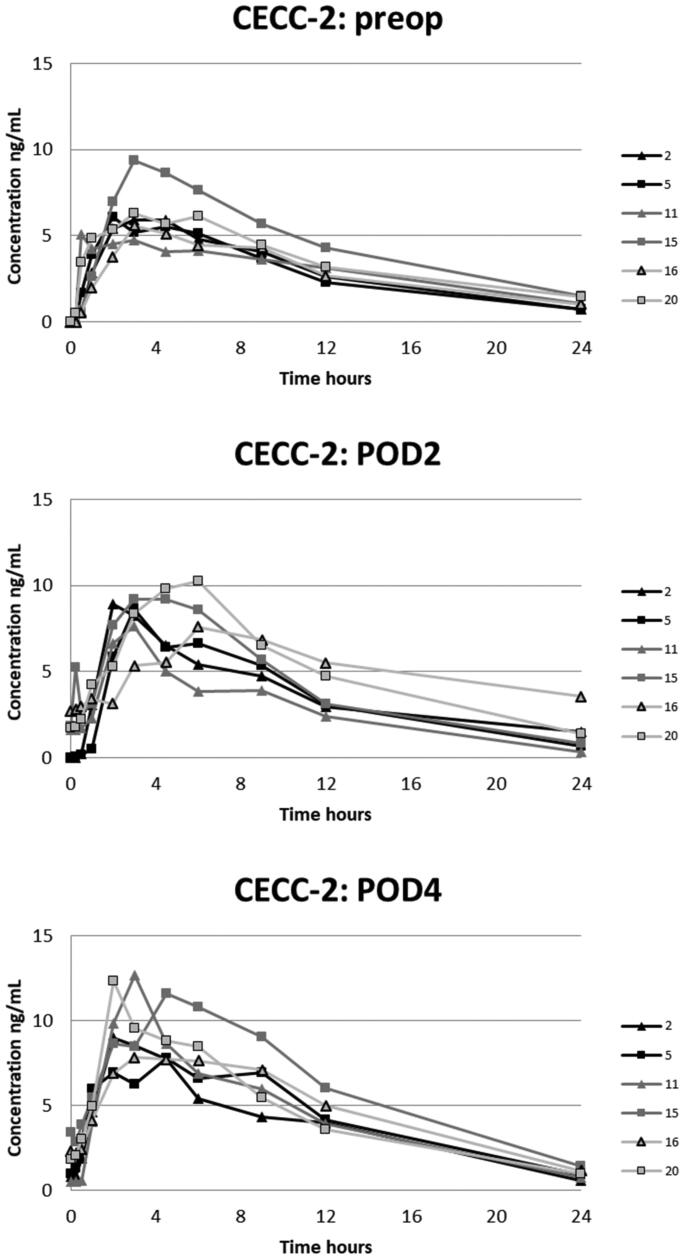
Figure 3.
Plasma concentrations of oxycodone in the CECC-group with blood samples in the preoperative, and 2nd and 4th postoperative days.
Figure 4.
Plasma concentrations of oxycodone in the OPCAB-group with blood samples in the preoperative, and 1st and 3rd postoperative days.
Figure 5.
Plasma concentrations of oxycodone in the OPCAB-group with blood samples in the preoperative, and 2nd and 4th postoperative days.
Table 2.
Pharmacokinetic parameters for 10 mg oxycodone hydrochloride (9.0 mg free base) after coronary artery by-pass surgery in the different treatment groups.
| Group | Study day | n | AUC0–24a | Corrected AUC0–24b | Cmaxa | tmax | t1/2 |
|---|---|---|---|---|---|---|---|
| (ng·min/mL) | (ng·min/mL) | (ng/mL) | (min) | (min) | |||
| 1 (CECC) | Preoperative | 6 | 4250 [3590–6570] | 4250 [3590–6570] | 6.4 [5.7–10.0] | 180 [120–270] | 387 [299–481] |
| POD1 | 6 | 3650 [2760-4550] | 3620 [2760–4490] | 6.9 [4.2–9.8] | 630 [270–1420] | – | |
| POD3 | 6 | 7060 [6450-7270]§§ | 6130 [5610–6800]§ | 10.6 [7.7–11.8]*,§ | 120 [60–720] | 437 [376–475] (n = 5) | |
| 2 (CECC) | Preoperative | 6 | 3830 [3560-6070] | 3830 [3560–6070] | 6.0 [5.1–9.4] | 180 [30–180] | 394 [313–496] |
| POD2 | 6 | 5600 [3790-7250] | 4960 [3140–6070] | 8.8 [7.6–10.3] | 180 [120–360] | 346 [286–968] | |
| POD4 | 6 | 6220 [5270-8610]* | 5500 [4920–6990] | 10.3 [7.8–12.7]* | 180 [120–270] | 330 [293–348] | |
| 3 (OPCAB) | Preoperative | 6 | 4560 [4280-6480] | 4560 [4280–6480] | 7.2 [5.7–9.3] | 225 [120–360] | 417 [378–531] |
| POD1 | 6 | 3740 [1390-5400] | 3690 [1360–5320] | 5.6 [1.7–10.2] | 1020 [720–1410] | – | |
| POD3 | 6 | 8000 [5620-9520]§§ | 5990 [3990–8330]§ | 10.1 [8.1–13.2]§ | 315 [180–720] | 462 [311–463] (n = 5) | |
| 4 (OPCAB) | Preoperative | 6 | 3720 [1930-4870] | 3720 [1930–4870] | 6.5 [4.0–7.1] | 150 [60–270] | 309 [244–497] |
| POD2 | 6 | 4570 [3370-8570] | 4030 [2100–5990] | 8.4 [3.6–12.7] | 230 [120–720] | 333 [263–394] (n = 4) | |
| POD4 | 5 | 4640 [2190-8170] | 4410 [2040–7150] | 7.3 [5.0–12.8] | 270 [120–540] | 374 [195–472] |
Data are presented as the median [minimum–maximum] (n for t1/2 if different from the group size).
aBased on the observed concentrations, bthe carry-over from the previous doses were subtracted as described in Materials and methods. Abbreviations: POD, postoperative day; CECC, conventional extracorporeal circulation; OPCAB, off-pump coronary artery bypass surgery. Significances: *p < .05 vs. Preoperative day; §p < .05 vs. POD1; §§p < .01 vs. POD1.
The corrected AUC0–24 on the PODs did not differ statistically from the preoperative values (Table 2). However, the corrected AUC0–24 and the observed AUC0–24 and Cmax were all higher on POD3 compared with those on POD1 after CECC (group 1) and OPCAB surgery (group 3), respectively. Additionally, Cmax on POD3 and POD4 was higher than the preoperative value after CECC surgery (groups 1 and 2), as was the observed AUC0–24 on POD4 compared with the preoperative value (group 2).
The median observed AUC0–24 on POD3 and POD4 was 62–75% higher than the preoperative value in groups 1–3 and 25% higher in group 4, respectively (Table 2). This observed accumulation after the surgery was markedly higher than the theoretical 7% steady-state accumulation calculated with the mean Kel of 0.112 (SD 0.025) 1/h (n = 24) on the preoperative day [1/(EXP(−0.112 1/h × 24 h) = 1.07]. A similar trend was seen in Cmax.
Noroxycodone was the main metabolite and oxymorphone and noroxymorphone were detected only in low concentrations (Table 3).
Table 3.
Peak concentrations of main oxycodone metabolites in plasma.
| Group | Study day | Cmax (ng/mL) |
||
|---|---|---|---|---|
| Noroxycodone | Oxymorphone | Noroxymorphone | ||
| 1 (CECC) | Preoperative | 6.7 [5.0–10.4] | 0.10 [0.07–0.15] | 1.3 [0.5–3.1] |
| POD1 | 6.3 [2.7–12.5] | 0.12 [0.10–0.19] | 1.5 [0.7–1.9] | |
| POD3 | 6.0 [3.5–10.1] | 0.23 [0.13–0.42] | 1.3 [0.8–3.3] | |
| 2 (CECC) | Preoperative | 7.0 [4.6–8.9] | 0.13 [0.0–0.18] | 1.0 [0.5–1.5] |
| POD2 | 6.0 [3.2–11.9] | 0.21 [0.0–0.30] | 1.4 [0.27–1.8] | |
| POD4 | 6.3 [2.7–12.5] | 0.12 [0.10–0.19] | 1.5 [0.7–1.9] | |
| 3 (OPCAB) | Preoperative | 6.5 [4.5–11.8] | 0.18 [0.0–0.26] | 1.9 [0.27–3.0] |
| POD1 | 5.3 [1.7–6.8] | 0.14 [0.0–0.23] | 1.1 [0.18–1.9] | |
| POD3 | 5.0 [2.5–9.9] | 0.39 [0.0–0.86] | 2.1 [0.5–2.9] | |
| 4 (OPCAB) | Preoperative | 8.9 [4.6–10.4] | 0.14 [0.0–0.29] | 1.6 [0.27–3.1] |
| POD2 | 9.4 [2.3–17.5] | 0.18 [0.0–0.54] | 1.7 [0.27–3.8] | |
| POD4 | 5.3 [2.5–9.2] | 0.28 [0.0–0.69] | 1.4 [0.45–2.0] | |
Data are presented as the median [minimum–maximum].
Abbreviations: Cmax, peak concentration; POD, postoperative day; CECC, conventional extracorporeal circulation; OPCAB, off-pump coronary artery bypass surgery.
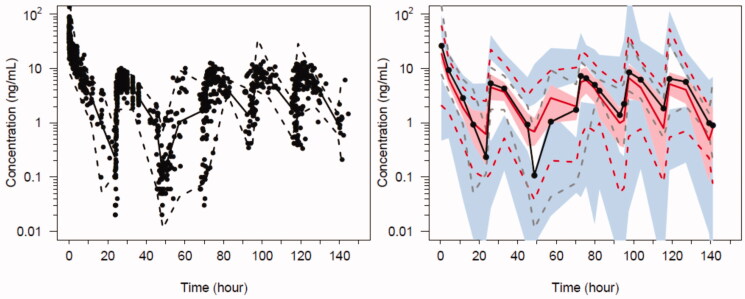
Population parameter estimates for the oxycodone 2-compartment analysis are shown in Table 4 and PC-VPC for the final oxycodone PK model in Figure 6. Data for PK analyses comprised of 1072 oxycodone plasma concentrations. Absorption was slow on POD1 (Fabs= 3.91) as was the lag time (Flag = 11.8) that rapidly resolved (lagT = 1.25 days). We were unable to define a difference in these parameter estimates in those who had surgery with cardiopulmonary bypass and those without cardiopulmonary bypass.
Table 4.
Oxycodone population pharmacokinetic parameter estimates.
| PPV (%) |
||||
|---|---|---|---|---|
| Parameter | Estimate | 95%CI | BSV | BOV |
| V1 (L/70 kg) | 134 | 90.9, 157 | 176 | 70.9 |
| V2 (L/70kg) | 81.9 | 72.3, 105 | 83.6 | |
| CL (L/h/70 kg) | 43.3 | 38.5, 49.5 | 37.0 | 32.1 |
| Q (L/h/70kg) | 155 | 151, 182 | 86.7 | |
| Relative bioavailability | 0.53 | 0.48, 0.60 | 56.6 | |
| tlag (hours) | 0.27 | 0.25, 0.33 | – | |
| Flag | 11.8 | 10.1, 15.1 | – | |
| LagT (days) | 1.25 | 1.2, 1.4 | – | |
| tabs (hours) | 1.55 | 1.4, 2.2 | – | |
| Fabs | 3.91 | 2.6, 4.6 | – | |
| RUV additive (ng/mL) | 0.082 | 0.035, 0.089 | ηPPV_RUV 0.44 | |
| RUV proportional (%) | 21.4 | 19, 33 | – | |
Parameter estimates are presented as the medians.
Abbreviations: V, volume of distribution; CL, clearance; Q, intercompartmental clearance; tlag, absorption lag-time; Flag is a scaling parameter applicable to tlag on POD1; tabs, absorption half-time; Fabs is a scaling parameter applicable to tabs on POD1; RUV, residual unidentified variability; CI, confident interval; PPV% (PPV%=√variance × 100), population parameter variability; BSV, between subject variability; BOV, between subject variability.
Figure 6.
Predicted-corrected visual predictive check (PC-VPC) for the final oxycodone PK model. Plots show median (solid) and 90% intervals (dashed lines). The y axis is presented on a logarithmic scale. Left hand plot shows all prediction corrected observed oxycodone concentrations. Right hand plot shows prediction corrected percentiles (10%, 50%, and 90%) for observations (grey dashed lines) and predictions (red dashed lines) with 95% confidence intervals for prediction percentiles (median, pink shading; 5th and 95th blue shading).
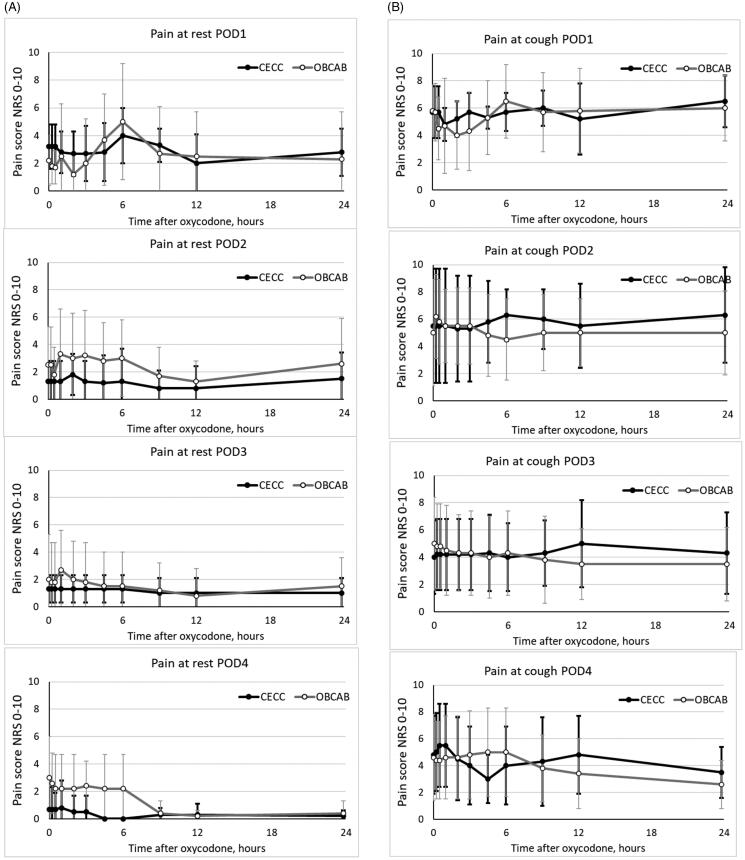
Pain scores were similar in patients after CECC and OPCAB surgery (Figure 7(A,B)).
Figure 7.
Pain scores in the two groups at rest (A) and with cough (B) in the two groups. Data are presented as the mean and standard deviation.
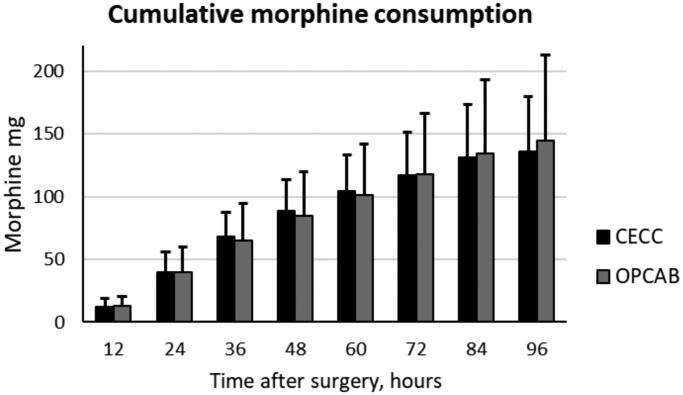
Morphine consumption via an IV PCA pump was similar among patients after CECC and OPCAB surgery. However, the between-subjects variation was large; after CECC surgery, it was between 59 and 199 mg/96 h, and after OPCAB surgery, it was between 69 and 324 mg/96 h (Figure 8).
Figure 8.
Morphine consumption via IV PCA pump in the two groups. Data are presented as the mean and standard deviation.
Twenty-one subjects (11 in the CECC group and ten in the OPCAB group) developed a total of 38 AEs (n = 18 and n = 20 in the two groups, respectively), most of which were probably opioid-related. Two patients had low SpO2 (86 and 89% at 2 and 6 h), and one had a low respiratory rate (7/min at 9 h) after preoperative oxycodone-naloxone administration, but all had normal ETCO2. They were given supplementary oxygen and recovery was uneventful thereafter. Postoperatively, there were no cases of low respiratory rates or high ETCO2 values, but eight subjects in each group had at least a single SpO2 value below 90% (the lowest recorded value was 81%) and thus, were given oxygen supplementation. Eight subjects had constipation, four were somnolent, three had postoperative atrial fibrillation, two were confused and two had nausea.
Discussion
In contrast to our hypothesis, the data of the present study suggest that the recovery of gastrointestinal function and the absorption of ingested oxycodone is similar after OPCAB surgery and after CAB surgery with CECC. The novelty of this study is that the observed and corrected AUC0–24 and Cmax of oxycodone equaled or exceeded the preoperative values on POD2 in four out of six patients in both groups, and it was similar to or higher than the preoperative values on POD3 and POD4 in each patient. Oxycodone-naloxone CR tablets are not recommended within the first 12–24 h post-operatively.18,21 Consistent with that, our data indicate that the appropriate time to start oxycodone administration by mouth is on POD2 in most patients with CAB surgery.
Oxycodone absorption was markedly delayed in most patients on POD1 after CAB surgery. The median tmax values on POD1 were 3- to 6-fold longer than those observed preoperatively or on POD2-POD4. We assume that this was caused by a slow gastric emptying time in the early phase of recovery. Oxycodone is absorbed mainly in the small intestine, and the majority of oxycodone is absorbed before the tablet reaches the colon.18 Following surgery, gastrointestinal track motility is impaired as a result of an ileus, pseudo-obstruction and the use of pharmacologic agents. Opioids per se also delay gastric emptying.14,22 However, the co-administration of naloxone reduces the oxycodone induced slowing of gastrointestinal transit.22 Our data suppose that this was also the case in the present study except on POD1, as the rate and extent of oxycodone absorption had already returned to similar or higher than the preoperative values in most patients on POD2. To support the early recovery of gastrointestinal function in the present study, on POD2, POD3 and POD4, the PK data of oxycodone-naloxone CR tablets were dose-proportional to those reported in healthy subjects12,22 and those found in our earlier study with oxycodone CR tablets in elderly patients undergoing cystoscopy under regional anesthesia.18
On POD1 oxycodone absorption was markedly delayed in eleven of twelve subjects. There were no differences in AUC0–24 and Cmax between the preoperative day and POD1, but the estimated AUC0–24, Cmax and tmax values on POD1 are likely to be somewhat inaccurate due to the delayed absorption and the fact that blood samples were not collected during the night (the last two samples were taken at 12 and 24 h). However, even with the inaccurate estimate of AUC0–24 on POD1, it is clear that AUC0–24 on POD3 was significantly higher than on POD1 as the median observed AUC0–24 was approximately twofold. The most likely explanation is that a portion of the doses given on POD1 and POD2 was absorbed with a significant delay leading to an increased drug exposure on POD3 that clearly exceeded the theoretical accumulation factor calculated from the preoperative day data (7% increase). A plausible mechanism is the surgery- and opioid-related pylorus closure.14 The delayed absorption after the surgery would also explain the 23–75% increase in median observed AUC0–24 on POD2, POD3 and POD4 compared with the preoperative day, respectively, even though a statistical significance was found only in the CECC group 2 between POD4 and the preoperative day.
A 2-compartment PK model with a lag-time, first-order absorption and first-order elimination was used to describe oxycodone disposition and parameter estimates align with findings from other studies where 2-compartment models have been used to describe oxycodone disposition. Use of a 2-compartment model allowed comparison with estimates published by others.17–19 Cardiac surgery is associated with profound changes to physiology (e.g. altered volume of distribution and changes in plasma protein concentrations), changes in gut, renal and hepatic activity; these factors can alter perioperative drug PK.23,24 We assume these factors contribute to the high between subject variability observed on V1, V2 and Q and the BOV on V1. The lag in absorption is likely caused by delayed gastric emptying following cardiac surgery. There was an increase in the absorption half-time on POD1. The lag time was also prolonged on POD1 (Flag = 11.8) but recovered rapidly with a half-time (LagT) of 1.25 days. This is likely attributable to the return of normal gut function in the days following cardiac surgery.
In the present study two subjects had nausea, but none had postoperative vomiting, and eight out of the 24 patients had constipation. Opioid induced bowel dysfunction can affect oxycodone absorption by several mechanisms. Postoperative nausea and vomiting are common complaints and the use of perioperative use of opioids is a known risk factor for postoperative vomiting. If a patient vomits within the first hour after oxycodone CR tablet intake, it is unlikely that any meaningful amount of oxycodone has been absorbed. However, it is not recommended to repeat or to take an extra dose. Pain management should just follow regular dosing schedule.21 Since oxycodone is used for postoperative pain, patients should have rescue analgesia available for breakthrough pain in any case. Opioid induced bowel dysfunction inhibits gastric emptying and peristalsis in the gastrointestinal tract. The first may result in delayed absorption of oxycodone due to delayed emptying (see above). Slowing intestinal peristalsis may also increase the retention time of oxycodone in small intestine.14 A prolonged transit in small intestine could have been one mechanism for higher extent of oxycodone absorption of oxycodone most evident on POD3.13 Other mechanism could also be involved, e.g. increased absorption of water, less gastric secretion and saliva production induced by oxycodone. Diet does not affect the PK of CR oxycodone tablets.10,12,21
Our data are consistent with data that were previously reported in CAB surgery patients who were administered postoperative metoprolol tablets.7,8 The data of those two studies showed that the rate and extent of metoprolol absorption after administration by mouth was markedly decreased in the early phase after CAB surgery. Similar to the results found in the present study, it was shown that the use of CECC in CAB surgery or performing OPCAB surgery does not affect the postoperative absorption of metoprolol, i.e. the restoration of gastrointestinal function.
The absorption of oxycodone from an oxycodone-naloxone CR tablet is biphasic. The initial absorption occurs from the surface of the tablet, following the dissolution of the film coating. The remaining drug substance is absorbed from the matrix, either by dissolution or diffusion from or through the tablet matrix. PK models indicate that the faster absorption accounts for one-third and the slower absorption two-thirds of the total absorption, so that 95% of oxycodone absorption in completed at 24 h after administration.18,25 Consistent with that finding, in the present study, most patients had substantial trough concentrations on POD2 and POD3 at 24 h after the previous dose, but on POD1, 48 h after the previous dose, trough concentrations were low (0.17 ng/mL or lower). A second peak could be explained, in theory, by enterohepatic circulation. It is not known whether oxycodone undergoes enterohepatic circulation. Morphine and its active metabolites are assumed to undergo substantial enterohepatic circulation and this may account a second peak in serum concentration over time after administration by mouth and increase in relatively potency of repeated doses compared to that of single morphine dose by mouth.26 The main route of oxycodone excretion is the kidney, 8% as oxycodone and 65% as metabolites, and oxycodone undergoes some first-pass metabolism in the intestine.10 Biliary excretion of oxycodone in humans is not known, but animal data indicate that small amount of oxycodone and its metabolites are excreted in feces.27
In the present PK study oxycodone-naloxone CR tablets were administered 24-hourly. In clinical practice, oxycodone-naloxone CR tablets are intended for scheduled administration, and the usual starting dose for opioid-naïve patients is 10/5 mg 12-hourly.21 In healthy volunteers, steady-state PK of oxycodone CR tablets is achieved after two or three 12-hourly doses.28 In the present study, the median of the oxycodone C12 ranged between 3.7 and 5.2 ng/mL on POD2, POD3 and POD4. Thus, a higher oxycodone accumulation would have been seen and higher trough concentrations observed if oxycodone-naloxone CR tablets were administered 12-hourly.
In consistent to PK data showing negligible absorption of oxycodone by mouth on POD1, morphine consumption to comfort was similar high to that reported earlier after CAB surgery.4,29,30 Thereafter, as PK data of indicate that oxycodone absorption had restored to preoperative values a parallel decline on the need for rescue PCA-analgesia was observed. Postoperative pain was most severe on POD1, but thereafter pain was rather well controlled. On POD2 and thereafter, majority of patients had just mild pain at rest. However, after thoracic surgery dynamic pain, pain provoked by movement, such as deep breathing or coughing, getting out of bed, or walking are more important parameters than pain at rest.1,4,29,30 In the present study dynamic pain was modestly well controlled with co-administration of opioid-analgesics and paracetamol. However, the between-subjects variation on dynamic pain scores was large, and in clinical practice these patients should be identified, have more attention and prescribed personalized pain management with adjuvant analgesia techniques and close follow-up in order to minimize the risk of persistent postoperative pain and other postoperative complications.1–3
The main limitations in the present study are a small number of subjects and that the postoperative blood samples were obtained on alternate PODs. However, due to logistical reasons, we were not able to enroll more patients. Moreover, it was considered unethical to obtain several blood samples daily. On each of three study days, a total of eleven blood samples were collected for this study, in addition to those collected for the clinical purposes. Another limitation is that allow the data enabled a standard non-compartmental statistical approach only. A modeling-based approach would have been far more informative and allow for robust conclusions and the potential for simulations to explore alternative dosing practices. Building a population PK model was evaluated but the data on POD1 especially were that heterogeneous that the concentration curves could not be reliably predicted or explained with PK model. Thus, the use of non-compartmental statistical approach is justified, and we believe that non-compartmental data are sufficient to show that administration by mouth on POD1 cannot be recommended in this context.31
One of the limitations is that this PK study does not enable us to draw any pharmacodynamic conclusions because there was no control group; each patient was administered oxycodone-naloxone 10/5 mg CR tablets 24-hourly, and there were not follow-up data collected after the first postoperative week. However, similar pain scores and postoperative morphine consumption support the contention that bioavailability was similar between both groups. Opioid-induced bowel dysfunction is one of the concerns with taking postoperative opioids. Previously, we showed that even seven days use of oxycodone-naloxone CR tablets administration may prevent the development or reduce the severity of opioid-induced bowel dysfunction without interfering with the analgesic efficacy of oxycodone compared to oxycodone CR tablets. Moreover, it was found that oxycodone-naloxone tablets had a carry-over effect after seven days use of oxycodone two times a day; surveyed two weeks after opioid cessation, bowel function was superior after seven days of taking oxycodone-naloxone tablets compared to oxycodone tablets.32 In the present study the opioid consumption and pain scores were similar or lower compared to our previous studies in similar settings.4,29,30 Thus, it is unlikely that naloxone interfered with the analgesic efficacy of oxycodone.
In conclusion, the postoperative bioavailability of oxycodone from oxycodone-naloxone CR tablets seems to be similar after CAB with CECC and after OPCAB surgery. Second, oxycodone administration by mouth is not recommended on POD1 because the absorption is markedly delayed in the majority of patients, but the rate and extent of oxycodone absorption resumed or exceeded the preoperative values on POD2 and beyond. Thus, the appropriate time to start oxycodone administration by mouth is on POD2 in most patients with CAB surgery.
Acknowledgements
The authors thank RN Petri Toroi for his help in conducting this research.
Transparency
Declaration of funding
This work was supported by the governmental VTR-grant by the Hospital District of Northern Savo, Kuopio, Finland; the Research Foundation of Kuopio University Hospital, Kuopio Finland; and Olvi Säätiö, Iisalmi, Finland.
Declaration of financial/other relationships
No potential conflict of interest was reported by the authors.
Authors contributions
Principal investigator: HK; study design: AV, VPR, HK; data collection: AV, PF, PL, TM; analysis and interpretation of data: AV, VPR, HK; pharmacometric analyses: JDM, BJA; contributed new methods and analyzed data: HH; wrote the paper: HK; critical evaluation of the intelligent content of the final version: AV, JDM, PF, HH, PL, TM, BJA, VPR.
Data availability statement
The data that support the findings of this study are available from the corresponding author, HK, upon reasonable request.
References
- 1.Lahtinen P, Kokki H, Hynynen M.. Pain after cardiac surgery: a prospective cohort study of 1-year incidence and intensity. Anesthesiology. 2006;105:794–800. [DOI] [PubMed] [Google Scholar]
- 2.Liu SS, Wu CL.. Effect of postoperative analgesia on major postoperative complications: a systematic update of the evidence. Anesth Analg. 2007;104:689–702. [DOI] [PubMed] [Google Scholar]
- 3.Fletcher D, Stamer UM, Pogatzki-Zahn E, et al. Chronic postsurgical pain in Europe: An observational study. Eur J Anaesthesiol. 2015;32:725–734. [DOI] [PubMed] [Google Scholar]
- 4.Lahtinen P, Kokki H, Hendolin H, et al. Propacetamol as adjunctive treatment for postoperative pain after cardiac surgery. Anesth Analg. 2002;95:813–819. [DOI] [PubMed] [Google Scholar]
- 5.Van Driest SL, Jooste EH, Shi Y, et al. Association between early postoperative acetaminophen exposure and acute kidney injury in pediatric patients undergoing cardiac surgery. JAMA Pediatr. 2018;172:655–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nussmeier NA, Whelton AA, Brown MT, et al. Complications of the COX-2 inhibitors parecoxib and valdecoxib after cardiac surgery. N Engl J Med. 2005;352:1081–1091. [DOI] [PubMed] [Google Scholar]
- 7.Valtola A, Kokki H, Gergov M, et al. Does coronary artery bypass surgery affect metoprolol bioavailability. Eur J Clin Pharmacol. 2007;63:471–478. [DOI] [PubMed] [Google Scholar]
- 8.Kokki H, Maaroos M, Ellam S, et al. How do different extracorporeal circulation systems affect metoprolol bioavailability in coronary artery bypass surgery patients. Eur J Clin Pharmacol. 2018;74:785–792. [DOI] [PubMed] [Google Scholar]
- 9.Porela-Tiihonen S, Kokki M, Kokki H.. Sufentanil sublingual formulation for the treatment of acute, moderate to severe postoperative pain in adult patients . Expert Rev Neurother. 2017;17:101–111. [DOI] [PubMed] [Google Scholar]
- 10.Kinnunen M, Piirainen P, Kokki H, et al. Updated clinical pharmacokinetics and pharmacodynamics of oxycodone. Clin Pharmacokinet. 2019;58:705–725. [DOI] [PubMed] [Google Scholar]
- 11.Ruetzler K, Blome CJ, Nabecker S, et al. A randomised trial of oral versus intravenous opioids for treatment of pain after cardiac surgery. J Anesth. 2014;28:580–586. [DOI] [PubMed] [Google Scholar]
- 12.Smith K, Hopp M, Mundin G, et al. Single- and multiple-dose pharmacokinetic evaluation of oxycodone and naloxone in an opioid agonist/antagonist prolonged-release combination in healthy adult volunteers. Clin Ther. 2008;30:2051–2068. [DOI] [PubMed] [Google Scholar]
- 13.Mundin GE, Smith KJ, Mysicka J, et al. Validated in vitro/in vivo correlation of prolonged-release oxycodone/naloxone with differing dissolution rates in relation to gastrointestinal transit times. Expert Opin Drug Metab Toxicol. 2012;8:1495–1503. [DOI] [PubMed] [Google Scholar]
- 14.Berger MM, Berger-Gryllaki M, Wiesel PH, et al. Intestinal absorption in patients after cardiac surgery. Crit Care Med. 2000;28:2217–2223. [DOI] [PubMed] [Google Scholar]
- 15.Parolari A, Alamanni F, Cannata A, et al. Off-pump versus on-pump coronary artery bypass: meta-analysis of currently available randomized trials. Ann Thorac Surg. 2003;76:37–40. [DOI] [PubMed] [Google Scholar]
- 16.Kokki M, Heikkinen M, Välitalo P, et al. Maturation of oxycodone pharmacokinetics in neonates and infants: Oxycodone and its metabolites in plasma and urine. Br J Clin Pharmacol. 2017;83:791–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Piirainen P, Kokki H, Anderson BJ, et al. Analgesic efficacy and pharmacokinetics of epidural oxycodone in pain management after gynaecological laparoscopy – a randomised, double blind, active control, double-dummy clinical comparison with intravenous administration . Br J Clin Pharmacol. 2019;85:1798–1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kokki M, Välitalo P, Rasanen I, et al. Absorption of different oral dosage forms of oxycodone in the elderly: a cross-over clinical trial in patients undergoing cystoscopy. Eur J Clin Pharmacol. 2012;68:1357–1363. [DOI] [PubMed] [Google Scholar]
- 19.Lamminsalo M, Piirainen P, Kokki H, et al. Population pharmacokinetics of oxycodone in plasma and cerebrospinal fluid after epidural and intravenous administration. Expert Opin Drug Deliv. 2019;16:649–656. [DOI] [PubMed] [Google Scholar]
- 20.Bergstrand M, Hooker AC, Wallin JE, et al. Prediction-corrected visual predictive checks for diagnosing nonlinear mixed-effects models. AAPS J. 2011;13:143–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.FIMEA . Databeases and Registers: [Inretnet]. Kuopio (FI): Finnish Medicine Agency, FIMEA. SPC. TARGINIQ. [Cited 2019 Dec 19]. Available from: http://spc.fimea.fi/indox/nam/html/nam/humspc/4/10878364.pdf
- 22.Smith K, Hopp M, Mundin G, et al. Naloxone as part of a prolonged release oxycodone/naloxone combination reduces oxycodone-induced slowing of gastrointestinal transit in healthy volunteers. Expert Opin Investig Drugs. 2011;20:427–439. [DOI] [PubMed] [Google Scholar]
- 23.Hall R. The pharmacokinetic behaviour of opioids administered during cardiac surgery. Can J Anaesth. 1991;38:747–756. [DOI] [PubMed] [Google Scholar]
- 24.Mets B. The pharmacokinetics of anesthetic drugs and adjuvants during cardiopulmonary bypass. Acta Anaesthesiol Scand. 2000; 44:261–273. [DOI] [PubMed] [Google Scholar]
- 25.Mandema JW, Kaiko RF, Oshlack B, et al. Characterization and validation of a pharmacokinetic model for controlled-release oxycodone. Br J Clin Pharmacol. 1996;42:747–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hasselström J, Säwe J.. Morphine pharmacokinetics and metabolism in humans. Enterohepatic cycling and relative contribution of metabolites to active opioid concentrations. Clin Pharmacokinet. 1993;24:344–354. [DOI] [PubMed] [Google Scholar]
- 27.Ishida T, Oguri K, Yoshimura H.. Determination of oxycodone metabolites in urines and feces of several mammalian species. J Pharmacobio-Dyn. 1982;5:521–525. [DOI] [PubMed] [Google Scholar]
- 28.Reder RF, Oshlack B, Miotto JB, et al. Steady-state bioavailability of controlled-release oxycodone in normal subjects. Clin Ther. 1996;18:95–105. [DOI] [PubMed] [Google Scholar]
- 29.Lahtinen P, Kokki H, Hakala T, et al. S(+)-ketamine as analgesic adjunct reduces opioid consumption after cardiac surgery. Anest Analg. 2004;99:1295–1301. [DOI] [PubMed] [Google Scholar]
- 30.Lahtinen P, Kokki H, Hynynen M.. Remifentanil infusion does not induce opioid tolerance after cardiac surgery. J Cardiothorac Vasc Anesth. 2008;22:225–229. [DOI] [PubMed] [Google Scholar]
- 31.Välitalo P, Ranta VP, Hooker AC, et al. Population pharmacometrics in support of analgesics studies. Acta Anaesthesiol Scand. 2014;58:143–156. [DOI] [PubMed] [Google Scholar]
- 32.Kokki M, Kuronen M, Naaranlahti T, et al. Opioid-induced bowel dysfunction in patients undergoing spine surgery: comparison of oxycodone and oxycodone-naloxone treatment. Adv Ther. 2017;34:236–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, HK, upon reasonable request.