Abstract
Context
Kalanchoe species (Crassulaceae) are widely used in traditional medicine as remedies in infectious diseases and cancer treatment.
Objective
Cytotoxic and antimicrobial activities of Kalanchoe daigremontiana Raym.-Hamet & H. Perrier, K. pinnata (Lam.) Pers., and K. blossfeldiana Poelln. extracts were determined. The relationship between biological activities and the extracts bufadienolides content was also investigated.
Materials and methods
Fresh leaves of Kalanchoe species were macerated with 95% ethanol or water. The quantitative analysis of bufadienolides in the extracts was carried out with mass spectrometry. Cytotoxicity tests were performed on human cancer cell lines – HeLa, SKOV-3, MCF-7, and A375 by MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide) assay and Real-Time Cell Analysis system. The microbiological study was done using a few bacteria strains (β-hemolytic Streptococcus, Corynebacterium diphtheriae, Staphylococcus aureus, Staphylococcus epidermidis, Enterococcus hirae, Escherichia coli) and Candida albicans.
Results
The K. blossfeldiana ethanol extract and K. daigremontiana water extract exhibited the most potent cytotoxic activity (IC50 < 19 µg/mL for HeLa and SKOV-3 cells). The strongest antibacterial effects showed ethanol extract of K. blossfeldiana and K. pinnata (MIC values were 8.45, 8.45, 0.25 and <33.75 µg/mL for S. aureus, S. epidermidis, and E. hirae, respectively). The highest total amount of bufadienolides was in K. daigremontiana ethanol extract. In contrast, K. blossfeldiana ethanol extract did not show the presence of these compounds.
Conclusions
Kalanchoe blossfeldiana ethanol extract is a potential candidate for cancer and bacterial infection treatment. Additionally, the biological effects of Kalanchoe extracts are not dependent on the presence and amount of bufadienolides in the plant extracts.
Keywords: Kalanchoe daigremontiana, Kalanchoe pinnata, Kalanchoe blossfeldiana, RTCA system, cytotoxicity, human cancer cells, Candida albicans
Introduction
Kalanchoe species (Crassulaceae) are succulents found in tropical and subtropical regions, commonly cultivated as household and garden plants. The specific interest in some species corresponds to the health properties. The whole aerial parts and juice are used externally to treat inflammation, allergies, and different skin disorders (Nassis et al. 1992; Ojewole 2005; Nayak et al. 2010). Kalanchoe extracts are also a popular internal remedy for stomach ulcers (Pal and Chaudhuri 1991), asthma (Salami et al. 2013), infections (Willcox and Bodeker 2004), tumours (Supratman et al. 2001), and regulation of blood sugar (Ojewole 2005; Majaz et al. 2011; Khooshbu and Ansari 2019). A wide range of ethnomedical applications of Kalanchoe are linked to the chemical composition. Despite the rich contents of flavonoids (Liu et al. 1989; Nielsen et al. 2005), alkaloids (Gaind and Gupta 1972; Biswas et al. 2012), phenolic acids (Singab et al. 2011; El-Shamy et al. 2013), saponins, and tannins (Pattewar 2012; El-Shamy et al. 2013), bufadienolides have been postulated as responsible for many pharmacological activities of Kalanchoe extracts (El Abdellaoui et al. 2010; Kolodziejczyk-Czepas and Stochmal 2017). Bufadienolide compounds possess anticancer, antiviral, antimicrobial, antioxidant, and cardiotonic effects (Scholtysik et al. 1986; Yamagishi et al. 1989; Supratman et al. 2001; Cunha Filho et al. 2005; Kolodziejczyk-Czepas and Stochmal 2017; Wu et al. 2006). According to their toxicity and cardiac side effects (Puschett et al. 2010), the identification and quantification of bufadienolides are essential.
Recent years have brought increased interest in Kalanchoe species, although there are still limited literature data concerning antiproliferative, antimicrobial, and antifungal properties of the three popular household species: K. pinnata (Lam.) Pers., K. blossfeldiana Poelln., and K. daigremontiana Raym.-Hamet & H. Perrier. Among these plant species, K. pinnata has been best known. Chloroform and ethanol extracts of K. pinnata were tested on human cervical cancer cells, and human acute lymphoblastic leukaemia T cells (Mahata et al. 2012; Bogucka-Kocka et al. 2018). Methanol, ethyl acetate, n-hexane extracts, and leaf juice of K. pinnata were tested on bacteria strains Staphylococcus aureus, Pseudomonas aeruginosa, Shigella sp., Bacillus sp., and Salmonella typhi (Joseph et al. 2011; Tatsimo et al. 2012). Water and methanol extracts of this plant also demonstrated the activity on several bacteria strains and fungi (Akinpelu 2000; Ofokansi et al. 2005; Chowdhury et al. 2011). In contrast, data on biological activities of K. blossfeldiana and K. daigremontiana extracts are scarce (Nahar et al. 2008; Sarkar et al. 2015; Stefanowicz-Hajduk et al. 2019, 2020).
This study evaluates and compares the cytotoxic and antimicrobial activities of Kalanchoe species extracts (K. daigremontiana, K. pinnata, and K. blossfeldiana) against different human cancer cell lines (cervical HeLa, ovarian SKOV-3, breast MCF-7, and melanoma A375) and bacteria and yeast strains (β-hemolytic Streptococcus, Staphylococcus aureus, Corynebacterium diphtheriae, Enterococcus hirae, Escherichia coli, and Candida albicans). We also estimated the presence and amount of particular bufadienolide compounds in the extracts and assessed the biological activity in combination with quantitative phytochemical results. Undertaking this kind of research is extremely important due to the growing popularity and use of Kalanchoe species which are still little known in relation to different activities in cells. Moreover, determination of the content of plant compounds with strong toxicity, such as bufadienolides, is crucial for plant extracts and closely related to the safe application of medicinal plants during long treatment.
Materials and methods
Plant material and preparation of Kalanchoe extracts
Leaves of K. daigremontiana, K. pinnata, and K. blossfeldiana were purchased (April 2019) from a commercial garden source (Garden Centre Justyna, Gdańsk, Poland). Botanical identification was carried out by the authors. Genetic identification of plant species was performed by the A&A Biotechnology Company (Poland). Voucher specimens (No. 21761-21763 for K. daigremontiana, K. pinnata, and K. blossfeldiana, respectively) were deposited in the Herbarium of the Medical University of Gdańsk (GDMA Herbarium). All the ethanol and water extracts of the plant species were prepared from fresh leaves (100 g), which were macerated and stirred with 95% ethanol or water (0.5 L), respectively, for 24 h at room temperature. Then, the extracts were filtered, concentrated under reduced pressure at 40 °C, and lyophilized. The ethanol and water extracts were dissolved in a sterile dimethyl sulfoxide (DMSO) or distilled water, respectively, for cytotoxic and microbiological tests.
Cell lines
The human ovarian cancer (SKOV-3), cervical adenocarcinoma (HeLa S3), malignant melanoma (A375), and breast cancer (MCF-7) cell lines were obtained from the American Type Culture Collection (ATCC). The HeLa S3, A375, and MCF-7 cell lines were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM). The SKOV-3 line was cultured in McCoy’s Medium. Both media were supplemented with 100 units/mL of penicillin, 100 µg/mL of streptomycin, and 10% (v/v) foetal bovine serum (FBS). The cells were incubated at 37 °C and 5% CO2.
Microorganism species
β-Hemolytic Streptococcus group A PCM 465, Streptococcus group G and Corynebacterium diphtheriae came from Department of Pharmaceutical Microbiology of Medical University of Gdańsk collection, Staphylococcus aureus ATCC6538, Staphylococcus aureus MRSA ATCC43300, Staphylococcus epidermidis ATCC14990, Enterococcus hirae ATCC10541, Escherichia coli ATCC8739 and Candida albicans ATCC10231 were obtained from the ATCC collection (ATCC, Manassas, VA, USA).
MTT assay
To estimate the inhibition of cell proliferation and viability, we performed MTT assay. The cell lines were seeded in 96-well plates (5 × 103 cells/well) and treated with the ethanol or water plant extracts at a concentration range of 0.1–150 µg/mL. The DMSO concentration used in the control sample was 0.75% (v/v). After 24 h, the cells were incubated with MTT (0.5 mg/mL) for 3 h and obtained formazan crystals were dissolved in DMSO. The absorbance of this solution was measured on a microtiter plate reader (Epoch, BioTek Instruments, Winooski, VT, USA). All the data were analysed in GraFit software v.7 and are expressed as IC50 mean values (± standard deviation, SD) of three independent experiments (in six repetitions, n = 18).
Real-Time xCELLigence system
To confirm the results from MTT assay, we performed RTCA analysis using the xCELLigence Real-Time Cell Analyzer Dual Plate instrument (RTCA DP). The system enables monitoring of adhesion, proliferation, and viability of tested cells growing on E-plates with microsensor electrodes. The results of the experiments are generated in real-time and are obtained continuously.
The cell lines were seeded at a density of 2 × 104 cells/well into E-plate 16. After 24 h, the ethanol or water extracts were added at a concentration range of 2–150 µg/mL. DMSO concentration used in a control sample was 0.75% (v/v). Vinblastine sulphate was dissolved in sterile water and was used as a positive control in the concentration range of 0.9−03–18.0−03 µg/mL. The IC50 values were calculated using the RTCA software v.1.2.1. All the experiments were independently repeated three times in duplicate.
Antibacterial assay
The antibacterial assay was performed according to a previously established method (Kula et al. 2013). Dry water extracts (20 mg) were dissolved in sterile distilled water (1 mL), and dry ethanol extracts (108, 105, and 315 mg for K. blossfeldiana, K. pinnata, and K. daigremontiana, respectively) were dissolved in DMSO. The final concentrations of all the water extracts used to the antimicrobial activity ranged from 50 to 0.05 µg/mL, and ethanol extracts from 270 to 0.13 µg/mL (K. blossfeldiana and K. pinnata, respectively) and from 787 to 0.375 µg/mL (K. daigremontiana). The concentration of DMSO in a control sample was not higher than 2.5% (v/v). The lowest concentration of the extracts at which there was no visible growth of microorganisms was taken as the MIC (minimal inhibitory concentration). As a positive control, we used ampicillin for bacteria and amphotericin B for yeast.
Quantitative analysis of bufadienolides from the extracts of Kalanchoe leaves
Reagents and the plant extracts
Methanol and acetonitrile HPLC grade were purchased from Merck Millipore (Darmstadt, Germany). Formic acid LC-MS grade was purchased from Sigma-Aldrich (St. Louis, MO, USA). Ultrapure water was obtained in-house with a purification system (Milli-Q-Simplicity-185, Millipore Corp.). The bufadienolide reference standards, purified from the roots of K. daigremontiana using chromatographic techniques (Moniuszko-Szajwaj et al. 2016), were kindly donated by Dr. Barbara Moniuszko-Szajwaj from the Institute of Soil Science and Plant Cultivation (IUNG) in Puławy, Poland.
The four crude, lyophilized extracts (K. daigremontiana ethanol and water extracts, K. pinnata ethanol extract, and K. blossfeldiana ethanol extract) from leaves of the investigated Kalanchoe species have been deposited at the Department of Biochemistry and Crop Quality of the IUNG. Prior to the analysis the extracts were stored in −20 °C.
Quantitative analysis of bufadienolides
Lyophilized extracts of leaves from the three Kalanchoe species were dissolved in suitable solvents: three ethanol extracts, K. daigremontiana (K.d. EtOH), K. pinnata (K.p. EtOH), and K. blossfeldiana (K.b. EtOH), were dissolved in 1 mL methanol whereas water extract of K. daigremontiana (K.d. H2O) was dissolved in 1 mL ultrapure water, using class A volumetric glassware. The samples were sonicated and then filtered in the syringeless filters (Whatman, Mini-UniPrep, Sigma-Aldrich). The final concentration of samples was 10 mg/mL.
Quantitative analyses of those extracts were carried out on UHRMS (ultrahigh-resolution mass spectrometry) using a Dionex UltiMate 3000RS (Thermo Scientific, Darmstadt, Germany) system, containing a charged aerosol detector (CAD, Thermo Corona Veo RS), and interfaced with a high-resolution quadrupole time-of-flight mass spectrometer (HR/Q-TOF/MS, Impact II, Bruker Daltonik GmbH, Bremen, Germany). The chromatographic separation was performed on an Acquity UPLC HSS C18 column (150 × 2.1 mm, 1.8 μm, Waters, Manchester, UK), the column temperature was maintained at 50 °C. The mobile phases were acidified (0.1% formic acid) water (solvent A) and acidified (0.1% formic acid) acetonitrile (solvent B), the chromatographic method consisted of in the following linear gradient: 10% B from 0 to 0.31 min, and the concentration of B was then increased to 40% from 0.31 to 21.01 min. The sample injection volume was 5.0 μL, and the flow rate was set at 500 μL/min. The column effluent was divided between the CAD and the mass spectrometer with a fixed split ratio of 3:1. Compounds identification was based on retention time data as well as calculated formulae and the fragmentation mass spectra of the NMR-confirmed reference standards. Atmospheric-pressure chemical ionization (APCI) was performed in positive ion mode. The mass scan range was set at 100–2000 m/z units. Ions source parameters; capillary voltage 4.0 kV, dry gas 3.0 L/min, and dry temperature 250 °C. Concentrations of bufadienolides were estimated using signals from the CAD. The response of CAD was calibrated from 10 to 500 ng/μL using a series of dilutions from 2.5 mg/mL solution of 11α,19-dihydroxytelocinobufagin. Data processing was performed using DataAnalysis 4.3 (Bruker Daltonik GmbH, Bremen, Germany).
Results
To estimate the cytotoxic effects of Kalanchoe species ethanol and water extracts, we prepared the MTT assay and the RTCA analysis. Both assays showed that the highest activity on all the tested cell lines revealed K. blossfeldiana ethanol extract and water extract of K. daigremontiana. The values of IC50 for K. blossfeldiana ethanol extract were 8.28 ± 0.29, 8.98 ± 0.1, 53.27 ± 5.37, and 52.08 ± 9.67 μg/mL on HeLa, SKOV-3, MCF-7, and A375 cells, respectively (according to the RTCA results). For water extract of K. daigremontiana, the values of IC50 were 18.86 ± 0.29, 14.39 ± 3.59, >100, and 35.32 ± 0.33 μg/mL for HeLa, SKOV-3, MCF-7, and A375 cells, respectively. In the case of both K. pinnata extracts, we did not observe the significant cytotoxic activity on the cancer cell lines. All the MTT and RTCA results are presented in Table 1 and Figures 1 and 2.
Table 1.
The IC50 values (µg/mL) of ethanol and water Kalanchoe species extracts on different cell lines.
| Cell line | Extracts | RTCA K. daigremontiana |
MTT K. daigremontiana |
RTCA K. pinnata |
MTT K. pinnata |
RTCA K. blossfeldiana |
MTT K. blossfeldiana |
RTCA Vinblastine |
|---|---|---|---|---|---|---|---|---|
| HeLa | Ethanol | >100* R2 = 0.86 |
>100* | 61.67 ± 2.34 R2 = 0.96 |
>100 |
8.28 ± 0.29* R2 = 0.97 |
9.63 ± 1.07* | 4.55−03 R2 = 0.94 |
| Water |
18.86 ± 0.29 R2 = 0.86 |
11.48 ± 0.43 | >100 R2 = 0.79 |
>100 | >100 R2 = 0.87 |
>100 | ||
| SKOV-3 | Ethanol | >100* R2 = 0.83 |
>100* | 49.03 ± 2.63 R2 = 0.97 |
>50 |
8.98 ± 0.1 R2 = 0.98 |
11.44 ± 0.35 | 7.64−03 R2 = 0.93 |
| Water |
14.39 ± 3.59 R2 = 0.86 |
10.12 ± 0.28 | >100 R2 = 0.86 |
>100 | >100 R2 = 0.68 |
>100 | ||
| MCF-7 | Ethanol | 45.93 ± 2.94* R2 = 0.90 |
>50* |
>100 R2 = 0.98 |
>100 | 53.27 ± 5.37 R2 = 0.96 |
>50 | 7.49−03 R2 = 0.95 |
| Water | >100 R2 = 0.96 |
>100 | >100 R2 = 0.81 |
>100 | >100 R2 = 0.76 |
>100 | ||
| A375 | Ethanol | >100* R2 = 0.83 |
>100* |
>100 R2 = 0.99 |
>100 | 52.08 ± 9.67 R2 = 0.97 |
36.79 ± 1.27 | 7.72−03 R2 = 0.92 |
| Water | 35.32 ± 0.33 R2 = 0.98 |
36.05 ± 3.15 | >100 R2 = 0.98 |
>100 | >100 R2 = 0.75 |
>100 |
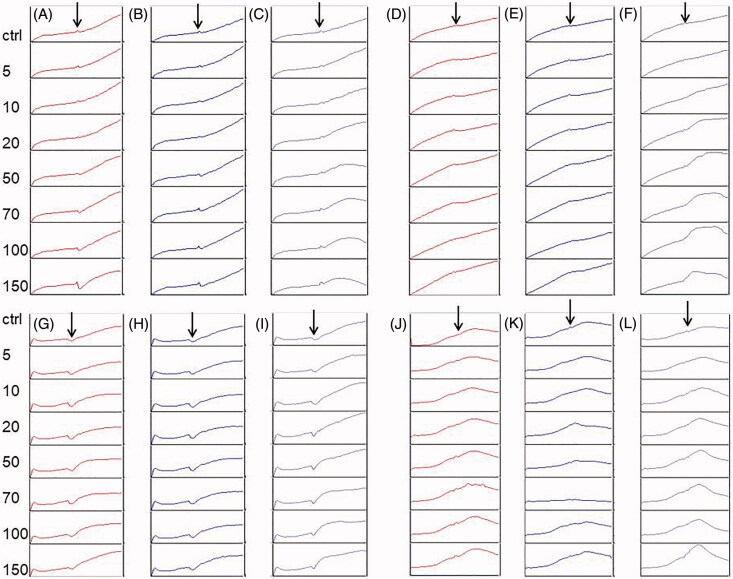
Figure 1.
The effect of Kalanchoe species water extracts on cancer cell lines. The HeLa (A–C), SKOV-3 (D–F), MCF-7 (G–I), and A375 (J–L) cells were treated with water extract of K. pinnata (A, D, G, J), K. blossfeldiana (B, E, H, K), and K. daigremontiana (C, F, I, L) at concentrations of 5–150 µg/mL for 24 h. The untreated cells were a control sample. Arrows represent the time point when the extracts were added to the cells.
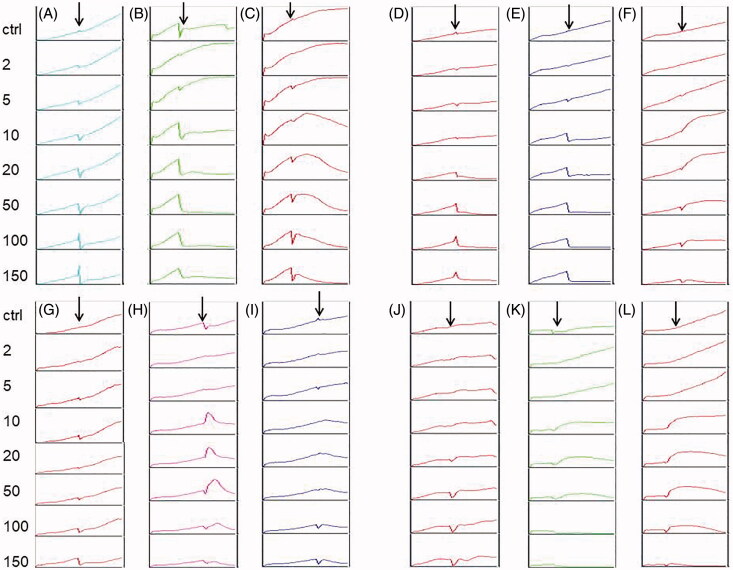
Figure 2.
The effect of Kalanchoe species ethanol extracts on cancer cell lines. The HeLa (A–C), SKOV-3 (D–F), MCF-7 (G–I), and A375 (J–L) cells were treated with ethanol extract of K. pinnata (A, D, G, J), K. blossfeldiana (B, E, H, K), and K. daigremontiana (C, F, I, L) at concentrations of 2–150 µg/mL for 24 h. DMSO (0.75%) was a control sample. Arrows represent the time point when the extracts were added to the cells.
The microbiological study revealed that the highest activity on different species of bacteria showed K. blossfeldiana ethanol extract. The lowest MIC for this extract was 8.45, 16.9, 8.45, and 0.25 µg/mL on three tested Staphylococcus species and Enterococcus hirae, respectively. Next, K. pinnata ethanol extract revealed the strongest microbiological activity on Staphylococcus aureus, S. epidermidis, and Enterococcus hirae with the MIC values of 16.9 and 33.75 µg/mL, respectively. Among all the tested ethanol extracts, the weakest activity showed K. daigremontiana. In the case of water Kalanchoe extracts, all the species revealed the MIC values ≥50 µg/mL towards the bacteria strains. We also did not observe the significant activity of the extracts on Candida albicans. All the results of the microbiological activities of Kalanchoe extracts are presented in Table 2.
Table 2.
The values of MIC/MBC (µg/mL) of Kalanchoe species ethanol and water extracts on different species of bacteria and Candida albicans.
| Microorganism | K. daigremontiana ethanol extract | K. pinnata ethanol extract | K. blossfeldiana ethanol extract | K. daigremontiana water extract | K. pinnata water extract | K. blossfeldiana water extract | Ampicillin/Amphotericina |
|---|---|---|---|---|---|---|---|
| β-Hemolytic Streptococcus group A | >787 | >270 | >270 | >50 | >50 | >50 | 0.3125 |
| β-Hemolytic Streptococcus group G | >787 | >270 | >270 | >50 | >50 | >50 | 0.16 |
| Corynebacterium diphtheriae | 196.75 | 135 | 67.5 | >50 | >50 | >50 | 0.025 |
| Staphylococcus aureus ATCC6538 | 196.75/>787 | 16.9/>270 | 8.45/>270 | >50 | 50/>50 | 50/>50 | <0.03 |
| Staphylococcus aureus MRSA ATCC43300 | 196.75/>787 | 67.5/>270 | 16.9/>270 | >50 | >50 | >50 | 0.24 |
| Staphylococcus epidermidis ATCC14990 | 98.4/393.5 | 33.75/135 | 8.45/67.5 | 50/>50 | 50/>50 | 50/>50 | 0.025 |
| Enterococcus hirae ATCC10541 | 393/>787 | 33.75/>270 | 0.25/270 | >50 | >50 | >50 | 0.1 |
| Escherichia coli ATCC8739 | >787 | >270 | >270 | >50 | >50 | >50 | 0.1 |
| Candida albicans ATCC10231 | >787 | >270 | >270 | >50 | >50 | >50 | 0.06a |
MIC: minimum inhibitory concentration; MBC: minimum bactericidal concentration.
aamphotericin as a positive control in tests with Candida albicans. Bold values indicate the highest activity of Kalanchoe species extracts.
Phytochemical analysis of bufadienolides in Kalanchoe extracts
Based on the cytotoxic and microbiological results, the extracts of Kalanchoe with the most potent cytotoxic and microbiological activities were analysed by mass spectrometry. The quantity of bufadienolides was assessed in K. daigremontiana water and ethanol extracts, K. pinnata, and K. blossfeldiana ethanol extracts. Qualitative analysis showed the presence of ten bufadienolides in the three samples analysed, and no compounds belonging to this class were found in the extract of leaves from K. blossfeldiana. Furthermore, the study revealed that the total content of all the identified bufadienolides was the highest in K. daigremontiana ethanol extract. On the other hand, the water extract from this species contained 2.5 times lower amount of bufadienolides. The amount of bersaldegenin-1,3,5-orthoacetate was the highest in K. daigremontiana extracts, whereas in the ethanol extract of K. pinnata, bersaldegenin-2-acetate and bersaldegenin-5-acetate were predominant. The results are presented in Table 3 and Figures 3 and 4.
Table 3.
Bufadienolides identified and quantified in the tested extracts of leaves from different Kalanchoe species using HR-QTOF-MS analysis.
| No. | Compound | Rt (min) | Meas. m/z [M + H] | Ion Formula | Presence/Content [mg/g] (Mean ± SD) |
|||
|---|---|---|---|---|---|---|---|---|
| K.p. EtOH | K.d. EtOH | K.d. H2O | K.b. EtOH | |||||
| 1 | Bersaldegenin acetate-3 | 6.81 | 475.2341 | C26H34O8 | 0.59 ± 0.17 | (–) | (–) | (–) |
| 2 | 16-Hydroxybersaldegenin acetate | 10.02 | 491.2273 | C26H34O9 | 0.97 ± 0.04 | 0.77 ± 0.09 | 0.13 ± 0.03 | (–) |
| 3 | Bersaldegenin acetate-4 | 11.44 | 475.2321 | C26H34O8 | 0.15 ± 0.05 | (–) | (–) | (–) |
| 4 | Bersaldegenin acetate-5 | 13.69 | 475.2335 | C26H34O8 | 1.14 ± 0.11 | (–) | (–) | (–) |
| 5 | Bryophyllin b | 13.88 | 489.2107 | C26H32O9 | (–) | (<LLOD) | (<LLOD) | (–) |
| 6 | Bryophyllin a | 14.66 | 473.2174 | C26H32O8 | 0.97 ± 0.07 | 0.34 ± 0.06 | 0.20 ± 0.01 | (–) |
| 7 | Bersaldegenin acetate-1 | 15.04 | 475.2332 | C26H34O8 | 0.14 ± 0.04 | 0.81 ± 0.03 | (–) | (–) |
| 8 | Bersaldegenin acetate-2 | 16.40 | 475.2331 | C26H34O9 | 1.11 ± 0.05 | 0.34 ± 0.04 | 0.18 ± 0.01 | (–) |
| 9 | Daigremontianin | 17.50 | 487.1944 | C26H30O9 | (–) | 0.53 ± 0.04 | 0.24 ± 0.02 | (–) |
| 10 | Bersaldegenin-1,3,5-orthoacetate | 20.62 | 457.2220 | C26H32O7 | 0.17 ± 0.04 | 4.33 ± 0.15 | 2.12 ± 0.04 | (–) |
| Total content | 5.24 ± 0.23 | 7.12 ± 0.20 | 2.87 ± 0.05 | (–) | ||||
(–) not present.
(<LLOD) below lower limit of detection.
K. p. EtOH: Kalanchoe pinnata ethanol extract; K. d. EtOH: Kalanchoe daigremontiana ethanol extract; K. d. H2O: Kalanchoe daigremontiana water extract; K. b. EtOH: Kalanchoe blossfeldiana ethanol extract.
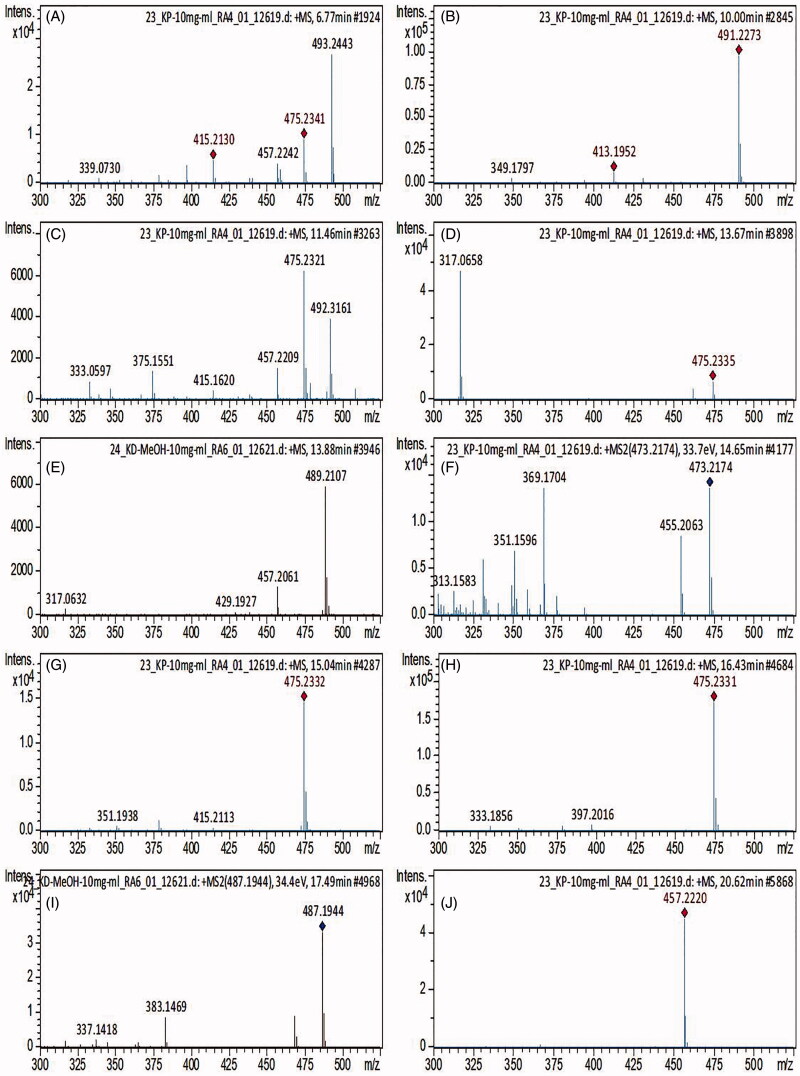
Figure 3.
MS spectra of identified compounds. (A) Bersaldegenin-3-acetate, (B) 16-hydroxybersaldegenin acetate, (C) bersaldegenin-4-acetate, (D) bersaldegenin-5-acetate, (E) bryophyllin B, (F) bryophyllin A, (G) bersaldegenin-1-acetate, (H) bersaldegenin-2-acetate, (I) daigremontianin, (J) bersaldegenin-1,3,5-orthoacetate.
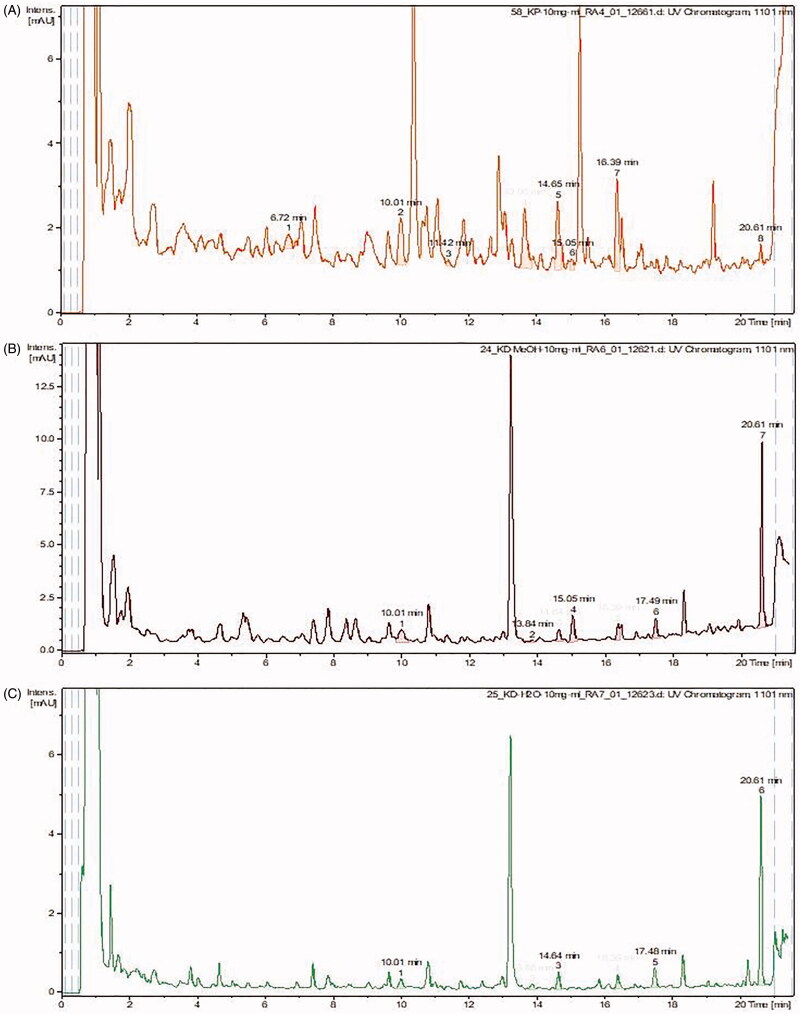
Figure 4.
CAD spectra of analysed samples (quantification analysis). (A) K. pinnata ethanol extract, (B) K. daigremontiana ethanol extract, (C) K. daigremontiana water extract.
Discussion
In our study, we showed the cytotoxic and microbiological effects of different extracts of Kalanchoe species. Additionally, we analysed the content of bufadienolide compounds in the water and ethanol extracts exhibiting significant biological effects. Our results revealed that the most potent cytotoxic and microbiological activity had the ethanol extract of K. blossfeldiana. Significant activity was also observed for the ethanol extract of K. pinnata. However, this extract showed only significant microbiological effect, while the cytotoxic effect towards the cancer cell lines was weak. In contrast, the water extract of K. daigremontiana exhibited significant cytotoxic activity on the cells. The strongest effects we observed on HeLa, SKOV-3, and A375 cells. Additionally, the ethanol extract of this species did not show strong activity on the cancer and bacteria lines, as well as Candida albicans.
The quantitative analysis of bufadienolides in the selected extracts from Kalanchoe species showed that the amount of bufadienolides is not a factor determining the cytotoxic and microbiological activity of these extracts. Generally, bufadienolide compounds are known as toxic components with strong cardiac and cytotoxic properties. A lot of them have been investigated up to now. Bryophyllin A and C were tested on Raji cells and showed antitumour promoting activities (Supratman et al. 2001). Kalantuboside A and B, together with bryotoxin C, bersaldegenin-1,3,5-orthoacetate, and bersaldegenin-1-acetate showed potent cytotoxicity against A549, Cal-27, A2058, and HL-60 cells (Huang et al. 2013). Also, kalanchosides A–C from K. gracilis exhibited cytotoxic activity against a panel of human tumour cell lines (Wu et al. 2006).
Bufadienolides have also been recently investigated on bacteria strains and yeast species. For example, marinobufagin and telocinobufagin inhibited the growth of Staphylococcus aureus and Escherichia coli. The MIC values for these compounds were 16 and 64 µg/mL for E. coli, respectively, and both 128 µg/mL for S. aureus (Cunha Filho et al. 2005). Telocinobufagin was also studied by Wu et al. (2015). They showed that the compound enhanced the immune response and protected against Salmonella typhimurium infection. Another bufadienolide, cinobufagin, was tested by Xie et al. (2016). The authors indicated that this compound modulated human innate immune responses and triggered antibacterial activity. Arenobufagin and gamabufotalin also exhibited antimicrobial properties (Barnhart et al. 2017).
It is well known that compounds other than bufadienolides contained in Kalanchoe species can influence the final biological activity of the extracts. Kalanchoe plants contain flavonoid glycosides, anthocyanins, phenolic acids, sterols, and fatty acids (Milad et al. 2014). An important group of compounds comprises flavonoids. In our previous study, we identified 19 flavonoids from K. daigremontiana extract (Stefanowicz-Hajduk et al. 2020). They were quercetin, kaempferol, myricetin, isorhamnetin, and patuletin derivatives. Flavonoids have also been identified in other studies with K. pinnata and K. blossfeldiana (Nielsen et al. 2005; Muzitano et al. 2006; Tatsimo et al. 2012; El-Shamy et al. 2013; Fürer et al. 2016). It is known that quercetin, kaempferol, and myricetin glycosides have documented antitumour and antimicrobial effects (Tatsimo et al. 2012; Devi et al. 2015; Jaisinghani 2017; Rauf et al. 2018; Wang et al. 2018; Imran et al. 2019).
The extract of K. blossfeldiana, which exhibited the strongest biological activities, did not contain bufadienolides. The compounds identified in this plant, apart from flavonoids and flavonoid glycosides, were carbohydrates, phenolic acids, tannins, sterols, and triterpenes (El-Shamy et al. 2013). Among phenolic compounds, four constituents have been described: methyl gallate, gallic acid, quercetin 3-O-β-galactopyranoside, and kaempferitin (El-Shamy et al. 2013). Palmitic acid was the major fatty acid, n-eicosane and n-octacosane were the primary hydrocarbons. The alcoholic extract of K. blossfeldiana showed significant activity on Staphylococcus aureus, Bacillus subtilis, Escherichia coli, and Pseudomonas aeruginosa (El-Shamy et al. 2013). In another study, the methanol extract of K. blossfeldiana also exhibited antimicrobial activities on clinical isolates and standard reference strains (Sarkar et al. 2015). Pseudomonas aeruginosa exposed to K. blossfeldiana extract displayed reduced biofilm formation and secretion of virulence factors (Sarkar et al. 2015). In our study, we observed a significant effect of the K. blossfeldiana ethanol extract on Corynebacterium diphtheria, Staphylococcus aureus, Staphylococcus epidermidis, and Enterococcus hirae but no effect on Escherichia coli Streptococcus and Candida albicans. Furthermore, our cytotoxic results revealed that K. blossfeldiana extract had the highest antitumour activity on HeLa and SKOV-3 cells. Similarly, in the investigation of El-Shamy et al. (2013), the K. blossfeldiana extract showed the highest activity on HeLa cells and also on liver cancer HEPG2, colon carcinoma HCT-116, head and neck squamous HEP-2, and breast cancer MCF-7 cell lines. In comparison to K. blossfeldiana extracts, K. pinnata did not have strong activity on cancer cells, although the ethanol extract showed a significant effect on selected bacteria strains. The study of K. pinnata chloroform extract performed by Mahata et al. (2012) demonstrated weak activity on human cervical cancer HeLa cells and much higher apoptotic effect of the petroleum ether: ethyl acetate (50/50) fraction. The microbiological studies revealed that 60% methanol extract of K. pinnata and leaf juice inhibited the growth of Bacillus subtilis, Escherichia coli, Proteus vulgaris, Shigella dysenteriae, and Staphylococcus aureus (Akinpelu 2000; Akinsulire et al. 2007; Joseph et al. 2011). Furthermore, Tatsimo et al. (2012) showed the higher antimicrobial activity of ethyl acetate fraction than whole methanol extract of K. pinnata on Staphylococcus aureus, Pseudomonas aeruginosa, Salmonella typhi, Candida albicans, and Cryptococcus neoformans.
In our previous investigation, we estimated the cytotoxic effects of ethanol extract and fractions of K. daigremontiana on tumour cells (Stefanowicz-Hajduk et al. 2020). In the present study, we additionally show the cytotoxic activity of water extract from K. daigremontiana leaves, as well as the microbiological effect of the ethanol and water plant extracts. The ethanol extract from this species did not have significant activity on the tested cancer cells. In contrast, the water extract was much more active than the ethanol extract, which had two times higher content of bufadienolides. Nevertheless, in the study on the action of K. daigremontiana extracts on bacteria strains and Candida albicans, we did not observe the strong effects of both extracts. Nahar et al. (2008) indicated, however, that fractions of K. daigremontiana methanol extract can have antimicrobial activity. They showed that the carbon tetrachloride-soluble fraction of the extract exhibited a significant effect on bacteria species.
Conclusions
Kalanchoe species exhibited different biological activities towards cancer cell lines and microorganisms. Among the three tested plant extracts, the most potent was K. blossfeldiana ethanol extract, in which no bufadienolide compounds were identified. The highest amount of bufadienolides was recorded in K. daigremontiana ethanol extract, which did not exhibit significant cytotoxic and antimicrobial activities. In contrast, K. daigremontiana water extract, with much lower content of these compounds, showed potent activity on cancer cells. This study reveals for the first time that biological activities of Kalanchoe extracts on human cancer cell lines and microorganism species do not depend on the content of bufadienolide compounds which are considered to have strong toxic properties. It seems interesting to examine individual fractions of Kalanchoe compounds and determine their cytotoxic and antibacterial activities in the future. Further studies should be conducted on these relationships in the context of the action of the whole plant extracts, especially with regard to the ethanol and water extracts of K. blossfeldiana and K. daigremontiana, respectively.
Funding Statement
This research was supported by statutory funds of Medical University of Gdańsk.
Disclosure statement
The authors report no declarations of interest.
Data availability statement
The datasets are available from the corresponding author upon reasonable request.
References
- Akinpelu DA. 2000. Antimicrobial activity of Bryophyllum pinnatum leaves. Fitoterapia. 71:193–194. [DOI] [PubMed] [Google Scholar]
- Akinsulire OR, Aibinu IE, Adenipekun T, Adelowotan T, Odugbemi T.. 2007. In vitro antimicrobial activity of crude extracts from plants Bryophyllum pinnatum and Kalanchoe crenata. Afr J Tradit Complement Altern Med. 4:338–344. [PMC free article] [PubMed] [Google Scholar]
- Barnhart K, Forman ME, Umile TP, Kueneman J, McKenzie V, Salinas I, Minbiole KPC, Woodhams DC.. 2017. Identification of bufadienolides from the boreal toad, Anaxyrus boreas, active against a fungal pathogen. Microb Ecol. 74:990–1000. [DOI] [PubMed] [Google Scholar]
- Biswas SK, Chowdhury A, Raihan SZ, Muhit MA, Akbar MA, Mowla R.. 2012. Phytochemical investigation with assessment of cytotoxicity and antibacterial activities of chloroform extract of the leaves of Kalanchoe pinnata. Am J Plant Physiol. 7:41–46. [Google Scholar]
- Bogucka-Kocka A, Zidorn C, Kasprzycka M, Szymczak G, Szewczyk K.. 2018. Phenolic acid content, antioxidant and cytotoxic activities of four Kalanchoë species. Saudi J Biol Sci. 25:622–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury A, Biswas SK, Das J, Karmakar UK, Shill MC, Dutta N.. 2011. Investigation of cytotoxicity and antifungal activities of petroleum ether and aqueous extracts of leaves and stems of Kalanchoe pinnata L. (Crassulaceae). Asian J Plant Sci. 10:274–277. [Google Scholar]
- Cunha Filho GA, Schwartz CA, Resck IS, Murta MM, Lemos SS, Castro MS, Kyaw C, Pires OR, Leite JR, Bloch C, et al. 2005. Antimicrobial activity of the bufadienolides marinobufagin and telocinobufagin isolated as major components from skin secretion of the toad Bufo rubescens. Toxicon. 45:777–782. [DOI] [PubMed] [Google Scholar]
- Devi KP, Rajavel T, Habtemariam S, Nabavi SF, Nabavi SM.. 2015. Molecular mechanisms underlying anticancer effects of myricetin. Life Sci. 142:19–25. [DOI] [PubMed] [Google Scholar]
- El Abdellaoui S, Destandau E, Toribio A, Elfakir C, Lafosse M, Renimel I, André P, Cancellieri P, Landemarre L.. 2010. Bioactive molecules in Kalanchoe pinnata leaves: extraction, purification, and identification. Anal Bioanal Chem. 398:1329–1338. [DOI] [PubMed] [Google Scholar]
- El-Shamy A, Fathy FI, Abdel-Rahman EH, Sabry MM.. 2013. Phytochemical, biological and botanical studies of Kalanchoe blossfeldiana Poelln. Int J Pharm Photon. 104:189–205. [Google Scholar]
- Fürer K, Simões-Wüst AP, von Mandach U, Hamburger M, Potterat O.. 2016. Bryophyllum pinnatum and related species used in anthroposophic medicine: constituents, pharmacological activities, and clinical efficacy. Planta Med. 82:930–941. [DOI] [PubMed] [Google Scholar]
- Gaind K, Gupta R.. 1972. Alkanes, alkanols, triterpenes, and sterols of Kalanchoe pinnata. Phytochemistry. 11:1500–1502. [Google Scholar]
- Huang HC, Lin MK, Yang HL, Hseu YC, Liaw CC, Tseng YH, Tsuzuki M, Kuo YH.. 2013. Cardenolides and bufadienolide glycosides from Kalanchoe tubiflora and evaluation of cytotoxicity. Planta Med. 79:1362–1369. [DOI] [PubMed] [Google Scholar]
- Imran M, Salehi B, Sharifi-Rad J, Gondal TA, Saeed F, Imran A, Shahbaz M, Fokou P, Arshad MU, Khan H, et al. 2019. Kaempferol: a key emphasis to its anticancer potential. Molecules. 24:2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaisinghani RN. 2017. Antibacterial properties of quercetin. Microbiol Res. 8:6877. [Google Scholar]
- Joseph B, Sridhar SSJ, Edwin BT.. 2011. Rare medicinal plant – Kalanchoe pinnata. Res J Microbiol. 6:322–327. [Google Scholar]
- Khooshbu P, Ansari I.. 2019. A pharmacognostical and pharmacological review on Bryophyllum pinnatum (Panphuti). Asian J Pharm Clin Res. 12:34–39. [Google Scholar]
- Kolodziejczyk-Czepas J, Stochmal A.. 2017. Bufadienolides of Kalanchoe species: an overview of chemical structure, biological activity and prospects for pharmacological use. Phytochem Rev. 16:1155–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kula M, Majdan M, Radwańska A, Nasal A, Hałasa R, Głód D, Matkowski A, Krauze-Baranowska M.. 2013. Chemical composition and biological activity of the fruits from Lonicera caerulea var. edulis ‘Wojtek’. Acad J Med Plants. 1:141–148. [Google Scholar]
- Liu KCS, Yang SL, Roberts MF, Phillipson JD.. 1989. Eupafolin rhamnoides from Kalanchoe gracilis. J Nat Prod. 52:970–974. [Google Scholar]
- Mahata S, Maru S, Shukla S, Pandey A, Mugesh G, Das B, Bharti AC.. 2012. Anticancer property of Bryophylum pinnata (Lam.) Oken. leaf on human cervical cancer cells. BMC Complem Alter Med. 12:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majaz Q, Tatiya AU, Khurshid M, Nazim S, Siraj S.. 2011. The miracle plant (Kalanchoe pinnata): a phytochemical and pharmacological review. IRAP. 2:1478–1482. [Google Scholar]
- Milad R, El-Ahmady S, Singab AN.. 2014. Genus Kalanchoe (Crassulaceae): a review of its ethnomedicinal, botanical, chemical and pharmacological properties. EJMP. 4:86–104. [Google Scholar]
- Moniuszko-Szajwaj B, Pecio Ł, Kowalczyk M, Stochmal A.. 2016. New bufadienolides isolated from the roots of Kalanchoe daigremontiana (Crassulaceae). Molecules. 21:243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzitano MF, Cruz EA, de Almeida AP, Da Silva SAG, Kaiser CR, Guette C, Rossi-Bergmann B, Costa SS.. 2006. Quercitrin: an antileishmanial flavonoid glycoside from Kalanchoe pinnata. Planta Med. 72:81–83. [DOI] [PubMed] [Google Scholar]
- Nahar K, Khan MGU, Rahman MS, Begum B, Rashid MA.. 2008. Antimicrobial and cytotoxic activities of Bryophyllum daigremontianum. Dhaka Univ J Pharm Sci. 7:99–101. [Google Scholar]
- Nassis CZ, Haebisch EM, Giesbrecht AM.. 1992. Antihistamine activity of Bryophyllum calycinum. Braz J Med Biol Res. 25:929–936. [PubMed] [Google Scholar]
- Nayak BS, Marshall JR, Isitor G.. 2010. Wound healing potential of ethanolic extract of Kalanchoe pinnata Lam. leaf – a preliminary study. Indian J Exp Biol. 48:572–576. [PubMed] [Google Scholar]
- Nielsen AH, Olsen CE, Moller BL.. 2005. Flavonoids in flowers of 16 Kalanchoë blossfeldiana varieties. Phytochemistry. 66:2829–2835. [DOI] [PubMed] [Google Scholar]
- Ofokansi KC, Esimone CO, Anele CR.. 2005. Evaluation of the in vitro combined antibacterial effect of the leaf extracts of Bryophyllum pinnatum (Fam: Crassulaceae) and Ocimum gratissimum (Fam: Labiatae). Plant Prod Res J. 9:23–27. [Google Scholar]
- Ojewole JA. 2005. Antinociceptive, anti-inflammatory and antidiabetic effects of Bryophyllum pinnatum (Crassulaceae) leaf aqueous extract. J Ethnopharmacol. 99:13–19. [DOI] [PubMed] [Google Scholar]
- Pal S, Chaudhuri A.. 1991. Studies on the anti-ulcer activity of a Bryophyllum pinnatum leaf extract in experimental animals. J Ethnopharmacol. 33:97–102. [DOI] [PubMed] [Google Scholar]
- Pattewar SV. 2012. Kalanchoe pinnata: phytochemical and pharmacological profile. Int J Phytopharm. 2:1–8. [Google Scholar]
- Puschett JB, Agunanne E, Uddin MN.. 2010. Emerging role of the bufadienolides in cardiovascular and kidney diseases. Am J Kidney Dis. 56:359–370. [DOI] [PubMed] [Google Scholar]
- Rauf A, Imran M, Khan IA, Ur-Rehman M, Gilani SA, Mehmood Z, Mubarak MS.. 2018. Anticancer potential of quercetin: a comprehensive review. Phytother Res. 32:2109–2130. [DOI] [PubMed] [Google Scholar]
- Salami EO, Ozolua RI, Okpo SO, Eze GI, Uwaya DO.. 2013. Studies on the anti-asthmatic and antitussive properties of aqueous leaf extract of Bryophyllum pinnatum in rodent species. Asian Pac J Trop Med. 6:421–425. [DOI] [PubMed] [Google Scholar]
- Sarkar R, Mondal C, Bera R, Chakraborty S, Barik R, Roy P, Kumar A, Yadav KK, Choudhury J, Chaudhary SK, et al. 2015. Antimicrobial properties of Kalanchoe blossfeldiana: a focus on drug resistance with particular reference to quorum sensing-mediated bacterial biofilm formation. J Pharm Pharmacol. 67:951–962. [DOI] [PubMed] [Google Scholar]
- Scholtysik G, Wagner H, Fischer M, Rüegg U.. 1986. Cardiac glycoside-like effects of a bufadienolide extracted from Kalanchoe daigremontiana. In: Erdmann E, Greef K, Skou JC, editors. Cardiac glycosides. Heidelberg: Steinkopff; p. 1785–1985. [Google Scholar]
- Singab ANB, El-Ahmady SH, Labib RM, Fekry SS.. 2011. Phenolics from Kalanchoe marmorata Baker, family Crassulaceae. Bull Fac Pharm Cairo Univ. 49:1–5. [Google Scholar]
- Stefanowicz-Hajduk J, Asztemborska M, Krauze-Baranowska M, Godlewska S, Gucwa M, Moniuszko-Szajwaj B, Stochmal A, Ochocka JR.. 2020. Identification of flavonoids and bufadienolides and cytotoxic effects of Kalanchoe daigremontiana extracts on human cancer cell lines. Planta Med. 86:239–238. [DOI] [PubMed] [Google Scholar]
- Stefanowicz-Hajduk J, Gucwa M, Hajduk A, Ochocka JR.. 2019. Kalanchoe blossfeldiana extract induces cell cycle arrest and necrosis in human cervical cancer cells. Phcog Mag. 15:527–537. [Google Scholar]
- Supratman U, Fujita T, Akiyama K, Hayashi H, Murakami A, Sakai H, Koshimizu K, Ohigashi H.. 2001. Anti-tumor promoting activity of bufadienolides from Kalanchoe pinnata and K. daigremontiana x tubiflora. Biosci Biotechnol Biochem. 65:947–949. [DOI] [PubMed] [Google Scholar]
- Tatsimo SJN, Tamokou JD, Havyarimana L, Csupor D, Forgo P, Hohmann J, Kuiate JR, Tane P.. 2012. Antimicrobial and antioxidant activity of kaempferol rhamnoside derivatives from Bryophyllum pinnatum. BMC Res Notes. 5:158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Fang X, Ge L, Cao F, Zhao L, Wang Z, Xiao W.. 2018. Antitumor, antioxidant and anti-inflammatory activities of kaempferol and its corresponding glycosides and the enzymatic preparation of kaempferol. PLoS One. 13:e0197563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willcox ML, Bodeker G.. 2004. Traditional herbal medicines for malaria. BMJ. 329:1156–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu SC, Fu BD, Shen HQ, Yi PF, Zhang LY, Lv S, Guo X, Xia F, Wu YL, Wei XB.. 2015. Telocinobufagin enhances the Th1 immune response and protects against Salmonella typhimurium infection. Int Immunopharmacol. 25:353–362. [DOI] [PubMed] [Google Scholar]
- Wu PL, Hsu YL, Wu TS, Bastow KF, Lee KH.. 2006. Kalanchosides A-C, new cytotoxic bufadienolides from the aerial parts of Kalanchoe gracilis. Org Lett. 8:5207–5210. [DOI] [PubMed] [Google Scholar]
- Xie S, Spelmink L, Codemo M, Subramanian K, Pütsep K, Henriques-Normark B, Olliver M.. 2016. Cinobufagin modulates human innate immune responses and triggers antibacterial activity. PLoS One. 11:e0160734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagishi T, Haruna M, Yan XZ, Chang JJ, Lee KH.. 1989. Antitumor agents, 110. Bryophyllin B, a novel potent cytotoxic bufadienolide from Bryophyllum pinnatum. J Nat Prod. 52:1071–1079. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets are available from the corresponding author upon reasonable request.