Abstract
Objective
Treatment of dyslipidemia lowers cardiovascular (CV) risk. Although statin use is a cornerstone therapy, many patients are not achieving their risk-specific low-density lipoprotein cholesterol (LDL-C) goals. The proprotein convertase subtilisin/kexin type 9 (PCSK9) monoclonal antibodies have been extensively studied as lipid-lowering therapy (LLT). Herein, we present an updated evidence-based review of the efficacy and safety of PCSK9 monoclonal antibodies in the treatment of familial and non-familial hypercholesterolemia.
Methods
PubMed database was searched to review Phase III studies on PCSK9 monoclonal antibodies. Then, the US National Institutes of Health Registry and the WHO International Clinical Trial Registry Platform were searched to identify and present the ongoing research.
Results
PCSK9 monoclonal antibodies were investigated for the treatment of dyslipidemia, as a single therapeutic agent or as an add-on therapy to the traditional LLT. They proved effective and safe in the treatment of familial and non-familial hypercholesterolemia, and in the prevention of adverse CV events.
Conclusions
The use of PCSK9 monoclonal antibodies in the treatment of dyslipidemia is currently recommended to achieve risk-specific LDL-C goal to reduce adverse CV events. Future results of the ongoing research might expand their clinical generalizability to broader patient populations.
Keywords: Alirocumab, anti-drug antibody, bococizumab, cardiovascular diseases, evolocumab, hypercholesterolemia, neurocognitive, PCSK9
Introduction
It has been well established that lipid-lowering therapy (LLT) targeting low-density lipoprotein cholesterol (LDL-C) lowers cardiovascular (CV) risk. This is largely based on significant benefits observed with statin therapy in the prevention of primary and secondary atherosclerotic cardiovascular disease (ASCVD)1,2. Although statins remain the cornerstone of LLT, yet patients on statin therapy may still experience CV events2, statin intolerance, or inability to achieve target LDL-C levels despite maximally-tolerated doses. Thus, alternative LLT to further decrease LDL-C levels and improve CV outcomes has been investigated1. Ezetimibe in combination with statin therapy has shown incremental lowering of LDL-C levels and improvement in CV outcomes. However, such improvements are modest and the overall outcome data on ezetimibe monotherapy are scarce1,3. Monoclonal antibodies are novel lipid-lowering agents that reduce LDL-C levels by inhibiting proprotein convertase subtilisin/kexin type 9 (PCSK9). The currently available antibodies, alirocumab and evolocumab, are fully human immunoglobulin-G (IgG) subtypes4 and are approved by the Food and Drug Administration (FDA) for heterozygous familial hypercholesterolemia (HeFH) and for the prevention of CV events in patients with established cardiovascular disease (CVD). Evolocumab is also indicated for homozygous familial hypercholesterolemia (HoFH)5. International guidelines have been updated to include PCSK9 antibodies in their recommendations6–8. The development of a third PCSK9 antibody, bococizumab, in advanced phase III clinical trials was abandoned in 2016. After the completion of six bococizumab studies, an unexpected attenuation of effect on LDL-C over time was observed, in addition to high rates of injection-site reaction due to high immunogenicity9. However, the development of new investigational monoclonal antibodies to inhibit PCSK9 is well under way with promising initial results10–13. This review will discuss the efficacy and safety profile of PCSK9 monoclonal antibodies or inhibitors in the treatment of familial and non-familial hypercholesterolemia. For the purpose of this review, PCSK9 monoclonal antibodies or inhibitors will be used interchangeably.
Materials and methods
Search strategy for studies on PCSK9 monoclonal antibodies
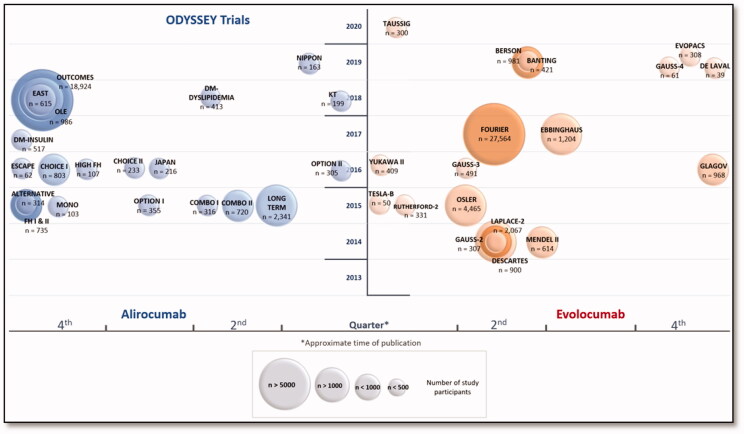
PubMed literature search was carried out on 31 December 2019 for original trials, using broad MeSH terms in the following search thread: ("alirocumab" [Supplementary Concept] OR "evolocumab" [Supplementary Concept] OR "bococizumab" [Supplementary Concept]). The search was limited to "Humans" and "Clinical Trial". The references’ lists of the identified trials were manually searched to identify further potentially useful articles. Several meta-analyses, pre-specified, post hoc and pooled analyses of PSCK9 inhibitors trials have been included to emphasize their findings. The literature search was updated on March 1, 2020. Figure 1 presents the approximate publication period of time of Phase III clinical trials discussed in the review.
Figure 1.
Timeline of phase III studies of alirocumab and evolocumab.
Search strategy for registered clinical trials (ReCTs)
Literature search on the ongoing active research on PCSK9 monoclonal antibodies was conducted on 15 March 2020. The search strategy included the individual agents separately: alirocumab and evolocumab. The United States (US) National Institutes of Health Registry (http://clinicaltrials.gov/) was searched for Phase III trials and recruitment status of “Active, not recruiting”, “Recruiting” and “Not yet recruiting” and the World Health Organization (WHO) International Clinical Trial Registry Platform (ICTRP) (http://www.who.int/ictrp/network/en/) for Phase III trials.
Mechanism of action of PCSK9 monoclonal antibodies
The PCSK9 is a low-abundance circulating plasma protein that is synthetized and secreted by hepatocytes14. PCSK9 regulates LDL receptor (LDLR) on hepatocytes surfaces, which is the primary receptor that is responsible for clearing the circulating LDL particles by endocytosis. PCSK9 binds to LDLR, stabilizes the LDL/LDLR complex and prevents LDLR from being released from the endocytosed vesicle causing lysosomal destruction of the LDLR and thus preventing its recycling5,15. Subsequently, there is reduction in the LDLR density on the hepatocytes surfaces which affects the ability to eliminate the circulating LDL particles leading to an elevated plasma cholesterol level14. Preclinical trials have shown that PCSK9 has direct proinflammatory action on the vessel walls which could be explained by its effect on LDLR-related protein 1 (LRP1) which regulates plaque macrophages5. PCSK9 circulating in the plasma has also been postulated to flow through the arterial plaque adding up to the plaque PCSK9 concentration which controls LDLR expression in the atheroma and reduces levels of LRP15. Increased LDLR expression through the inhibition of PCSK9 will result in profound uptake of lipoprotein cholesterol into the atheroma theoretically while the reduction of LRP1 levels results in induction of inflammation4,5. Monoclonal antibodies such as alirocumab and evolocumab, self-administered subcutaneously every two or four weeks, bind to PCSK9 preventing its binding to LDLR. When PCSK9 is absent or its binding to LDLR is inhibited, LDL is degraded while the LDLR can be recycled then accumulate on the hepatocyte’s membrane leading to an accelerated LDL clearance and reduced levels in blood5. Accordingly, PCSK9 inhibition have two counteracting actions with a net effect of reduction of LDL-C levels and atheroma volume4. An injection of monoclonal antibodies can completely inactivate all the circulating PCSK9 within hours5,14, and all the newly secreted PCSK9 after several days5. Marked reduction in LDL-C levels starts one day after an injection14. Inhibition of PCSK9 can also be achieved by targeting PCSK9 synthesis in hepatocytes. Inclisiran, a long-acting small interfering RNA (siRNA), that inhibits translation of PCSK9 mRNA leading to a reduced PCSK9 synthesis. Inclisiran is a valid alternative to PCSK9 inhibitors with an advantage of twice-a-year injection to produce an LDL-C reduction by 50% or more16. Bempedoic acid is another promising agent that could be a potential alternative to statins. It is an oral once-daily molecule that acts as an inhibitor to adenosine triphosphate (ATP) citrate lyase. The latter is an enzyme that upstream from 3-hydroxy-3-methylglutaryl-coenzyme A. Thus, reducing cholesterol synthesis16,17.
Efficacy of PCSK9 monoclonal antibodies
The Phase I studies of evolocumab, alirocumab, and bococizumab have shown reduction in LDL-C levels in healthy volunteers and patients with familial and non-familial hypercholesterolemia. They were generally effective, safe and well-tolerated18–20. Similar results have been obtained from Phase II studies, which are usually dose-finding, of alirocumab21–24, bococizumab25–27, and evolocumab28–31. The PCSK9 monoclonal antibodies have been studied in Phase III clinical trials for lipid, cardiovascular, and safety outcomes. Data in HoFH are limited in general.
Lipid outcomes
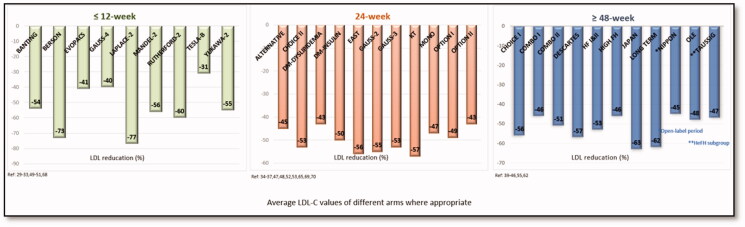
For the purpose of Figure 2, when the monoclonal antibody was tested in several arms i.e. in different strengths and/or in combination with other LLT, the LDL-C values were averaged using the values of different arms of the Phase III trials presented in the review.
Figure 2.
Approximate LDL-C reductions in phase III trials.
Primary hypercholesterolemia
Primary hypercholesterolemia is defined as blood cholesterol measurement >5.17 mmol/L (200 mg/dL) that could be attributed to genetics, obesity, or dietary intake32. In patients with primary hypercholesteremia in the absence or presence of various CV risk levels, who have not reached their CV risk-specific LDL-C goals, the effect of PCSK9 inhibitors on LDL-C levels has been extensively studied as monotherapy, in statin intolerance, or as an add-on to diet-alone or other LLT such as statins, ezetimibe, fenofibrate. Several studies have shown that alirocumab and evolocumab significantly reduce LDL-C levels at Weeks 1233–37, 2438–42, 5243–45, 5646, 7847,48, 9649, and 10450 of therapy. In most trials38,46,50–52, authors adopted the 201153 and 201254 European guidelines’ definitions of CV risk categories. Patients with very high CV risk are defined, as per studies, to have a documented coronary heart disease (CHD) or CHD risk equivalents such as type 1 (T1DM) or 2 (T2DM) diabetes mellitus (DM) with target organ damage, ischemic stroke, peripheral artery disease (PAD), or carotid artery occlusion >50% without symptoms, carotid endarterectomy or carotid artery stent procedure, or renal artery stenosis or renal artery stent procedure. High CV risk are defined as patients with no history of CVD or CHD but with other risk factors such as calculated 10-year risk of fatal CVD using the European Systematic Coronary Risk Evaluation (SCORE) score of ≥5% (although the guidelines specified ≥5% and < 10%), T1DM or T2DM without target organ damage, moderate chronic kidney disease (CKD). Moderate CV risk is defined as SCORE score of ≥1 and < 5%38,46. On the other hand, the 2018 American guidelines7 defined very high-risk patients to have a history of multiple major ASCVD events or one major ASCVD event and multiple high-risk conditions such as age ≥65 years, HeFH, history of coronary artery bypass surgery or percutaneous coronary intervention outside of the major ASCVD event(s), DM, hypertension, CKD (eGFR 15–59 mL/min/1.73 m2) , current smoking persistently elevated LDL-C (LDL-C ≥ 2.6 mmol/L (100 mg/dL) despite maximally tolerated statin therapy and ezetimibe or history of congestive heart failure. It is worth nothing that the 2019 European guidelines8 re-defined very high risk CV category to include family history with ASCVD or with another major risk factor, and moderate risk CV category to include young patients (T1DM < 35 years, T2DM < 50 years) with DM duration of < 10 years and without other risk factors.
Short-term trials (≤12 weeks)
The LAPLACE-233 study (n = 2067) enrolled patients with primary hypercholesterolemia and mixed dyslipidemia. Evolocumab 140 mg every 2 weeks (Q2W) or 420 mg monthly (QM), on top of moderate-intensity statins reduced LDL-C by 59–66% or by 62–65%, respectively. When added to high-intensity statins, Q2W dose reduced LDL-C by 86–90%, while QM dose reduced the levels by 93–95%. Around 86–94% of the patients on evolocumab and 17–62% on ezetimibe achieved LDL-C level < 1.8 mmol/L (70 mg/dL) regardless of the statin therapy used. In the large MENDEL-237 monotherapy trial (n = 612), the biweekly and monthly evolocumab decreased LDL-C levels from baseline by an average of 57 and 56.1% compared to 17.8 and 18.6% for ezetimibe or to 0.1 and 1.3% for placebo (p < .001, all comparisons), respectively. There were higher rates in patients achieving LDL-C level < 1.8 mmol/L (70 mg/dL) in the evolocumab groups (69%) than ezetimibe (1%) or placebo (1%) groups. Kiyosue et al.34 in the Japanese YUKAWA II study (n = 409), recruited high CV risk patients with hyperlipidemia and mixed dyslipidemia. Evolocumab 140 mg Q2W or 420 mg QM on top of atorvastatin 5 or 20 mg reduced LDL-C by an average of 67–76%. When studied in patients during hospitalization due to acute coronary syndrome (ACS) in the EVOPACS55 study (n = 308), evolocumab 420 mg QM for 2 doses added to high-dose statin resulted in a difference in mean percentage change from baseline of −40.7% (p < .001). In the evolocumab group, around 95.7% of patients achieved LDL-C levels < 1.8 mmol/L (70 mg/dL) by Week 8 versus 37.6% in the placebo group. The BERSON56 trial (n = 981) evaluated the efficacy and safety of evolocumab (140 mg Q2W or 420 mg QM) combined with atorvastatin in T2DM patients with hyperlipidemia and mixed dyslipidemia. Evolocumab was well-tolerated and significantly decreased LDL-C levels by ≥70% in both dosing regimens (p < .0001). In a similar patient population in the BANTING57 trial (n = 421), evolocumab (420 mg QM) on top of statins reduced LDL-C by a mean of 54.3% compared to 1.1% in placebo group (p < .0001) with significantly more patients achieved LDL-C < 1.8 mmol/L (70 mg/dL); 84.5 versus 15.4%, respectively. Evolocumab had no negative impact on glycemic parameters in both trials after 12 weeks of treatment56,57.
24-Week trials
In ODYSSEY EAST58 (n = 615), ODYSSEY KT41 (n = 199), ODYSSEY OPTIONS I51 (n = 355) and ODYSSEY OPTIONS II52 (n = 305) trials, alirocumab 75 mg Q2W (75Q2W), increased to 150 mg Q2W (150Q2W) at Week 12 if LDL-D was ≥ 1.8 mmol/L (70 mg/dL) at Week 8, was studied in patients with very high CV risk and LDL-C levels ≥1.8 mmol/L (70 mg/dL) or with high CV risk and LDL-C levels ≥2.6 mmol/L (100 mg/dL) despite maximumly tolerated statin therapy. In ODYSSEY EAST58 trial that was conducted in three Asian countries (China, India, Thailand), there were reductions in LDL-C levels by 56 versus 20.3% with alirocumab versus ezetimibe (p < .0001). The ODYSSEY KT41 trial enrolled patients from South Korea and Taiwan who were on atorvastatin 40 to 80 mg, rosuvastatin 20, or simvastatin 40 mg. The least-squares mean percentage change in LDL-C from baseline was −57.1% in alirocumab group and +6.3% in placebo group, with a difference between groups of −63.4% (p < .0001). In ODYSSEY OPTIONS I51 trial, alirocumab as an add-on to atorvastatin 20 mg and 40 mg reduced LDL-C levels by 44.1 and 54.0% compared to 20.5 and 22.6% with ezetimibe (p < .001, both comparisons), respectively. Switching atorvastatin 40 mg to rosuvastatin 40 mg in a third arm resulted in 21.4% reduction, while doubling atorvastatin dose in the fourth arm yielded an average of 5% reduction in baseline LDL-C levels. In ODYSSEY OPTIONS II52 trial, alirocumab was added to either rosuvastatin 10 mg or 20 mg. In rosuvastatin 10 mg arm, alirocumab reduced LDL-C levels by 50.6% compared to 14.4% in ezetimibe group or 16.3% by doubling rosuvastatin dose (p < .0001). In rosuvastatin 20 mg arm, alirocumab reduced LDL-C levels by 36.3% compared to 11% in ezetimibe group (p = .0136) or to 15.9% in double-dose rosuvastatin group (p = .0453). In the four aforementioned studies41,51,52,58, the reductions were observed at Week 4 and maintained through the study duration with an average of 80% or more of the patients on alirocumab achieving LDL–C < 1.8 mmol/L (70 mg/dL). ODYSSEY DM-INSULIN39 trial (n = 517), in diabetics (types 1 and 2) with hypercholesterolemia and high CV risk on insulin and statins with or without other LLT, showed a least-squares mean percentage change in baseline LDL-C of −50.1% with alirocumab (75Q2W) versus −1.3% with placebo (p < .0001). The difference between the groups was −49.0% in T2DM and −47.8% in T1DM (p < .0001). The reductions were similar regardless of disease state or age. In ODYSSEY DM-DYSLIPIDEMIA40 trial (n = 413), patients with T2DM and mixed dyslipidaemia with ASCVD or at least one additional CV risk factor were randomized to alirocumab (75Q2W) or usual care (maximally tolerated statins with other LLT; fenofibrate, ezetimibe, omega‐3 fatty acid, nicotinic acid). The mean non-high-density lipoprotein cholesterol (non-HDL-C) reduction was −37.3% in alirocumab group versus −4.7% (difference of −32.5%, p < .0001) with >66% of patients achieving non‐HDL-C level of 2.6 mmol/L (100 mg/dL). Alirocumab also reduced LDL-C levels by 43%. In the trials that evaluated the every-4-week (Q4W) dose, alirocumab has shown similar outcomes. In ODYSSEY CHOICE II38 trial (n = 233), moderate, high, and very high CV risk patients with hypercholesterolemia receiving fenofibrate or ezetimibe or diet-alone, were randomized to alirocumab 150 mg Q4W (150Q4W) or 75Q2W increased to 150Q2W at Week 12, if target LDL-C was not achieved at Week 8 (75/150 mg). The least-squares mean LDL-C changes was −51.7% (150Q4W) and −53.5% (75Q2W) versus placebo +4.7% (p < .0001, both comparisons). The reductions were observed at Week 4 and maintained through the study duration with 63.9% (150Q4W) and 70.3% (75Q2W) of patients on alirocumab achieving LDL–C target versus 1.8% on placebo. ODYSSEY MONO59 trial (n = 103), the first study to test alirocumab in patients on no LLT, showed that patients on alirocumab (75Q2W) had a 47.2% reduction in LDL-C compared with a 15.6% with ezetimibe (p < .0001). A post-hoc pooled analysis60 from nine randomized, double-blind, placebo- or ezetimibe-controlled, 24- to 104-week ODYSSEY Phase III trials evaluated add-on alirocumab to statins in regimens of 150Q2W and 75/150 mg. The observed change in LDL-C level was −61.5% with alirocumab 150Q2W versus −1.0% with placebo, −46.4% with alirocumab 75/150 mg versus +6.3% with placebo, and −48.7% with alirocumab 75/150 mg versus −20.6% with ezetimibe. Without statins, an LDL-C change of −54.9% was observed with alirocumab versus +4% with ezetimibe. Patients on alirocumab were more likely to achieve LDL-C < 1.8 mmol/L (70 mg/dL) and < 1.42 mmol/L (55 mg/dL). At Week 8, around 16.3% of patients on alirocumab 75Q2W and statins required dose increase to 150Q2W, while 34.8% alirocumab 75Q2W without statins required dose adjustment.
Longer-term trials (≥48 weeks)
Evolocumab 420 mg Q4W combined with diet-alone or with atorvastatin 10 mg or 80 mg (with or without ezetimibe), showed an average of 57% (p < .001) reduction in LDL-C at Week 12 maintained through Week 52 when studied in hyperlipidemic patients with CV risk in the DESCARTES44 trial (n = 900). Around 82.3% of patients on evolocumab achieved LDL-C level < 1.8 mmol/L (70 mg/dL) versus 6.4% on placebo. In the diet-alone group, the least-square mean reduction in LDL-C was 55.7%, in the atorvastatin 10 mg group was 61.6%, in the atorvastatin 80 mg group was 56.8% and in the atorvastatin 80 mg plus ezetimibe 10 mg group was 48.5% (p < .001, all comparisons). The ODYSSEY COMBO I43 trial (n = 316) in hypercholesterolemic patients at high CV risk, showed estimate mean difference in baseline LDL-C of −45.9% (p < .0001) with alirocumab 75Q2W at 24 weeks. Furthermore, 75% of patients achieved LDL-C < 1.8 mmol/L (70 mg/dL) in alirocumab group compared to 9% in the placebo group. The reductions were sustained till Week 52. In ODYSSEY CHOICE I46 trial (n = 803) in hypercholesterolemic patients at moderate-to-very-high CV risk, alirocumab 300 mg Q4W without statins showed mean LDL-C change from baseline by −52.7% compared to +0.3% with placebo (p < .0001) at Week 24. While those on statins showed a mean change of −58.8% compared to −0.1% with placebo (p < .0001). The results were maintained through Week 48 in both groups. When compared to ezetimibe in hypercholesterolemic patients at high CV risk in the ODYSSEY COMBO II50 trial (n = 720), alirocumab 75Q2W reduced LDL-C by a mean of 50.6% as compared to a mean of 20.7% with a difference of −29.8% (p < .0001) at Week 24. The reductions were sustained till Week 52 and the percentage of patients who achieved LDL-C < 1.8 mmol/L (70 mg/dL) was 77% on alirocumab versus 45.6% on ezetimibe (p < .0001).
Familial hypercholesterolemia
Alirocumab 150Q2W when added to statin therapy in ODYSSEY HIGH FH47 trial (n = 107) in patients with HeFH and LDL-C ≥ 4.14 mmol/L (160 mg/dL), resulted in a percent change in LDL-C from baseline of −45.7% compared to −6.6% by placebo with least-square mean difference between the groups of −39.1% (p < .0001). Forty one percent of the patients on alirocumab achieved LDL-C risk-specific goals versus 5.7% in the placebo group at Week 24. These reductions were maintained through the treatment phase of 78 weeks. At a dose of 75/150 mg in patients with HeFH in ODYSSEY FH I and FH II48, alirocumab on top of maximally tolerated statins led to 57.9 and 51.4% (p < .0001) reduction in LDL-C at Week 24, respectively, and were maintained up to week 78. After 78 weeks, the reductions were 51.8 and 52.1% from baseline LDL-C levels. The percentage of patients who achieved LDL-C levels < 1.8 mmol/L (70 mg/dL) was 59.8 and 68.2% versus 0.8 and 1.2% in placebo, respectively. Evolocumab 420 mg Q4W on top of stable LLT, reduced baseline LDL-C by 30·9% (p < .0001) in 50 patients with HoFH at 12 weeks in the TESLA-B trial35. In an open-label single-arm TAUSSIG61 trial (n = 300), evolocumab over 4 years was well-tolerated and effective in patients with HoFH (35%) and severe HeFH (65%) who completed Part A or B of the TESLA study. At Week 12 and 216, relative reductions in LDL-C were smaller in patients with HoFH (-21.2 and −24%, respectively) as compared with that in patients with severe HeFH (-54.9 and −47.2%, respectively). When studied in RUTHERFORD-236 trial (n = 331) which recruited patients with HeFH and LDL-C ≥ 2.6 mmol/L (100 mg/dL), evolocumab reduced baseline LDL-C by 61·3% with 420 mg QM and 59·2% with 140 mg Q2W (p < .0001, for both against placebo) at Week 12. In an analysis62 of patients with HeFH (n = 1257) in 4 double-blind, randomized, placebo-controlled, 78-week ODYSSEY studies (HIGH FH47, FH I and II48, and LONG TERM63) a mean percent change in baseline LDL-C of −43.6% was observed with alirocumab 75Q2W at Week 12, increased to −48.8% with alirocumab (75/150 mg) at Week 24. Mean changes of −57.1% at Week 12 and −55.0% at Week 24 were observed with 150Q2W (p < .0001). Around 75.3% (75/150 mg) or 64.5% (150 mg) of patients achieved their LDL-C goal of 1.8 or 2.6 mmol/L (70 or 100 mg/dL) based on CV risk by Week 24 and maintained it up to Week 78. In ODYSSEY OLE49 trial (n = 986), the mean LDL-C level reduction of 44.2% was observed in patients in alirocumab groups (75 mg or 150 mg Q2W) by Week 8 which increased to 47.9% by Week 96. Alirocumab use for 18 weeks in apheresis-treated patients with HeFH was safe and effective in ODYSSEY ESCAPE64 trial (n = 62). Lipoprotein apheresis can decrease lipoproteins’ levels but is an invasive procedure. As compared to the placebo, there was a 75% additional reduction in the standardized rate of apheresis. A percentage of 63.4% of patients avoided all and 92.7% avoided at least half of the lipoprotein apheresis treatment. The open-label DE LAVAL65 study (n = 36) showed that evolocumab significantly reduced apheresis requirement as well. The patients were randomized to either discontinue pheresis and receive evolocumab (140 mg Q2W) or continue pheresis frequency. At Week 6, 84% versus 10% avoided apheresis (treatment difference, 74%, 95% confidence interval (CI) 45 − 87; p < .0001). Furthermore, in the recent TAUSSIG61 trial (n = 300), lipoprotein apheresis was used by 61 patients. Of the 34 patients with HoFH and the 27 with HeFH 9% and 48% were able to stop apheresis treatment when evolocumab was used, respectively.
Mixed populations
In Alirocumab expanded-use program42, an open-label single arm trial, in patients with HeFH and/or CHD and baseline LDL-C of ≥4.14 mmol/L (160 mg/dL) on LLT, a reduction of 55% in LDL-C level was observed with alirocumab 150Q2W arm by Week 24. The single-arm ODYSSEY APPRISE66 study (n = 955) is the first study in a real life setting prior commercial availability of alirocumab in subjects (63% with HeFH; 68% with a history of CVD) inadequately controlled on their maximally-tolerated LLT. In the interim data of the first 843 patients, LDL-C level reduction from baseline was 56% at Week 12. The ODYSSEY JAPAN45 trial (n = 216) was a randomized double-blind trial in Japanese patients with HeFH or non-FH and at high CV risk who were on stable statins. Alirocumab (75/150 mg) at Week 24, showed a least-square mean change in baseline LDL-C of −62.5% versus +1.6% in placebo with 96.7% of patients achieving their LDL-C goals. An average reduction of 62.5% was maintained for 52 weeks. A post hoc analysis67 of the diabetic patients in ODYSSEY JAPAN showed a least-square mean change in baseline LDL-C of −63.1% in DM versus −60.8% in non-DM at Week 24, which was maintained for 52 weeks with similar percentage of patients attaining their LDL-C goals as in the parent trial. The ODYSSEY NIPPON68 trial (n = 163) was another one conducted in Japanese patients with either HeFH or non-HeFH with CHD who were on lowest-strength atorvastatin (5 mg per day) or non-statin LLT. At Week 12, the least-square mean percent change in LDL-C from baseline was −43.8% with alirocumab 150Q4W, −70.1% with 150Q2W, and −4.3% with placebo. The study continued as 52-week open-label treatment period and the patients received 150Q4W with possible up-titration to 150Q2W at Week 24. The mean LDL-C change from baseline was −45.1% at Week 20, with a further reduction at Week 36, with achieved levels maintained to Week 64. In a pooled analysis69 of eight ODYSSEY Phase III trials (n = 4629) in patients with HeFH at high CV risk on maximally tolerated statin therapy, LDL-C reduction by 48.9% was observed in 75/150 mg arms versus 9.3% with ezetimibe and 60.4% in 150Q2W arms versus 0.5% increase with placebo (p < .0001, all comparisons) at Week 24. The LDL-C reductions appeared as early as Week 4 and maintained up to the studies’ durations. The percentage of patients who achieved risk-based LDL-C goals of 1.8 or 2.6 mmol/L (70 or 100 mg/dL) by Week 24 was 75–79% on alirocumab versus 52% on ezetimibe and 6–8% on placebo. In PROFICIO70 (n = 3146), a pooled analysis of four randomized Phase III evolocumab 12‐week trials (LAPLACE-233, RUTHERFORD-236, MENDEL-237, GAUSS-271) that included patients with FH, primary hypercholesterolemia with different CV risks and prior statin intolerance, evolocumab as compared to placebo resulted in mean percent changes in LDL‐C of −65.7% for the Q2W dose and −65.0% for the QM dose. Compared to ezetimibe, the mean percent changes in LDL‐C was −38.9% for Q2W and −40.3% for QM. There were no differences between evolocumab and placebo or ezetimibe across the various subgroups i.e. age, gender, race, ethnicity, region, glucose tolerance status, or risk categories. In an analysis72 of the efficacy of biweekly and monthly evolocumab in hypercholesterolemic patients (n = 1148) from four Phase III randomized trials, LAPLACE-233, RUTHERFORD-236, MENDEL-237, GAUSS-271) who had fasting triglyceride (TG) levels of ≥1.7 mmol/L (150 mg/dL), a mean percentage change in LDL-C of −67% versus placebo and −42% versus ezetimibe was observed (p < .001, all comparisons), which was similar to the observed LDL-C reductions in patients without elevated TG levels.
Statin intolerance
It is estimated that about 10 of patients73,74 may be unable to tolerate any dose of statins (i.e. complete intolerance) or the dose that effectively reduces LDL-C (i.e. partial intolerance). More than half of the patients who experience statin-related adverse effects, discontinue their statin therapy. Thus, this precludes patients from achieving the target LDL-C level4, and exposing high CV risk patients to increased risk of mortality or adverse CV events5. Statin adverse muscle symptoms are considered the most predominant cause for statin discontinuation4,5.
Therapy with PCSK9 inhibitors has been effective and well tolerated in statin-intolerant patients in the short-term (12–24 weeks)71,74–77 or longer-term (up to two years)78 pivotal trials. Statin-intolerance in such trials was defined as the inability to tolerate two or more statins at the lowest available dosage71,76,77. Evolocumab therapy in three randomized double-blind trials, the 12-week Phase II (GAUSS; n = 236)74, the Japanese 12-week Phase III (GAUSS-4; n = 61)75 and the 24-week Phase III (GAUSS-2; n = 307)71, significantly reduced LDL-C levels and was associated with short-term tolerability. The efficacy and safety persisted over 24 weeks then up to two years, as shown in the two-stage GAUSS-376 (n = 491) trial and the subset analysis78 of the OSLER open-label extension studies, respectively. In GAUSS-271 and GAUSS-376 trials, evolocumab reduced LDL-C by 53 to 56 and 52.8% (p < .001, both comparisons), respectively. Adverse muscle events occurred in 12 and 20.7% of patients on evolocumab as compared to 23 and 28.8% of patients on ezetimibe, respectively. In the GUASS-475 trial, the percent change in mean LDL-C was −40.1% as compared to ezetimibe (adjusted p < .0001) with diarrhea (9.5%) and nasopharyngitis (12.5%) being the most common adverse events. Similarly, alirocumab in the ODYSSEY ALTERNATIVE77 trial (n = 314) was well-tolerated and produced significant reductions in LDL-C levels by 45% (p < .0001) at Week 24 in patients with statin intolerance due to muscle symptoms. Skeletal muscles adverse events were less frequent with alirocumab as compared to ezetimibe (hazard ratio (HR), 0.71, 95% CI 0.47 − 1.06, p = .096).
Cardiovascular outcomes
The use PCSK9 inhibitors has been associated with a benefit in CV outcomes. One interpretation of the potential mechanism is their ability to lower the atherogenic lipids levels (namely LDL-C, non-HDL-C, lipoprotein(a) (Lp(a))79. A number of studies has shown that PCSK9 inhibitors had significant reduction in atherogenic lipid fractions i.e. LDL-C, non-HDL-C, apolipoprotein-B (apo-B), and Lp(a) levels. In a post hoc analysis of a Phase II trial, alirocumab reduced total LDL particle (LDL-P) and very-low-density lipoprotein (VLDL), and increased HDL particles by 63.3, 36.4, and 11.2% (p < .01, all), respectively80. Evolocumab in the BERSON56 trial, BANTING trial57 and a post hoc analysis81 of the DESCARTES trial significantly decreased the serum levels and size of the lipoprotein particles. Both alirocumab and evolocumab caused significant reduction in LDL-apoB58,82,83 by 56.3% each82 and Lp(a)58,84–86 by 23.0–30.30%84,85 and 24.5–29.5%86, respectively. In a recent pre-specified analysis87 of the ODYSSEY OUTCOMES trial, a reduction in Lp(a) was an independent factor of lowering the risk of major adverse CV events (MACE), and that a 1 mg/dL reduction in Lp(a) was associated with an HR of 0.994 (95% CI, 0.990 − 0.999, p = .0081). In addition, there has been a positive correlation between plasma levels of PCSK9 and the carotid intima media wall thickness88, the platelet count in patients with coronary artery disease (CAD)89, and the necrotic core tissue fraction within the coronary plaques90, suggesting that the PCSK9 protein may have a key modulating effect in atherosclerosis91. The GLAGOV92 trial (n = 968) has confirmed that evolocumab in addition to statin therapy led to significant reduction in atheroma volume and induced plaque regression in higher percentage of patients. Alirocumab was evaluated in the Japanese Phase IV ODYSSEY J-IVUS93 study (n = 206) which enrolled hypercholesterolemic patients recently hospitalized with ACS. Using intravascular ultrasound imaging analysis, there was a numerically more percent reduction in normalized total atheroma volume which did not reach the statistical significance. It remains to be established whether PCSK9 inhibition has important clinical effects on arterial stiffness, endothelial function, and inflammatory responses. Circulating PCSK9 was significantly associated with arterial stiffness94 and drove a pro-inflammatory response on macrophage that stimulates cytokines95. To date, two patient cases have reported with impressive results on the use of PCSK9 inhibitors through improvement in carotid-femoral pulse wave velocity96. Two-month use of alirocumab has improved endothelial function as assessed by brachial artery vasoreactivity test97.
Prior to the major clinical outcome trials, FOURIER98 and ODYSSEY OUTCOMES99, findings from the published evidence gave an important signal that the reduction of LDL-C using PCSK9 inhibitors lowered the adverse CV events. In the OSLER100 study (n = 4465), evolocumab as compared to the control group reduced LDL-C by 61% (p < .001) and adverse CV events rate by 53% (HR, 0.47, 95% CI 0.28 − 0.78; p = .003) after one year of treatment. Alirocumab in the ODYSSEY LONG TERM63 trial (n = 2341) over a period of 78 weeks, significantly reduced LDL-C by 62% (p < .001) and in a post hoc analysis of it, there was an evidence of a reduction in the rate of MACE (HR, 0.52, 95% CI 0.31 − 0.90; nominal p = .02). A post hoc analysis101 from ten ODYSSEY trials (n = 4974), showed that the incidence of MACE was reduced by 24% for every 39 mg/dL reduction in LDL-C (adjusted HR, 0.76, 95% CI 0.63 − 0.91; p = .0025) and by 29% for every additional 50% reduction in LDL-C from baseline (HR, 0.71, 95% CI 0.57 − 0.89; p = .003). A meta-analysis102 of 24 randomized control trials (RCTs) (n = 10,159) showed that the use of PCSK9 inhibitors was associated with reductions in LDL-C by 47.49% (p < .001), all-cause mortality (odds ratio (OR), 0.45, 95% CI 95% 0.23 − 0.86; p = .015; heterogeneity p = .63; I2 = 0%), MI (OR, 0.49, 95% CI, 0.26 − 0.93; p = .03; heterogeneity p = .45; I2 = 0%), and CV mortality (OR, 0.50, 95% CI, 0.23 − 1.10; p = .084; heterogeneity p = .78; I2 = 0%) compared with no PCSK9 inhibitors treatment. A study103 predicted the individual lifetime benefit of PCSK9 inhibition, expressed in terms of gain in life expectancy free of (recurrent) stroke or MI, in statin-treated patients with stable CAD based on competing two risk models developed in data from the Treating to New Targets (TNT) trial. The individual lifetime benefit varied substantially, ranging from < 6 months free of stroke or MI in 61% of patients to ≥12 months in 10% of patients. The expected benefit was the highest in younger patients (aged 40 − 60 years) with risk factors, especially if LDL-C level is >1.8 mmol/L (70 mg/dL).
Three Phase III trials, FOURIER98, ODYSSEY OUTCOMES99, and SPIRE104, investigated PCSK9 inhibitors in patients with a history of clinically evident CVD. The FOURIER trial (n = 27,564) randomized patients with stable ASCVD and fasting LDL-C level ≥ 1.8 mmol/L (70 mg/dL) or non-HDL ≥2.6 mmol/L (100 mg/dL) who were on statin therapy. At Week 48, evolocumab 140 mg Q2W or 420 mg QM, reduced LDL-C by 59% compared to placebo. The primary outcome of a composite of CV death, MI, stroke, hospitalization for unstable angina (UA), or coronary revascularization was significantly lower (HR, 0.85; 95% CI, 0.79 − 0.92; p < .001) at a median duration of follow-up of 2.2 years98 (Table 1). The reduction of the composite of CV events was greater in patients with high-risk features i.e. more recent MI (HR, 0.80, 95% CI, 0.71 − 0.9), multiple prior MI (HR, 0.82; 95% CI, 0.72 − 0.93), and residual multivessel CAD (HR, 0.79; 95% CI, 0.69 − 0.91) with absolute risk reductions that exceeded 3% (3.4, 3.7, and 3.6%, respectively) versus around 1% in the low-risk groups (0.8, 1.3, and 1.2%)105. A Pre-specified secondary analysis106 of the FOURIER trial evaluated the effect of evolocumab on total CV events. The total CV events were reduced by 18% (incidence rate ratio (RR), 0.82; 95% CI, 0.75-0.90; p < .001) which was driven by decreases in MI, stroke, and coronary revascularization. In another secondary ad hoc analysis107 of the FOURIER trial, the reduction of adverse CV events was equally effective in patients with stable ASCVD regardless of the baseline LDL-C whether it was ≤1.8 mmol/L (70 mg/dL) or the background statins whether it was of a maximal or submaximal potency. Thirteen percent of patients in the FOURIER trial had PAD; evolocumab significantly lowered the primary end point in PAD patients (HR, 0.79; 95% CI 0.66 − 0.94; p = .0098) and the risk of major adverse limb events in all patients (HR, 0.58; 95% CI, 0.38 − 0.88; p = .0093). The reduction in LDL-C levels lowered the risk of major adverse limb events108. Evolocumab induced a relative reduction in venous thromboembolism (VTE) events by 29% (HR, 0.71; 95% CI, 0.50–1.00; p = .05) in the first year and by 46% (HR, 0.54; 95% CI, 0.33–0.88; p = .014) thereafter, in a pre-specified analysis of the FOURIER trial109. Moreover, evolocumab in the FOURIER trial reduced adverse CV events across the strata of baseline high-sensitivity C-reactive protein (hs-CRP) ( < 1, 1 − 3, and >3 mg/dL) with more absolute risk reductions in the patients with higher-baseline hs-CRP110. A meta-analysis111 found that PCSK9 inhibitors do not impact the circulating hs-CRP levels in contrast to statins112 or bempedoic acid17,113. A recent post hoc analysis of the FOURIER trial found that after the first 12 months of evolocumab use, there was a 52% relative reduction (HR, 0.48; 95% CI, 0.25-0.93) in aortic stenosis (AS) events rates in evolocumab group, which was attributed to the reduction in Lp(a) levels114. A previous study have suggested that PCSK9 inhibitors may potentially have a role in reducing the risk of AS based on the finding that PCSK9 R46L loss-of-function mutation was associated with lower Lp(a) and LDL-C level as well as reduced risk of AS115. The ODYSSEY OUTCOMES99 trial (n = 18,924) randomized patients on statin therapy with inadequate LDL-C control who had a recent ACS event rather than the more stable ones enrolled in the FOURIER trial. Alirocumab 75 mg or 150 mg Q2W over a median follow up of 2.8 years, significantly reduced the four-point composite of CV death, nonfatal MI, fatal or nonfatal ischemic stroke, or UA requiring hospitalization (HR, 0.85, 95% CI, 0.78 − 0.93; p < 0.001) (Table 1). The absolute benefit was greater among patients who had a baseline LDL-C of ≥ 2.6 mmol/L (100 mg/dL) than among patients with lower baseline LDL-C level. In a pre-specified analysis116, alirocumab prevented total (first and recurrent) non-fatal CV events (HR, 0.87, 95% CI, 0.82 − 0.93) and all-cause mortality (HR, 0.83, 95% CI, 0.71 − 0.97) in the presence of a strong association between nonfatal and fatal event risk. In another pre-specified analysis117 , alirocumab has also reduced the total (first and subsequent) hospitalizations (HR, 0.96 [95% CI, 0.92-1.00]; p = 0.04) and slightly increased days alive and out of hospital (RR, 1.003 [95% CI, 1.000-1.007]; p = 0.05) due to a decrease in days dead (RR, 0.847 [95% CI, 0.728-0.986]; p = 0.03), defined as the time from a patient’s death to the study end date. A third pre-specified analysis118 of the ODYSSEY OUTCOMES trial investigated the effect of alirocumab in ACS patients with a history of coronary artery bypass grafting. Alirocumab was associated with large absolute reductions in MACE and death. In a prespecified analysis of other vascular events in the ODYSSEY OUTCOMES trial, alirocumab lowered PAD events (HR, 0.69; 95% CI, 0.54–0.89; p = .004) significantly, but missed the statistical significance in reducing VTE events (HR, 0.67; 95% CI, 0.44–1.01; p = .06)119. When combined data from the FOURIER and ODYSSEY OUTCOMES trials (n = 46,488) were analyzed, it resulted in a 31% reduction in VTE events [HR, 0.69; 95% CI, 0.53–0.90; p = .007]109. Evidence also suggests the association with Lp(a) levels reduction109,119. The two parallel, SPIRE-1 and SPIRE-2, CV outcome trials (n = 27,438)104 were conducted in 2013 to assess the efficacy and safety of bococizumab, at a dose of 150 mg Q2W, in patients at high CV risk. However, the trials were terminated early by the sponsor after a median follow up of 10 months. The sponsor opted to stop the development of bococizumab due to the high rates of the anti-drug antibodies (ADAs), as detected in the data from other studies in the SPIRE program that consisted of a total of eight studies (i.e. six lipid-lowering and two CV outcome trials). In a combined analysis of SPIRE-1 and SPIRE-2 trials, bococizumab showed no benefit in term of MACE9,104 (Table 1).
Table 1.
Cardiovascular outcomes.
| Study | MACE | CV death | MI | Stroke | UA | Revascularization |
|---|---|---|---|---|---|---|
| SPIRE 1&2104(combined)2017 | 0.88 (0.76–1.02) 0.08 | 1.00 (0.71–1.41)> 0.99 | 0.91 (0.75–1.11) 0.35 | 0.60 (0.41–0.86) 0.006 | 0.89 (0.64–1.24) 0.49 | – |
| FOURIER982017 | 0.85 (0.79–0.92) <0.001 | 1.05 (0.88–1.25) 0.62 | 0.73 (0.65–0.82) <0.001 | 0.79 (0.66–0.95) 0.01 | 0.99 (0.82–1.18) 0.89 | 0.78 (0.71–0.86) <0.001 |
| ODYSSEY OUTCOMES992018 | 0.85 (0.78–0.93) <0.001 | 0.88 (0.74–1.05) NR* | 0.86 (0.77–0.96) NR* | 0.73 (0.57–0.93) NR* | 0.61 (0.41–0.92) NR* | 0.88 (0.79–0.97) NR* |
[HR (95%CI) p value].
Abbreviations. CV, cardiovascular; HR, hazard ration; MACE, major adverse CV events; MI, myocardial infarction; NR, not reported; UA, unstable angina.
*Hierarchical analysis was stopped after the first nonsignificant p value was observed, in accordance with the hierarchical testing plan.
Safety of PCSK9 monoclonal antibodies
In general, the safety profile of both evolocumab and alirocumab is excellent. Nasopharyngitis and mild injection-site reactions are considered the most common adverse reactions5.
Adverse effects
In two meta-analyses1,79 of RCTs, treatment with PCSK9 inhibitors was not associated with the adverse effects commonly described with statin therapy such as myalgia and elevations in serum aminotransferases or creatine kinase, with overall serious adverse events that were comparable to the control group. The aforementioned meta-analyses did not report the injection site reactions which are the most frequent adverse events with the PCSK9 inhibitors use4. The allergic local injection-site reactions (e.g. itching, redness, swelling) rates were 3.8% in alirocumab group versus 2.1% in placebo (p < .001)99 and 5.9 versus 4.2% over a period of 78 weeks63. Injection-site reactions are usually mild and self-limited99. The reactions rates with evolocumab were 2.1% versus 1.6% in the placebo group98, and there was no increase in hypersensitivity with longer treatment120. Bococizumab caused higher injection-site reactions as compared to placebo (10.4 versus 1.3%, p < .001)104 and 12.7 per 100 person-years in six trials evaluating bococizumab. However, the rates did not increase with longer time121.
Anti-drug antibodies
Therapeutic molecules may have an immunogenicity potential that may generate ADAs. The ADAs can be generated in response to fully human (e.g. alirocumab and evolocumab) or humanized (e.g. murine-derived bococizumab) monoclonal antibodies. High-titer ADAs have substantially developed in response to treatment with bococizumab (i.e. high degree of immunogenicity), while treatment with either alirocumab or evolocumab has not significantly been associated with ADAs generation104,121. This immunologic difference explains the higher injection-site reactions rate reported with bococizumab104. ADAs can be either neutralizing or binding122. Binding ADAs bind to therapeutic proteins without affecting the molecules’ function120, while neutralizing ADAs can directly impair their function122. In the ODYSSEY OUTCOMES99 trial, neutralizing ADAs were observed in 0.5% of patients on alirocumab versus 0.1% of those on placebo. Roth et al.123 reported data from 10 trials and found that ADAs against alirocumab were observed in 5.1% of patients as compared to 1.0% in the control group with persistent ADAs found in 1.4% versus 0.2% after 12 weeks or more, respectively. Neutralizing ADAs were reported in only 1.3% of the patients in alirocumab group. In the FOURIER98 trial, evolocumab generated binding ADAs in 0.3% of the patients and no neutralizing ADAs have been detected. These findings were consistent over a period of four years as observed in the OSLER-1120 study. In four RCTs that reported data regarding ADAs for evolocumab and alirocumab, the ADAs were transient and their titers decreased over time79. Almost half of the patients who received bococizumab in the SPIRE studies had high-titer ADAs with neutralizing antibodies developed in 29% of the patients104. Comparisons of ADAs incidence among the different PCSK9 inhibitors may be misleading because immunogenicity potential is dependent on various factors123. Cross-reactivity between PCSK9 inhibitors is unlikely to occur due to the specificity of the binding domains121. ADAs directed against PCSK9 inhibitors are one of the theoretical mechanisms for the PCSK9 inhibitors hyporesponsiveness (i.e. < 15% reduction in LDL-C level) due to the physiological impairment of monoclonal antibodies once absorbed into the circulation14. The occurrence of ADAs with PCSK9 inhibitors has attracted widespread attention. Although autoantibodies were detected in some patients on alirocumab or evolocumab, reductions in LDL-C levels induced by either monoclonal antibody were not attenuated79. ADAs did not appear to significantly attenuate the lipid-lowering effect of alirocumab99,123 or evolocumab98, unlike that of bococizumab121. The high immunogenicity rates and the wide variation in LDL-C response among patients on bococizumab led to the discontinuation of further clinical development of bococizumab9,121.
Neurocognitive events
Low LDL-C levels in the clinical trials of PCSK9 inhibitors have raised a concern about the association of their use with cognitive impairment124. Neurocognitive adverse events were reported as delirium, attention disorders, amnesia, dementia, disturbances in thinking and perception, or mental impairment disorders4,5. Neurocognitive adverse events reported in the clinical trials of alirocumab and evolocumab were not significantly different from those reported in the control groups63,98,99. Furthermore, similar findings were found in two meta-analyses of RCTs1,79 and in the EBBINGHAUS124 study (n = 1204), a sub-study of the FOURIER trial, that evaluated the cognitive deficits. The findings of the latter study were supported by a Mendelian randomization study which found no causal relationship between inhibition of PCSK9 function and neurodegenerative diseases125.
Diabetes
It has been hypothesized that the significant LDL-C-lowering effect of the PCSK9 inhibitors may negatively affect the glycemic status, namely the new-onset diabetes, as suggested with statin therapy to be associated with an increase in diabetes incidence104,126. It has been postulated that the genetic variations in the targets of statins and PCSK9 inhibitors i.e. 3-hydroxy-3-methylglutaryl-CoA reductase (HMGCR) and PCSK9 respectively, have been associated with desirable protective effect against atherosclerosis but on the other hand with adverse effects in term of diabetes104. The currently available evidence did not suggest significant increase in new-onset diabetes or worsening of preexisting diabetes on shorter or longer terms with either alirocumab39,63,99,127 or evolocumab56,57,98,120,128–130. In the SPIRE104 studies, although bococizumab use was not associated with significant increase in new-diabetes, the early termination of the trials precluded the ability to assess adverse effect on glycemic control. The results of three recent meta-analyses1,79,126 are in line with the findings of the previous studies. However, one126 of the aforementioned meta-analyses has elaborated that the imbalance in the background LLT of the control arms may have masked the effect of PCSK9 inhibitors on diabetes.
Fat-soluble vitamins and steroid hormones
Fat-soluble vitamins and steroid hormones levels were measured as part of prespecified safety analyses in trials with PCSK9 inhibitors such as ODYSSEY LONG TERM63 and DESCARTES131. In the alirocumab group, although the levels of vitamin E or vitamin K were below the lower limit of the normal range, there were no clinically important changes. Moreover, there was no clinically meaningful effect with regards the changes in the levels of cortisol or other fat-soluble vitamins63. Similarly, vitamin E levels were lower in evolocumab-treated subjects but the results were not statistically significant after the adjustment of LDL-C level, and there were no adverse effects reported for steroid or gonadal hormones131.
Cost-effectiveness of PCSK9 monoclonal antibodies
Evaluation of cost effectiveness of PCSK9 inhibitors has been of interest to several trials. In 2015, they were not proven to be cost effective as their use increased annual prescription spending by $125 billion over ezetimibe in patients with HeFH or ASCVD132. Another study133 recommended price reduction ($14,000–15,000) by 70% as they possessed incremental cost of about $350,000 per quality adjusted life years (QALYs) when compared to statins. A treatment cost analysis134 of evolocumab versus ezetimibe to prevent one MACE in T2DM using data from the FOURIER and IMPROVE-IT trials had similar conclusion. In all analyses performed, ezetimibe was consistently a cost-saving strategy compared with evolocumab except for the case where evolocumab price is significantly reduced and the branded ezetimibe is used. In a review135 of 10 health economic evaluations from United States of America (USA) and Europe, cost effectiveness of PCSK9 inhibitors remained uncertain and price reduction was recommended. Following the CV outcome trials, a study showed that relevant costs saved per CV event annually remained very small in Apulia, Italy136. In response to 2018 price reductions, National Lipid Association issued a statement to highlight in which subgroups of patients PCSK9 monoclonal antibodies are currently considered a cost-effective reasonable option ( < USD 100,000/QALY). The subgroups included (a) extremely high–risk patients with ASCVD of >40% 10-year ASCVD risk and LDL-C ≥ 1.8 mmol/L (70 mg/dL), (b) very-high-risk patients with ASCVD of 30–39% 10-y ASCVD risk and LDL-C ≥ 2.6 mmol/L (100 mg/dL) and (c) high-risk patients with 20–29% 10-year ASCVD risk and LDL-C ≥ 3.36 mmol/L (130 mg/dL). All patients should receive a maximally tolerated statin therapy and ezetimibe prior starting PCSK9 monoclonal antibodies therapy137.
Registered clinical trials
The documented significant benefit of the PCSK9 monoclonal antibodies in decreasing lipid parameters and adverse CV events encouraged further investigations on their efficacy and safety. Research is ongoing in patients with familial and non-familial hypercholesterolemia. New research is targeting special patient populations such as pediatrics and human immunodeficiency virus (HIV) subjects. Relevant ongoing Phase III trials are listed in Table 2.
Table 2.
Phase III registered clinical trials.
| Trial ID* | Agent | Trial brief title (acronym) | Target size | Primary outcome (time frame) | Start date | Status |
|---|---|---|---|---|---|---|
| Hypercholesterolemia | ||||||
| NCT02476006 | Alirocumab | Safety, tolerability, and effect of alirocumab in high cardiovascular risk patients with severe hypercholesterolemia not adequately controlled with conventional lipid-modifying therapies (ODYSSEY APPRISE) | 998 | Proportion of patients with adverse events and percent change in lipid levels (30 months) | June 2015 | Completed |
| NCT03433755 | Evolocumab | Safety and efficacy of evolocumab in addition to optimal stable background statin therapy in chinese subjects with primary hypercholesterolemia and mixed dyslipidemia | 450 | Mean percent change in LDL-C from baseline (10 and 12 weeks) | May 2019 | Recruiting |
| NCT03156621 | Alirocumab | Study in participants HoFH (ODYSSEY HoFH) | 69 | Percent change in LDL-C from baseline (12 weeks) | October 2017 | Active, not recruiting |
| Cardiovascular outcomes | ||||||
| NCT03872401 | Evolocumab | Effect of evolocumab in patients at high cardiovascular risk without prior MI or stroke (VESALIUS-CV) | 13,000 | Time to coronary heart disease death, MI, or ischemic stroke (4.5 years) | June 2019 | Recruiting |
| NCT02867813 | Evolocumab | Further cardiovascular outcomes research with PCSK9 inhibition in subjects with elevated risk open-label extension (FOURIER OLE) | 5,037 | Subject incidence of adverse events (5 years) | September 2016 | Active, not recruiting |
| NCT03080935 | Evolocumab | Fourier open-label extension study in subjects with clinically evident cardiovascular disease in selected European countries | 1,600 | Adverse event measurement (5 years) | March 2017 | Active, not recruiting |
| NCT03570697 | Evolocumab | Imaging of coronary plaques in subjects treated with evolocumab (HUYGENS) | 164 | Absolute change in minimum FCT (50 weeks) | November 2018 | Active, not recruiting |
| NCT03689946 | Evolocumab | Effect of evolocumab on coronary artery plaque volume and composition by CCTA and microcalcification by F18-NaF PET | 55 | Change in NCPV from baseline (18 months) | March 2019 | Recruiting |
| NCT02729025 | Evolocumab | Effects of proprotein convertase subtilisin/Kexin Type 9 (PCSK9) inhibition on arterial wall inflammation in patients with elevated Lp(a) (ANITSCHKOW) | 129 | Percent change in Lp(a) in target-to-background ratio of an index vessel by FDG-PET/CT (16 weeks) | April 2016 | Completed |
| JPRN-jRCT1051180064 | Alirocumab | Effect of alirocumab and rosuvastatin or rosuvastatin alone on lipid core plaques in coronary artery disease evaluated by near-infrared spectroscopy intravascular ultrasound (ANTARES) | 30 | Absolute change of maxLCBI (4 mm) from baseline (36 weeks) | April 2018 | Recruiting |
| JPRN-jRCT1051180063 | Alirocumab | Efficacy of Alirocumab for thin-cap fibroatheroma in patients with coronary artery disease estImated by optical coherence tomography trial (ALTAIR) | 24 | Change in minimum FCT between baseline (36 weeks) | September 2017 | Completed |
| NCT03067844 | Alirocumab | Vascular effects of alirocumab in acute MI-patients (PACMAN-AMI) | 294 | Change in PAV from baseline (52 weeks) | April 2017 | Recruiting |
| Special patient populations | ||||||
|
NCT03510884 [pediatrics] |
Alirocumab | An efficacy and safety study of alirocumab in children and adolescents with HeFH | 150 | Percent change in LDL-C from baseline (24 weeks) | May 2018 | Recruiting |
|
NCT03510715 [pediatrics] |
Alirocumab | An efficacy and safety study of alirocumab in children and adolescents with HoFH | 18 | Percent change in LDL-C from baseline (12 weeks) | August 2018 | Completed |
|
NCT02392559 [pediatrics] |
Evolocumab | Trial assessing efficacy, safety and tolerability of PCSK9 inhibition in pediatric subjects with genetic LDL disorders (HAUSER-RCT) | 159 | Percentage change LDL-C levels from baseline (24 weeks) | February 2016 | Completed |
|
NCT02624869 [pediatrics] |
Evolocumab | Open label study to evaluate safety, tolerability and efficacy of evolocumab (AMG 145) in pediatric subjects (10–17 years of age) with HeFH or HoFH(HAUSER-OLE) | 163 | Number of participants with treatment-related Adverse Events as assessed by CTCAE V4.0 (80 weeks) | September 2016 | Active, not recruiting |
|
NCT03207945 [HIV subjects] |
Alirocumab | Effect of PCSK9 inhibition on cardiovascular risk in treated HIV infection (EPIC-HIV) | 140 | FDG PET/CT Endpoint. Change in Target-to-background ratio from baseline. The main arterial endpoint is the most diseased segment of the index vessel (52 weeks) | April 2018 | Recruiting |
|
NCT02833844 [HIV subjects] |
Evolocumab | Safety, tolerability & efficacy on LDL-C of evolocumab in subjects with HIV & hyperlipidemia/mixed dyslipidemia (BEIJERINCK) | 467 | Percent change of LDL-C from baseline (24 weeks) | May 2017 | Completed |
|
NCT03734211 [Heart transplant recipients] |
Evolocumab | Cholesterol lowering with evolocumab to prevent cardiac allograft vasculopathy in de-novo heart transplant recipients (EVOLVD) | 130 | Maximal intimal thickness measured by coronary IVUS; defined as the largest distance (in mm) from the intimal leading edge to the external elastic membrane (12 months) | June 2019 | Recruiting |
|
NCT03480568 [dialysis patients] |
Alirocumab | Alirocumab in patients on a stable dialysis regimen | 20 | Change in LDL cholesterol levels from baseline (4 ,8, 12 weeks) | May 2018 | Recruiting |
|
NCT03344692 [diabetics] |
Alirocumab | Effect of alirocumab on postprandial hyperlipemia in patients with type 2 diabetes (EUTERPE) |
24 | Total AUC of post-prandial TG concentration-time from meal-time until 8 h after standardized high fat meal (During 8 hours at week 10 after first injection) | February 2019 | Recruiting |
Abbreviations. AUC, area under curve; CTCAE, Common Terminology Criteria for Adverse Events; FCT, fibrous-cap thickness; FDG PET/CT, fluoro-deoxyglucose positron-emission tomography/computed tomography; HeFH, heterozygous familial hypercholesterolemia; HoFH, homozygous FH; HIV, human immunodeficiency virus; IVUS, intravascular ultrasound; LDL-C, low-density lipoprotein cholesterol (LDL-C); Lp, Lp(a); maxLCBI, maximal lipid-core burden index; MI, myocardial infarction; NCPV, noncalcified coronary artery plaque volume; PAV, percent atheroma volume; PET, positron emission tomography (PET); TG, triglycerides.
Conclusions
The currently-available PCSK9 monoclonal antibodies, alirocumab and evolocumab, are safe and effective when used as an add-on or as monotherapy. They are effective in lowering LDL-C levels by more than 50% in pooled populations. The outcome studies have confirmed that the significant LDL-C-lowering capacity was translated into a reduction in CV events without causing excessive adverse effects. The benefit in CV outcomes was attributed to the ability of PCSK9 inhibitors to lower atherogenic lipid fractions such as LDL-C, non-HDL-C, apo-B, and Lp(a) levels and atheroma volume. The ability to lower Lp(a) levels could potentially reduce PAD, VTE, and AS events as well.
Acknowledgements
None reported.
Transparency
Declaration of funding
Nothing to declare.
Declaration of financial/other relationships
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
References
- 1.Karatasakis A, Danek BA, Karacsonyi J, et al. Effect of PCSk9 inhibitors on clinical outcomes in patients with hypercholesterolemia: a meta-analysis of 35 randomized controlled trials. J Am Heart Assoc. 2017;6(12)pii:e006910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jones PH, Nair R, Thakker KM.. Prevalence of dyslipidemia and lipid goal attainment in statin-treated subjects from 3 data sources: a retrospective analysis. J Am Heart Assoc. 2012;1(6):e001800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cannon CP, Blazing MA, Giugliano RP, et al. IMPROVE-IT investigators. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372(25):2387–2397. [DOI] [PubMed] [Google Scholar]
- 4.Rosenson RS, Hegele RA, Koenig W.. Cholesterol-lowering agents PCSK9 inhibitors today and tomorrow. Circ Res. 2019;124(3):364–385. [DOI] [PubMed] [Google Scholar]
- 5.Rosenson RS, Hegele RA, Fazio S, et al. The evolving future of PCSK9 inhibitors. J Am Coll Cardiol. 2018;72(3):314–329. [DOI] [PubMed] [Google Scholar]
- 6.Landmesser U, Chapman MJ, Stock JK, et al. 2017 Update of ESC/EAS Task Force on practical clinical guidance for proprotein convertase subtilisin/kexin type 9 inhibition in patients with atherosclerotic cardiovascular disease or in familial hypercholesterolaemia. Eur Heart J. 2018;39(14):1131–1143. [DOI] [PubMed] [Google Scholar]
- 7.Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. J Am Coll Cardiol. 2019;73(24):e285–e350; Circulation. 2019;139(25): e1082–e1143. [DOI] [PubMed] [Google Scholar]
- 8.Mach F, Baigent C, Catapano AL, ESC Scientific Document Group, et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41(1):111–188. [DOI] [PubMed] [Google Scholar]
- 9.Pfizer press release . Pfizer discontinues global development of bococizumab, its investigational PCSK9 inhibitor; November, 2016. [cited 2020 Feb 11]. Available from: https://www.pfizer.com/news/press-release/press-releasedetail/pfizer_discontinues_global_development_of_bococizumab_its_investigational_pcsk9_inhibitor..
- 10.Levisetti M, Joh T, Wan H, et al. A phase I randomized study of a specifically engineered, pH-sensitive PCSK9 inhibitor RN317 (PF-05335810) in hypercholesterolemic subjects on statin therapy. Clin Transl Sci. 2017;10(1):3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shen T, James DE, Krueger KA.. Population pharmacokinetics (PK) and pharmacodynamics (PD) analysis of LY3015014, a monoclonal antibody to protein convertase subtilisin/kexin type 9 (PCSK9) in healthy subjects and hypercholesterolemia patients. Pharm Res. 2017;34(1):185–192. [DOI] [PubMed] [Google Scholar]
- 12.Budha NR, Leabman M, Jin JY, et al. Modeling and simulation to support phase 2 dose selection for RG7652, a fully human monoclonal antibody against proprotein convertase subtilisin/kexin type 9. Aaps J. 2015;17(4):881–890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baruch A, Luca D, Kahn RS, et al. A phase 1 study to evaluate the safety and LDL cholesterol-lowering effects of RG7652, a fully human monoclonal antibody against proprotein convertase subtilisin/kexin type 9. Clin Cardiol. 2017;40(7):503–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Warden BA, Fazio S, Shapir MD.. The PCSK9 revolution: current status, controversies, and future directions. Trends Cardiovasc Med. 2019; 30(3):179–185. [DOI] [PubMed] [Google Scholar]
- 15.Salam AM. The therapeutic potential of PCSK9 inhibition in primary dyslipidemia, the example from SAR236553/REGN727. Expert Opin Investig Drugs. 2012;21(10):1585–1588. [DOI] [PubMed] [Google Scholar]
- 16.Bove M, Cicero AFG, Borghi C.. Emerging drugs for the treatment of hypercholesterolemia. Expert Opin Emerg Drugs. 2019;24(1):63–69. [DOI] [PubMed] [Google Scholar]
- 17.Ballantyne CM, Banach M, Mancini GBJ, et al. Efficacy and safety of bempedoic acid added to ezetimibe in statin-intolerant patients with hypercholesterolemia: a randomized, placebo-controlled study. Atherosclerosis. 2018;277:195–203. [DOI] [PubMed] [Google Scholar]
- 18.Stein EA, Mellis S, Yancopoulos GD, et al. Effect of a monoclonal antibody to PCSK9 on LDL cholesterol. N Engl J Med. 2012;366(12):1108–1118. [DOI] [PubMed] [Google Scholar]
- 19.Dias CS, Shaywitz AJ, Wasserman SM, et al. Effects of AMG 145 on low-density lipoprotein cholesterol levels: results from 2 randomized, double-blind, placebo-controlled, ascending-dose phase 1 studies in healthy volunteers and hypercholesterolemic subjects on statins. J Am Coll Cardiol. 2012;60(19):1888–1898. [DOI] [PubMed] [Google Scholar]
- 20.Gumbiner B, Joh T, Liang H, et al. The effects of single- and multiple-dose administration of bococizumab (RN316/PF-04950615), a humanized IgG2Δa monoclonal antibody binding proprotein convertase subtilisin/kexin type 9, in hypercholesterolemic subjects treated with and without atorvastatin: results from four phase I studies. Cardiovasc Ther. 2018;36(1):e12309. [DOI] [PubMed] [Google Scholar]
- 21.Roth EM, McKenney JM, Hanotin C, et al. Atorvastatin with or without an antibody to PCSK9 in primary hypercholesterolemia. N Engl J Med. 2012;367(20):1891–1900. [DOI] [PubMed] [Google Scholar]
- 22.McKenney JM, Koren MJ, Kereiakes DJ, et al. Safety and efficacy of a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 serine protease, SAR236553/REGN727, in patients with primary hypercholesterolemia receiving ongoing stable atorvastatin therapy. J Am Coll Cardiol. 2012;59(25):2344–2353. [DOI] [PubMed] [Google Scholar]
- 23.Stein EA, Gipe D, Bergeron J, et al. Effect of a monoclonal antibody to PCSK9, REGN727/SAR236553, to reduce low-density lipoprotein cholesterol in patients with heterozygous familial hypercholesterolaemia on stable statin dose with or without ezetimibe therapy: a phase 2 randomised controlled trial. Lancet. 2012;380(9836):29–36. [DOI] [PubMed] [Google Scholar]
- 24.Teramoto T, Kobayashi M, Uno K, et al. Efficacy and Safety of Alirocumab in Japanese Subjects (Phase 1 and 2 Studies). Am J Cardiol. 2016;118(1):56–63. [DOI] [PubMed] [Google Scholar]
- 25.Ballantyne CM, Neutel J, Cropp A, et al. Results of bococizumab, a monoclonal antibody against proprotein convertase subtilisin/kexin type 9, from a randomized, placebo-controlled, dose-ranging study in statin-treated subjects with hypercholesterolemia. Am J Cardiol. 2015;115(9):1212–1221. [DOI] [PubMed] [Google Scholar]
- 26.Fazio S, Robertson DG, Joh T, et al. Effects of 12 weeks of treatment with intravenously administered bococizumab, a humanized monoclonal antibody blocking proprotein convertase subtilisin/kexin type 9, in. Cardiovasc Ther. 2018;36(1):e12308. [DOI] [PubMed] [Google Scholar]
- 27.Yokote K, Kanada S, Matsuoka O, et al. Efficacy and safety of bococizumab (RN316/PF-04950615), a monoclonal antibody against proprotein convertase subtilisin/kexin type 9, in. Circ J. 2017;81(10):1496–1505. [DOI] [PubMed] [Google Scholar]
- 28.Koren MJ, Scott R, Kim JB, et al. Efficacy, safety, and tolerability of a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 as monotherapy in patients with hypercholesterolaemia (MENDEL): a randomised, double-blind, placebo-controlled, phase 2 study. Lancet. 2012;380(9858):1995–2006. [DOI] [PubMed] [Google Scholar]
- 29.Giugliano RP, Desai NR, Kohli P, et al. LAPLACE-TIMI 57 Investigators. Efficacy, safety, and tolerability of a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 in combination with a statin in patients with hypercholesterolaemia (LAPLACE-TIMI 57): a randomised, placebo-controlled, dose-ranging, phase 2 study. Lancet. 2012;380(9858):2007–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Raal F, Scott R, Somaratne R, et al. Low-density lipoprotein cholesterol-lowering effects of AMG 145, a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 serine protease in patients with heterozygous familial hypercholesterolemia: the reduction of LDL-C with PCSK9 inhibition in heterozygous familial hypercholesterolemia disorder (RUTHERFORD) randomized trial. Circulation. 2012;126(20):2408–2417. [DOI] [PubMed] [Google Scholar]
- 31.Hirayama A, Honarpour N, Yoshida M, et al. Effects of evolocumab (AMG 145), a monoclonal antibody to PCSK9, in hypercholesterolemic, statin-treated Japanese patients at high cardiovascular risk–primary results from the phase 2 YUKAWA study. Circ J. 2014;78(5):1073–1082. [DOI] [PubMed] [Google Scholar]
- 32.Martinez-Hervas S, Ascaso JF.. Hypercholesterolemia [Internet]. Encyclopedia of Endocrine Diseases. 2nd ed. Cambridge (MA): Academic Press; 2018. [cited 2020 May 31]. Available from: https://www.sciencedirect.com/science/article/pii/B9780128012383653400. [Google Scholar]
- 33.Robinson JG, Nedergaard BS, Rogers WJ, et al. LAPLACE-2 Investigators: Effect of evolocumab or ezetimibe added to moderate-or high-intensity statin therapy on LDL-C lowering in patients with hypercholesterolemia: The LAPLACE-2 randomized clinical trial. JAMA. 2014;311(18):1870–1882. [DOI] [PubMed] [Google Scholar]
- 34.Kiyosue A, Honarpour N, Kurtz C, et al. A phase 3 study of evolocumab (AMG 145) in statin-treated Japanese patients at high cardiovascular risk. Am J Cardiol. 2016;117(1):40–47. [DOI] [PubMed] [Google Scholar]
- 35.Raal FJ, Honarpour N, Blom DJ, Tesla Investigators, et al. Inhibition of PCSK9 with evolocumab in homozygous familial hypercholesterolaemia (TESLA Part B): a randomised, double-blind, placebo-controlled trial. Lancet. 2015;385(9965):341–350. [DOI] [PubMed] [Google Scholar]
- 36.Raal FJ, Stein EA, Dufour R, et al. PCSK9 inhibition with evolocumab (AMG 145) in heterozygous familial hypercholesterolaemia (RUTHERFORD-2): a randomised, double-blind, placebo-controlled trial. Lancet. 2015;385(9965):331–340. [DOI] [PubMed] [Google Scholar]
- 37.Koren MJ, Lundqvist P, Bolognese M, MENDEL-2 Investigators. et al. Anti-PCSK9 monotherapy for hypercholesterolemia: the MENDEL-2 randomized, controlled phase III clinical trial of evolocumab. J Am Coll Cardiol. 2014;63(23):2531–2540. [DOI] [PubMed] [Google Scholar]
- 38.Stroes E, Guyton JR, Lepor N, Odyssey Choice II Investigators, et al. Efficacy and safety of alirocumab 150 mg every 4 weeks in patients with hypercholesterolemia not on statin therapy: the ODYSSEY CHOICE II study. J Am Heart Assoc. 2016;5(9)pii:e003421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Leiter LA, Cariou B, Müller‐Wieland D, et al. Efficacy and safety of alirocumab in insulin‐treated individuals with type 1 or type 2 diabetes and high cardiovascular risk: the ODYSSEY DM‐INSULIN randomized trial. Diabetes Obes Metab. 2017;19(12):1781–1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ray KK, Leiter LA, Müller‐Wieland D, et al. Alirocumab vs. usual lipid‐lowering care as add‐on to statin therapy in individuals with type 2 diabetes and mixed dyslipidaemia: the ODYSSEY DM‐DYSLIPIDEMIA randomized trial. Diabetes Obes Metab. 2018;20(6):1479–1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Koh KK, Nam CW, Chao TH, et al. A randomized trial evaluating the efficacy and safety of alirocumab in South Korea and Taiwan (ODYSSEY KT). J Clin Lipidol. 2018;12(1):162–172. [DOI] [PubMed] [Google Scholar]
- 42.Glueck CJ, Brown A, Goldberg AC, et al. Alirocumab in high-risk patients: observations from the open-label expanded use program. J Clin Lipidol. 2018;12(3):662–668. [DOI] [PubMed] [Google Scholar]
- 43.Kereiakes DJ, Robinson JG, Cannon CP, et al. Efficacy and safety of the proprotein convertase subtilisin/kexin type 9 inhibitor alirocumab among high cardiovascular risk patients on maximally tolerated statin therapy: the ODYSSEY COMBO I study. Am Heart J. 2015;169(6):906–915. [DOI] [PubMed] [Google Scholar]
- 44.Blom DJ, Hala T, Bolognese M, et al. A 52-week placebo-controlled trial of evolocumab in hyperlipidemia. N Engl J Med. 2014;370(19):1809–1819. [DOI] [PubMed] [Google Scholar]
- 45.Teramoto T, Kobayashi M, Tasaki H, et al. Efficacy and safety of alirocumab in Japanese patients with heterozygous familial hypercholesterolemia or at high cardiovascular risk with hypercholesterolemia not adequately controlled with statins–ODYSSEY JAPAN randomized controlled trial. Circ J. 2016;80(9):1980–1987. [DOI] [PubMed] [Google Scholar]
- 46.Roth EM, Moriarty PM, Bergeron J, et al. A phase III randomized trial evaluating alirocumab 300 mg every 4 weeks as monotherapy or add-on to statin: ODYSSEY CHOICE I. Atherosclerosis. 2016;254:254–262. [DOI] [PubMed] [Google Scholar]
- 47.Ginsberg HN, Rader DJ, Raal FJ, et al. Efficacy and safety of alirocumab in patients with heterozygous familial hypercholesterolemia and LDL-C of 160 mg/dl or higher. Cardiovasc Drugs Ther. 2016;30(5):473–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kastelein JJ, Ginsberg HN, Langslet G, et al. ODYSSEY FH I and FH II: 78 week results with alirocumab treatment in 735 patients with heterozygous familial hypercholesterolaemia. Eur Heart J. 2015;36(43):2996–3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Farnier M, Hovingh GK, Langslet G, et al. Long-term safety and efficacy of alirocumab in patients with heterozygous familial hypercholesterolemia: an open-label extension of the ODYSSEY program. Atherosclerosis. 2018;278:307–314. [DOI] [PubMed] [Google Scholar]
- 50.Cannon CP, Cariou B, Blom D, et al. Efficacy and safety of alirocumab in high cardiovascular risk patients with inadequately controlled hypercholesterolaemia on maximally tolerated doses of statins: the ODYSSEY COMBO II randomized controlled trial. Eur Heart J. 2015;36(19):1186–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bays H, Gaudet D, Weiss R, et al. Alirocumab as add-on to atorvastatin versus other lipid treatment strategies: ODYSSEY OPTIONS I randomized trial. J Clin Endocrinol Metab. 2015;100(8):3140–3148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Farnier M, Jones P, Severance R, et al. Efficacy and safety of adding alirocumab to rosuvastatin versus adding ezetimibe or doubling the rosuvastatin dose in high cardiovascular-risk patients: the ODYSSEY OPTIONS II randomized trial. Atherosclerosis. 2016;244:138–146. [DOI] [PubMed] [Google Scholar]
- 53.Catapano AL, Reiner Z, De Backer G, et al. ESC/EAS guidelines for the management of dyslipidaemias The Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS). Atherosclerosis. 2011;217(1):3–46. [DOI] [PubMed] [Google Scholar]
- 54.Perk J, De Backer G, Gohlke H, et al. European Guidelines on cardiovascular disease prevention in clinical practice (version 2012). The Fifth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of nine societies and by invited experts) [published correction appears in. Eur Heart J. 2012;33(17):1635–1701. [DOI] [PubMed] [Google Scholar]
- 55.Koskinas KC, Windecker S, Pedrazzini G, et al. Evolocumab for early reduction of LDL cholesterol levels in patients with acute coronary syndromes (EVOPACS). J Am Coll Cardiol. 2019;74(20):2452–2462. [DOI] [PubMed] [Google Scholar]
- 56.Lorenzatti AJ, Eliaschewitz FG, Chen Y, et al. Randomised study of evolocumab in patients with type 2 diabetes and dyslipidaemia on background statin: primary results of the BERSON clinical trial. Diabetes Obes Metab. 2019;21(6):1455–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rosenson RS, Daviglus ML, Handelsman Y, et al. Efficacy and safety of evolocumab in individuals with type 2 diabetes mellitus: primary results of the randomised controlled BANTING study. Diabetologia. 2019;62(6):948–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Han Y, Chen J, Chopra VK, et al. ODYSSEY EAST: Alirocumab efficacy and safety vs ezetimibe in high cardiovascular risk patients with hypercholesterolemia and on maximally tolerated statin in China, India, and Thailand. J Clin Lipidol. 2019;14(1):98–108. [DOI] [PubMed] [Google Scholar]
- 59.Roth EM, McKenney JM.. ODYSSEY MONO: effect of alirocumab 75 mg subcutaneously every 2 weeks as monotherapy versus ezetimibe over 24 weeks. Future Cardiol. 2015;11(1):27–37. [DOI] [PubMed] [Google Scholar]
- 60.Kastelein JJ, Kereiakes DJ, Cannon CP, et al. Effect of alirocumab dose increase on LDL lowering and lipid goal attainment in patients with dyslipidemia. Coron Artery Dis. 2017;28(3):190–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Santos RD, Stein EA, Hovingh GK, et al. Long-term evolocumab in patients with familial hypercholesterolemia. Am Coll Cardiol. 2020;75(6):565–574. [DOI] [PubMed] [Google Scholar]
- 62.Kastelein JJ, Hovingh GK, Langslet G, et al. Efficacy and safety of the proprotein convertase subtilisin/kexin type 9 monoclonal antibody alirocumab vs placebo in patients with heterozygous familial hypercholesterolemia. J Clin Lipidol. 2017;11(1):195–203. [DOI] [PubMed] [Google Scholar]
- 63.Robinson JG, Farnier M, Krempf M, ODYSSEY LONG Term Investigators, et al. Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372(16):1489–1499. [DOI] [PubMed] [Google Scholar]
- 64.Moriarty PM, Parhofer KG, Babirak SP, et al. Alirocumab in patients with heterozygous familial hypercholesterolaemia undergoing lipoprotein apheresis: the ODYSSEY ESCAPE trial. Eur Heart J. 2016;37(48):3588–3595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Baum SJ, Sampietro T, Datta D, et al. Effect of evolocumab on lipoprotein apheresis requirement and lipid levels: Results of the randomized, controlled, open-label DE LAVAL study. J Clin Lipidol. 2019;13(6):901–909.e3. [DOI] [PubMed] [Google Scholar]
- 66.Henry P, Cariou B, Averna M, et al. Open-label ODYSSEY APPRISE study: interim data from the first 843 participants. Arch of Cardiovasc Dis Suppl. 2018;10(1):127. [Google Scholar]
- 67.Teramoto T, Usami M, Takagi Y, ODYSSEY Japan Investigators, et al. Efficacy and safety of alirocumab in Japanese patients with diabetes mellitus: post-hoc subanalysis of ODYSSEY Japan. J Atheroscler Thromb. 2019;26(3):282–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Teramoto T, Kiyosue A, Ishigaki Y, et al. Efficacy and safety of alirocumab 150mg every 4 weeks in hypercholesterolemic patients on non-statin lipid-lowering therapy or lowest strength dose of statin: ODYSSEY NIPPON. J Cardiol. 2019;73(3):218–227. [DOI] [PubMed] [Google Scholar]
- 69.Farnier M, Gaudet D, Valcheva V, et al. Efficacy of alirocumab in high cardiovascular risk populations with or without heterozygous familial hypercholesterolemia: pooled analysis of eight ODYSSEY phase 3 clinical program trials. Int J Cardiol. 2016;223:750–757. [DOI] [PubMed] [Google Scholar]
- 70.Stroes E, Robinson JG, Raal FJ, et al. Consistent LDL‐C response with evolocumab among patient subgroups in PROFICIO: a pooled analysis of 3146 patients from phase 3 studies. Clin Cardiol. 2018;41(10):1328–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Stroes E, Colquhoun D, Sullivan D, et al. GAUSS-2 Investigators. Anti-PCSK9 antibody effectively lowers cholesterol in patients with statin intolerance: the GAUSS-2 randomized, placebo-controlled phase 3 clinical trial of evolocumab. J Am Coll Cardiol. 2014;63(23):2541–2548. [DOI] [PubMed] [Google Scholar]
- 72.Rosenson RS, Jacobson TA, Preiss D, et al. Efficacy and safety of the PCSK9 inhibitor evolocumab in patients with mixed hyperlipidemia. Cardiovasc Drugs Ther. 2016;30(3):305–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bruckert E, Hayem G, Dejager S, et al. Mild to moderate muscular symptoms with high-dosage statin therapy in hyperlipidemic patients–the PRIMO study. Cardiovasc Drugs Ther. 2005;19(6):403–414. [DOI] [PubMed] [Google Scholar]
- 74.Sullivan D, Olsson AG, Scott R, et al. Effect of a monoclonal antibody to PCSK9 on low-density lipoprotein cholesterol levels in statin-intolerant patients: the GAUSS randomized trial. JAMA. 2012;308(23):2497–2506. [DOI] [PubMed] [Google Scholar]
- 75.Koba S, Inoue I, Cyrille M, et al. Evolocumab vs. ezetimibe in statin-intolerant hyperlipidemic Japanese patients: phase 3 GAUSS-4 trial. J Atheroscler Thromb. 2019;27(5):471–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nissen SE, Stroes E, Dent-Acosta RE, for the GAUSS-3 Investigators, et al. Efficacy and tolerability of evolocumab vs ezetimibe in patients with muscle-related statin intolerance: the GAUSS-3 randomized clinical trial. JAMA. 2016;315(15):1580–1590. [DOI] [PubMed] [Google Scholar]
- 77.Moriarty PM, Thompson PD, Cannon CP, ODYSSEY Alternative Investigators, et al. Efficacy and safety of alirocumab vs ezetimibe in statin-intolerant patients, with a statin rechallenge arm: The ODYSSEY ALTERNATIVE randomized trial. J Clin Lipidol. 2015;9(6):758–769. [DOI] [PubMed] [Google Scholar]
- 78.Ho L, Dent R, Stroes ESG, et al. Persistent safety and efficacy of evolocumab in patients with statin intolerance: a subset analysis of the OSLER open-label extension studies. Cardiovasc Drugs Ther. 2018;32(4):365–372. [DOI] [PubMed] [Google Scholar]
- 79.Bai J, Gong LL, Li QF, et al. Long-term efficacy and safety of proprotein convertase subtilisin/kexin 9 monoclonal antibodies: a meta-analysis of 11 randomized controlled trials. J Clin Lipidol. 2018;12(2):277–291. [DOI] [PubMed] [Google Scholar]
- 80.Koren MJ, Kereiakes D, Pourfarzib R, et al. Effect of PCSK9 inhibition by alirocumab on lipoprotein particle concentrations determined by nuclear magnetic resonance spectroscopy. J Am Heart Assoc. 2015;4(11)pii:e002224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Toth PP, Sattar N, Blom DJ, et al. Effect of evolocumab on lipoprotein particles. Am J Cardiol. 2018;121(3):308–314. [DOI] [PubMed] [Google Scholar]
- 82.Reyes-Soffer G, Pavlyha M, Ngai C, et al. Effects of PCSK9 inhibition with alirocumab on lipoprotein metabolism in healthy humans. Circulation. 2017;135(4):352–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Watts GF, Chan DC, Somaratne R, et al. Controlled study of the effect of proprotein convertase subtilisin-kexin type 9 inhibition with evolocumab on lipoprotein(a) particle kinetics. Eur Heart J. 2018;39(27):2577–2585. [DOI] [PubMed] [Google Scholar]
- 84.Gaudet D, Watts GF, Robinson JG, et al. Effect of alirocumab on lipoprotein(a) over ≥1.5 years (from the phase 3 ODYSSEY Program). Am J Cardiol. 2017;119(1):40–46. [DOI] [PubMed] [Google Scholar]
- 85.Gaudet D, Kereiakes DJ, McKenney JM, et al. Effect of alirocumab, a monoclonal proprotein convertase subtilisin/kexin 9 antibody, on lipoprotein(a) concentrations (a pooled analysis of 150 mg every two weeks dosing from phase 2 trials). Am J Cardiol. 2014;114(5):711–715. [DOI] [PubMed] [Google Scholar]
- 86.Raal FJ, Giugliano RP, Sabatine MS, et al. Reduction in lipoprotein(a) with PCSK9 monoclonal antibody evolocumab (AMG 145): a pooled analysis of more than 1,300 patients in 4 phase II trials. J Am Coll Cardiol. 2014;63(13):1278–1288. [DOI] [PubMed] [Google Scholar]
- 87.Bittner VA, Szarek M, Aylward PE, ODYSSEY OUTCOMES Committees and Investigators, et al. Effect of alirocumab on lipoprotein(a) and cardiovascular risk after acute coronary syndrome. J Am Coll Cardiol. 2020;75(2):133–144. [DOI] [PubMed] [Google Scholar]
- 88.Huijgen R, Fouchier SW, Denoun M, et al. Plasma levels of PCSK9 and phenotypic variability in familial hypercholesterolemia. J Lipid Res. 2012;53(5):979–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Li S, Zhu CG, Guo YL, et al. The relationship between the plasma PCSK9 levels and platelet indices in patients with stable coronary artery disease. J Atheroscler Thromb. 2015;22(1):76–84. [DOI] [PubMed] [Google Scholar]
- 90.Cheng JM, Oemrawsingh RM, Garcia-Garcia HM, et al. PCSK9 in relation to coronary plaque inflammation: results of the ATHEROREMO-IVUS study. Atherosclerosis. 2016;248:117–122. [DOI] [PubMed] [Google Scholar]
- 91.Li S, Li JJ.. PCSK9: a key factor modulating atherosclerosis. J Atheroscler Thromb. 2015;22(3):221–230. [DOI] [PubMed] [Google Scholar]
- 92.Nicholls SJ, Puri R, Anderson T, et al. Effect of evolocumab on progression of coronary disease in statin-treated patients: the GLAGOV randomized clinical trial. JAMA. 2016;316(22):2373–2384. [DOI] [PubMed] [Google Scholar]
- 93.Ako J, Hibi K, Tsujita K, et al. Effect of alirocumab on coronary atheroma volume in Japanese patients with acute coronary syndrome-The ODYSSEY J-IVUS Trial. Circ J. 2019;83(10):2025–2033. [DOI] [PubMed] [Google Scholar]
- 94.Ruscica M, Ferri N, Fogacci F, et al. Circulating levels of proprotein convertase subtilisin/kexin type 9 and arterial stiffness in a large population sample: data from the Brisighella heart study. J Am Heart Assoc. 2017;6(5):e005764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ricci C, Ruscica M, Camera M, et al. PCSK9 induces a pro-inflammatory response in macrophages. Sci Rep. 2018;8(1):2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cicero AFG, Toth PP, Fogacci F, et al. Improvement in arterial stiffness after short-term treatment with PCSK9 inhibitors. Nutr Metab Cardiovasc Dis. 2019;29(5):527–529. [DOI] [PubMed] [Google Scholar]
- 97.Maulucci G, Cipriani F, Russo D, et al. Improved endothelial function after short-term therapy with evolocumab. J Clin Lipidol. 2018;12(3):669–673. [DOI] [PubMed] [Google Scholar]
- 98.Sabatine MS, Giugliano RP, Keech AC, FOURIER Steering Committee and Investigators, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376(18):1713–1122. [DOI] [PubMed] [Google Scholar]
- 99.Schwartz GG, Steg PG, Szarek M, ODYSSEY OUTCOMES Committees and Investigators, et al. Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med. 2018;379(22):2097–2107. [DOI] [PubMed] [Google Scholar]
- 100.Sabatine MS, Giugliano RP, Wiviott SD, Open-Label Study of Long-Term Evaluation against LDL Cholesterol (OSLER) Investigators, et al. Efficacy and safety of evolocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372(16):1500–1509. [DOI] [PubMed] [Google Scholar]
- 101.Ray KK, Ginsberg HN, Davidson MH, et al. Reductions in atherogenic lipids and major cardiovascular events: a pooled analysis of 10 ODYSSEY trials comparing alirocumab with control. Circulation. 2016;134(24):1931–1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Navarese EP, Kolodziejczak M, Schulze V, et al. Effects of proprotein convertase subtilisin/kexin type 9 antibodies in adults with hypercholesterolemia: a systematic review and metaanalysis. Ann Intern Med. 2015; 163(1):40–51. [DOI] [PubMed] [Google Scholar]
- 103.Kaasenbrood L, Ray KK, Boekholdt SM, et al. Estimated individual lifetime benefit from PCSK9 inhibition in statin-treated patients with coronary artery disease. Heart. 2018;104(20):1699–1705. [DOI] [PubMed] [Google Scholar]
- 104.Ridker PM, Revkin J, Amarenco P, SPIRE Cardiovascular Outcome Investigators, et al. Cardiovascular efficacy and safety of bococizumab in high-risk patients. N Engl J Med. 2017;376(16):1527–1539. [DOI] [PubMed] [Google Scholar]
- 105.Sabatine MS, De Ferrari GM, Giugliano RP, et al. Clinical benefit of evolocumab by severity and extent of coronary artery disease. Circulation. 2018;138(8):756–766. [DOI] [PubMed] [Google Scholar]
- 106.Murphy SA, Pedersen TR, Gaciong ZA, et al. Effect of the PCSK9 inhibitor evolocumab on total cardiovascular events in patients with cardiovascular disease: a prespecified analysis from the FOURIER trial. JAMA Cardiol. 2019;4(7):613–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Giugliano RP, Keech A, Murphy SA, et al. Clinical efficacy and safety of evolocumab in high-risk patients receiving a statin: secondary analysis of patients with low LDL cholesterol levels and in those already receiving a maximal-potency statin in a randomized clinical trial. JAMA Cardiol. 2017;2(12):1385–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bonaca MP, Nault P, Giugliano RP, et al. Low-density lipoprotein cholesterol lowering with evolocumab and outcomes in patients with peripheral artery disease: insights from the FOURIER Trial (further cardiovascular outcomes research with PCSK9 inhibition in subjects with elevated risk). Circulation. 2018;137(4):338–350. [DOI] [PubMed] [Google Scholar]
- 109.Marston NA, Gurmu Y, Melloni GEM, et al. The effect of PCSK9 (proprotein convertase subtilisin/kexin type 9) inhibition on the risk of venous thromboembolism. Circulation. 2020;141(20):1600–1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bohula EA, Giugliano RP, Leiter LA, et al. Inflammatory and cholesterol risk in the FOURIER trial. Circulation. 2018;138(2):131–140. [DOI] [PubMed] [Google Scholar]
- 111.Cao YX, Li S, Liu HH, et al. Impact of PCSK9 monoclonal antibodies on circulating hs-CRP levels: a systematic review and meta-analysis of randomised controlled trials. BMJ Open. 2018;8(9):e022348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ridker PM, Danielson E, Fonseca FA, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359(21):2195–2207. [DOI] [PubMed] [Google Scholar]
- 113.Laufs U, Banach M, Mancini GBJ, et al. Efficacy and safety of bempedoic acid in patients with hypercholesterolemia and statin intolerance. J Am Heart Assoc. 2019;8(7):e011662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bergmark B, O'Donoghue ML, Murphy S. PCSK9 inhibition and aortic stenosis in the FOURIER trial. Proceedings of American College of Cardiology 2020 Scientific Session (ACC.20)/World Congress of Cardiology (WCC); Mar 28–30; Chicago, Washington: ACC. 2020. [Google Scholar]
- 115.Langsted A, Nordestgaard BG, Benn M, et al. PCSK9 R46L loss-of-function mutation reduces lipoprotein(a), LDL cholesterol, and risk of aortic valve stenosis. J Clin Endocrinol Metab. 2016;101(9):3281–3287. [DOI] [PubMed] [Google Scholar]
- 116.Szarek M, White HD, Schwartz GG, ODYSSEY OUTCOMES Committees and Investigators, et al. Alirocumab reduces total nonfatal cardiovascular and fatal events: the ODYSSEY OUTCOMES Trial. J Am Coll Cardiol. 2019;73(4):387–396. [DOI] [PubMed] [Google Scholar]
- 117.Szarek M, Steg PG, DiCenso D, et al. Alirocumab reduces total hospitalizations and increases days alive and out of hospital in the ODYSSEY OUTCOMES trial. Circ Cardiovasc Qual Outcomes. 2019;12(11):e005858. [DOI] [PubMed] [Google Scholar]
- 118.Goodman SG, Aylward PE, Szarek M, ODYSSEY OUTCOMES Committees and Investigators, et al. Effects of alirocumab on cardiovascular events after coronary bypass surgery. J Am Coll Cardiol. 2019;74(9):1177–1186. [DOI] [PubMed] [Google Scholar]
- 119.Schwartz GG, Steg PG, Szarek M, ODYSSEY OUTCOMES Committees and Investigators*, et al. Peripheral artery disease and venous thromboembolic events after acute coronary syndrome: role of lipoprotein(a) and modification by alirocumab: prespecified analysis of the ODYSSEY OUTCOMES randomized clinical trial. Circulation. 2020;141(20):1608–1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Koren MJ, Sabatine MS, Giugliano RP, et al. Long-term efficacy and safety of evolocumab in patients with hypercholesterolemia. J Am Coll Cardiol. 2019;74(17):2132–2146. [DOI] [PubMed] [Google Scholar]
- 121.Ridker PM, Tardif JC, Amarenco P, SPIRE Investigators, et al. Lipid-reduction variability and antidrug-antibody formation with bococizumab. N Engl J Med. 2017;376(16):1517–1526. [DOI] [PubMed] [Google Scholar]
- 122.Kuriakose A, Chirmule N, Nair P.. Immunogenicity of biotherapeutics: causes and association with posttranslational modifications. J Immunol Res. 2016;2016:1298473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Roth EM, Goldberg AC, Catapano AL, et al. Antidrug antibodies in patients treated with alirocumab. N Engl J Med. 2017;376(16):1589–1590. [DOI] [PubMed] [Google Scholar]
- 124.Giugliano RP, Mach F, Zavitz K, EBBINGHAUS Investigators, et al. Cognitive function in a randomized trial of evolocumab. N Engl J Med. 2017;377(7):633–643. [DOI] [PubMed] [Google Scholar]
- 125.Benn M, Nordestgaard BG, Frikke-Schmidt R, et al. Low LDL cholesterol, PCSK9 and HMGCR genetic variation, and risk of Alzheimer's disease and Parkinson's disease: Mendelian randomisation study. BMJ. 2017;357:j1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Chen Q, Wu G, Li C, et al. Safety of proprotein convertase subtilisin/kexin type 9 monoclonal antibodies in regard to diabetes mellitus: a systematic review and meta-analysis of randomized controlled trials. Am J Cardiovasc Drugs. 2019. [DOI] [PubMed] [Google Scholar]
- 127.Colhoun HM, Ginsberg HN, Robinson JG, et al. No effect of PCSK9 inhibitor alirocumab on the incidence of diabetes in a pooled analysis from 10 ODYSSEY phase 3 studies. Eur Heart J. 2016;37(39):2981–2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Blom DJ, Koren MJ, Roth E, et al. Evaluation of the efficacy, safety and glycaemic effects of evolocumab (AMG 145) in hypercholesterolaemic patients stratified by glycaemic status and metabolic syndrome. Diabetes Obes Metab. 2017;19(1):98–107. [DOI] [PubMed] [Google Scholar]
- 129.Sattar N, Toth PP, Blom DJ, et al. Effect of the proprotein convertase subtilisin/kexin type 9 inhibitor evolocumab on glycemia, body weight, and new-onset diabetes mellitus. Am J Cardiol. 2017;120(9):1521–1527. [DOI] [PubMed] [Google Scholar]
- 130.Sabatine MS, Leiter LA, Wiviott SD, et al. Cardiovascular safety and efficacy of the PCSK9 inhibitor evolocumab in patients with and without diabetes and the effect of evolocumab on glycaemia and risk of new-onset diabetes: a prespecified analysis of the FOURIER randomised controlled trial. Lancet Diabetes Endocrinol. 2017;5(12):941–950. [DOI] [PubMed] [Google Scholar]
- 131.Blom DJ, Djedjos CS, Monsalvo ML, et al. Effects of evolocumab on vitamin E and steroid hormone levels: results from the 52-week, phase 3, double-blind, randomized, placebo-controlled DESCARTES Study. Circ Res. 2015;117(8):731–741. [DOI] [PubMed] [Google Scholar]
- 132.Kazi DS, Moran AE, Coxson PG, et al. Cost-effectiveness of PCSK9 inhibitor therapy in patients with heterozygous familial hypercholesterolemia or atherosclerotic cardiovascular disease. JAMA. 2016;316(7):743–753. [DOI] [PubMed] [Google Scholar]
- 133.Arrieta A, Page TF, Veledar E, et al. Economic evaluation of PCSK9 inhibitors in reducing cardiovascular risk from health system and private payer perspectives. PLOS One. 2017;12(1):e0169761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Arbel R, Hammerman A, Azuri J.. Usefulness of ezetimibe versus evolocumab as add-on therapy for secondary prevention of cardiovascular events in patients with type 2 diabetes mellitus. Am J Cardiol. 2019;123(8):1273–1276. [DOI] [PubMed] [Google Scholar]
- 135.Korman MJ, Retterstøl K, Kristiansen IS, et al. Are PCSK9 inhibitors cost effective? Pharmacoeconomics. 2018;36(9):1031–1041. [DOI] [PubMed] [Google Scholar]
- 136.Brunetti ND, De Gennaro L, Tricarico L, et al. Budget impact analysis of PCSK9 inhibitors costs from a community payers' perspective in Apulia, Italy. Open Heart. 2019;6(2):e001018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Robinson JG, Jayanna MB, Brown AS, et al. Enhancing the value of PCSK9 monoclonal antibodies by identifying patients most likely to benefit. A consensus statement from the National Lipid Association. J Clin Lipidol. 2019;13(4):525–‐537. [DOI] [PubMed] [Google Scholar]