Abstract
Context
Fructus Meliae toosendan extracts (FMTE) have a good therapeutic effect on coccidiosis, but there is no relevant research on its prophylactic effect on coccidiosis.
Objective
This study comprehensively evaluates the anticoccidial effect of FMTE.
Materials and methods
In vitro, the unsporulated oocysts were treated with serial dilutions of FMTE and incubated for 7 d, and the sporulated oocysts were counted for calculating the median lethal concentration (LC50) of FMTE. In vivo, 180 10-day-old broiler chickens free of coccidiosis were weighted and randomly distributed into six groups: normal group, untreated group, 4 protective groups (positive group and three FMTE groups). From day 10 to day 21, chickens in the three FMTE groups were pre-treated with FMTE at the dosage of 2.5, 5 and 10 g/kg/d, respectively, and chickens in the positive group were pre-treated with qiuliling (10 g/kg/d). On day 14, chickens in all groups except the normal group were orally infected with 1.5 × 104 sporulated oocysts. The clinical symptoms were observed from day 10 to day 21, the anticoccidial index (ACI), tissue lesions, and intestinal microflora were determined on day 21.
Results
FMTE showed anti-sporulation effect against E. tenella and the LC50 value was 245.83 µg/mL in vitro. In vivo, FMTE at the dosage of 10 g/kg/d was effective against E. tenella infection, and its ACI value was 162.56, which was higher than the value of positive drug qiuliling (128.81).
Discussion and conclusions: FMTE have potent anticoccidial effects, and it presents an alternative anticoccidial agent for avian coccidiosis control.
Keywords: Avian coccidiosis, anticoccidial drug, intestinal microflora
Introduction
Avian coccidiosis is a major intracellular parasitic disease caused by the genus Eimeria (Eimeriidae), leading to tremendous economic losses of poultry worldwide (Allen and Fetterer 2002; Dalloul and Lillehoj 2006). The life cycle of E. tenella is complex. It starts from the exogenous stage of unsporulated oocysts shedding in faeces, then sporulation and infection. In the endogenous phase, when the environmentally resistant oocysts infect chickens, the haploid sporozoites are released from sporocysts contained within each oocyst (Sharman et al. 2010), and subsequently invade intestinal epithelial cells. Eventually, the final generation of merozoites differentiates into either male or female microgametes and release from the host cells, and male gametes invade and fuse with intracellular female gametes to form zygotes. Zygotes mature into oocysts within the gut and are excreted into faeces (Kinnaird et al. 2004). Intestinal colonization can cause damage to the intestinal tract and caecum, which decreases feed conversion, leading to lower productivity and performance. Moreover, coccidiosis also causes the disbalance of intestinal microflora, such as increasing Enterobacteriaceae abundance, decreasing Bacillales and Lactobacillales abundance, and weakening the immune function, even boosting the susceptibility to secondary bacterial infections (Morris et al. 2007; Shirley et al. 2007; Tian et al. 2014; MacDonald et al. 2017).
Chemoprophylaxis, live vaccines and feed additives play an important role in the control of coccidiosis, but the problem of drug resistance has been commonly detected, due to the continuous use and abuse of anticoccidial drugs (Chapman 1999; Abbas et al. 2011). Consequently, there is a constant demand for safe and efficient innovative products.
It has been reported that many plants and their extracts had therapeutic effects on broiler chickens infected with E. tenella. For example, infected chickens treated with Musa paradisiaca L. (Musaceae) root extract, Artemisia annua Linn (Asteraceae) extract, herbal compound (leaves of Azadirachta indica A. Juss (Meliaceae) and Nicotiana tabacum Linn (Solanaceae), flowers of Calotropis procera (Aiton) W. T. Aiton (Apocynaceae), and seeds of Trachyspermum ammi (L.) Sprague) (Apiaceae) or guar meal may reduce the mortality and diarrhoea rate, improve the lesion score and production performance, and decrease the number of oocysts per gram (OPG) (Hassan et al. 2008; Anosa and Okoro 2011; Zaman et al. 2012; Kaboutari et al. 2014). Meanwhile, with the discovery of more cases of Chinese herbal medicines, as well as the advantages of their anticoccidial effects, the use of herbal medicines and natural products have become more and more popular with consumers in the domestic poultry industry (Hassan et al. 2008; Orengo et al. 2012).
Fructus Meliae toosendan, a traditional Chinese herbal medicine, is the fruit of Meliae toosendan Sieb et Zucc. (Meliaceae). Fructus Meliae toosendan extracts (FMTE) has been generally used as an insecticidal and medicinal plant to treat ascariasis, stomachache and inflammation (Xie et al. 2008). Thus, this study evaluates the anticoccidial effects of FMTE through prophylactic treatment, and provides a theoretical basis for further clinical trials.
Materials and methods
The preparation of FMTE
Dry powders of FMT (Sichuan Jinlingzi Biotechnology Co, Ltd, Suining, China) (200 g) were extracted with 2000 mL 70% ethanol by heated reflux, the same powder extracted twice (2 h per time). The combined extraction was then filtered and concentrated using a rotary evaporator (Yarong Biochemical Instrument Factory, Shanghai, China), and the final extract was stored in the dark at −20 °C.
Qiuliling (batch number: (2014)100205090) was purchased from the Jiangsu wei tai long Biotechnology Co, Ltd (Jiangsu, China).
Animals and parasites
Broiler chickens (1-day-old), free of coccidiosis, were bought from Guangdong Xinguang Nongmu Co, Ltd (Chengdu, China). The chickens were fed in clean wire cages under a hygienically controlled coccidian-free environment and provided with feed and water without any antibiotics. Chickens were killed by cervical dislocation. The entire procedures were executed as rapidly and painlessly as possible.
All procedures involving animals and their care in this study were approved by the Ethics Committee of Sichuan Agricultural University according to the Regulation of Experimental Animal Management (State Scientific and Technological Commission of the People’s Republic of China, No. 2, 1988) and the Interim Measures of Sichuan Province Experimental Animal Management (Science and Technology Bureau of Sichuan, China, No. 25, 2013).
The sporulated oocysts of E. tenella were provided by the China Agricultural University parasites teaching and research office (Beijing, China). Fourteen 2-week-old coccidian-free chickens were inoculated with 1 × 104 sporulated oocysts of E. tenella, and chickens caecal content were collected and soaked in water on the seventh day post-infection. The mixture was homogenized, then filtered through a fine sieve. The filtrate was centrifuged at 200 g for 3 min. The supernatant was discarded and the sediment was resuspended in saturated solution of sodium chloride and centrifuged at 200 g for 3 min. The supernatant was removed, mixed with water and kept overnight. Discarding the supernatant, and the oocysts were collected for in vitro assay. In vivo assay, the oocysts were stored in 2.5% potassium dichromate solution to induce sporulation at 28 °C for 72 h, and the sporulated oocysts were used to infect chickens.
LC50 assay
FMTE was dissolved in 4% dimethyl sulfoxide (DMSO) solution to give serial 2-fold dilutions (400, 200, 100, 50, 25, 12.5, and 6.25 µg/mL). The 4% DMSO, 25% ammonium hydroxide and 2.5% potassium dichromate were used as the normal, positive, and untreated groups, respectively, and each group was mixed with 1.0 × 106 unsporulated oocysts. After incubated at 27 °C for 7 d, the supernatant was removed by centrifugation and oocysts were washed 3 times with distilled water. In order to activate sporulation, oocysts were suspended in 2.5% (w/v) potassium dichromate for 72 h and then the numbers of sporulated oocysts were counted using a Neubauer haemocytometer (BOECO) (Williams 1997). The LC50 values were calculated by regression analysis (Zaman et al. 2015).
In vivo test
At 10 days of age, 180 10-day-old chickens free of coccidiosis were weighed and randomly distributed into six groups of 30 chickens each. From day 10 to day 22, the chickens in the FMTE-treated groups (L, M and H) were administrated with FMTE at the dosage of 2.5, 5, and 10 g/kg/d, respectively, through mixing with feed. Qiuliling composed of Artemisia annua herba, Agrimoniae herba, Polygoni multiflori radix, Pulsatillae radix and Cinnamomi cortex, also be called Jiqiuchong san, was an anticoccidiosis drug, which was recoded in Chinese Veterinary Pharmacopoeia (2005 Edition) (Commission 2005; Guo et al. 2013). Therefore, qiuliling was selected as positive control. The dosage of qiuliling was 10 g/kg/d. In the normal group and the untreated group, the chickens were administrated with equal weight of normal feed. On day 14, chickens in other groups except the normal group were orally infected with 1.5 × 104 sporulated oocysts. Clinical symptoms, anticoccidial index, tissue lesions and intestinal microflora were determined during the experiment or the end of the experiment.
Evaluation of anticoccidial effect
ACI was used to evaluate the anticoccidial efficacy. [ACI= (relative ratio of body weight gains + survival rate) - (oocyst value + median lesion scores)]. The ACI value below 120 means no anticoccidial efficiency; the value between 120 and 160 represents low efficiency; the value between 160 and 180 means medium efficiency; the value above 180 represents high efficiency on anticoccidiosis (De Pablos et al. 2010).
On day 22, the survival rate of each group was calculated and chickens were weighed to determine the relative growth ratio. Meanwhile, all chickens were sacrificed to determine the caecum lesions score of each group according to the standard assay (Johnson and Reid 1970). Based on the macroscopic general appearance of petechiae, thick or shrunk intestinal walls and bloody caecal contents, the lesion scores were ranked from 0 (absence of lesions) to 4 (extremely severe lesions and dead). The number of oocysts was counted by McMaster method (Hodgson 1970), the OPG value from 0 to 40, where 0, 5, 10, 20, 30 and 40 represented the number of OPG (×106) was 0 ∼ 0.1, 0.11 ∼ 1.0, 1.1 ∼ 1.9, 2.0 ∼ 5.9, 6.0 ∼ 10.9, and ≥11.0, respectively. The bloody diarrhoea scores of each group were recorded from day 4 to 7, and the scores were from 0 (normal) to 4 (complete bloody diarrhoea).
Histopathological examination
On day 22, part of the organs preserved in 4% paraformaldehyde from all the groups was subjected to histological examination. These organs were dehydrated with different concentrations of ethanol, and embedded in paraffin. Tissues were cut into thickness of 5-μm and stained with haematoxylin and eosin (HE). Each section was observed under an optical microscope.
Intestinal microflora analyses
On day 22, caecal contents were collected and frozen at −80 °C for further microbiota analysis. Bacterial DNA was extracted from the caecal digest medium according to the instructions of QIAamp DNA Stool Mini Kit (Qiagen Inc., Valencia, CA). The V3∼V4 region of the bacterial 16S rRNA gene was amplified with the barcoded primer pair 341 F/805R. After amplification, PCR products were analysed on a 2% agarose gel and purified using a QIAquick Gel Extraction Kit (Qiagen, Hilden, Germany). Pyrosequencing of 16S rDNA was performed on an Illumina HiSeq2500 PE250 platform at Beijing Baimaike Biotechnology Co, Ltd (Beijing, China).
Statistical analyses
The results were expressed as the mean ± standard deviation (M ± SD). The statistical significance was compared between the control and experimental groups by one-way analysis of variance (ANOVA) followed by the Student–Newman–Keuls test. Statistical difference was at the level of p < 0.05.
Results
Anticoccidial effects of FMTE in vitro
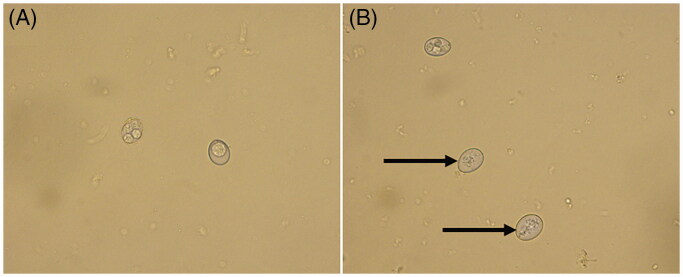
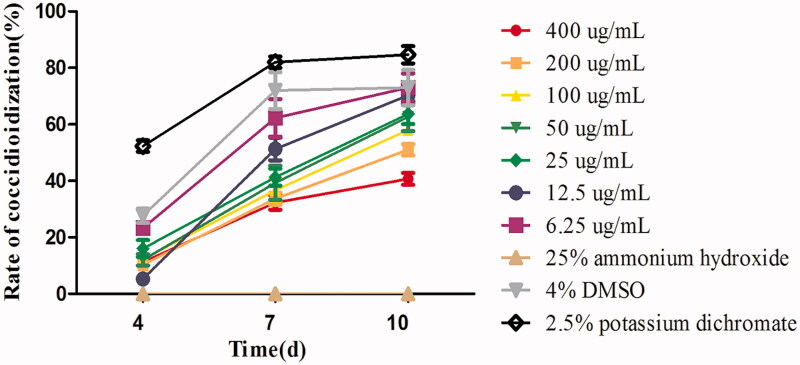
Compared with the normal group (Figure 1(A)), the internal structures of most oocysts after FMTE treatment (400 µg/mL) on 7d were damaged (Figure 1(B)). On days 4, 7, and 10, the rate of sporulated oocysts was determined (Figure 2). As the concentration of FMTE decreased, the rate of sporulated oocysts increased generally. FMTE at the concentration of 6.25 µg/mL had the highest percentage of sporulated oocysts.
Figure 1.
Photomicrographs of oocysts of E. tenella. (A) Morphology of oocysts in the normal group. (B) Morphology of oocysts after FMTE treatment (400 µg/mL) for 7 d, the internal structures of coccidioides were damaged (→).
Figure 2.
The sporulation rate of E. tenella under different concentrations of FMTE on days 4, 7, and 10. The 4% DMSO, 2.5% potassium dichromate and 25% ammonium hydroxide were used as the normal group, the untreated group and the positive group, and others group were different doses of FMTE groups.
After the treatment with 6.25 µg/mL FMTE, the percentage of sporulated oocysts on days 4, 7, and 10 was 23.33%, 62.33% and 73% respectively. Compared with 2.5% potassium dichromate (84.67%) and 6.25 µg/mL FMTE (73%), the rate of sporulated oocysts of 400 µg/mL FMTE (40.67%) was significantly decreased (p < 0.05). Thus, the high dose of FMTE strongly inhibited sporulation of oocyst, and the LC50 value was 245.83 µg/mL.
Anticoccidial effects of FMTE in vivo
Clinical signs
Chickens in the normal group were healthy and have no bloody faeces throughout the experiment. Compared with the normal group, chickens in the FMTE-H group showed slight listlessness and bloody diarrhoea, and chickens in the FMTE-L group, the FMTE-M group and the qiuliling-treated group showed severe coccidiosis clinical signs, such as weakness, listlessness, decreased appetites, ruffled feather, head fall, inability to stand and deaths. All chickens were euthanized by cervical dislocation at the end of the experiment, the caecum was examined to evaluate the lesion score immediately. The results are shown in Table 1. The most serious caecal injury was found in the untreated group, and the degree of caecal lesions in the pre-treated groups was alleviated. Compared with other pre-treated groups, the FMTE-H group (10 g/kg/d) had the best anticoccidial effects, it can significantly reduce the degree of caecal damage (p < 0.05).
Table 1.
Caecal lesion values (n = 3).
| Group | Lesion value |
|---|---|
| FMTE-L | 30e |
| FMTE-M | 20c |
| FMTE-H | 15b |
| Qiuliling | 25d |
| Untreated | 35f |
| Normal | 0a |
Chickens in the FMTE-treated groups (L, M and H) were administrated with FMTE at the dosage of 2.5, 5, and 10 g/kg/d, respectively, through mixing with feed, and the chickens in the qiuliling-treated group (positive control) were administrated with qiuliling at the dosage of 10 g/kg/d. Different letters mean significant difference (p < 0.05).
Bloody diarrhoea
Bloody diarrhoea was observed and recorded from day 4 to day 8 after chickens infected with E. tenella, and all infected chickens emerged bloody diarrhoea (Table 2). Compared with the pre-treated groups, the chickens with severe bloody diarrhoea were observed in the untreated group, which had the highest bloody diarrhoea scores (p < 0.05).
Table 2.
Bloody diarrhoea scores (n = 3).
| Group | Bloody diarrhoea scores |
||||
|---|---|---|---|---|---|
| 4d | 5d | 6d | 7d | 8d | |
| FMTE-L | 1 | 1 | 2 | 2 | 1b |
| FMTE-M | 1 | 2 | 2 | 1 | 2c |
| FMTE-H | 1 | 1 | 1 | 1 | 1b |
| Qiuliling | 1 | 2 | 1 | 1 | 1b |
| Untreated | 1 | 2 | 2 | 2 | 2c |
| Normal | 0 | 0 | 0 | 0 | 0a |
Chickens in the FMTE-treated groups (L, M and H) were administrated with FMTE at the dosage of 2.5, 5, and 10 g/kg/d, respectively, through mixing with feed, and the chickens in the qiuliling-treated group (positive control) were administrated with qiuliling at the dosage of 10 g/kg/d. Same letter means no significant difference (p > 0.05), different letters mean significant difference (p < 0.05).
Oocyst value
The faeces were collected and oocyst value was evaluated from day 4 to day 8 after chickens were infected by E. tenella (Table 3). The results were in consistent with bloody diarrhoea scores. The oocyst value of the untreated group was significantly higher than other groups (p < 0.05), but there was no significant difference between the pre-treated groups (p > 0.05).
Table 3.
Oocyst value (n = 3).
| Group | Oocyst value |
||||
|---|---|---|---|---|---|
| 4d | 5d | 6d | 7d | 8d | |
| FMTE-L | 5 | 0 | 0 | 0 | 5b |
| FMTE-M | 5 | 0 | 0 | 0 | 5b |
| FMTE-H | 0 | 5 | 0 | 0 | 5b |
| Qiuliling | 0 | 0 | 0 | 0 | 5b |
| Untreated | 5 | 5 | 5 | 5 | 20c |
| Normal | 0 | 0 | 0 | 0 | 0a |
Chickens in the FMTE-treated groups (L, M and H) were administrated with FMTE at the dosage of 2.5, 5, and 10 g/kg/d, respectively, through mixing with feed, and the chickens in the qiuliling-treated group (positive control) were administrated with qiuliling at the dosage of 10 g/kg/d. Same letter means no significant difference (p > 0.05), different letters mean significant difference (p < 0.05).
Anticoccidial index
Compared with the normal group, coccidiosis lead to the relative weight gain rate decreased (Table 4). Compared with the untreated group, the relative weight gain rate of the FMTE-treated group was 87.56%, which was higher than the qiuliling-treated group (63.81%). Meanwhile, the gross lesion score and oocyst value were declined after pre-treatment with FMTE. The change of the ACI value in the FMTE-treated group was dose-dependent. The ACI value of the FMTE-H group (10 g/kg/d) and the qiuliling-treated group was 162.56 and 128.81, respectively. The prophylactic effects of FMTE at the dosage of 10 g/kg/d on avian coccidiosis was superior to that of the positive drug qiuliling.
Table 4.
The anticoccidial index.
| Group | Average initial weight/g | Average final weight/g | Relative weight gain rate/% | Gross lesions score | oocyst value | Survival rate/% | ACI | Therapeutic evaluation |
|---|---|---|---|---|---|---|---|---|
| FMTE-L | 124.44 | 142.33 | 44.38 | 30 | 5 | 80 | 89.38 | No |
| FMTE-M | 121.24 | 152.67 | 77.95 | 20 | 5 | 90 | 142.95 | Low |
| FMTE-H | 129.02 | 164.33 | 87.56 | 15 | 5 | 95 | 162.56 | Medium |
| Qiuliling | 127.28 | 153.01 | 63.81 | 25 | 5 | 95 | 128.81 | Low |
| Untreated | 129.85 | 158.16 | 70.22 | 35 | 20 | 70 | 85.22 | No |
| Normal | 127.54 | 167.86 | 100.00 | 0 | 0 | 100 | – | – |
Chickens in the FMTE-treated groups (L, M and H) were administrated with FMTE at the dosage of 2.5, 5, and 10 g/kg/d, respectively, through mixing with feed, and the chickens in the qiuliling-treated group (positive control) were administrated with qiuliling at the dosage of 10 g/kg/d.
Histological examinations
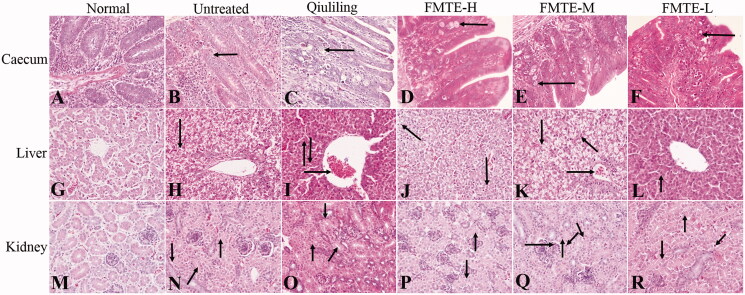
The structure of the caecum in the normal group was normal (Figure 3(A)), while the caecum in the untreated group had serious lesions. Due to the large number of oocysts in the intestinal mucosa, the intestinal gland was disorganized (Figure 3(B)). Compared with the untreated group, the number of oocysts in the intestinal mucosa of the pre-treated groups was decreased (Figure 3(C–F)), and that of the FMTE-H group was the least (Figure 3(D)). In the normal group, the appearance of the liver was normal (Figure 3(G)). In the untreated group, the hepatocytes were swollen, and serious vacuolar degeneration, disappeared hepatic cord and narrowed hepatic sinuses were observed (Figure 3(H)). In the pre-treatment group, there were varying degrees of granular and vacuolar degeneration (Figure 3(I–L)). In the FMTE-H group, mild vacuolar degeneration of hepatocytes and inflammatory cell infiltration were found in the marginal area of hepatic sinuses (Figure 3(J)). In the normal group, the structure of kidney was normal (Figure 3(M)), but in the untreated group, the epithelial cells of renal tubules were swollen, even with severe granular degeneration and vacuole degeneration, which resulted in the narrowing of renal tubules and the shedding of cells in the tubules (Figure 3(N)). In the pre-treatment group, there were different degrees of granular and vacuolar degeneration and inflammatory cell infiltration in kidney (Figure 3(O–R)). Compared with other pre-treatment groups, the appearance of kidney was normal, and the epithelial cells of renal tubules had slight granular degeneration and vacuole degeneration in the FMTE-H group (Figure 3(P)).
Figure 3.
The pathological lesions of tissues in the different groups. (←) in (B)–(F) indicated oocysts in the intestinal mucosa; (→) in (I), (K) and (Q) indicated congestion; (↓) in (H)–(K), (N)–(P) and (R) indicated vacuolar degeneration ; (↑) in (I), (L) and (N)–(R) indicated granular degeneration in renal tubular epithelial cells; (↖) in (J) and (K) indicated inflammatory cell infiltrated; (↗) in (N) and (O) indicated the tubular lumen of renal tubules was narrowed; (↙) in (Q) and (R) indicated eosinophils infiltration; (↘) in (Q) indicated neutrophil infiltration.
Intestinal microflora
The microbiota diversity
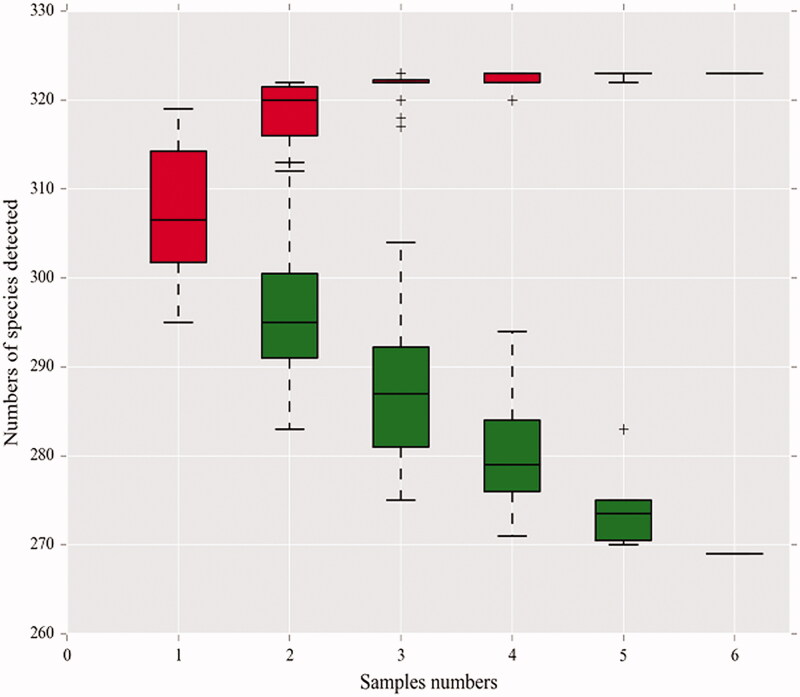
As the number of samples increased, the species accumulation curve of this test tended to be stable, indicating that the samples were qualified and could be used for subsequent tests (Figure 4).
Figure 4.
Species accumulation curves in the genus-level. A single red box reflects the total number of species in the sample, and the total red box forms a cumulative curve, reflecting the rate of new species emergence under continuous sampling; a single green box reflects the number of species shared in the sample. The total green box forms a common population curve, which reflects the rate of occurrence of common species in the sample under continuous sampling.
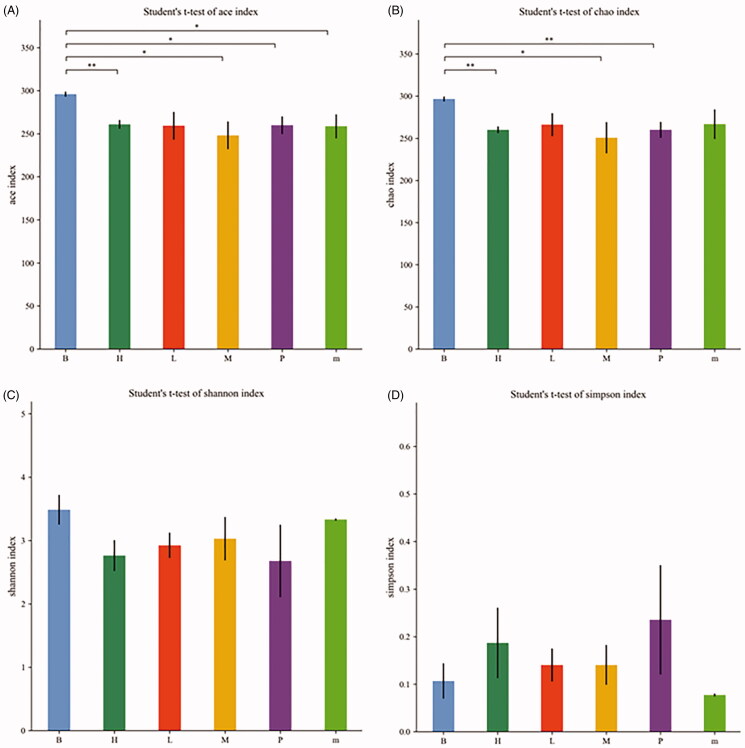
The alpha diversity analysis included the ACE, Chao, Shannon and Simpson indexes, which was intended to be representative of the species richness and species diversity (Figure 5). The ACE, Chao and Shannon indexes of the normal group were higher than those of the other treatment groups, but the Simpson index was lower than that of the other treatment groups, indicating that the species diversity of the normal group was higher. The ACE and Chao indexes of the FMTE-H group were similar to those of the untreated group. However, the ACE and Chao indexes of the FMTE-H group had significantly difference from those of the normal group (p < 0.01).
Figure 5.
The alpha diversity analysis of all groups. (A) Ace index; (B) Chao index; (C) Shannon index; (D) Simpson index. ‘B’ represented the normal group; ‘m’ represented the untreated group; Chickens in the group P were administrated with qiuliling at the dosage of 10 g/kg/d; Chickens in the FMTE-treated groups (L, M and H) were administrated with FMTE at the dosage of 2.5, 5, and 10 g/kg/d, respectively, through mixing with feed. Note: ‘*’ mean significant difference (p < 0.05), ‘**’ mean highly significant difference (p < 0.01).
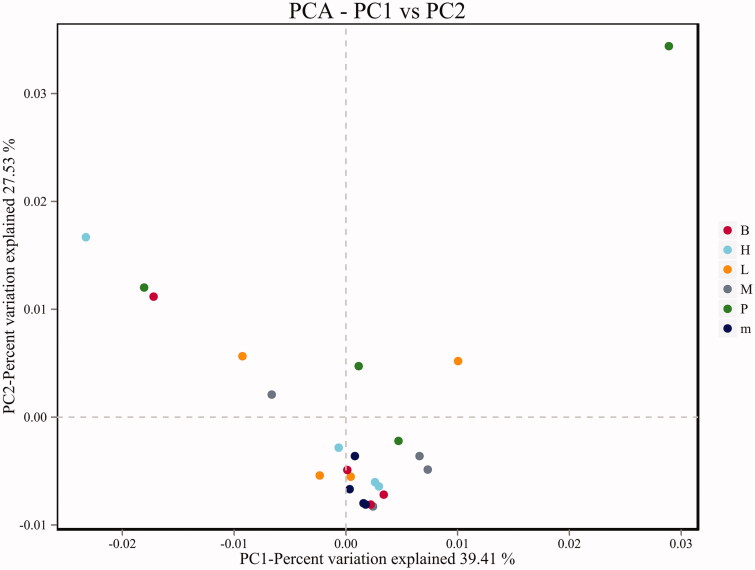
Principal component analysis (PCA) was included of the beta diversity analysis, which reflects the differences and distances between samples (Figure 6). There was no significant separation of PCA points between the normal group and the untreated group, but the principal component points of the FMTE-H group were closer to that of the normal group, indicating that the FMTE-H group had the best regulatory effect. However, the PCA points of some groups were discrete, which may be caused by the special physiological conditions of individual chickens.
Figure 6.
PCA analysis chart. ‘B’ represented the normal group; ‘m’ represented the untreated group; Chickens in the group P were administrated with qiuliling at the dosage of 10 g/kg/d; Chickens in the FMTE-treated groups (L, M and H) were administrated with FMTE at the dosage of 2.5, 5, and 10 g/kg/d, respectively, through mixing with feed. The points of the same colour indicated different samples in the same group. The closer the different points were, the more similar the principal components were; the farther away they were, the more different the principal components were.
Comparison of microbiota in the caecum
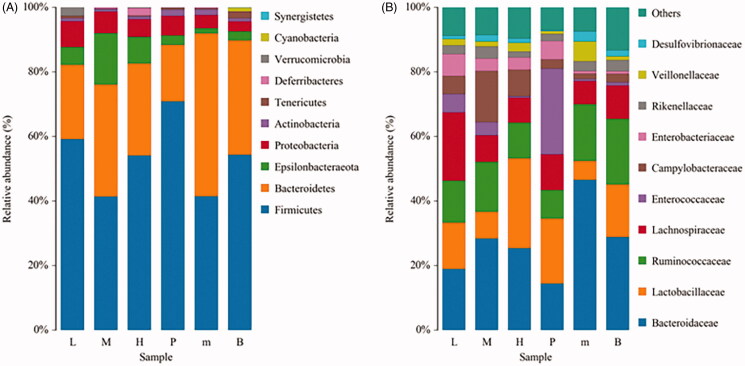
Histogram of species distribution showed the ratio of different species. As the results shown in Figure 7, the proportion of intestinal flora in the different groups was inconsistent. After infection with E. tenella, the proportion of Bacteroidetes in the untreated group was relatively increased, while the proportion of Firmicutes and Epsilonbacteraeota was decreased in the phylum level. Compared with the untreated group, the proportion of these bacteria in the FMTE-H group was increased and even close to the level of normal group (Figure 7(A)). In the family level, the proportion of Bacteroides in the untreated group was relatively increased, while the proportion of Lactobacillus and Ruminococcaceae was decreased. Compared with the untreated group, the proportion of these bacteria in the FMTE-H group was increased and even relatively close to that of the normal group (Figure 7(B)).
Figure 7.
Analysis of the abundance of bacteria in chicken faeces. (A) In the phylum level; (B) In the family level. ‘B’ represented the normal group; ‘m’ represented the untreated group; Chickens in the group P were administrated with qiuliling at the dosage of 10 g/kg/d; Chickens in the FMTE-treated groups (L, M and H) were administrated with FMTE at the dosage of 2.5, 5, and 10 g/kg/d, respectively, through mixing with feed. The same colour in each diagram represented the same bacteria, and different areas represented different proportions.
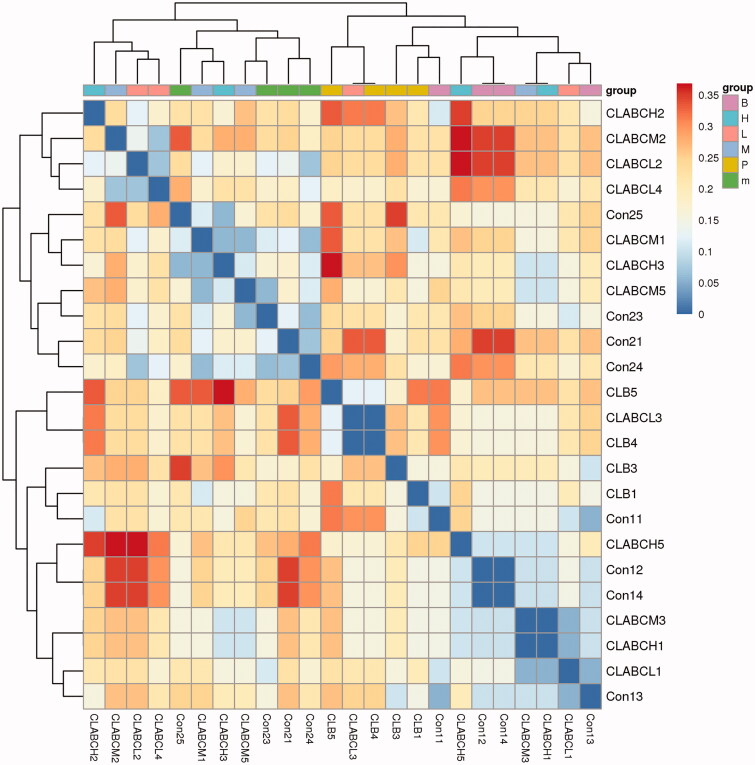
The heatmap in the different groups was shown in Figure 8. The close distance between the normal group and the normal group indicated that the intestinal flora was similar, while the close distance between the FMTE-H group and the normal group indicated that the microbial composition of the FMTE-H group was close to that of the normal group.
Figure 8.
Heatmap analysis. Con1 and con2 represented the normal group and the untreated group. Chickens in the group CLB were administrated with qiuliling at the dosage of 10 g/kg/d. Chickens in the groups CLABCL, CLABCM and CLABCH were administrated with FMTE at the dosage of 2.5, 5, and 10 g/kg/d, respectively, through mixing with feed. The same colour in the figure represented different samples in the same group, and the closer they were to each other, the more similar the colony composition were.
Discussion
It was reported that many plants could inhibit the sporulation of oocysts of E. tenella (Molan et al. 2009; Del Cacho et al. 2010). In this study, FMTE could decrease the ratio of sporulated oocysts and increase the proportion of degenerated oocysts. These results indicated that FMTE have strong inhibitory properties against oocysts sporulation. Meanwhile, the destruction of the internal structure of E. tenella oocysts was observed under an optical microscope, suggesting that FMTE may degrade the contents of oocysts and destroy the structure of oocysts. The similar phenomenon had been observed in the previous findings that the oocysts pre-treated with pine bark extract could maintain their original appearance, but the sporozoites were distorted into abnormal shapes (Shanker et al. 2004).
FMTE is rich in triterpenoids (Wu et al. 2010), steroids (Hu et al. 2018) and limonoids (Zhang et al. 2014; Hu et al. 2011). These compounds confer a variety of biological activities on the plant. In this study, our data confirmed that the prophylactic effects of FMTE at the dosage of 10 g/kg/d on avian coccidiosis was superior to that of the positive drug qiuliling. Reducing bleeding could keep the chickens infected with E. tenella from bacterial infection, even inflammatory response (Bozkurt et al. 2013). In vivo assay, FMTE relieved the degree of bloody diarrhoea and the output of oocysts and enhanced the relative weight gain rate, so as to improve the anticoccidial effect. To some extent, these results were similar to the anticoccidial effect of Artemisia herba-alba Asso, which have a good anticoccidial effect, especially reducing the output of oocysts and the mortality of animals (Messaï et al. 2014). The caecum is one of the most important digestive organs in chickens, and it plays an important role in improving the conditions of the chickens infected with E. tenella (Wunderlich et al. 2014). FMTE could decrease the degree of caecal lesions caused by E. tenella from the macroscopic perspective. At the same time, the histological examination of caecum also showed that the number of oocysts in the mucosa was decreased. These phenomenon suggested that the growth and development of E. tenella was inhibited or delayed by FMTE.
After the chickens were infected with E. tenella, the histological sections of liver and kidney showed severe granular and vacuolar degeneration occurred in hepatocytes and renal tubular epithelial cells, even inflammatory cell infiltration in interstitial. However, pre-treatment with FMTE could improve these lesions and restore the function of organs (Youssef et al. 2015; Mikail et al. 2019).
It has been known that intestinal microflora is important for the development and maturation of both innate defense mechanisms and adaptive immune responses (Muir et al. 2000; Brisbin et al. 2008), and it also has significant effects on host nutrient uptake, utilization, growth performance and health (Lei et al. 2015). The intestinal microflora of caecum showed that FMTE could improve the ratio of probiotics and restore the species and microorganism diversity efficiently, which occupied the common receptors on the epithelial cells resulting in reducing the colonization of pathogenic bacteria or oocysts of E. tenella in the intestinal tract and decreasing the damage of mucosal barrier (Dalloul et al. 2003). FMTE could decrease the susceptibility to secondary bacterial infections and enhance immune function of chickens infected with E. tenella further. Although some results in the analysis of intestinal microflora were unsatisfactory, for example, different samples in the same group in the PCA analysis results were inconsistent, which might be due to the special physiological conditions of individual chickens or indicated that there was no significant change in the structure of intestinal microflora. The ACI value in the FMTE-H group at the dosage of 10 g/kg/d was 162.56, which was higher than that of the qiuliling-treated group (positive control). These results suggested that FMTE was a medium effective anticoccidial drug.
Conclusions
The present study is the first to describe the prophylactic effects of FMTE on Eimeria parasites. These findings showed that FMTE could inhibit oocyst sporulation and prevent E. tenella infection in broiler chickens, presenting an alternative anticoccidial agent for the control of avian coccidiosis.
Funding Statement
This research was financially supported by the Program Sichuan Veterinary Medicine and Drug Innovation Group of China Agricultural Research System [SCCXTD-2020-18] and the Science and Technology Project of Sichuan Province [18ZDYF3246].
Disclosure statement
The authors declare that they have no conflict of interest.
References
- Abbas RZ, Iqbal Z, Blake D, Khan MN, Saleemi MK. 2011. Anticoccidial drug resistance in fowl coccidia: the state of play revisited. World Poult Sci J. 67(2):337–350. [Google Scholar]
- Allen PC, Fetterer RH. 2002. Recent advances in biology and immunobiology of Eimeria species and in diagnosis and control of infection with these coccidian parasites of poultry. Clin Microbiol Rev. 15(1):58–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anosa GN, Okoro OJ. 2011. Anticoccidial activity of the methanolic extract of Musa paradisiaca root in chickens. Trop Anim Health Prod. 43(1):245–248. [DOI] [PubMed] [Google Scholar]
- Bozkurt M, Giannenas I, Küçükyilmaz K, Christaki E, Florou-Paneri P. 2013. An update on approaches to controlling coccidia in poultry using botanical extracts. Br Poult Sci. 54(6):713–727. [DOI] [PubMed] [Google Scholar]
- Brisbin JT, Gong J, Sharif S. 2008. Interactions between commensal bacteria and the gut-associated immune system of the chicken. Anim Health Res Rev. 9(1):101–110. [DOI] [PubMed] [Google Scholar]
- Chapman HD. 1999. Anticoccidial drugs and their effects upon the development of immunity to Eimeria infections in poultry. Avian Pathol. 28(6):521–535. [DOI] [PubMed] [Google Scholar]
- Commission C. 2005. Veterinary pharmacopoeia of the People’s Republic of China (part II). Beijing: Agricultural Press of China. [Google Scholar]
- Dalloul RA, Lillehoj HS. 2006. Poultry coccidiosis: recent advancements in control measures and vaccine development. Expert Rev Vaccines. 5(1):143–163. [DOI] [PubMed] [Google Scholar]
- Dalloul RA, Lillehoj HS, Shellem TA, Doerr JA. 2003. Enhanced mucosal immunity against Eimeria acervulina in broilers fed a Lactobacillus-based probiotic. Poult Sci. 82(1):62–66. [DOI] [PubMed] [Google Scholar]
- De Pablos LM, dos Santos MFB, Montero E, Garcia-Granados A, Parra A, Osuna A. 2010. Anticoccidial activity of maslinic acid against infection with Eimeria tenella in chickens. Parasitol Res. 107(3):601–604. [DOI] [PubMed] [Google Scholar]
- Del Cacho E, Gallego M, Francesch M, Quílez J, Sánchez-Acedo C. 2010. Effect of artemisinin on oocyst wall formation and sporulation during Eimeria tenella infection. Parasitol Int. 59(4):506–511. [DOI] [PubMed] [Google Scholar]
- Guo ZT, Liang JP, Wei XB, Shang RF, Guo WZ, Wang XH, Hao BC. 2013. Curative effect of radix dichroa extractive on coccidiosis in chicken artificially caused by Eimeria tenella. Chin J Vet Sci. 33:1282–1287. [Google Scholar]
- Hassan SM, El-Gayar AK, Cadwell DJ, Bailey CA, Cartwright AL. 2008. Guar meal ameliorates Eimeria tenella infection in broiler chicks. Vet Parasitol. 157(1–2):133–138. [DOI] [PubMed] [Google Scholar]
- Hodgson JN. 1970. Coccidiosis: Oocyst counting technique for coccidiostat evaluation. Exp Parasitol. 28(1):99–102. [DOI] [PubMed] [Google Scholar]
- Hu JF, Fan H, Wang LJ, Wu SB, Zhao Y. 2011. Limonoids from the fruits of Melia toosendan. Phytochem Lett. 4(3):292–297. [Google Scholar]
- Hu YL, Li Y, Qiu L, Li JH, Heng L, Wei SS, Gao HL, Wang XB, Luo J, Kong LY. 2018. New triterpenoids with diverse side-chains from the barks of Melia toosendan. Fitoterapia. 127:62–68. [DOI] [PubMed] [Google Scholar]
- Johnson J, Reid WM. 1970. Anticoccidial drugs: lesion scoring techniques in battery and floor-pen experiments with chickens. Exp Parasitol. 28(1):30–36. [DOI] [PubMed] [Google Scholar]
- Kaboutari J, Arab HA, Ebrahimi K, Rahbari S. 2014. Prophylactic and therapeutic effects of a novel granulated formulation of Artemisia extract on broiler coccidiosis. Trop Anim Health Prod. 46(1):43–48. [DOI] [PubMed] [Google Scholar]
- Kinnaird JH, Bumstead JM, Mann DJ, Ryan R, Shirley MW, Shiels BR, Tomley FM. 2004. EtCRK2, a cyclin-dependent kinase gene expressed during the sexual and asexual phases of the Eimeria tenella life cycle. Int J Parasitol. 34(6):683–692. [DOI] [PubMed] [Google Scholar]
- Lei XJ, Piao XS, Ru YJ, Zhang HY, Péron A, Zhang HF. 2015. Effect of Bacillus amyloliquefaciens-based direct-fed microbial on performance, nutrient utilization, intestinal morphology and cecal microflora in broiler chickens. Asian Australas J Anim Sci. 28(2):239–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald SE, Nolan MJ, Harman K, Boulton K, Hume DA, Tomley FM, Stabler RA, Blake DP. 2017. Effects of Eimeria tenella infection on chicken caecal microbiome diversity, exploring variation associated with severity of pathology. PLoS One. 12(9):e0184890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messaï A, Bensegueni A, Abdeldjelil MC, Agabou A, Redouane-Salah S. 2014. Effects of white wormwood (Artemisia herba-alba Asso), during an experimental coccidiosis in broilers. Ann Biol Res. 5:61–66. [Google Scholar]
- Mikail HG, Abubakar IA, Mohammed BR, Yusuf M, Hussain G, Fatihu MY. 2019. Effects of methanolic leaf extract of Lannea schimperi on some organs histopathology in experimentally induced coccidiosis in broiler chickens. J Vet Sci Ani Husb. 6:602. [Google Scholar]
- Molan AL, Liu Z, De S. 2009. Effect of pine bark (Pinus radiata) extracts on sporulation of coccidian oocysts. Folia Parasitol. 56(1):1–5. [DOI] [PubMed] [Google Scholar]
- Morris GM, Woods WG, Richards DG, Gasser RB. 2007. Investigating a persistent coccidiosis problem on a commercial broiler-breeder farm utilising PCR-coupled capillary electrophoresis. Parasitol Res. 101(3):583–589. [DOI] [PubMed] [Google Scholar]
- Muir WI, Bryden WL, Husband AJ. 2000. Immunity, vaccination and the avian intestinal tract. Dev Comp Immunol. 24(2–3):325–342. [DOI] [PubMed] [Google Scholar]
- Orengo J, Buendía AJ, Ruiz-Ibáñez MR, Madrid J, Del Río L, Catalá-Gregori P, García V, Hernández F. 2012. Evaluating the efficacy of cinnamaldehyde and Echinacea purpurea plant extract in broilers against Eimeria acervulina. Vet Parasitol. 185(2–4):158–163. [DOI] [PubMed] [Google Scholar]
- Shanker S, Bustamante H, Karaman M, Pashley R. 2004. Method for the destruction of oocysts. Patent Application 10/477,839. [Google Scholar]
- Sharman PA, Smith NC, Wallach MG, Katrib M. 2010. Chasing the golden egg: vaccination against poultry coccidiosis. Parasite Immunol. 32(8):590–598. [DOI] [PubMed] [Google Scholar]
- Shirley MW, Smith AL, Blake DP. 2007. Challenges in the successful control of the avian coccidia. Vaccine. 25(30):5540–5547. [DOI] [PubMed] [Google Scholar]
- Tian EJ, Zhou BH, Wang XY, Zhao J, Deng W, Wang HW. 2014. Effect of diclazuril on intestinal morphology and SIgA expression in chicken infected with Eimeria tenella. Parasitol Res. 113(11):4057–4064. [DOI] [PubMed] [Google Scholar]
- Williams RB. 1997. Laboratory tests of phenolic disinfectants as oocysticides against the chicken coccidium Eimeria tenella. Vet Rec. 141(17):447–448. [DOI] [PubMed] [Google Scholar]
- Wu SB, Su JJ, Sun LH, Wang WX, Zhao Y, Li H, Zhang SP, Dai GH, Wang CG, Hu JF. 2010. Triterpenoids and steroids from the fruits of Melia toosendan and their cytotoxic effects on two human cancer cell lines. J Nat Prod. 73(11):1898–1906. [DOI] [PubMed] [Google Scholar]
- Wunderlich F, Al-Quraishy S, Steinbrenner H, Sies H, Dkhil M. 2014. Towards identifying novel anti-Eimeria agents: trace elements, vitamins, and plant-based natural products. Parasitol Res. 113(10):3547–3556. [DOI] [PubMed] [Google Scholar]
- Xie F, Zhang M, Zhang CF, Wang ZT, Yu BY, Kou JP. 2008. Anti-inflammatory and analgesic activities of ethanolic extract and two limonoids from Melia toosendan fruit. J Ethnopharmacol. 117(3):463–466. [DOI] [PubMed] [Google Scholar]
- Youssef FM, Abd El-Hamid HA, El Sheshtawy EA. 2015. Clinicopathological studies on the effect of Artemisia cina (Sheih Baladi) on coccidiosis in chickens. Egyptian J Vet Sci. 46:11–24. [Google Scholar]
- Zaman MA, Iqbal Z, Abbas RZ, Khan MN. 2012. Anticoccidial activity of herbal complex in broiler chickens challenged with Eimeria tenella. Parasitology. 139(2):237–243. [DOI] [PubMed] [Google Scholar]
- Zaman MA, Iqbal Z, Rao ZA, Ehtisham-Ul-Haque S. 2015. In vitro efficacy of herbal extracts against Eimeria tenella. IJAB. 17(4):848–850. [Google Scholar]
- Zhang Q, Liang JY, Li QS, Min ZD. 2010. New limonoids from the fruits of Melia toosendan. Chin Chem Lett. 21(7):838–841. [Google Scholar]