Abstract
The cerebellum is known for its crossed activation pattern with the contralateral cerebral hemisphere during language functional magnetic resonance imaging (fMRI) tasks in healthy patients. Crossed cerebro-cerebellar activation has been previously shown to occur in patients with brain tumors not affecting the activation areas. However, the presence of a tumor in left Broca’s area in the inferior frontal gyrus is known to disrupt cerebral activation during language tasks. This study investigated if crossed cerebro-cerebellar activation patterns for language tasks would still occur in such patients. A total of 43 right-handed patients with a glioma affecting left Broca’s area were examined for their cerebral and cerebellar activation during an fMRI language task. Only 13 of the 43 patients exhibited crossed cerebro-cerebellar activation patterns. Statistically significant differences of atypical cerebro-cerebellar activation patterns were found between cerebral right-dominant (RD) and cerebral co-dominant (CD) (p < 0.001) as well as cerebral RD and cerebral left-dominant (LD) patients (p < 0.01), while no differences were found when patients were divided based on cerebellar dominance (p > 0.75) or tumor grade (p > 0.5). No relation was found between the cerebellar and cerebral laterality index (LI) values (ρ = −0.20; p = 0.21). Atypical activation patterns are suspected to have been caused by the tumor, perhaps a result of contralateral reorganization in some cases and false negative activation in left Broca’s area from neurovascular uncoupling (NVU) in others. Cerebellar activation may also potentially indicate cerebral false negative behavior and future cerebral contralateral reorganization.
Keywords: fMRI, Cerebro-cerebellar network, contralateral reorganization, neovascular uncoupling, Broca’s area
Introduction
Functional magnetic resonance (fMRI) studies have been instrumental in revealing crossed activation patterns between the cerebellar hemispheres and the contralateral cerebral hemisphere [1, 2]. This knowledge has been advanced as part of recent discoveries highlighting the role of the cerebellum in higher order cognitive tasks such as timed motor skills [3], spatial processing [4, 5], working memory [4], and language [3–6], which are mainly attributed to the cerebrum. For language function, which is typically left dominant (LD) in the cerebral cortex of healthy right-handed humans [7, 8], fMRI studies have consistently shown right-cerebellar activation [3–6]. Additionally, crossed activation has been shown to occur in a pair of monozygotic twins where one of them was right-handed, cerebral LD, and cerebellar right-dominant (RD) while the other was left-handed, cerebral RD, and cerebellar LD [5].
Studies have shown that crossed activation of language function remains generally intact in patients with pediatric epilepsy [9], left-cerebral stroke [10], left-cerebral congenital brain lesion [11], and brain tumor [12]. However, there are some instances of atypical activation patterns in the cerebral cortex. In these studies, atypical activation patterns were thought to be caused by pathological effects such as contralateral brain reorganization [9–11] or false negative behavior [12].
Contralateral brain reorganization is presumed to be a manifestation of brain plasticity and represents the process in which a lesion causes proximal, eloquent brain areas to shift their activation to a contralateral, unaffected location [13]. This process has been suggested to occur in the cerebral cortex of patients with brain tumors in language areas [14–16] as well as in the cerebellum for motor areas [17]. Contralateral reorganization of the entire cerebro-cerebellar network has also been suggested in patients with stroke [10] and congenital brain lesion [11].
Accurate identification of cortical reorganization is complicated by false negative fMRI results, where eloquent brain areas proximal to a brain tumor appear to not be activated due to abnormal vasculature induced by the lesion potentially due to neurovascular uncoupling [18–22]. While false negative behavior has been shown to occur in patients with low-grade gliomas [21], their effect has been more pronounced in patients with high-grade gliomas [18–20]. This is of especial importance when tumors are in left Broca’s area since muted activation can lead to difficulty or even inaccurate determinations of cerebral language dominance [18]. As a result, activation in other structures, such as the middle frontal gyrus, has been proposed as an indicator of cerebral language dominance in Broca’s area when such determination is difficult because of a tumor affecting the region [23].
The purpose of this study was to examine if crossed cerebro-cerebellar activation persists in right-handed patients during language tasks with a brain tumor affecting the left Broca’s area. Our hypothesis was that tumors affecting the left Broca’s area in right-handed patients will cause atypical cerebro-cerebellar activation patterns, which may be due to contralateral reorganization or false negative activation in left Broca’s area depending on the cerebro-cerebellar activation pattern. In addition, this study aimed to investigate relationships between atypical cerebro-cerebellar activation patterns and clinical variables such as hemispheric dominance and tumor grade.
Patients and Methods
Patient Selection
This study was conducted in compliance with the Health Insurance Portability and Accountability Act. Our institutional review board approved this retrospective study and issued a waiver of informed consent. A total of 43 patients were included with the following inclusion criteria: 1) patients with a glioma affecting left Broca’s area, 2) patients who have obtained a pre-surgical language task paradigm fMRI scan that included the entire cerebellum and, 3) patients who are right-handed according to the Edinburgh Inventory [24]. The patients’ clinical data and pathology are summarized in Table 1.
Table 1.
Clinical Data and Pathology of Patients
| Clinical Data | |||
| Characteristic | Patients | ||
| Sex (Male / Female) | 22 / 21 | ||
| Average Age (yrs) | 49.5 ± 13.8 | ||
| Handedness (Right / Left) | 43 / 0 | ||
| Pathology | |||
| High-grade (III/IV): | Number of Patients | Low-grade (I/II): | Number of Patients |
| Glioblastoma | 11 | Astrocytoma | 7 |
| Anaplastic Tumor | 10 | Oligodendroglioma | 5 |
| Glioma | 4 | Glioma | 4 |
| Astrocytic Neoplasm | 1 | Oligoastrocytoma | 1 |
| Total: | 26 | Total: | 17 |
Table 1 summarizes the clinical data (sex, age, handedness) and pathology of the patients.
Image Acquisition
fMRI was performed as part of each patient’s pre-surgical MRI protocol. The scans were acquired on a 3T scanner (GE Healthcare, Chicago, IL) with an eight-channel head coil. Functional matching T1-weighted (TR/TE = 400/14 ms, 256 × 256 matrix), FLAIR (TR/TE = 10000/106 ms, inversion time = 220 ms, 256 × 256 matrix), T2-weighted (TR/TE = 4000/102 ms, 256 × 256 matrix), and T1 post-contrast (TR/TE = 600/20 ms, 256 × 256 matrix) images were obtained. 3D-T1-weighted anatomical images were acquired using a spoiled gradient recalled sequence (TR/TE = 22/4 ms, 256 × 256 matrix, 1.5 mm thickness). fMRI data were acquired with a single shot gradient echo echo-planar imaging sequence (TR/TE = 4000/35 ms; 128 × 128 matrix; 4.5 mm thickness; 90 degree flip angle; 24 cm field of view, 34–36 slices covering whole brain).
fMRI Task Paradigms
Each patient underwent the phonemic and semantic language fMRI task paradigms, and the task that yielded the more optimal activation map, as clinically determined by a neuroradiologist with 20 years of experience in fMRI (initials blinded for review), was used for analysis (phonemic, n = 40; semantic, n = 3). During the phonemic task, patients were given a letter, and then they were asked to silently generate words that began with that letter. During the semantic task, patients were given a general category, and then they were asked to silently generate words that fit in the given category. A block-designed paradigm of alternating rest (40 seconds) and active (20 seconds) periods was carried out in each patient. The task consisted of 90 volumes for a total of 6 cycles each of stimulation and rest periods.
Before going into the scanner, each patient was pre-tested for their respective task to ensure proper performance and accurate images. They were instructed to silently perform the tasks without moving their mouth and tongue. Each patient’s brain activity and head motion were monitored in real time using a real-time post-processing software called BrainWave (Brainwave RT, Medical Numerics, Germantown, MD).
fMRI Data Analysis
fMRI data were processed and analyzed using the software Analysis of Functional NeuroImages (AFNI) [25]. After spike signal removal, head motion correction was performed using 3D rigid-body registration, and spatial smoothing was applied to improve the signal-to-noise ratio using a Gaussian filter with 4 mm full width of half maximum. Linear trend was removed where necessary. Signal changes over time were cross-correlated with a mathematical Gaussian model of the hemodynamic response to neural activation. Cross-correlation involved convolving the modeled waveform corresponding to the task performance block with all pixel time courses on a pixel-by-pixel basis to generate functional activity data. Functional activation maps were generated at a threshold of p < 0.001. To reduce false positive activity from large venous structures or head motion, voxels in which the standard deviation of the acquired time series exceeded 8% of the mean signal intensity were set to zero.
Following the generation of functional activation maps, voxel region of interest (ROI) masks were drawn by a neuroradiologist (initials blinded for review) with over 20 years of experience. For analysis of the cerebellum, ROI masks were drawn on the left and right Broca’s areas in the inferior frontal gyrus while for analysis of the cerebellum, ROI masks were drawn on each hemisphere of the cerebellum as done in previous studies [3, 9, 11, 12]. All ROI masks were drawn using high resolution axial anatomical images. The laterality index (LI) was then measured from the activated voxels in the ROI masks for both cerebral and cerebellar dominance using the formula: , where L and R are the number of activated voxels per drawn ROI in the left and right hemisphere, respectively. Left-dominance (LD) was considered to be an LI ≥ 0.2; co-dominance (CD) was considered to be −0.2 < LI < 0.2; and right-dominance (RD) was considered to be an LI ≤ −0.2 [16, 26].
A cerebro-cerebellar activation pattern was considered to be either crossed (when the cerebral dominance was contralateral to the cerebellar dominance (i.e., cerebral LD / cerebellar RD, cerebral RD / cerebellar LD, cerebral CD / cerebellar CD)) or atypical (for any other lateralization pattern).
Statistical Analysis
Spearman’s rank correlation coefficient analysis was used to determine if there was a relationship between cerebral and cerebellar LI values. Then, the LI values were used to categorize each subject depending on their cerebral and cerebellar dominance (cerebral LD / cerebellar LD, cerebral LD / cerebellar CD, cerebral LD / cerebellar RD, etc.). Contingency tables were made to analyze any relationships between atypical cerebro-cerebellar activation patterns and the clinical variables in this study, which were hemispheric dominance and tumor grade. The Fisher’s exact test was performed to determine any statistically significant associations in the contingency tables. If overall significance was found in any contingency tables larger than 2 × 2, post-hoc Fisher’s exact tests with Holm-Sidak corrected p-values were performed to determine any specific associations. For all analyses, the significance level was set at α = 0.05.
Results
Cerebral and Cerebellar Lateralization
Each patient was categorized based on their cerebral and cerebellar lateralization as determined by their LI values for Broca’s area and the cerebellum, respectively (Table 2 and Figure 1). Only 13 of the 43 patients demonstrated crossed cerebro-cerebellar activation, of whom 5 were cerebral LD / cerebellar RD and 8 were cerebral CD / cerebellar CD. No patients demonstrated crossed cerebral RD / cerebellar LD activation.
Table 2.
Categorizations of Patient Cerebral and Cerebellar Lateralization Patterns
| Cerebellar LD | Cerebellar CD | Cerebellar RD | |||||||
| Cerebral LD | Cerebral LI | Cerebellar LI | Grade | Cerebral LI | Cerebellar LI | Grade | Cerebral LI | Cerebellar LI | Grade |
| 0.99 | 0.80 | Low | 0.80 | −0.03 | High | 1.00 | −0.95 | High | |
| 0.77 | 0.15 | High | 0.37 | −0.59 | High | ||||
| 0.37 | −0.18 | High | 0.31 | −0.37 | High | ||||
| 0.25 | −0.15 | High | 0.71 | −1.00 | High | ||||
| 0.52 | −0.17 | High | 0.33 | −0.33 | Low | ||||
| 0.41 | −0.05 | Low | |||||||
| Cerebral CD | Cerebral LI | Cerebellar LI | Grade | Cerebral LI | Cerebellar LI | Grade | Cerebral LI | Cerebellar LI | Grade |
| 0.11 | 0.21 | High | 0.16 | 0.00 | High | 0.10 | −0.70 | High | |
| 0.07 | −0.05 | High | −0.05 | −0.20 | High | ||||
| −0.05 | 0.05 | High | −0.17 | −0.25 | High | ||||
| −0.06 | −0.02 | Low | −0.13 | −0.75 | Low | ||||
| 0.09 | 0.05 | Low | −0.06 | −0.38 | Low | ||||
| 0.01 | 0.01 | Low | −0.04 | −0.32 | Low | ||||
| −0.16 | 0.01 | Low | |||||||
| −0.16 | 0.04 | Low | |||||||
| Cerebral RD | Cerebral LI | Cerebellar LI | Grade | Cerebral LI | Cerebellar LI | Grade | Cerebral LI | Cerebellar LI | Grade |
| −0.28 | 0.00 | High | −0.54 | −0.33 | High | ||||
| −0.87 | 0.06 | High | −0.50 | −0.70 | High | ||||
| −1.00 | 0.07 | High | −0.50 | −0.28 | High | ||||
| −0.81 | 0.10 | High | −1.00 | −0.37 | High | ||||
| −1.00 | 0.02 | High | |||||||
| −0.73 | 0.14 | High | |||||||
| −0.95 | −0.14 | High | |||||||
| −0.28 | −0.12 | Low | |||||||
| −0.62 | −0.12 | Low | |||||||
| −0.33 | −0.03 | Low | |||||||
| −0.26 | 0.16 | Low | |||||||
| −0.67 | 0.13 | Low | |||||||
Table 2 summarizes the distribution of lateralization patterns observed in this patient group. The shaded boxes represent the categorizations of crossed cerebro-cerebellar activation, and only 13 out of 43 patients exhibited this pattern. No patients exhibited crossed cerebral RD / cerebellar LD activation.
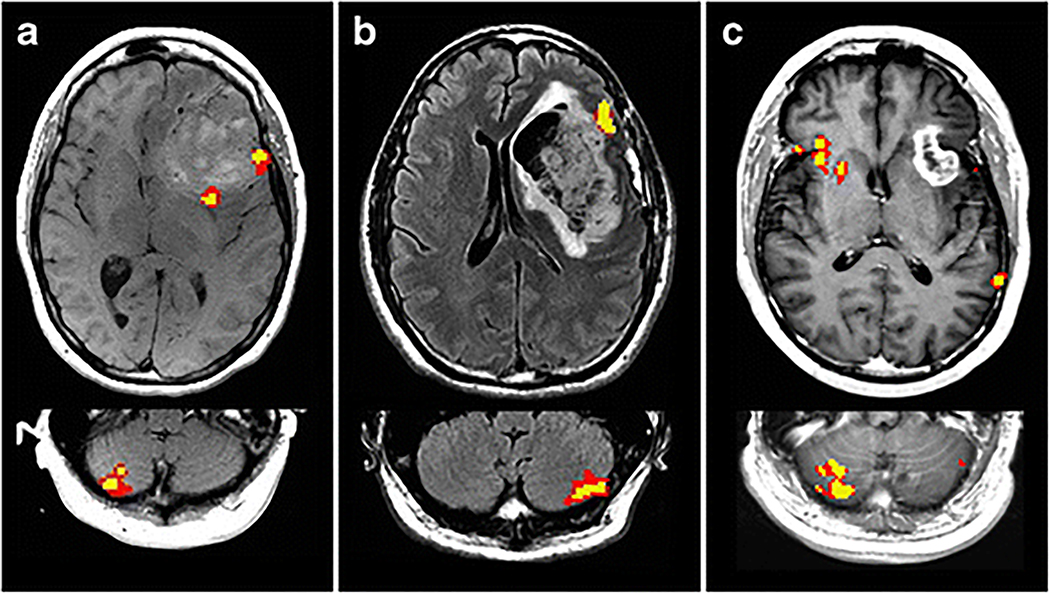
Fig 1.
fMRI scans of the cerebral cortex and cerebellum for representative cases of the following observed language lateralization patterns: (a) crossed Cerebral LD / Cerebellar RD, (b) atypical Cerebral LD / Cerebellar LD, and (c) atypical Cerebral RD / Cerebellar RD.
The remaining 30 of the 43 patients demonstrated atypical cerebro-cerebellar activation, as follows: 1 cerebral LD / cerebellar LD, 6 cerebral LD / cerebellar CD, 1 cerebral CD / cerebellar LD, 6 cerebral CD / cerebellar RD, 12 cerebral RD / cerebellar CD, and 4 cerebral RD / cerebellar RD.
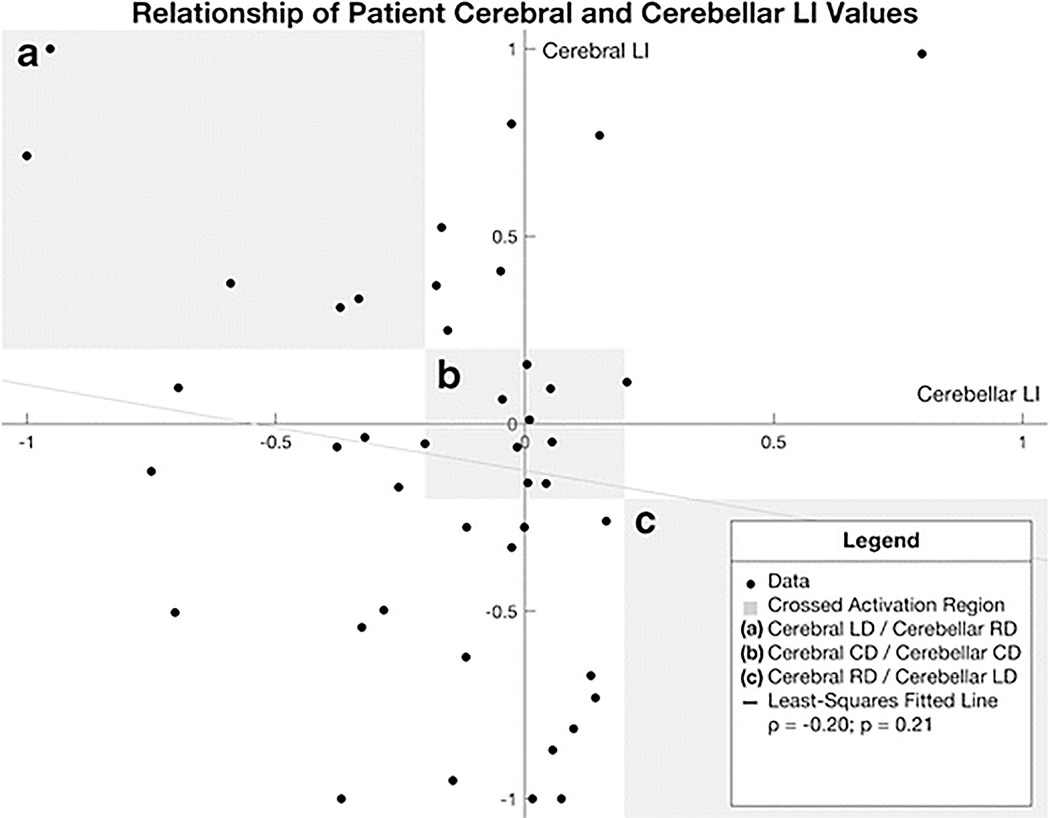
As a result of the large number of patients not exhibiting crossed cerebro-cerebellar activation, no significant correlation was found between the cerebral and cerebellar LI values (ρ = −0.20; p = 0.21) (Figure 2).
Fig. 2.
Scatterplot of patient cerebral (Broca’s) LI values plotted against cerebellar LI values. The shaded regions correspond to regions where the data reflect crossed activation: (a) Cerebral LD / Cerebellar RD (b) Cerebral CD / Cerebellar CD (c) Cerebral RD / Cerebellar LD. There was no relationship found between the two LI values (ρ = −0.20; p = 0.21).
Relationship between Cerebro-Cerebellar Activation Patterns and Language Dominance
There was an overall significant difference in frequencies of crossed and atypical cerebro-cerebellar activation based on each patient’s language dominance in Broca’s area (LD, CD, or RD) (p < 0.002) (Table 3). In the cerebral LD group, 7 out of 12 patients demonstrated atypical activation patterns, and in the cerebral CD group, 7 out of 16 patients demonstrated atypical activation patterns. However, in the cerebral RD group, all 16 patients demonstrated atypical activation patterns.
Table 3.
Observed Frequencies of Cerebro-Cerebellar Activation Patterns and Language Dominance
| Crossed | Atypical | |
|---|---|---|
| Cerebral LD | 5 | 7 |
| Cerebral CD | 8 | 7 |
| Cerebral RD | 0 | 16 |
Table 3 shows the frequencies of crossed and atypical cerebro-cerebellar activation patterns across between the three language dominance groups. An overall statistically significant difference was found (p < 0.002).
There were significant differences between the cerebral CD and cerebral RD groups (p < 0.001) and the cerebral LD and cerebral RD groups (p < 0.01), while no significant difference was found between the cerebral LD and cerebral CD groups (p > 0.5).
Relationship between Cerebro-Cerebellar Activation Patterns and Cerebellar Dominance or Tumor Grade
When grouping the patients based on cerebellar dominance and tumor grade, no statistically significant differences were found based on either categorization (p > 0.75, p > 0.5, respectively) (Table 4).
Table 4.
Observed Frequencies of Cerebro-Cerebellar Activation Patterns Based on Cerebellar Dominance and Tumor Grade
| Categorized by Cerebellar Dominance | ||
| Crossed | Atypical | |
| Cerebellar LD | 0 | 2 |
| Cerebellar CD | 8 | 18 |
| Cerebellar RD | 5 | 10 |
| Categorized by Tumor Grade | ||
| Crossed | Atypical | |
| High Grade | 7 | 20 |
| Low Grade | 6 | 10 |
Table 4 shows the frequencies of crossed and atypical cerebro-cerebellar activation patterns across between the three cerebellar dominance two tumor grade groups. No statistically significant difference was found (p > 0.75, p > 0.5, respectively).
Discussion
This study investigated the cerebro-cerebellar language functional activation patterns in right-handed patients with a brain tumor affecting left Broca’s area. Only 13 of the 43 patients in this study exhibited crossed activation patterns, which is the typical activation in healthy patients, while the majority of patients exhibited atypical activation patterns. When assessing whether cerebral dominance affected frequencies of atypical cerebro-cerebellar activation patterns between groups, there were statistically significant differences between the cerebral CD and cerebral RD groups (p < 0.001), as well as the cerebral LD and cerebral RD groups (p < 0.01). Conversely, there were no significant findings based on either cerebellar dominance (p > 0.75) or tumor grade (p > 0.5). In addition, no correlation was found between the LI values of the cerebellum and cerebral cortex (p > 0.2).
In order to understand the observed data, a number of explanations are proposed in this paper, each of which matches a specific pattern of activation. These explanations are based on the present data as well as on what has previously been documented in brain tumors in terms of 1) contralateral reorganization and 2) false negative activations caused by neurovascular uncoupling.
Contralateral Brain Reorganization of the Entire Cerebro-Cerebellar Network
The cerebellum is known to have a role during language tasks [3–6], and its functional activation pattern is characterized by its crossed activation with the cerebral cortex regardless of cerebral dominance [27–29]. While 5 of the 13 patients with crossed activation patterns exhibited typical cerebral LD / cerebellar RD behavior, the other 8 patients exhibited atypical cerebral CD / cerebellar CD behavior. All of our patients were right-handed, and since language dominance is usually cerebral LD for right-handed people [7, 8], the cerebral CD activation suggests at least a partial reorganization of language function to the contralateral frontal lobe. Cerebral CD language behavior has already been attributed to contralateral brain reorganization in right-handed patients with brain tumor in language areas, with the suggestion that this process is a means of recruiting additional brain regions to compensate for regions impacted by the tumor [16].
The results in these 8 patients also imply cerebellar reorganization because of these patients’ cerebellar CD behavior, which further suggests reorganization of the entire cerebro-cerebellar network. Contralateral reorganization of the entire cerebro-cerebellar network has been previously suggested in patients with stroke [10] and congenital brain lesion [11].
Atypical Cerebro-Cerebellar Activation Patterns: Neurovascular Uncoupling and False Negative fMRI Activation
Thirty two of the 43 patients, or nearly 75% of all patients in this study, did not exhibit the typical crossed activation pattern seen in healthy patients. One potential reason for the infrequent observation of crossed cerebro-cerebellar activation patterns in this study is the presence of abnormal neovasculature in left Broca’s area leading to false negative fMRI activation. Based on our data, it appears that the presence of false negative activation may be implied by the observation that right cerebellar dominance, which was never reciprocated in the left cerebral hemisphere where the tumor was located. However, cerebellar RD instead demonstrated activation with cerebral CD (n = 6) and cerebral RD (n = 4).
Because tumors can cause false negative results in their vicinity [20, 22, 30], this raises the possibility that the activation of the cerebellum as detected by fMRI might indicate the true positive activation of Broca’s area, whereas the lack of activation of Broca’s area could indicate a false negative. The reduced activation signal in the left Broca’s area might indeed be due to neurovascular uncoupling, in which a brain region appears to be not activated according to fMRI activation due to tumor vasculature or other factors that impact blood flow to the region [18–22]. False negative behavior has been used to explain a similar finding in a previous study of cerebro-cerebellar activation patterns in patients with brain tumor [12].
A previous study involving patients with a brain tumor at a non-specific location in the brain suggested that the crossed cerebro-cerebellar activation pattern can be preserved in all variations, including cerebral RD / cerebellar LD activation patterns [12]. However, in this present study, there were no patients exhibiting crossed cerebral RD / cerebellar LD activation despite observations of other variations of crossed cerebro-cerebellar activation patterns. Instead, all cerebral RD patients exhibited cerebellar CD or cerebellar RD activation, which suggests there was undetected, false negative left cerebral activation in the area of the tumor that would account for the substantial right cerebellar activation. While false negative behavior in the left Broca’s area would not impact the observed dominance pattern of patients who are cerebral LD, this would greatly impact that of patients who are cerebral RD. This hypothesis is further supported by our results showing that the frequencies of atypical cerebro-cerebellar activation were statistically significant in comparisons involving cerebral RD patients (cerebral CD and cerebral RD, p < 0.001; cerebral LD and cerebral RD, p < 0.01) but not in comparisons involving cerebellar LD with either cerebellar CD or RD patients. Thus, the difference between these comparisons are likely to have come from fMRI activation anomalies affecting only the cerebral fMRI activation map, which in this case would be false negative behavior.
If cerebellar dominance might indicate the true cerebral dominance, then cerebellar dominance may have the potential to assist in the clinical determination of false negative activation occurring proximal to a lesion in the cerebral cortex in difficult cases. A similar suggestion has been made by Dong et al., stating that activation of the middle frontal gyrus might indicate the true activation of Broca’s area when a tumor was affecting Broca’s area [23]. This has important implications for image-guided neurosurgery, where caution must be taken when interpreting fMRI activation for areas proximal to a lesion since reduced fMRI activation by false negative behavior can lead to incorrect pre-surgical planning, which poses a higher risk for post-surgical deficits [18].
Cerebellar Contralateral Reorganization
There were also cases in this study in which there appeared to be more left cerebellar activation than expected. Six patients were cerebral LD / cerebellar CD, one patient was cerebral LD / cerebellar LD, and one patient was cerebral CD / cerebellar LD. These results suggest that contralateral reorganization has occurred more in the cerebellum than in the cerebral cortex. A similar finding was presented in a previous study investigating cerebro-cerebellar motor activation patterns in patients with brain tumor where the authors reported an overall increase in contralateral cerebellar activation as well as cerebellar activation [17].
If cerebellar contralateral reorganization could occur without cerebral contralateral reorganization, despite the typical crossed activation of the cerebral cortex and cerebellum, then cerebellar contralateral reorganization may occur before the onset of cerebral contralateral reorganization. Thus, contralateral cerebellar activation may be an indicator of future cerebral contralateral reorganization as cerebellar regions are immediately recruited to compensate for the damaged cerebral regions proximal to the lesion before the entire cerebro-cerebellar network can be reorganized. However, future studies would be required to support this claim.
No Detected Role of Tumor Grade
There were no significant differences when grouping the subjects by tumor grade (p > 0.5). However, the high-grade glioma patients in this study may have potentially conflicting impacts on the study analyses. High-grade gliomas have been suggested to induce more contralateral brain reorganization than low-grade gliomas since the former initially present as low-grade and then progress over time [16]. This would allow more time for the brain to recruit contralateral areas, including those in the cerebellum, to compensate for the affected region as compared with newly formed low-grade gliomas [16]. However, high-grade gliomas are also known to induce more false negative behavior than low-grade gliomas [18–20]. If false negative behavior occurs in a mixed population of patients with and without contralateral brain reorganization, then this in itself would consequently produce a mixed population of patients with crossed and atypical cerebro-cerebellar activation. This could have confounded our results and prevented significant differences to be found.
Limitations
There were several limitations to this study. One limitation was the small sample size, which caused some of the frequencies of certain lateralization patterns to be less than five. In the future, it would be favorable to study a larger and more diverse patient population to further study the potential impacts of tumor type and location on cerebro-cerebellar activation patterns. Additionally, LI values were used to determine each patient’s language dominance, but it has been suggested that this metric may not fully and accurately describe functional dominance because the LI value does not take into account the voxel intensity and because it is dependent on the statistical threshold [31]. Another limitation was the lack of longitudinal data in our study. Having longitudinal data would allow for a stronger claim about the potential role of cerebellar fMRI activation as an indicator of future cerebral contralateral brain reorganization and the impact of false negative behavior on the readings of the cerebral activation maps. Electrocortical stimulation may also be conducted to further investigate potential observations of contralateral brain reorganization and false negative behavior.
Conclusion
This study investigated the activation patterns in the cerebellum and cerebral cortex for language tasks in right-handed patients with a tumor in the left Broca’s area using fMRI. While some patients exhibited the typical crossed cerebro-cerebellar activation pattern, nearly 75% of patients in this study exhibited atypical cerebro-cerebellar activation patterns. These results may demonstrate how cerebellar activation can be used to potentially identify instances of false negative behavior in the cerebral cortex and serve as an indicator for future contralateral brain reorganization. More studies will need to be conducted to better understand our results.
Acknowledgements
We would like to thank Joanne Chin for her editorial assistance.
Funding: This research was funded in part through the National Institutes of Health / National Cancer Institute Cancer Center Support Grant P30 CA008748.
Footnotes
Conflict of Interest: The authors declare that they have no conflict of interest.
Compliance with Ethical Standards: For this type of study formal consent is not required. A waiver of informed consent was issued by our institutional review board. This article does not contain any studies with animals performed by any of the authors.
References
- 1.Hubrich-Ungureanu P, Kaemmerer N, Henn FA and Braus DF. Lateralized organization of the cerebellum in a silent verbal fluency task: a functional magnetic resonance imaging study in healthy volunteers. Neuroscience Letters 2002: 319:91–4. doi 10.1016/S0304-3940(01)02566-6 [DOI] [PubMed] [Google Scholar]
- 2.Jansen A, Flöel A, Van Randenborgh J, Konrad C, Rotte M, Förster A-F, Deppe M and Knecht S. Crossed cerebro–cerebellar language dominance. Human Brain Mapping 2005: 24:165–72. doi 10.1002/hbm.20077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Riecker A, Ackermann H, Wildgruber D, Dogil G and Grodd W. Opposite hemispheric lateralization effects during speaking and singing at motor cortex, insula and cerebellum. Neuroreport 2000: 11:1997–2000. [DOI] [PubMed] [Google Scholar]
- 4.Stoodley CJ, Valera EM and Schmahmann JD. Functional topography of the cerebellum for motor and cognitive tasks: an fMRI study. Neuroimage 2011: 59:1560–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lux S, Keller S, Mackay C, Ebers G, Marshall JC, Cherkas L, Rezaie R, Roberts N, Fink GR and Gurd JM. Crossed cerebral lateralization for verbal and visuo-spatial function in a pair of handedness discordant monozygotic twins: MRI and fMRI brain imaging. Journal of Anatomy 2008: 212:235–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Binder JR, Frost JA, Hammeke TA, Cox RW, Rao SM and Prieto T. Human brain language areas identified by functional magnetic resonance imaging. Journal of Neuroscience 1997: 17:353–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Isaacs KL, Barr WB, Nelson PK and Devinsky O. Degree of handedness and cerebral dominance. Neurology 2006: 66:1855–8. [DOI] [PubMed] [Google Scholar]
- 8.Knecht S, Drager B, Deppe M, Bobe L, Lohmann H, Floel A, Ringelstein EB and Henningsen H. Handedness and hemispheric language dominance in healthy humans. Brain 2000: 123:2512–8. [DOI] [PubMed] [Google Scholar]
- 9.Gelinas JN, Fitzpatrick KP, Kim HC and Bjornson BH. Cerebellar language mapping and cerebral language dominance in pediatric epilepsy surgery patients. NeuroImage: Clinical 2014: 6:296–306. doi D - NLM: PMC4215475 OTO - NOTNLM [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Connor LT, DeShazo Braby T, Snyder AZ, Lewis C, Blasi V and Corbetta M. Cerebellar activity switches hemispheres with cerebral recovery in aphasia. Neuropsychologia 2006: 44:171–7. [DOI] [PubMed] [Google Scholar]
- 11.Lidzba K, Wilke M, Staudt M, Krageloh-Mann I and Grodd W. Reorganization of the cerebro-cerebellar network of language production in patients with congenital left-hemispheric brain lesions. Brain and Language 2008: 106:204–10. [DOI] [PubMed] [Google Scholar]
- 12.Mendez Orellana C, Visch-Brink E, Vernooij M, Kalloe S, Satoer D, Vincent A, van der Lugt A and Smits M. Crossed cerebrocerebellar language lateralization: an additional diagnostic feature for assessing atypical language representation in presurgical functional MR imaging. American Journal of Neuroradiology 2015: 36:518–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duffau H Brain Mapping: From Neural Basis of Cognition to Surgical Applications Springer; 2011. [Google Scholar]
- 14.Partovi S, BJ, Rapps N, Zipp L, Karimi S, Rengier F, Lyo JK and Stippich C. Clinical standardized fMRI reveals altered language lateralization in patients with brain tumor. American Journal of Neuroradiology 2012: 33:2151–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thiel A, Habedank B, Winhuisen L, Herholz K, Kessler J, Haupt WF and Heiss WD. Essential language function of the right hemisphere in brain tumor patients. Annals of Neurology 2005: 57:128–31. [DOI] [PubMed] [Google Scholar]
- 16.Tantillo G, Peck KK, Arevalo-Perez J, Lyo JK, Chou JF, Young RJ, Brennan NP and Holodny AI. Corpus Callosum Diffusion and Language Lateralization in Patients with Brain Tumors: A DTI and fMRI Study. Journal of neuroimaging : official journal of the American Society of Neuroimaging 2015: 26:224–31. doi 10.1111/jon.12275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kurabe S, Itoh K, Nakada T and Fujii Y. Evidence for cerebellar motor functional reorganization in brain tumor patients: An fMRI study. Neuroscience Letters 2016: 622:45–8. doi 10.1016/j.neulet.2016.04.036 [DOI] [PubMed] [Google Scholar]
- 18.Fraga de Abreu VH, Peck KK, Petrovich-Brennan NM, Woo KM and Holodny AI. Brain Tumors: The Influence of Tumor Type and Routine MR Imaging Characteristics at BOLD Functional MR Imaging in the Primary Motor Gyrus. Radiology 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hou BL, Bradbury M, Peck KK, Petrovich NM and Gutin PHH, A. I. Effect of brain tumor neovasculature defined by rCBV on BOLD fMRI activation volume in the primary motor cortex. Neuroimage 2006: 32:489–97. [DOI] [PubMed] [Google Scholar]
- 20.Holodny AI, Schulder M, Liu WC, Wolko J, Maldjian JA and Kalnin AJ. The effect of brain tumors on BOLD functional MR imaging activation in the adjacent motor cortex: implications for image-guided neurosurgery. American Journal of Neuroradiology 2000: 21:1415–22. [PMC free article] [PubMed] [Google Scholar]
- 21.Zaca D, Jovicich J, Nadar SR, Voyvodic JT and Pillai JJ. Cerebrovascular reactivity mapping in patients with low grade gliomas undergoing presurgical sensorimotor mapping with BOLD fMRI. Journal of Magnetic Resonance Imaging 2014: 40:383–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ulmer JL, Hacein-Bey L, Mathews VP, Mueller WM, DeYoe EA, Prost RW, Meyer GA, Krouwer HG and Schmainda KM. Lesion-induced pseudo-dominance at functional magnetic resonance imaging: implications for preoperative assessments. Neurosurgery 2004: 55:569–79. [DOI] [PubMed] [Google Scholar]
- 23.Dong JW, Brennan NMP, Izzo G, Peck KK and Holodny AI. fMRI activation in the middle frontal gyrus as an indicator of hemispheric dominance for language in brain tumor patients: a comparison with Broca’s area. Neuroradiology 2016: 58:513–20. doi 10.1007/s00234-016-1655-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 1971: 9:97–113. [DOI] [PubMed] [Google Scholar]
- 25.Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res 1996: 29:162–73. [DOI] [PubMed] [Google Scholar]
- 26.Peck KK, Bradbury M, Petrovich NM, Hou BL, Ishill N, Brennan C, Tabar V and Holodny AI. Presurgical Evaluation of Language Using Functional Magnetic Resonance Imaging in Brain Tumor Patients with Previous Surgery. Neurosurgery 2009: 64:644–53. doi 10.1227/01.NEU.0000339122.01957.0A [DOI] [PubMed] [Google Scholar]
- 27.Middleton FA and Strick PL. Cerebellar projections to the prefrontal cortex of the primate. Journal of Neuroscience 2001: 21:700–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krienen FM and Buckner RL. Segregated fronto-cerebellar circuits revealed by intrinsic functional connectivity. Cereb Cortex 2009: 19:2485–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schmahmann JD. From movement to thought: anatomic substrates of the cerebellar contribution to cognitive processing. Human Brain Mapping 1996: 4:174–98. [DOI] [PubMed] [Google Scholar]
- 30.Ulmer JL, Krouwer HG, Mueller WM, Ugurel MS, Kocak M and Mark LP. Pseudo-Reorganization of Language Cortical Function at fMR Imaging: A Consequence of Tumor-Induced Neurovascular Uncoupling. American Journal of Neuroradiology 2003: 24:213. [PMC free article] [PubMed] [Google Scholar]
- 31.Seghier ML. Laterality index in functional MRI: methodological issues. Magnetic Resonance Imaging 2008: 26:594–601. doi 10.1016/j.mri.2007.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]