ABSTRACT
Bladder cancer is the ninth most frequent-diagnosed disease worldwide, bearing high morbidity and mortality rates. Studies have shown that a particular population of CXCR5+CD8+ T cells was associated with superior prognosis in various tumor types, and yet its role in muscle-invasive bladder cancer (MIBC) remains unclear. In this study, 662 MIBC patients from 3 cohorts (Zhongshan Hospital, n = 141; Shanghai Cancer Center, n = 108; The Cancer Genome Atlas, n = 403) were analyzed retrospectively. 11 fresh resected samples of MIBC were examined to characterize the phenotype of CXCR5+CD8+ T cells and 402 MIBC patients from TCGA were applied for bioinformatics analysis. It was explored that the abundance of intratumoral CXCR5+CD8+ T cells indicated superior overall survival and disease-free survival. Patients with a higher infiltration of CXCR5+CD8+ T cells in tumor tissue benefit more from adjuvant chemotherapy (ACT). Intratumoral CXCR5+CD8+ T cells displayed cytolytic and self-renewal features. Remarkably, CXCR5+CD8+ T cells were mainly presented in the basal and stromal-rich subtypes of MIBC and tumors with enriched CXCR5+CD8+ T cells showed limited FGFR3 signaling signature and activated immunotherapeutic and EGFR associated pathway. In conclusion, we identified an excellent prognosis and ACT sensitive subtype of MIBC with intratumoral CXCR5+CD8+ T cell abundance. Tumors with high density of CXCR5+CD8+ T cells possessed potential sensitivity to immunotherapy and EGFR-targeted therapy. CXCR5+CD8+ T cells provide a new potential biomarker as well as a therapeutic target in MIBC.
KEYWORDS: Muscle-invasive bladder cancer, CXCR5+CD8+ T cells, prognosis, adjuvant chemotherapy, molecular subtype
Introduction
As one of the most common cancer types, bladder cancer bears high morbidity and mortality rates. In 2012 alone, approximately 430,000 patients were newly diagnosed with bladder cancer.1 As a rather aggressive subtype of bladder cancer, muscle-invasive bladder cancer (MIBC) is intractable and radical cystectomy (RC) is applied as the major treatment, but there is still an approximately 50% rate of relapse or metastasis.2 Hence, MIBC patients treated with RC are recommended to take additional chemotherapy.3 However, it is reported that only a fraction of patients gains benefits from adjuvant chemotherapy (ACT).1 Meanwhile, most of the existing biomarkers lack robust prognostic accuracy owning to the high molecular heterogeneity of MIBC.4 Therefore, for MIBC patients, novel and efficient predictive indicators for clinical prognosis and chemotherapeutic responsiveness is in urgent need.
In addition to cytotoxic chemotherapy, the medical field has witnessed the emergence of new effective therapies for MIBC in recent years.5,6 The immune checkpoint blockade therapy targeting PD-1/PD-L1 axis and CTLA-4 has been approved of clinical practice and exhibited certain therapeutic effect,7 while only a fraction of people obtained benefits.8,9 Besides, developments were made on therapies targeting fibroblast growth factor receptor (FGFR),10 and epidermal growth factor receptor (EGFR) as well.11 Advances in molecular pathology contributes to reclassifying MIBC subgroups, which could possibly support the choice of ACT, targeted therapy and ICK blockade therapy.4,12 Further development in individualized therapy still seeks for new predictive biomarkers.
CD8+ T cells are normally believed to take on a major antitumor role. Studies has reported the association between intratumoral CD8+ T cell abundance and the therapeutic effect of ACT and certain immune therapies.13 However, it has been revealed that intratumoral CD8+ T cells are a highly heterogeneous population awaiting further exploration.14 CXCR5+CD8+ T cell, also known as TFC cell, is a distinct subset of CD8+ T cells identified to present high effector functions in infectious disease such as chronic lymphocytic choriomeningitis virus (LCMV) infection.15,16 Remarkably, CXCR5+CD8+ T cells are reported to display a rather important role in immune response against tumor after further investigation.17 In cases of B cell lymphoma, a trend of CXCR5+CD8+ T cells directly targeting tumor cells was observed.18 In HBV-related liver cancer, CXCR5+CD8+ T cells are likely to be initially induced by viral responses, and then act on tumor cells.19 As it is demonstrated in previous studies, CXCR5+CD8+ T cell frequency is positively correlated with the overall survival of patients with various malignancies, including the malignancies mentioned above along with colorectal,20 pancreas,21 lung22 and thyroid23 cancer. Intriguingly, a study revealed that CXCR5+CD8+ T cells provide a proliferation burst after PD-1 blockade in LCMV infection, suggesting its sensitivity to anti-PD-1 therapy.24 Herein, we intend to further explore the potential therapeutic effect and prognostic value of targeting CXCR5+CD8+ T cells in bladder cancer.
In this research, we identified and validated the prognostic value of CXCR5+CD8+ T cell and its predictive value for responsiveness to adjuvant chemotherapy (ACT) in MIBC patients within three different cohorts, covering 662 MIBC patients. We explored and uncovered the association among CXCR5+CD8+ T signature score, MIBC molecular subtypes and therapy-related signature in The Cancer Genome Atlas (TCGA). Our results highlighted the clinical significance of CXCR5+CD8+ T cells as well as indicated its potential predictive ability for individualized treatment for MIBC.
Methods and materials
Study population
This study began with 393 bladder cancer patients who underwent radical cystectomy from 2002 to 2014 at Zhongshan Hospital of Fudan University (n = 215, ZS Cohort) and from 2008 to 2012 Fudan University Shanghai Cancer Center (n = 178, FUSCC Cohort) respectively. The whole procedure was performed with the approval of the Clinical Research Ethics Committee of Zhongshan Hospital and the Clinical Research Ethics Committee of Fudan University Shanghai Cancer Center. Formalin-fixed paraffin-embedded samples of the two cohorts were constituted by tissue microarray (TMA) technology. 74 patients from ZS Cohort and 70 patients from FUSCC Cohort were excluded based on the following exclusion criteria: (1) without information consent; (2) unavailable clinical data and follow-up information (18 excluded); (3) diagnosed as nonurothelial carcinoma or nonmuscle invasive bladder cancer (125 excluded); (4) with other therapies besides cisplatin-based ACT and RC; (5) detachment in TMA (1 excluded). As a result, ZS Cohort and FUSCC Cohort are comprised of 141 and 108 patients for analysis respectively. All data were gathered retrospectively and patients with ACT were defined as patients who received cisplatin-based ACT lasting one therapeutic cycle at least. Patients received follow-up visits every 3 months in the first year, every 6 months for 2 years and once per year afterward, which included clinical history, physical examination and laboratory measurement. All follow-up data were collected until July 2016.
Patient characteristics of The Cancer Genome Atlas (TCGA) data set were obtained from http://www.cbioportal.org at Jan 2020. A total of 403 MIBC patients were feasible for analysis with available clinical data and follow-up information. The overall survival (OS) and the disease-free survival (DFS) were defined as the time from the date of RC to the date of death and the first recurrence, or to the last visit date.
Immunohistochemistry
Immunohistochemistry staining was practiced according to the protocols described previously.25 The TMA sections were scanned with image Pro plus 6.0 under high-power magnification filed (HPF, *200 magnification). The density of targeted cells was evaluated as the mean number of cells/HPF from sxi randomized fields counted by two independent pathologists (each with three fields) who were blinded from the clinical data. The IHC antibodies were listed in Supplementary Table 1.
Flow cytometry
Fresh samples, including MIBC tumor (n = 11) and distant normal bladder tissue (n = 8), were collected from five different clinical centers (Zhongshan Hospital of Fudan University, Fudan University Shanghai Cancer Center, Ruijin Hospital, Shanghai General Hospital and Shanghai Ninth People’s Hospital). All patients signed the informed consent.
Fresh resected tumor and peritumor tissues were digested into single-cell suspension with collagenase V (Sigma) for 2 hours at 37°C. Then, samples of approximately 1 × 106 cells were centrifuged and incubated in RBC lysis buffer on ice for 5 minutes. After incubated with Human Fc receptor blocking reagents (BD Pharmingen), cells were stained with membrane markers for 40 minutes at 4°C in the dark. Cells were stimulated for 5 hours with phorbol myristate acetate (50 ng/ml) and ionomycin (1 µg/ml) in the presence of GolgiStop Protein Transport Inhibitor (1:1000) for intracellular cytokine measurement. Cells were fixed and permeabilized with Fixation/Permeabilization Solution Kit (BD Biosciences) for 30 minutes at 4°C before intracellular staining. Stained cells were then analyzed with a FACSCelesta flow cytometer (BD Biosciences), and data were analyzed via FlowJo software V10 (TreeStar). All flow cytometry antibodies were listed in Supplementary Table 2.
Bioinformatics analysis
In the TCGA Cohort, the CXCR5+CD8+ T signature score was comprised of the mean value of the mRNA expression of CD8A, CXCR5, CXCR3, ICOS, CD27, IL21, TNF, TNFRSF6B, PDCD1, TBX21, SLAMF6, and IL27RA.17,26 To control the range of the CXCR5+CD8+ T signature score, the mRNA expression was pre-calculated with log2 (x + 1). The gene constitution of multiple signatures was provided in Supplementary Table 3.
The cutoff values of CXCR5+CD8+ T cells for the ZS Cohort and TCGA Cohort were determined automatically by the X-Tile Version 3.4.7 (offered by Robert L Camp, M.D., PhD, Yale University, http://tissuearray.org). The cutoff value of CXCR5+CD8+ T cells for the FUSCC Cohort was determined by the one of ZS Cohort for validation. The cutoff values of CXCR5+CD8+ T cell infiltration in ZS Cohort and FUSCC Cohort were both 15 cells/HPF and the cutoff value of CXCR5+CD8+ T signature score in the TCGA cohort was 0.120.
Statistical analysis
Survival outcomes including overall survival and disease-free survival were analyzed with Kaplan-Meier curves, log-rank test and univariate/multivariate Cox regression analysis. Data of immunohistochemistry evaluation and flow cytometry was analyzed by Pearson correlation test and Student t test. Kruskal-Wallis H test and Mann-Whiney U test were applied for bioinformatics analysis. Two-sided P < .05 was considered statistically significant. IBM SPSS Statistics v25.0 for windows (SPSS Inc., Chicago, IL), Medcalc v12.7.0, and GraphPad Prism v5.01 were used for statistical analysis.
Results
Intratumoral CXCR5+CD8+ T cell infiltration adversely correlates with tumor stage in MIBC patients
To determine the presence of CXCR5+CD8+ T cells in the tumor environment, we performed dual immunohistochemistry staining and flow cytometry. (Figure 1(a,b) and Supplementary Figure 2). The clinical-pathologic characteristics of patients were presented in Supplementary Tables 4,5, which presented a positive association between AJCC stage and CXCR5+CD8+ T cell infiltration. We observed significantly declining CXCR5+CD8+ T cell infiltration within the advanced AJCC stage in MIBC (Figure 1(c)). Correspondingly, a strong correlation was observed between tumors with high/low CXCR5+CD8+ T cell groups and AJCC stages (Figure 1(d) and Supplementary Table 5). Also, there was a higher infiltration of CXCR5+CD8+ T cells inside tumor tissue compared to the peritumoral tissue (Figure 1(e)). These data suggested that CXCR5+CD8+ T cells were presented in MIBC and adversely correlated with disease progression in MIBC patients.
Figure 1.
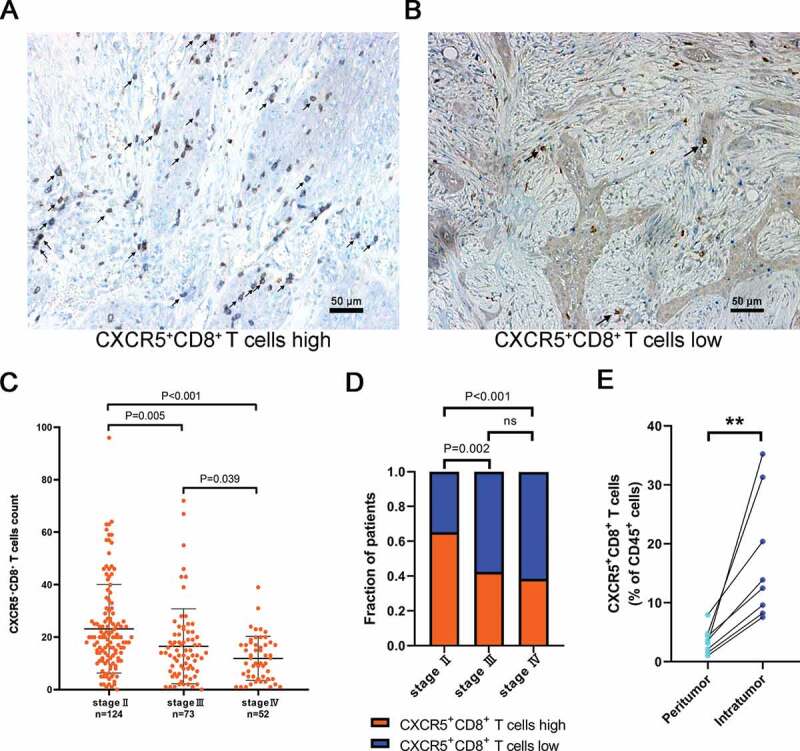
Intratumoral CXCR5+CD8+ T cell infiltration correlates with tumor stage.
Representative IHC images of high (a) and low (b) CXCR5+CD8+ T cells infiltrated in MIBC with black arrowheads pointing the target cells. (c) Comparison of intratumoral CXCR5+CD8+ T cell count according to different AJCC stages. The data were based on Supplementary Table 5. (d) Distribution of tumors with CXCR5+CD8+ T cells high/low subgroups according to different AJCC stages. The data were based on Supplementary Table 5. ns refers to no statistical significance. (e) Different infiltration levels of CXCR5+CD8+ T cells in tumor tissue and peritumoral tissue. *P < .05, **P < .01.
Intratumoral CXCR5+CD8+ T cells abundance indicates superior clinical outcomes in MIBC patients
To clarify the relevance of CXCR5+CD8+ T cells and clinical outcomes, we conducted Kaplan-Meier curves and log-rank test in three different cohorts. In ZS Cohort (n = 141), high infiltration of CXCR5+CD8+ T cells was associated with significantly superior OS and DFS (OS: P < .0001, DFS: P = .0096; Figure 2(a,b)). Prolonged OS and DFS in high CXCR5+CD8+ T cells group were also observed in FUSCC Cohort (n = 108, OS: P = .0031, DFS: P = .0005; Figure 2(c,d)) and in TCGA Cohort (OS: n = 403, P = .0137; Figure 2(e)) than the ones in low CXCR5+CD8+ T cells group. However, the improvement of DFS in high vs. low CXCR5+CD8+ T cells group was on the boundary of significance in TCGA cohort (n = 184, P = .0582; figure 2(f)). Univariate and multivariate Cox regression analysis revealed CXCR5+CD8+ T cells possessed strong prognostic potential in both ZS cohort and FUSCC cohort (Supplementary Tables 6,7). Furthermore, the comparison of prognostic value further elucidated that CXCR5+CD8+ T cells could act as a more powerful prognosticator than CD8+ T cells in all MIBC or different AJCC stage subgroups (Table 1). Taken together, intratumoral CXCR5+CD8+ T cells itself could serve as an independent and significant prognosticator whose enrichment indicates superior clinical outcomes in MIBC patients.
Figure 2.
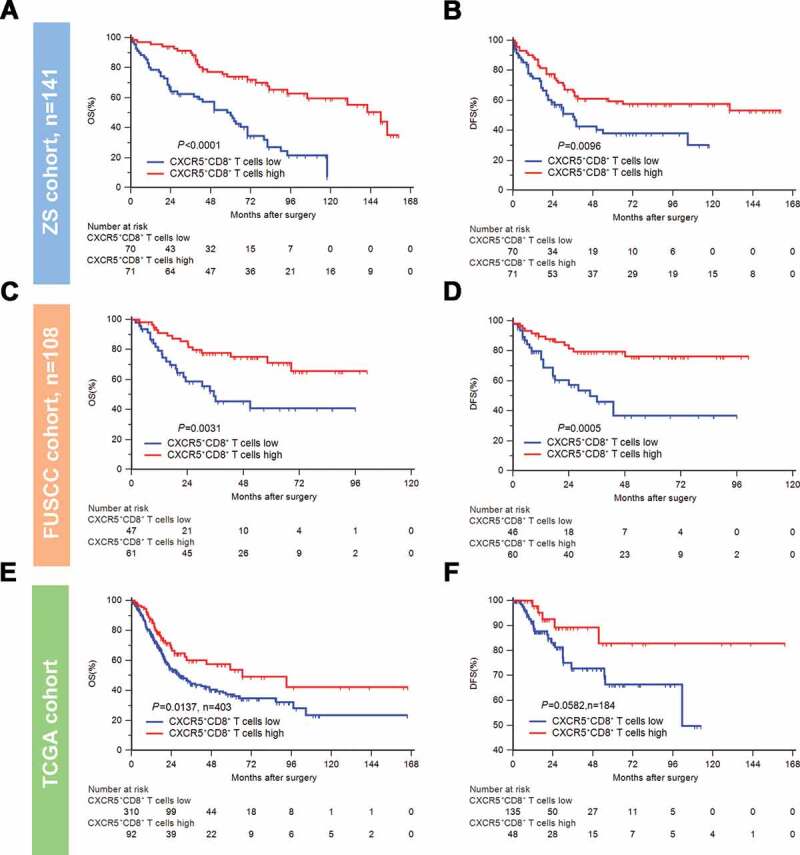
Intratumoral CXCR5+CD8+ T cell enrichment indicates superior clinical outcomes.
Kaplan-Meier curves of OS (left) and DFS (right) for CXCR5+CD8+ T cell infiltration in the ZS Cohort (A-B, n = 141), FUSCC Cohort (C-D, n = 108), and TCGA Cohort (E-F, n = 403, n = 184). Log-rank P values were shown.
Table 1.
CXCR5+CD8+ T cells act as a better prognosticator than CD8+ T cells in MIBC patients.
| Factor | Patients | HR (95% CI) | P | Pinteraction |
|---|---|---|---|---|
| Overall survival | ||||
| All patients | CD8+ T cells (high vs low) | 0.550 (0.377–0.803) | 0.002 | <0.001 |
| CXCR5+CD8+ T cells (high vs low) | 0.279 (0.186–0.420) | <0.001 | ||
| Stage II | CD8+ T cells (high vs low) | 0.775 (0.431–1.395) | 0.396 | 0.008 |
| CXCR5+CD8+ T cells (high vs low) | 0.340 (0.186–0.623) | <0.001 | ||
| Stage III+IV | CD8+ T cells (high vs low) | 0.539 (0.327–0.888) | 0.015 | 0.001 |
| CXCR5+CD8+ T cells (high vs low) | 0.286 (0.161–0.508) | <0.001 | ||
| Disease-free survival | ||||
| All patients | CD8+ T cells (high vs low) | 0.757 (0.513–1.116) | 0.160 | 0.004 |
| CXCR5+CD8+ T cells (high vs low) | 0.431 (0.289–0.644) | <0.001 | ||
| Stage II | CD8+ T cells (high vs low) | 0.818 (0.434–1.543) | 0.536 | 0.044 |
| CXCR5+CD8+ T cells (high vs low) | 0.383 (0.203–0.723) | 0.003 | ||
| Stage III+IV | CD8+ T cells (high vs low) | 0.811 (0.493–1.334) | 0.410 | 0.241 |
| CXCR5+CD8+ T cells (high vs low) | 0.575 (0.338–0.976) | 0.041 | ||
Abbreviations: HR, hazard ratio; CI, confidence interval;
P value < .05 marked in bold font shows statistical significant.
Intratumoral CXCR5+CD8+ T cells abundance yields optimal ACT responsive-ness in MIBC patients
To evaluate the benefit of cisplatin-based adjuvant chemotherapy according to different infiltration levels of CXCR5+CD8+ T cells, we compared the prognosis of patients stratified by ACT application in different CXCR5+CD8+ T cell infiltration subgroups. In the combined cohort, the infiltration level of CXCR5+CD8+ T cells was not associated with OS (P = .2713; Figure 3(a)) and DFS (P = .5192; Figure 3(c)) in patients without ACT application (n = 136). However, the infiltration level of CXCR5+CD8+ T cells was significantly associated with superior OS (P < .0001; Figure 3(b)) and DFS (P < .0001; Figure 3(d)) in patients with ACT application (n = 113). Similarly, in TCGA cohort, the infiltration level of CXCR5+CD8+ T cells was not associated with OS (P = .2713; Figure 3(e)) in patients without ACT application (n = 179), while the infiltration level of CXCR5+CD8+ T cells was significantly associated with improved OS (P = .0179; figure 3(f)) in patients with ACT application (n = 76). These results indicated that the cisplatin-based chemotherapy was potentially associated with the activation of the antitumor CXCR5+CD8+ T cells. Moreover, CXCR5+CD8+ T cell enrichment could predict an optimal ACT responsiveness in MIBC.
Figure 3.
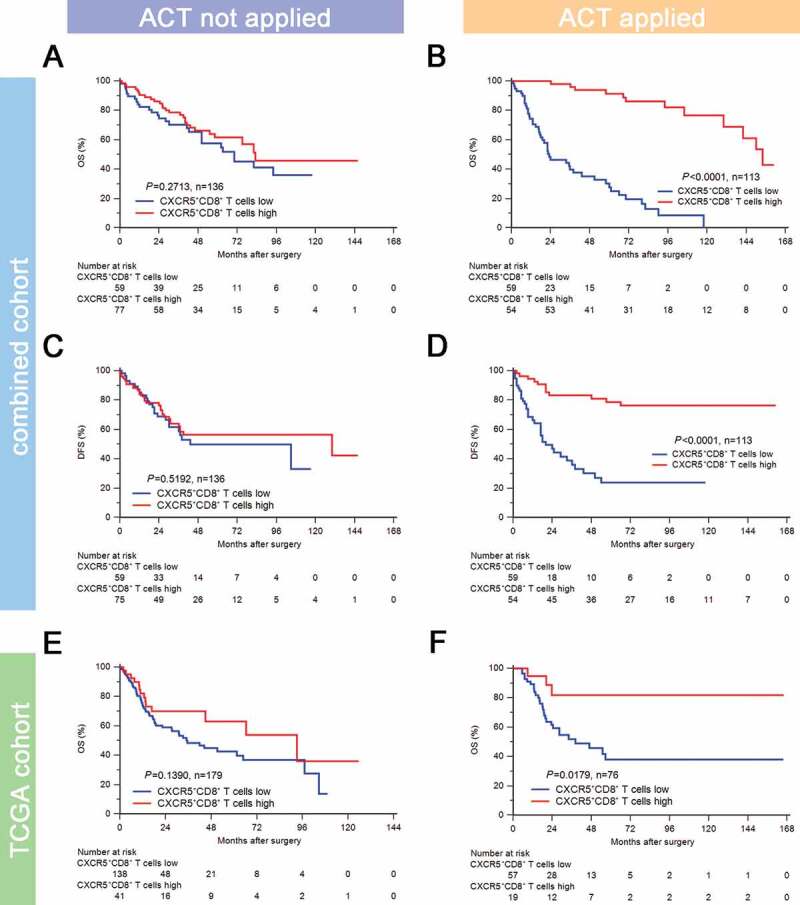
Intratumoral CXCR5+CD8+ T cell abundance yields optimal ACT responsiveness.
Kaplan-Meier curves for OS in ACT applied (a) and ACT not applied (b) subgroups of combined cohort according to CXCR5+CD8+ T cell infiltration strata. Kaplan-Meier curves for DFS in ACT applied (c) and ACT not applied (d) subgroups of combined cohort according to CXCR5+CD8+ T cell infiltration strata. Kaplan-Meier curves for OS in ACT applied (e) and ACT not applied (f) subgroups of TCGA cohort according to CXCR5+CD8+ T signature score strata. Log-rank test was performed for Kaplan-Meier curves.
Intratumoral CXCR5+CD8+ Tcells display tumor suppression characteristics
Since the abundance of intratumoral CXCR5+CD8+ T cells was associated with superior prognosis and better ACT responsiveness, we attempted to explore the principle beneath what was found. The study started with a set of IHC staining. A positive correlation between the expression of GZMB, IFN-γ, PRF1 in the tumor environment and CXCR5+CD8+ T cells infiltration was observed (Supplementary Figure 1(a)). We also noticed that the expression of immune checkpoints CTLA-4, LAG-3 and TIGIT was elevated in the tumor environment in high CXCR5+CD8+ T cells group compared with the one in low CXCR5+CD8+ T cells group (Supplementary Figure 1(b)). The phenotype of CXCR5+CD8+ T cells was next investigated through flow cytometry analysis of fresh MIBC surgical specimens. Notably, intratumoral CXCR5+CD8+ T cells expressed elevated PD-1, LAG-3, CTLA-4 and reduced TIM-3 compared with their CXCR5−CD8+ T cells counterparts (Figure 4(a)). A higher level of IFN-γ, GZMB, TNFα, CD107a and IL-17A was presented in CXCR5+CD8+ T cells (Figure 4(b,c)), which suggests CXCR5+CD8+ T cell possess robust cytotoxicity activity.27,28 Interestingly, while comparable Ki-67 was expressed in the two subsets, CXCR5+CD8+ T cells had higher expression of Tcf-1 and CD62L, indicating the self-renewal and effector memory characteristics as previously reported26 (Figure 4(d)).
Figure 4.
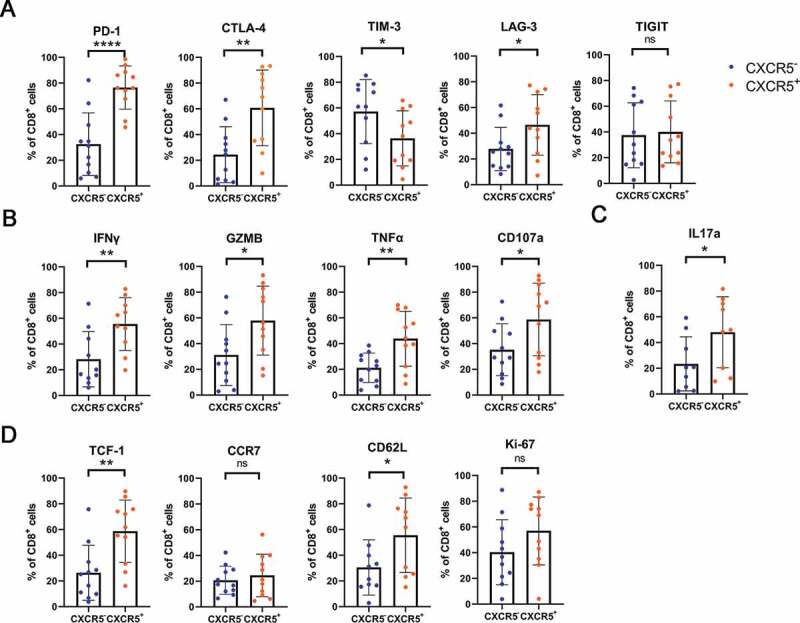
Phenotyping intratumoral CXCR5+CD8+ T cells in MIBC patients.
(a) Comparison of immune checkpoints (PD-1, CTLA-4, TIM-3, LAG-3 and TIGIT) expression on CXCR5+CD8+ T cell vs. CXCR5− CD8+ T cell in MIBC. (b) Comparison of effector cytokines (IFN-γ, GZMB, TNF-α and CD107a) expression on CXCR5+CD8+ T cell vs. CXCR5− CD8+ T cell in MIBC. (c) Comparison of IL-17A expression on CXCR5+CD8+ T cell vs. CXCR5− CD8+ T cell in MIBC. (d) Comparison of TCF-1, CCR7, CD62L and Ki-67 expression on CXCR5+CD8+ T cell vs. CXCR5− CD8+ T cell in MIBC. Data was analyzed by Pearson correlation test and Student t test. *P < .05, **P < .01, ***P< .001, ****P < .0001.
Association of CXCR5+CD8+ T cells with molecular subtypes in MIBC patients
To study the association of CXCR5+CD8+ T cells with molecular subtypes in MIBC patients, we used mRNA expression of several molecules from TCGA to constitute the CXCR5+CD8+ T signature score (Figure 5(a)). CXCR5+CD8+ T signature score was abundant in the Ba/Sq subtype of the consensus classification and both basal subtypes in UNC ad MDA classification (Figure 5(b–d), P < .0001). Interestingly, in the consensus classification, CXCR5+CD8+ T cells signature score was high in both basal/squamous subtype and stroma-rich subtype. Furthermore, tumors with high CXCR5+CD8+ T signature score were featured with high basal signature score (P < .0001; Figure 5(e)) and p53-like signature score (P < .05; Figure 5(e)) and low luminal signature score (P < .0001; Figure 5(e)). Also, proliferation score was superior in tumors with high CXCR5+CD8+ T signature score (P < .05; figure 5(f)). Additionally, tumors with high density of CXCR5+CD8+ T cells were strongly enriched with EGFR signature, antigen-presenting and immune checkpoints signature (P < .0001, P < .0001, P < .0001 and P < .0001, respectively; Figure 5(g)), indicating the value of applying EGFR-targeted drugs and immunotherapy in tumors with high CXCR5+CD8+ T cells. Collectively, our results revealed that CXCR5+CD8+ T signature accumulation was associated with basal and stroma-rich subtypes of MIBC, and CXCR5+CD8+ T cells density could possibly be an independent indicator for individualized targeted therapies.
Figure 5.
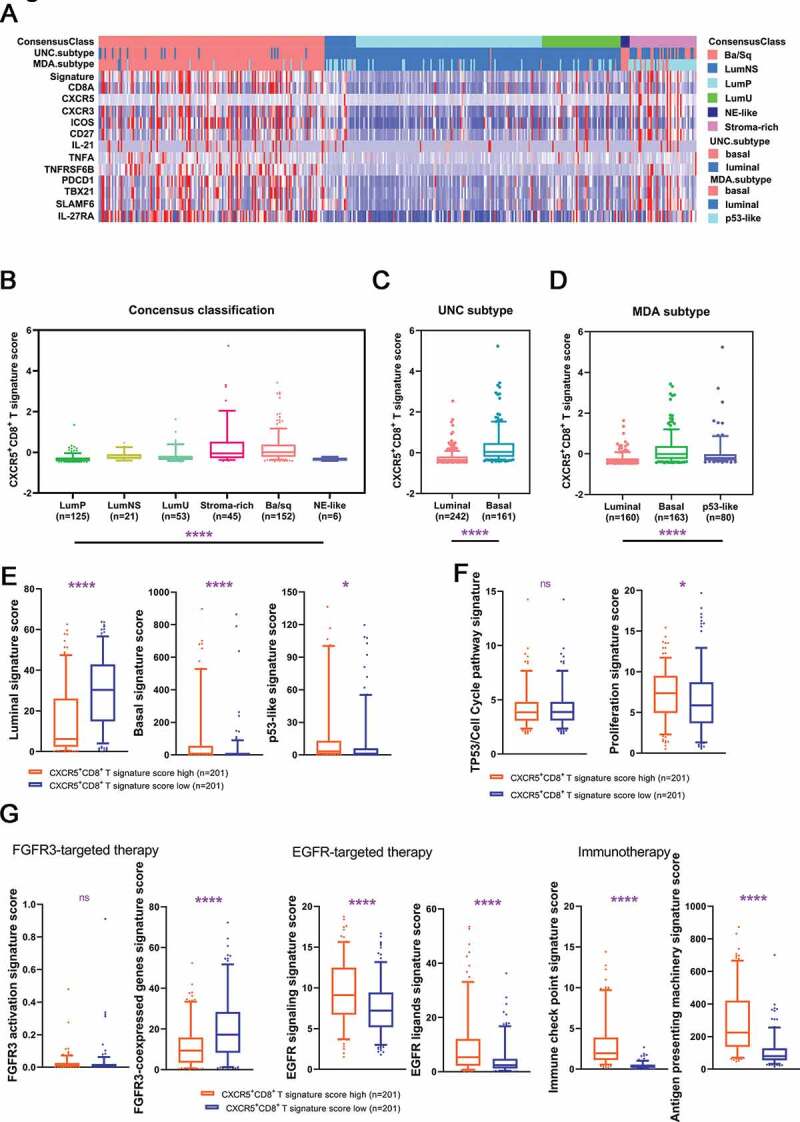
Characteristics of CXCR5+CD8+ T cells across MIBC molecular classifications.
(a) The overall constitution of CXCR5+CD8+ T signature score and the corresponding molecular classification. (b–d) Quantification analyses of CXCR5+CD8+ T signature score in TCGA database across molecular classification systems. (e) Quantification analyses of molecular subtype signatures between low/high CXCR5+CD8+ T signature score subgroup. Quantification analyses of cell cycle-associated (f) and therapy-associated (g) gene signatures between low/high CXCR5+CD8+ T signature score subgroup. Data was analyzed by Kruskal-Wallis H test and Mann-Whiney U test. *P < .05, **P < .01, ***P < .001, ****P < .0001.
Discussion
In this study, we firstly demonstrated that high density of intratumoral CXCR5+CD8+ T cells was associated with improved OS and DFS by analyzing three different patient cohorts, covering 662 MIBC patients. Researches showed a majority of CD8+ T cells act as bystanders in human tumor infiltrates, and these bystander CD8+ T cells were a highly heterogeneous population.14 Although intratumoral CD8+ T cells were regarded as a protective factor against tumor,29 studies also showed an association of intratumoral CD8+ T cells and poor clinical outcomes.30 Based on the studies, it is reasonable to assume that the highly heterogeneous population of intratumoral CD8+ T cells could be further divided into different subtypes with distinct phenotypes, even antagonistic to each other. Intriguingly, we found CXCR5+CD8+ T cells could serve as a more powerful prognosticator than CD8+ T cells in MIBC, which was in line with its impressive anti-tumor function as discovered in other tumor types.18–23 We have previously reported several immune cells infiltration associated with ACT benefit in MIBC, such as tumor-infiltrating neutrophils, mast cells, B cells, and IL-22+ cell.25,31-34 Remarkably, we revealed the outstanding predictive ability of CXCR5+CD8+ T cells in response to ACT in MIBC. It is reported that CD8+ T cell-related pathways are involved in mediating response to chemotherapy35 leading to tumor suppression. Our result unveiled that CXCR5+CD8+ T cell might be a crucial CD8+ T cell subtype responding to cisplatin-based ACT, of which the intrinsic mechanism worth further exploration.
Corresponding to the previous studies in circumstances of viral infection and tumor infiltration, we confirm the self-renewal and effector memory-like phenotype of CXCR5+CD8+ T cells in MIBC.17,26 Intriguingly, we discovered that CXCR5+CD8+ T cells expressed activated TCF1 (transcripted by TCF7) compared to its CXCR5−CD8+ T cells counterpart. Meanwhile, it is reported that TCF7+ identified a subset of superior survival-indicating CD8+ T cells in melanoma,36 which is consistent with our results. Moreover, our results showed an enriched production of both immune checkpoints especially PD1 and effector cytokines in CXCR5+CD8+ T cells. Recent studies identified a progenitor exhausted subset CD8+ T cells featured with high level of CXCR5 and PD1 expression and low level of TIM3 expression,37 which is in line with our results and implied CXCR5+CD8+ T cells in MIBC also possessed a progenitor exhausted-like characterization. Progenitor exhausted CD8+ T cells could mediate long-term tumor eradication and show response to PD-1.37 It was also reported that CXCR5+CD8+ T cells provided proliferation burst of CD8+ T cells after PD-1/PD-L1 blockade therapies and contributed to therapeutic benefits in viral infection.24 Together with our phenotype analysis, we suspected that CXCR5+CD8+ T cells could exert a durable anti-tumor function and would play an essential part responding to PD-1/PD-L1 blockade in MIBC.
Increasing numbers of studies shed light on the importance of molecular features in MIBC that it could provide new opportunities for prognostic application, disease monitoring and personalized therapy.38 We discovered that tumors with high density of CXCR5+CD8+ T cells presented basal subtype, and these high CXCR5+CD8+ T cell density tumors were endowed with potential EGFR-targeted sensitivity and immunotherapeutic indication. Meanwhile, low CXCR5+CD8+ T cell density tumors appear luminal subtype with possible FGFR3-targeted potency. The relation between CXCR5+CD8+ T cell density and molecular subtype created new possibilities for individualized therapy, indicating that intratumoral CXCR5+CD8+ T cell density could be taken as a biomarker for clinical utilization of EGFR-targeted therapy as well as immunotherapy. However, further investigation was needed on a practical level for these finding.
Conclusion
In this study, we identified an excellent prognosis and ACT sensitive subtype of MIBC with intratumoral CXCR5+CD8+ T cell abundance, highlighting its clinical significance. CXCR5+CD8+ T cells correlated with long-term tumor eradication capacity. Furthermore, our results implied that tumors with CXCR5+CD8+ T cell abundance possessed potential sensitivity to immunotherapy and EGFR-targeted therapy while resistance to FGFR3-targeted therapy. Future studies could put more concentration on this compelling CD8+ T cell subtype to discover its predictive role and intrinsic relationship with certain therapeutic approach.
Supplementary Material
Acknowledgments
We thank Dr. Lingli Chen (Department of Pathology, Zhongshan Hospital, Fudan University, Shanghai, China) and Dr. Yunyi Kong (Department of Pathology, Fudan University Shanghai Cancer Center, Shanghai, China) for their excellent pathological technology help.
Funding Statement
This work was supported by grants from National Natural Science Foundation of China [81671628, 81702496, 81702497, 81702805, 81772696, 81871306, 81872082, 81902556, 81902563, 81902898, 81974393], National Key R&D Program of China [2017YFC0114303], Shanghai Municipal Natural Science Foundation [16ZR1406500, 17ZR1405100, 19ZR1431800], Guide Project of Science and Technology Commission of Shanghai Municipality [17411963100], Shanghai Sailing Program [18YF1404500, 19YF1407900, 19YF1427200, 20YF1406100, 20YF1406200], Shanghai Municipal Commission of Health and Family Planning Program [20174Y0042, 201840168, 20184Y0151], Fudan University Shanghai Cancer Center for Outstanding Youth Scholars Foundation [YJYQ201802] and Shanghai Cancer Research Charity Center. All these study sponsors have no roles in the study design, in the collection, analysis and interpretation of data.
Author contributions
Q. Huang, Q. Zhou, H. Zhang, Z. Liu and H. Zeng for acquisition of data, analysis and interpretation of data, statistical analysis, and drafting of the manuscript; Y. Chen, Y. Qu, Y. Xiong, J. Wang, Y. Chang, Y. Xia, Y. Wang, L. Liu, Y. Zhu, L. Xu, B. Dai, and J. Guo for technical and material support; Z. Wang, Q. Bai, and W. Zhang for study concept and design, analysis and interpretation of data, drafting of the manuscript, obtained funding, and study supervision. All authors read and approved the final manuscript.
Data availability
All data generated that are relevant to the results presented in this article are included in this article. Other data that were not relevant for the results presented here are available from the corresponding author Dr. Zhang upon reasonable request.
Disclosure of potential conflicts of interest
The authors declare no conflict of interest.
Consent for publication
All authors provide their consent for publication of the manuscript.
Ethics approval and consent to participate
Written informed consent was obtained from each patient included. The study was conducted and performed after approval by the Clinical Research Ethics Committee of Zhongshan Hospital, the Ethics Committee of Fudan University Shanghai Cancer Center, the Ethics Committee of Ruijin Hospital, the Ethics Committee of Shanghai General Hospital and the Ethics Committee of Shanghai Ninth People’s Hospital.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
References
- 1.Antoni S, Ferlay J, Soerjomataram I, Znaor A, Jemal A, Bray F.. Bladder cancer incidence and mortality: a global overview and recent trends. Eur Urol. 2017;71(1):96–8. doi: 10.1016/j.eururo.2016.06.010. [DOI] [PubMed] [Google Scholar]
- 2.Witjes JA, Comperat E, Cowan NC, De Santis M, Gakis G, Lebret T, Ribal MJ, Van der Heijden AG, Sherif A. EAU guidelines on muscle-invasive and metastatic bladder cancer: summary of the 2013 guidelines. Eur Urol. 2014;65(4):778–792. doi: 10.1016/j.eururo.2013.11.046. [DOI] [PubMed] [Google Scholar]
- 3.Alfred Witjes J, Lebret T, Comperat EM, Cowan NC, De Santis M, Bruins HM, Hernández V, Espinós EL, Dunn J, Rouanne M, et al. Updated 2016 EAU guidelines on muscle-invasive and metastatic bladder cancer. Eur Urol. 2017;71(3):462–475. doi: 10.1016/j.eururo.2016.06.020. [DOI] [PubMed] [Google Scholar]
- 4.Kamoun A, de Reynies A, Allory Y, Sjodahl G, Robertson AG, Seiler R, Hoadley KA, Groeneveld CS, Al-Ahmadie H, Choi W, Castro MA. A consensus molecular classification of muscle-invasive bladder cancer. Eur Urol. 2020;77(4):420–433. doi:10.1016/j.eururo.2019.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alifrangis C, McGovern U, Freeman A, Powles T, Linch M. Molecular and histopathology directed therapy for advanced bladder cancer. Nat Rev Urol. 2019;16(8):465–483. doi: 10.1038/s41585-019-0208-0. [DOI] [PubMed] [Google Scholar]
- 6.Felsenstein KM, Theodorescu D. Precision medicine for urothelial bladder cancer: update on tumour genomics and immunotherapy. Nat Rev Urol. 2018;15(2):92–111. doi: 10.1038/nrurol.2017.179. [DOI] [PubMed] [Google Scholar]
- 7.Carosella ED, Ploussard G, LeMaoult J, Desgrandchamps F. A systematic review of immunotherapy in urologic cancer: evolving roles for targeting of CTLA-4, PD-1/PD-L1, and HLA-G. Eur Urol. 2015;68(2):267–279. doi: 10.1016/j.eururo.2015.02.032. [DOI] [PubMed] [Google Scholar]
- 8.Sharma P, Retz M, Siefker-Radtke A, Baron A, Necchi A, Bedke J, Plimack ER, Vaena D, Grimm M-O, Bracarda S, et al. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): a multicentre, single-arm, phase 2 trial. Lancet Oncol. 2017;18(3):312–322. doi: 10.1016/S1470-2045(17)30065-7. [DOI] [PubMed] [Google Scholar]
- 9.Balar AV, Galsky MD, Rosenberg JE, Powles T, Petrylak DP, Bellmunt J, Loriot Y, Necchi A, Hoffman-Censits J, Perez-Gracia JL, et al. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: a single-arm, multicentre, phase 2 trial. Lancet (London, England). 2017;389(10064):67–76. doi: 10.1016/S0140-6736(16)32455-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Loriot Y, Necchi A, Park SH, Garcia-Donas J, Huddart R, Burgess E, Fleming M, Rezazadeh A, Mellado B, Varlamov S, et al. Erdafitinib in locally advanced or metastatic urothelial carcinoma. N Engl J Med. 2019;381(4):338–348. doi: 10.1056/NEJMoa1817323. [DOI] [PubMed] [Google Scholar]
- 11.Powles T, Huddart RA, Elliott T, Sarker SJ, Ackerman C, Jones R, Hussain S, Crabb S, Jagdev S, Chester J, et al. Phase III, double-blind, randomized trial that compared maintenance lapatinib versus placebo after first-line chemotherapy in patients with human epidermal growth factor receptor 1/2-positive metastatic bladder cancer. J clin oncol. 2017;35(1):48–55. doi: 10.1200/JCO.2015.66.3468. [DOI] [PubMed] [Google Scholar]
- 12.Robertson AG, Kim J, Al-Ahmadie H, Bellmunt J, Guo G, Cherniack AD, Hinoue T, Laird PW, Hoadley KA, Akbani R, et al. Comprehensive molecular characterization of muscle-invasive bladder cancer. Cell. 2017;171(3):540–56.e25. doi: 10.1016/j.cell.2017.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vesely MD, Kershaw MH, Schreiber RD, Smyth MJ. Natural innate and adaptive immunity to cancer. Annu Rev Immunol. 2011;29:235–271. doi: 10.1146/annurev-immunol-031210-101324. [DOI] [PubMed] [Google Scholar]
- 14.Simoni Y, Becht E, Fehlings M, Loh CY, Koo SL, Teng KWW, Yeong JPS, Nahar R, Zhang T, Kared H, et al. Bystander CD8(+) T cells are abundant and phenotypically distinct in human tumour infiltrates. Nature. 2018;557(7706):575–579. doi: 10.1038/s41586-018-0130-2. [DOI] [PubMed] [Google Scholar]
- 15.He R, Hou S, Liu C, Zhang A, Bai Q, Han M, Yang Y, Wei G, Shen T, Yang X, et al. Follicular CXCR5- expressing CD8(+) T cells curtail chronic viral infection. Nature. 2016;537(7620):412–428. doi: 10.1038/nature19317. [DOI] [PubMed] [Google Scholar]
- 16.Leong YA, Chen Y, Ong HS, Wu D, Man K, Deleage C, Minnich M, Meckiff BJ, Wei Y, Hou Z, et al. CXCR5(+) follicular cytotoxic T cells control viral infection in B cell follicles. Nat Immunol. 2016;17(10):1187–1196. doi: 10.1038/ni.3543. [DOI] [PubMed] [Google Scholar]
- 17.Valentine KM, Hoyer KK. CXCR5+ CD8 T cells: protective or pathogenic? Front Immunol. 2019;10:1322. doi: 10.3389/fimmu.2019.01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chu F, Li HS, Liu X, Cao J, Ma W, Ma Y, Weng J, Zhu Z, Cheng X, Wang Z, et al. CXCR5(+)CD8(+) T cells are a distinct functional subset with an antitumor activity. Leukemia. 2019;33(11):2640–2653. doi: 10.1038/s41375-019-0464-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jin Y, Lang C, Tang J, Geng J, Song HK, Sun Z, Wang J. CXCR5(+)CD8(+) T cells could induce the death of tumor cells in HBV-related hepatocellular carcinoma. Int Immunopharmacol. 2017;53:42–48. doi: 10.1016/j.intimp.2017.10.009. [DOI] [PubMed] [Google Scholar]
- 20.Jifu E, Yan F, Kang Z, Zhu L, Xing J, Yu E. CD8(+)CXCR5(+) T cells in tumor-draining lymph nodes are highly activated and predict better prognosis in colorectal cancer. Hum Immunol. 2018;79(6):446–452. doi: 10.1016/j.humimm.2018.03.003. [DOI] [PubMed] [Google Scholar]
- 21.Bai M, Zheng Y, Liu H, Su B, Zhan Y, He H. CXCR5(+) CD8(+) T cells potently infiltrate pancreatic tumors and present high functionality. Exp Cell Res. 2017;361(1):39–45. doi: 10.1016/j.yexcr.2017.09.039. [DOI] [PubMed] [Google Scholar]
- 22.Brummelman J, Mazza EMC, Alvisi G, Colombo FS, Grilli A, Mikulak J, Mavilio D, Alloisio M, Ferrari F, Lopci E, et al. High-dimensional single cell analysis identifies stem-like cytotoxic CD8(+) T cells infiltrating human tumors. J Exp Med. 2018;215(10):2520–2535. doi: 10.1084/jem.20180684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou Y, Guo L, Sun H, Xu J, Ba T. CXCR5(+) CD8 T cells displayed higher activation potential despite high PD-1 expression, in tumor-involved lymph nodes from patients with thyroid cancer. Int Immunopharmacol. 2018;62:114–119. doi: 10.1016/j.intimp.2018.07.002. [DOI] [PubMed] [Google Scholar]
- 24.Im SJ, Hashimoto M, Gerner MY, Lee J, Kissick HT, Burger MC, Shan Q, Hale JS, Lee J, Nasti TH, et al. Defining CD8+ T cells that provide the proliferative burst after PD-1 therapy. Nature. 2016;537(7620):417–421. doi: 10.1038/nature19330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hu B, Wang Z, Zeng H, Qi Y, Chen Y, Wang T, Wang J, Chang Y, Bai Q, Xia Y, et al. Blockade of DC-SIGN+ tumor-associated macrophages reactivates anti-tumor immunity and improves immunotherapy in muscle-invasive bladder cancer. Cancer Res. 2020. doi: 10.1158/0008-5472.CAN-19-2254. [DOI] [PubMed] [Google Scholar]
- 26.Yu D, Ye L. A portrait of CXCR5(+) follicular cytotoxic CD8(+) T cells. Trends Immunol. 2018;39(12):965–979. doi: 10.1016/j.it.2018.10.002. [DOI] [PubMed] [Google Scholar]
- 27.Wherry EJ, Kurachi M. Molecular and cellular insights into T cell exhaustion. Nat Rev Immunol. 2015;15(8):486–499. doi: 10.1038/nri3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pauken KE, Wherry EJ. Overcoming T cell exhaustion in infection and cancer. Trends Immunol. 2015;36(4):265–276. doi: 10.1016/j.it.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Glaser AP, Fantini D, Shilatifard A, Schaeffer EM, Meeks JJ. The evolving genomic landscape of urothelial carcinoma. Nat Rev Urol. 2017;14(4):215–229. doi: 10.1038/nrurol.2017.11. [DOI] [PubMed] [Google Scholar]
- 30.Thompson ED, Zahurak M, Murphy A, Cornish T, Cuka N, Abdelfatah E, Yang S, Duncan M, Ahuja N, Taube JM, et al. Patterns of PD-L1 expression and CD8 T cell infiltration in gastric adenocarcinomas and associated immune stroma. Gut. 2017;66(5):794–801. doi: 10.1136/gutjnl-2015-310839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou L, Xu L, Chen L, Fu Q, Liu Z, Chang Y, Lin Z, Xu J. Tumor-infiltrating neutrophils predict benefit from adjuvant chemotherapy in patients with muscle invasive bladder cancer. Oncoimmunology. 2017;6(4):e1293211. doi: 10.1080/2162402X.2017.1293211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu Z, Zhu Y, Xu L, Zhang J, Xie H, Fu H, Zhou Q, Chang Y, Dai B, Xu J, et al. Tumor stroma-infiltrating mast cells predict prognosis and adjuvant chemotherapeutic benefits in patients with muscle invasive bladder cancer. Oncoimmunology. 2018;7(9):e1474317. doi: 10.1080/2162402X.2018.1474317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jiang Q, Fu Q, Chang Y, Liu Z, Zhang J, Xu L, Zhu Y, Wang Y, Zhang W, Xu J, et al. CD19+ tumor-infiltrating B-cells prime CD4+ T-cell immunity and predict platinum-based chemotherapy efficacy in muscle-invasive bladder cancer. Cancer Immunol Immun. 2019;68(1):45–56. doi: 10.1007/s00262-018-2250-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fu H, Zhu Y, Wang Y, Liu Z, Zhang J, Xie H, Fu Q, Dai B, Ye D, Xu J, et al. Identification and validation of stromal immunotype predict survival and benefit from adjuvant chemotherapy in patients with muscle-invasive bladder cancer. Clin Cancer Res. 2018;24(13):3069–3078. doi: 10.1158/1078-0432.CCR-17-2687. [DOI] [PubMed] [Google Scholar]
- 35.Ruffell B, Chang-Strachan D, Chan V, Rosenbusch A, Ho CM, Pryer N, Daniel D, Hwang E, Rugo H, Coussens L, et al. Macrophage IL-10 blocks CD8+ T cell-dependent responses to chemotherapy by suppressing IL-12 expression in intratumoral dendritic cells. Cancer Cell. 2014;26(5):623–637. doi: 10.1016/j.ccell.2014.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sade-Feldman M, Yizhak K, Bjorgaard SL, Ray JP, de Boer CG, Jenkins RW, Lieb DJ, Chen JH, Frederick DT, Barzily-Rokni M, et al. Defining T cell states associated with response to checkpoint immunotherapy in melanoma. Cell. 2018;175(4):998–1013.e20. doi: 10.1016/j.cell.2018.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miller BC, Sen DR, Al Abosy R, Bi K, Virkud YV, LaFleur MW, Yates KB, Lako A, Felt K, Naik GS, et al. Subsets of exhausted CD8+ T cells differentially mediate tumor control and respond to checkpoint blockade. Nat Immunol. 2019;20(3):326–336. doi: 10.1038/s41590-019-0312-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Knowles MA, Hurst CD. Molecular biology of bladder cancer: new insights into pathogenesis and clinical diversity. Nat Rev Cancer. 2015;15(1):25–41. doi: 10.1038/nrc3817. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated that are relevant to the results presented in this article are included in this article. Other data that were not relevant for the results presented here are available from the corresponding author Dr. Zhang upon reasonable request.


